Abstract
Temperature is an important modulator of longevity and aging in both poikilotherms and homeotherm animals. In homeotherms, temperature homeostasis is regulated primarily in the preoptic area (POA) of the hypothalamus. This region receives and integrates peripheral, central and environmental signals and maintains a nearly constant core body temperature (Tcore) by regulating the autonomic and hormonal control of heat production and heat dissipation. Temperature sensitive neurons found in the POA are considered key elements of the neuronal circuitry modulating these effects. Nutrient homeostasis is also a hypothalamically regulated modulator of aging as well as one of the signals that can influence Tcore in homeotherms. Investigating the mechanisms of the regulation of nutrient and temperature homeostasis in the hypothalamus is important to understand how these two elements of energy homeostasis influence longevity and aging as well as how aging can affect hypothalamic homeostatic mechanisms.
Keywords: hypothalamus, thermoregulation, aging, preoptic neurons, calorie restriction
Introduction
Energy homeostasis has emerged as an important regulator of longevity and aging. Reduction of calorie intake, also known as calorie restriction (CR), has been known for over 70 years to prolong lifespan and retard aging. Its action appears to be “universal” as CR was effective in all organisms and species it was tested on, from yeast to primates (Bishop et al. 2007; Mair et al. 2008). The most recent identification of genes and pathways that can prolong lifespan also participate in energy homeostasis, primarily in the response to the amount of nutrients or energy available (Bishop and Guarente 2007; Mair and Dillin 2008).
In addition to CR, core body temperature (Tcore) was also demonstrated to influence longevity and aging. Temperature can be considered as one component of energy homeostasis but investigating its effects and mechanisms of action on aging across species proved more challenging than for CR. Animals can be distinguished into two categories based on the ability to regulate their temperature. Homeotherms are generally referred to as warm-blooded animals and maintain their Tcore at nearly constant values at different ambient temperatures. This review covers homeotherm research in mouse and humans. By contrast, poikilotherms lack this ability and their Tcore fluctuates with that of the environment; they are often referred to as cold-blooded animals. The two “model” organisms most commonly utilized in the study of aging, the worm Caenorabditis elegans and the fruit fly Drosophyla melanogaster, are poikilotherms. Modest temperature reduction prolonged lifespan in both poikilotherms and homeotherms (Liu et al. 1966). While testing the effects of temperature on aging in poikilotherms was achieved simply by maintaining these organisms at a specific ambient temperature, this experimental paradigm was not possible in homeotherms as they maintain a constant Tcore when exposed to different ambient external temperatures. To date only one experimental model of a transgenic mouse with reduced Tcore has demonstrated that a lower Tcore prolongs lifespan in homeotherms (Conti et al. 2006). Interestingly, comparative analysis of the mortality curves suggested that temperature may affect longevity by different mechanisms in homeotherms than in and poikilotherms. Specifically, it was proposed that temperature reduction may influence aging primarily by thermodynamic effects in poikilotherms and by mechanisms similar to that mediating the effects of CR in homeotherms. When considering this possibility it should be emphasized that temperature homeostasis has profound implications on energy homeostasis. Temperature homeostasis is in fact possible in homeotherms due to their ability to regulate endogenous heat production (metabolic rate) and heat dissipation. These processes, of key importance for determining Tcore, are controlled centrally, primarily by the hypothalamus.
Located in the rostral part of the brain stem in the diencephalon, the hypothalamus is the major regulator of homeostatic and vital functions including reproduction, metabolism, osmoregulation, growth, stress response and circadian rhythms. These functions are mediated primarily via the hypothalamic control of the pituitary gland and subsequent regulation of endocrine responses. The hypothalamus also functions as an integrator of inputs from the sensory and autonomic systems conveying information from the organism as well as from the environment. Thus, different signals can influence each other and ultimately the hypothalamic regulatory responses. This is particularly relevant when considering the aforementioned interaction between nutrient and temperature homeostasis as these components of energy homeostasis can influence each other and both can influence longevity and aging. For instance, in homeotherms CR and feeding induce a reduction and a transient increase of Tcore, respectively. The functional link between temperature and nutrient homeostasis is surprisingly poorly investigated and to date is primarily described as correlational.
A review of the hypothalamus and its functions is beyond the goal of the present review. We will however present a detailed description of the components and the mechanisms of temperature regulation by of the hypothalamic preoptic area (POA) and will refer to other hypothalamic nuclei, neuro-hormones or pathways when required to present our arguments. We will primarily refer to work carried out in homeotherms since poikilotherms lack the neuronal complexity found in the hypothalamus of vertebrates and do not have comparable temperature homeostasis. A review of the effects of temperature on life span in poikilotherms is presented by Godon Lithgow in this special issue on thermodynamics and aging. Yet, studies carried out in these organisms will be referred to when considered appropriate for the topic discussed here. In presenting the relevance of hypothalamic regulation of temperature with respect to longevity and aging we will also often consider its correlation with calorie intake and energy expenditure both modulated by the hypothalamus. Extensive review of these subjects is also presented in other articles of this special issue by Juli Mattison and John Speakman. Similarly, only a brief description of the effects of aging on hypothalamic temperature regulation as a circadian phenomenon will be provided as the subject is treated in detail by Dietmar Weinert.
Calorie restriction reduces core body temperature and retards aging
Restriction of food intake (termed here as calorie restriction) is a very well-established, widely and long-used means to prolong the life of laboratory animals. In fact, the first well-controlled report of lifespan extension in laboratory rats by CR and was described by McCay and colleagues some 74 years ago (McCay et al. 1935). The most common means of calorically restricting rodents is by simply lowering food intake by 30 - 40% through reducing food availability. This was reported to yield approximately a 50 - 65% increase in lifespan (reviewed in (Masoro 2005)). Similarly, intermittent feeding (every other day) extends lifespan in mice by 27% (Goodrick et al. 1990).
Interest in CR as it pertains to thermoregulation-related aging, stems from the observation that CR causes a reduction in core body temperature (Tcore) in homeotherms including mice, primates and humans (Liu et al. 1972; Leto et al. 1976). For instance in mice, it has been reported that a 40% reduction in food intake results in a lower Tcore for six of six strains examined. It should be noted however, that not all six strains of inbred mice used in this study exhibited CR-associated Tcore reductions to the same extent. In fact, 129 mice showed a rather modest 1.5 °C reduction while BALB/c mice had a robust mean decrease of 4 °C under CR indicating that subtle genetic variation can lead to substantial effects on Tcore (Rikke et al. 2003). Given the close association of reduced Tcore with CR and longevity, efforts have been made to better define this relationship empirically. Experiments in fish revealed, at least in this poikilotherms system, that CR instituted during the first half of life and lower Tcore applied during second half resulted in an additive effect increasing life span by 300% (Walford 1983). Evidence was also presented using thermoneutrally maintained mice that suggested approximately 50% of life span increase imparted by CR is dependent on lowering Tcore (Koizumi et al. 1996). Although, it should be noted that this research relied on a small number of mice and statistical significance could not be obtained. These studies indicate that only part of the longevity conferred by CR depend upon a reduction in Tcore and that the remainder of the observed increase in life span is due to other mechanisms.
Two ongoing studies in rhesus monkeys were initiated in the late 1980's with the focus of examining the effects of CR on longevity and on general health. While these studies are not expected to officially conclude for approximately another 10 years (as the maximum lifespan of rhesus monkeys is roughly 40 years) analysis thus far has shown that monkeys on 30% caloric restriction exhibit 70% less body fat and higher insulin sensitivity. Researchers also found that early data from one of these studies indicates that CR provides complete protection from Type 2 diabetes and confers an emerging survival advantage versus controls and exhibit the stereotypical CR-associated drop in Tcore of approximately 0.5 °C measured via radiotelemetry (reviewed in (Lane et al. 1996; Mattison et al. 2003; Anderson et al. 2006)).
There are few human studies into CR, longevity and aging. On September 26, 1991 eight people entered an enclosed ecological space known as Biosphere 2. During their two-year stint within this self-contained eco-system the inhabitants were placed on a low-calorie diet (1750-2100 kcal/day) and blood samples periodically taken and analyzed. While Tcore was not measured researchers did report a decrease in blood cholesterol, blood pressure, fasting blood sugar, and lower white blood cell counts over the duration of the subjects' stay which also correlates well with rodents under CR (Walford et al. 1995; Paglia et al. 2005). The most ambitious CR trial to date in humans, known as the CALERIE project, is currently being sponsored by the National Institute of Aging. Approximately 140 healthy, non-obese participants have been enrolled and divided into groups with different CR regimes with CR ranging from 10 - 30%. Human subjects were under CR for 6 or 12 months. Preliminary results from the 6 month group of participants on CR showed a decrease in insulin levels of about 2 μU/mL and body temperature reduction of approximately 0.2 °C consistent with observations in rodent and nonhuman primate models (Heilbronn et al. 2006).
A correlation between CR and lowered Tcore was also collected earlier by Roy Walford who led several pioneering studies into the effects of lower Tcore on longevity in humans. Most notably, Dr. Walford collected data on Indian yoga masters that subsisted on a low-calorie diet perhaps mimicking CR and reported a 1-2 °C reduction of their Tcore (Walford 1983).
Reducing Tcore during CR can be regarded as a survival strategy to cope with a condition of limited nutrients. Since homeotherms maintain a Tcore that is normally higher than the ambient temperature, its maintenance requires a considerable amount of energy. Reducing its absolute value when nutritional resources are limited is an effective way to save energy and prolong survival in time of food scarcity. These mechanisms are believed to be similar to those allowing some animals to undergo torpor or hibernation in extreme conditions and indicate the existence of a functional link between nutrient and temperature homeostasis. In fact, Tcore reduction during CR is maintained by reduction of metabolic rate.
Yet, the possibility existed that Tcore reduction could not only be a consequence of CR but also contribute to its beneficial effects on longevity and aging was explored. Such a possibility was supported by the demonstration that in poikilotherms, a modest temperature reduction prolonged lifespan. This hypothesis was tested using a transgenic mouse model with reduced Tcore (Conti et al. 2006). These animals were generated hypothesizing that local heat production in the proximity of the “central thermostat” located in the preoptic area of the hypothalamus would mimic an increase of Tcore activating thermoregulatory compensatory mechanisms that would result in an actual reduction of Tcore. This goal was achieved by overexpressing the uncoupling protein 2 (UCP2) exclusively in the small subpopulation of hypocretin (Hcrt)-expressing hypothalamic neurons (hence, Hcrt-UCP2 mice). UCP2 is an inner mitochondrial membrane protein that uncouples oxidative phosphorylation from respiration, dissipating the proton gradient energy in the form of heat. Thus, UCP2 over expression produces excess heat in the POA. Hypocretins (Hypocretin 1 and 2) participate in the regulation of autonomic functions and are exclusively expressed in ca 3,000 neurons in the lateral hypothalamus, at 0.8 mm from the POA. Hcrt-UCP2 mice had elevated hypothalamic temperature. This resulted in a modest (0.3-0.56 °C less than their wild-type littermates) but prolonged reduction of Tcore. Hcrt-UCP2 mice had a calorie intake that was similar to that of their wild-type littermates, increased metabolic efficiency and 12-20% increased median life expectancy in male and female, respectively. This experiment demonstrated that reduction of Tcore can contribute to the beneficial effects of CR in homeotherms.
Inspection of the complementary log mortality plots between groups was used to gain information on the possible mechanisms of prolonged lifespan in Hcrt-UCP2 mice. In poikilotherms, where both CR and reduction of temperature were demonstrated to increase lifespan, the effects of CR and temperature on longevity can be clearly distinguished by the slopes of the mortality curves (Tatar et al. 1993; Promislow et al. 1999; Tatar 2001; Mair et al. 2003)(Tatar et al. 1993; Promislow et al. 1999; Tatar 2001; Mair et al. 2003)(Tatar et al. 1993; Promislow et al. 1999; Tatar 2001; Mair et al. 2003)(Tatar et al. 1993; Promislow et al. 1999; Tatar 2001; Mair et al. 2003)(Tatar et al. 1993; Promislow et al. 1999; Tatar 2001; Mair et al. 2003). For instance, in Drosophila, chronic CR results in a delay in the onset of a detectable aging-related increase in mortality (Mair et al. 2003). However, once the mortality increase is detected, it proceeds at roughly the same rate (same slope) in DR and control flies. This is in sharp contrast to the effects of lowered temperature, which increases life-span in drosophila, but also reduces the slope of the mortality trajectory. Compared to their wild-type littermates Hcrt-UCP2 mice had a parallel, proportional shift in the mortality rate trajectory similar to the effects of CR in both poikilotherms and homeotherms and not to to the effects of lowered temperature tested in poikilotherms (Conti et al. 2006). This similarity between CR and Tcore reduction in homeotherms as opposed to poikilotherms may be due the tight correlation between temperature and energy homeostasis and their hypothalamic integration and regulation found in homeotherms but not in poikilotherms.
The hypothalamus and the endocrine regulation of lifespan
In mammals, the hypothalamus is an important regulator of lifespan. Such recognition comes primarily from the discovery that repressing the somatotropic axis regulating the production or the action of growth hormone (GH) and IGF-1, can affect lifespan. The Ames and Snell dwarf mice carrying a mutation in different loci causing a reduced GH, thyroid stimulating hormone (TSH) and prolactin levels are long-lived. Similarly, mice with mutation in the GH-releasing hormone receptor and mice null for the GH receptor binding protein have increased lifespan. Interestingly, GHRKO and Ames mutants exhibit decreased Tcore (Hunter et al. 1999; Bartke et al. 2001; Hauck et al. 2001; Hauck et al. 2001). Increased lifespan was also observed in insulin-like growth factor 1 (IGF1) heterozygous knockout mice (Holzenberger et al. 2003), fat-specific insulin receptor knockout mice (Bluher et al. 2003) and insulin receptor substrate 2 knockout mice (Taguchi et al. 2007).
The relevance of the role of central control of endocrine functions on aging was evident also in simpler organisms like Drosophila melanogaster and Caenorabditis elegans although these animals have a neuronal regulation of the endocrine responses that is far less complex than that found in mammals (For review see (Broughton et al. 2009)). For instance, in the fly, ablation or specific targeting of the median neurosecretory cells (the Drosophila equivalent of mammalian pancreatic insulin producing β-cells) conferred prolonged lifespan (Broughton et al. 2005; Wang et al. 2005). Work with Caenorabditis elegans, also demonstrated the relevance of the neuroendocrine system in modulating longevity in the worm. For instance, neuronal expression of PI3K (age-1), a component of insulin signaling pathway, was sufficient to rescue the life-extending phenotype conferred by its ectopic deletion (Wolkow et al. 2000). More recently, the search for factors mediating the effects of calorie restriction in worm led to the finding that the transcription factor SNK-1, similar to the mammalian NRF2, can modulate worm lifespan upon specific neuronal expression, probably by regulating energy homeostasis (Bishop et al. 2007). An additional remarkable finding with these organisms was that gustatory and olfactory neurons can regulate longevity and aging. For instance in drosophila exposure to nutrient-derived odorants partially reversed the longevity-extending effects of dietary restriction and ablation of a specific odorant receptor prolonged lifespan. These actions are believed to be mediated by neuronal dependent modulation of metabolism in response to nutrient perception (Alcedo et al. 2004; Lans et al. 2007; Libert et al. 2007).
Although this remains to be demonstrated in homeotherms, increasing attention is being paid to the role of central mechanisms of calorie homeostasis in influencing longevity and aging. In mammals feeding is regulated through the autonomic nervous system. The hypothalamus, as well as the limbic forebrain, play an important role in caloric intake by receiving signals about ingested food and adiposity and integrating them with other signals (for instance taste, environmental factors, emotional factors, memory or taste of food) to determine behavioral responses regulating food intake (Leibowitz et al. 2004; Morrison et al. 2007). These processes are mediated by specific hypothalamic nuclei and are modulated by several neuropeptides. For instance, the arcuate nucleus (ARC) and the paraventricular nucleus (PVN) as well as the Ventro- and dorsomedial nuclei (VMN and DMN) are the hypothalamic areas believed to be primarily involved in the regulation of calorie intake. The ARC is regarded as the sensory nucleus of energy availability. Neurons in the ARC can in fact respond to insulin and to leptin and produce both anabolic and catabolic peptides influencing feeding and energy expenditure. These include the α-melanocyte-stimulating hormone and the cocaine-amphetamine-related transcript, both catabolic, and the neuropeptide Y (NPY) and agouti-related protein, both anabolic. NPY neurons of the ARC project to the PVN containing parvocellular neurosecretory neurons including those synthesizing thyrotropin-releasing hormone (TRH), which regulates TSH secretion thus influencing metabolic rates. In addition, lesions of the VMN in young rats resulted in increased body fat, glucose intolerance, hyperlipidemia, and reduced renal function. Conversely, ablation of the hypothalamic DMN in young rats resulted in decreased size and body fat, normal glucose and lipid metabolism, decreased risk of renal malfunction and lower IGF-I serum levels, all of which resembles the phenotype of a rodent under CR (Bernardis et al. 1996).
Through these mechanisms, information about nutrient level can be sensed and utilized by the hypothalamus to modulate feeding as well as metabolic rate thus affecting also temperature. However, much remains to be understood primarily with respect to the mechanisms that are responsible for the maintenance of temperature reduction over time as well as for understanding the bidirectional correlation between temperature and nutrient homeostasis. The limited amount of information on this subject is due to difficulty of investigating the hypothalamic regulation of temperature homeostasis mostly for lack of specific molecular markers.
Preoptic area and thermoregulation
The core body temperature is the temperature of an organism in deep structures of the body (such as the gut, or the liver), in comparison to temperatures of peripheral tissues. Tcore is normally maintained within a narrow range so that essential enzymatic reactions can occur. Prolonged and large core temperature elevation (hyperthermia) or depression (hypothermia) is incompatible with life with few exceptions (e.g. hibernation).
The key role played by the hypothalamus in the regulation of Tcore began to be recognized more than a 100 years ago, based on observations in human patients having suffered brain damage and experiments using brain lesions made in animals (Keller 1938; Clark et al. 1939; Ranson et al. 1939; Bligh 1973). These studies have also demonstrated that in addition to the hypothalamus, lower brain stem and spinal structures also participate in the mechanisms of thermoregulation in a hierarchical way, possibly through the medial forebrain bundle (Bligh 1973; Simon et al. 1986). The initial lesion studies were limited by the course spatial resolution of the methods, and have been revisited and refined by numerous later studies (see below).
The thermoregulatory importance of the preoptic area and anterior hypothalamus (PO/AH) has been conclusively demonstrated in the seminal experiments of Barbour (Barbour 1912) by using implanted thermodes to selectively warm and cool this brain area. Sustained or alternating PO/AH cooling and heating induced thermoregulatory activities (physiological or behavioral), causing Tcore to change in the direction opposite to that of the hypothalamic temperature (Thy). The latter experiments identified the PO/AH as an important thermosensitive region. The quantitative studies of Hammel and colleagues (Hammel et al. 1960) continued the qualitative observations by Barbour. Selective PO/AH cooling and heating with chronically implanted thermodes was carried out in mammals under defined ambient thermal conditions while effects on Tcore, shivering, vasoconstriction and panting were observed. Based on these studies Hammel (Hammel et al. 1963) proposed the concept of hypothalamic proportional control, where a particular net thermoregulatory response (i.e. skin blood flow, shivering) was proportional to (Thy–Tset), where Tset represented a hypothetical set reference. Tset was viewed as a complex parameter representing the state of activity of neuronal populations rather than a set point temperature (as it is frequently assumed mistakenly). Each variable (thermal or non-thermal) known to affect thermoregulation was assumed to do so by altering Tset.
Nakayama and colleagues (Nakayama et al. 1961; Nakayama et al. 1963) published the first extracellular single-unit studies in which they reported that some PO/AH neurons, termed “high Q10” neurons and later “warm-sensitive” neurons, increased their firing rates when Thy increased, it has been considered that they represent the central thermoreceptors. The other PO/AH neurons, which display little temperature-dependent changes in firing rate or a decrease in firing rate with warming, were termed “low Q10” neurons. The latter were termed “cold-sensitive” while the former were termed “temperature-insensitive”. Nakayama et al (1961, 1963) also found that PO/AH neurons responded to peripheral thermal stimulation, suggesting that in addition to sensing local changes of temperature, the preoptic region also serves as an integrator of thermal information from the organism and the environment by receiving afferent sensory input from the body and the skin. Based on these studies Hammel proposed a neural model of temperature regulation (Fig. 1 (Hammel 1965)).
Figure 1.
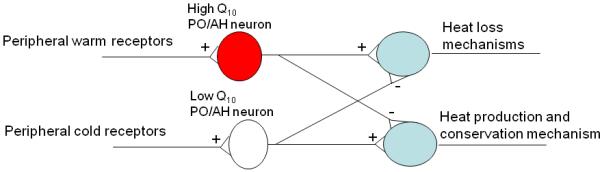
Hammel's model of neuronal temperature regulation (Hammel 1965).
This simple neural circuit is still a working model today and many variations have been proposed (Bligh 1973; Boulant et al. 1974; Boulant 1981). More recent studies (see detailed descriptions below) have shed some light on the assumptions on which the model is based, while others remain to be verified. Briefly, we know now that the output of PO/AH neurons involved in thermoregulation is mainly GABAergic (Morrison et al. 2008), and that warm-sensitive PO/AH neurons play a more important role in the various thermoregulatory responses than cold-sensitive neurons (Zhang et al. 1995; Chen et al. 1998). The peripheral inputs to the PO/AH neurons are not via the thalamus, as suggested by Hammel (1965), but via the lateral parabrachial nucleus (LPB) (Nakamura et al. 2008). Distinct sets of thermosensitive PO/AH neurons may be controlling the different thermoregulatory responses (Nagashima et al. 2000). Finally, it is not established whether PO/AH thermosensitive neurons are projecting directly to downstream thermoregulatory centers or via other PO/AH neurons.
Cellular properties of thermosensitive of PO/AH neurons
Following the first papers of Nakayama (Nakayama et al. 1961; Nakayama et al. 1963), numerous extracellular studies have directed their efforts to the characterization of the firing properties of thermosensitive PO/AH neurons with the assumption that thermosensitivity is a distinctive property of neurons involved in thermoregulation. However, neurons displaying thermosensitive action potential firing could be recorded also in other brain regions, e.g. in the cortex (Barker et al. 1970). While these findings suggest that thermosensitivity per se does not imply a role in the control of body temperature, a thermoregulatory network must include thermosensitive cells. In the analysis of thermosensitive PO/AH neurons recorded in vivo and in vitro, many attempts have been made to correlate their response patterns (e.g. temperature “thresholds” of activation) to defined functions in temperature homeostasis (Bligh 1973; Boulant 1981) but such classifications have remained circumstantial due to the extreme variability of response patterns recorded in vivo.
Besides the specificity of the thermosensitive phenotype, another difficulty encountered was how to define thermosensitivity quantitatively. Thermosensitivity of PO/AH neurons is measured as the thermal coefficient (the slope factor of the firing rate vs temperature plot) or a similar measure like Q10. However, there is little agreement between authors as to how to define warm-sensitive cells versus insensitive ones on the basis of such a coefficient. The value of this parameter varies from one report to the next, e.g. 0.6 impulses s−1 °C−1 (Kobayashi et al. 1993; Vasilenko et al. 2000), 0.7 impulses s−1 °C−1 (Matsuda et al. 1992), or 0.8 impulses s−1 °C−1 (Kelso et al. 1982). Thus, it seems that the separation of warm-sensitive from insensitive neurons is still a convention. Cold-sensitive neurons are those with coefficients smaller than −0.6 impulses s−1 °C−1, value which is less controversial. One in vivo study in anaesthetized rabbits has found that thermosensitive PO/AH neurons (specifically those neurons with thermal coefficient above 0.8 impulses s−1 °C−1 or below −0.6 impulses s−1 °C−1) were also sensitive to peripheral thermal stimuli, in contrast to temperature-insensitive PO/AH neurons (Boulant and Hardy 1974). This study argued that these results indicated a functional specificity of thermosensitive PO/AH neurons and provided a rationale for the 0.8 impulses s−1 °C−1 as the cutoff value for warm-sensitivity. However, it is not clear if these results can be extrapolated to all homeotherms. Later studies in rat brain slices have confirmed a percentage of warm-sensitive PO/AH neurons (∼20%) value similar with that observed in vivo (Boulant 1998). It must be noted however, that the slice studies were performed in extracellular solutions containing 6.25 mM K+ a concentration above the physiological range.
Several studies have addressed the question whether thermosensitivity is an intrinsic property of a neuron or it is due to the neuron's synaptic input. Recordings in “high Mg low Ca” bath solutions that inhibit neurotransmitter release have indicated that warm-sensitivity is an intrinsic property for a majority of PO/AH warm-sensitive neurons (Kelso and Boulant 1982; Hays et al. 1997). In contrast, cold-sensitivity disappeared in “high Mg low Ca” bath solutions strongly indicating that it does not represent an intrinsic property of the neurons (Kelso and Boulant 1982; Hays et al. 1997). It has been proposed that warm-sensitive neurons exert synaptic inhibition on the so-called “cold sensitive” neurons. Warm-sensitive neurons decrease their firing rate when the temperature drops, thus reducing their synaptic inhibition and allowing an increase of the firing rate of the cold sensitive neurons (Boulant 1981; Curras et al. 1991). Indeed, cold-sensitive neurons are rarely recorded in vitro, and usually represent a minor proportion of the neurons (3% or less). In contrast, most in vivo studies report cold-sensitive units, which may represent up to 14% of all the neurons (Alam et al. 1995). These results point to a role of synaptic inputs in cold-sensitivity, since they are the most affected by the slicing procedure.
Intracellular recordings in hypothalamic slices containing the PO/AH have revealed further details regarding the spontaneous activity of PO/AH neurons. Most PO/AH neurons display spontaneous firing activity which is driven by either EPSPs or “pacemaker potentials” (Boulant 1998). Warming induces a depolarization and inward currents in PO/AH neurons (Kobayashi and Takahashi 1993; Tabarean et al. 2005; Zhao et al. 2005). All PO/AH neurons receive both excitatory and inhibitory synaptic activity. Warming reduces the amplitude and duration of IPSPs, mechanism which may modulate warm-sensitivity in some neurons (reviewed in (Boulant 1998)).
Patch clamp recordings have provided the opportunity to fill the recorded neurons with biocytin and study their morphology. However, this approach has not succeeded to unravel any distinguishing characteristic morphology of warm-sensitive PO/AH neurons, other than that, on average, warm-sensitive neurons tend to send their dendrites perpendicularly to the third ventricle, while temperature-insensitive neurons tend to send their dendrites in all directions (Griffin et al. 2001). The axonal projections of the biocytin-filled neurons could not be determined, a fact which may reflect sparse local projections and/or a technical limitation. Warm-sensitive PO/AH neurons in culture also could not be distinguished from temperature-insensitive neurons based on their soma size or shape or number of dendrites (Tabarean et al. 2005), thus the identification of warm sensitive PO/ AH neurons relies on electrophysiological recordings at several temperatures.
Mechanisms of thermosensitivity
The mechanism of intrinsic warm-sensitivity of PO/AH neurons is controversial. Thus, Boulant and colleagues consider that the increased firing rate is solely due to an increased rate of rise of the prepotential which precedes an action potential (reviewed in (Boulant 1998)). On the other hand, Kobayashi and colleagues describe strong depolarizations (10 mV or larger) in response to heating which cause the increased firing rate in warm-sensitive neurons (Kobayashi and Takahashi 1993). The recordings of Kobayashi and colleagues were performed during hyperpolarizing current injection and therefore the measured depolarizations are exacerbated by the warming-induced decrease in the input resistance of the neuron. Another questionable aspect of the conclusions we can draw from these studies is that all the studies of Boulant and colleagues utilize a non-physiological 6.25 mM external K concentration, which itself depolarizes the PO/AH neurons and favors the “pacemaker” (prepotential-driven) mode of action potential firing (discussion in (Tabarean et al. 2004)). In cultured PO/AH neurons both phenomena are present, however they occur also in temperature-insensitive neurons (Tabarean et al. 2005). Finally, the warming-activated inward current was found to be tetrodotoxin (TTX)-insensitive in some studies (Tabarean et al. 2005; Zhao and Boulant 2005) and TTX-sensitive (i.e. mediated by voltage-gated Na channels) in others (Kiyohara et al. 1990).
Synaptic mechanisms in thermosensitivity
In vivo block of synaptic inputs to PO/AH neurons potently modulate thermoregulation. Blocking (with bicuculline) or activating GABA-A receptors (with muscimol) results in rapid hypothermia or hyperthermia, respectively (Osborne et al. 1994; Osaka 2006), while increasing glutamatergic drive in the PO/AH results in decreased thermogenesis (Chen et al. 1998). The effect of these treatments on the thermosensitivity of PO/AH neurons have not been studied. To date no evidence has been presented that “true” thermoreceptors might exist in the central nervous system, i.e. neurons which do not receive synaptic inputs, are intrinsically thermosensitive, and feed their signals into the appropriate pathways. On the contrary, in all the intracellular studies the presence of inhibitory postsynaptic potentials (IPSPs) and/or excitatory postsynaptic potentials (EPSPs) is reported in every PO/AH neuron (Curras et al. 1991; Griffin et al. 2001; Tabarean et al. 2005).
The use of “high Mg low Ca” extracellular solution to separate intrinsic versus synaptic mechanisms is problematic because of two reasons. Firstly, it drastically reduces somatic, not only presynaptic, Ca currents. T-type Ca currents, which are present in PO/AH neurons (Tabarean et al. 2005), are involved in the spontaneous firing mechanisms of central neurons (e.g. (Llinas et al. 1986)). The question remains open as to whether all warm-sensitive PO/AH neurons have some intrinsic thermosensitivity or if they can be also of another type, namely their firing rate is purely synaptically-driven (Tabarean et al. 2005).
We have recently shown that prostaglandin E2 (PGE2), a well established endogenous pyrogen, that is known to activate thermogenesis, increased thermosensitivity and firing rates of PO/AH neurons by decreasing the frequency of IPSPs (Tabarean et al. 2004). In contrast, IL-1β hyperpolarizes a different set of PO/AH neurons and reduces their thermosensitivity by increasing the frequency of IPSPs and of miniature IPSPs (Sanchez-Alavez et al. 2006; Tabarean et al. 2006). We would also like to note that besides the acute changes in thermosensitivity caused by pyrogens, synaptic mechanisms could also provide the basis for longer-term plasticity of this neuronal property, as encountered during sleep (Alam et al. 1995) or cold acclimation (Sun et al. 1997).
Synaptic events display temperature-dependent frequency in some PO/AH neurons (Griffin et al. 2001). The thermosensitivity of spontaneous release (minis) at various synapses is well documented (starting with (Katz et al. 1965)) and reflects a general biophysical property. As it is the case at most synaptic terminals (reviewed in (Dodson et al. 2004)), A-type K channels potently influence the frequency of IPSPs and EPSPs (Diem et al. 2003; Tabarean et al. 2006). The probability of synaptic release displays heterogeneous characteristics in different PO/AH neurons, and involves various combinations of L, N, P, Q -type Ca channels (Druzin et al. 2002). Since the A-type K currents and Ca currents are also modulated by IL-1 and PGE2 (Diem et al. 2003; Tabarean et al. 2006) they may have a role in the mechanism of synaptic release and its thermosensitivity.
Thermosensitivity of PO/AH neurons is a plastic property
The thermosensitivity of PO/AH neurons is not a constant characteristic of a neuron but displays plasticity both in vivo and in vitro. Thus thermosensitivity can change rapidly in the presence of the pyrogens PGE2 (Tabarean et al. 2004) or IL-1β (Vasilenko et al. 2000; Sanchez-Alavez et al. 2006). Slower changes are observed in some warm-sensitive PO/AH neurons which decrease their thermosensitivity during NREM sleep (Alam et al. 1995). Finally, the proportion of thermosensitive neurons and their thermal coefficients are different in warm- and cold-acclimated animals (Sun et al. 1997) when compared with their littermates, suggesting that afferents to PO/AH neurons can affect the expression of thermosensitivity.
Neuronal mechanisms of mechanism for fever
Fever represents the elevation of Tcore that occurs in “defensive” response to the entry into the body of pathogenic agents. It is thus distinct from hyperthermia, the distinction being that the Tcore rise of fever is the deliberate result of the regulated operation of active thermogenic effectors, whereas that of hyperthermia is the consequence of the passive gain of heat in excess of the capability of active thermolytic effectors to dissipate it. Many studies have addressed the effect of pyrogens (substances that induce fever such as LPS, IL-1β, TNFα, IL-6, PGE2) on the activity of PO/AH neurons. PGE2 appears to mediate the effects of most endotoxins and cytokines. Early data showed that PGE2 could affect the firing rate of thermosensitive PO/AH neurons (Gordon et al. 1980; Morimoto et al. 1988; Matsuda et al. 1992; Sutarmo Setiadji et al. 1998; Ranels et al. 2003). The most common effect was a reduction in firing rate (of the warm sensitive neurons) which, according to the Hammel model, results in increased heat production. More recently it was shown that firing rates and thermosensitivity of a different set of PO/AH neurons is enhanced by PGE2 application, effect associated with a decrease in the frequency of IPSPs (Tabarean et al. 2004). While many endotoxins and pyrogenic cytokines act via PGE2, the endogenous pyrogen IL-1β can increase Tcore in a PGE2-independent manner, by activating the N-sphingomyelinase pathway which results in the production of ceramide and subsequent activation of the src pathway (Davis et al. 2006; Sanchez-Alavez et al. 2006; Tabarean et al. 2006).
Multimodality of PO/AH thermosensitive neurons
PO/AH thermosensitive neurons respond not only to changes in local and peripheral temperature, but also to hormones, osmolarity, and glucose concentration. These findings suggest that these neurons, or a subgroup of them, also play a role in the integration of thermoregulation with other homeostatic processes such as control of metabolic rate (glucose sensing). Several studies have addressed the influence of osmolality on the firing activity of thermosensitive PO/AH neurons (Silva et al. 1984; Travis et al. 1993) in an attempt to explain known osmoregulatory influences on thermoregulation. Warm-sensitive neurons inhibited by hyperosmolality would mediate the well-known inhibition of evaporative water loss by hyperosmolality. Similarly, a decrease in glucose concentrations increased the firing activity of PO/AH neurons ((Silva and Boulant 1984)) action which may explain the hypothermia induced by hypoglycemia. PO/AH neurons were found to be sensitive to the steroid hormones estradiol or testosterone suggesting a mechanism for the interaction between reproductive behavior and thermoregulation (Silva et al. 1986; Boulant et al. 1987). It should be noted however that the same modulation by osmolality, glucose or steroid hormones was also observed in temperature-insensitive PO/AH neurons. Thermosensitive PO/AH neurons also appear to be involved in regulation of sleep: PO/AH warm-sensitive neurons exhibit increased discharge rate during NREM sleep, suggesting a role in its onset and regulation (Alam et al. 1995).
Thermoregulatory neuronal networks comprising PO/AH thermosensitive neurons
As mentioned above the local axonal projections of thermosensitive PO/AH neurons are not known. One study has reported temperature-dependent changes in the frequency of EPSCs recorded in some PO/AH neurons (Griffin et al. 2001). However, it is not clear whether they reflect changes in firing rate of PO/AH neurons, or that of neurons projecting to this area. Also it is not sure if this thermosensitive glutamate release is an intrinsic property of the presynaptic terminals, i.e. not related to the firing activity of the presynaptic neuron. Thus, the local networks (i.e. within the PO/AH) comprising warm-sensitive neurons are not yet understood.
Neuronal network controlling brown adipose tissue (BAT) thermogenesis
Studies utilizing thermal and chemical stimulation in the PO/AH as well as selective hypothalamic transections have found that warm-sensitive PO/AH neurons send efferent signals to loci involved in the control of heat production (e.g. brown adipose tissue thermogenesis) (Zhang et al. 1995; Chen et al. 1998). The PO/AH contributes a tonic inhibitory input to these loci via lateral hypothalamic pathways. These studies have also analyzed the relative contributions to the control of BAT thermogenesis by measuring the effect of PO/AH warming or cooling on this mechanism. PO/AH warming or injection of glutamate suppressed BAT thermogenesis thus suggesting that it is controlled by warm-sensitive neurons (Chen et al. 1998). Studies using retrograde tracers and immunocytochemistry revealed that EP3 prostanoid receptor - positive GABAergic PO/AH neurons project to the sympathetic premotor neurons in the rostral raphe pallidus (rRPA) directly or via the dorsomedial hypothalamus (DMH) (Nakamura et al. 2002; Nakamura et al. 2005). These studies also involved bilateral microinjection of GABA-A receptor agonists or antagonists into the rRPa or DMH, which blocked the fever induced by intra-PO/AH PGE2 applications. A role of the DMH, downstream of the PO/AH, in the control of BAT thermogenesis was suggested also by direct electrical and chemical stimulation (Zaretskaia et al. 2003). A proposed diagram for neuronal control of BAT is illustrated in Figure 2. These findings suggest that the PO/AH sends GABAergic efferents to the DMH and rRPA and thus controls thermoregulation and febrile responses. However, the physiological characteristics (spontaneous firing characteristics, synaptic inputs, thermosensitivity etc) of PO/AH neurons sending these efferents are not known. A recent study in mice showed the importance of EP3-receptor expression specifically in neurons of the median and medial preoptic nuclei in the fever response, further indicating their central role in the control of thermoregulation (Lazarus et al. 2007).
Figure 2.
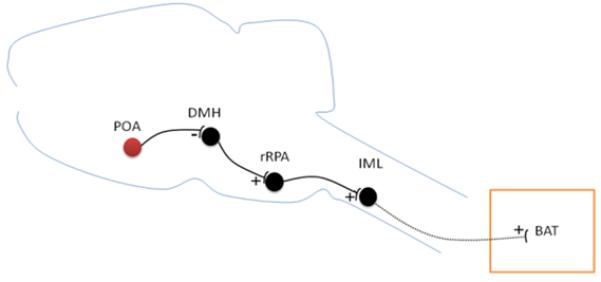
Diagram of neural pathway controlling BAT thermogenesis (redrawn from (Nakamura et al. 2007)). GABAergic neurons in the POA tonically inhibit neurons in the DMH. DMH neurons activate BAT-controlling sympathetic premotor neurons in the rRPa. This results in stimulation of the BAT-controlling sympathetic output system. IML, intermediolateral cell column.
Neuronal network controlling shivering
Injections of excitatory amino acids as well as PO/AH warming inhibited cold-induced shivering suggesting that this mechanism, similar to BAT thermogenesis was controlled by PO/AH warm-sensitive neurons (Zhang et al. 1995). In contrast, cooling of the PO/AH had little effect on cold-induced shivering. The efferent signals mediating shivering descend in the medial forebrain bundle (Kanosue et al. 1994).
Neuronal network controlling evaporative heat loss
The only brain region that induces salivary secretion when warmed is the PO/AH (Kanosue et al. 1994). Preoptic warming, glutamate injections as well as electrical stimulation facilitate salivary secretion (Zhang et al. 1995) as well as body extension (Tanaka et al. 1986), another aspect of evaporative heat loss.
Neuronal network controlling cutaneous blood flow
Warming the PO/AH elicits skin vasodilation (Ishikawa et al. 1984), by activation of warm-sensitive neurons (Zhang et al. 1995). The efferent pathway descends through the medial forebrain bundle (Kanosue et al. 1994). PO/AH neurons controlling cutaneous blood flow project to the ventral tegmental area (VTA) or to the periaqueductal gray (PAG) (Zhang et al. 1997). The former contains neurons that are excited by preoptic warming while the latter contains neurons that are inhibited by it (Zhang et al. 1997). It is thought that warm-sensitive neurons in PO/AH send excitatory signals to vasodilator neurons and inhibitory signals to vasoconstrictor neurons.
In summary, it appears that PO/AH warm-sensitive neurons are the major sensors of hypothalamic temperature as well as the recipient of information form cutaneous thermoreceptors. These neurons appear to be important for both heat production and heat loss mechanisms. They send mostly inhibitory signals to downstream thermoregulatory neurons, with the possible exception of those controlling vasodillation, which receive excitatory input. Studies measuring simultaneously several thermoregulatory mechanisms have indicated that each may have different “threshold” temperatures for activation and that these values can change independently. Such observations have led to arguments for a thermoregulatory system with multiple controllers rather than one with a single controller (Romanovsky 2007). Basic coordination between thermoeffector mechanisms is likely to be achieved through their dependence on Tcore. Each thermoeffector is activated in function of Tcore, and its activation affects this common variable (Romanovsky 2007).
Afferent networks to thermoregulatory PO/AH neurons
The most important thermal somatosensory ascending pathway is the spinothalamocortical pathway (Craig 2002), however it does not appear to convey thermal information to the thermoregulatory controller(s), since even after removal of the neocortex, dorsal hippocampus and most of the striatum, animal still initiate a metabolic increase in response to skin cooling (Osaka 2004). The spinothalamocortical pathway, may however play a role in the activation of behavioral thermoeffector mechanisms, such as seeking a warm or cold environment. Recent studies utilizing retrograde tracer injections in the PO/AH (specifically in the median preoptic neucleus, MnPO) and c-fos immunocytotochemistry have identified neurons in the external lateral subnucleus of the mesencephalic lateral parabrachial nucleus (LPB) that are projecting directly to MnPO neurons and are activated during cold exposure (Nakamura and Morrison 2008). These LPB neurons receive synaptic imputs from dorsal horn lamina I neurons (Nakamura and Morrison 2008). Stimulation of LPB neurons with NMDA induces similar responses with those induced by skin cooling: BAT thermogenesis, increased metabolism and heart rate (Nakamura and Morrison 2008). Blocking glutamate receptors in the MnPO blocked these responses, suggesting that LPB neurons send glutamatergic inputs to MnPO neurons. Cutaneous warm signals appear to be sent to the PO/AH via a different LPB subnucleus (Nakamura et al. 2007).
A direct communication between thermoregulatory PO/AH neurons and hypothalamic neurons in nuclei regulating energy homeostasis remains to be investigated.
‘Old is Cold’
The term “old is cold’ is often used by clinicians to refer to the observation that the fever response to infection is generally reduced in the elderly compared to young individuals, and often is completely absent (Norman 2000). It is also reported that the elderly have a diminished ability to cope with both cold and heat stress indicating age-associated reduction of thermoregulation. Studies on the effects of age on temperature in humans have indicated that aging is associated with either a decrease of Tcore baseline value and of its rhythmicity or to no change at all (Reviewed in (Kenney et al. 2003; Weinert et al. 2007)). Seemingly trivial to perform, these studies are not easy to interpret for the several variables that may be present including the time and methods of temperature recordings and the heterogeneity of the individuals investigated with respect to general health, history, chronic diseases and pharmacology therapy, exercise and nutritional regimen. In addition, most of these studies are not longitudinal.
Experiments carried out in our laboratory (unpublished) and by others showed that in mice the absolute Tcore value is reduced with age as is the amplitude of the circadian Tcore fluctuation. It is likely that these differences reflect changes in both central and peripheral mechanisms of thermoregulation. For instance, age associated reduction of vasoconstriction and skin blood flow, sweat gland output, metabolic heat production and cardiac output have all been reported. In addition, aging of the hypothalamus has also been proposed to be implicated. Most studies investigating the effects of age in the hypothalamus have so far investigated the age dependent changes in the number of specific hypothalamic neuronal populations. However, it is important to consider that the different hypothalamic cell populations showed different patterns of aging. For instance, in humans, while many neuronal populations decrease with age, the neurons producing vasopressin and oxytocin of the supraoptic nucleus do not show any reduction in their number with age and the number of corticotrophin-releasing hormone producing neurons is increased in old age (Reviewed in (Hofman 1997)).
As unequivocal morphological markers for temperature sensitive neurons of the POA do not yet exist it is still not possible to determine their possible decline with time. However, the POA is sexually dimorphic in humans and is larger in man than in women (a difference arising at childhood and puberty). This hypothalamic region undergoes a gender specific reduction during aging reaching 10-15% of the number at childhood (Hofman 1997).
Of particular interest is also that the suprachiasmatic nucleus (SCN) of the hypothalamus, regulating the rhythmicity of biological processes, undergoes age-dependent degeneration possibly contributing to the reduced amplitude of the circadian temperature profile and contributing to compromised temperature homeostasis. These effects have been studied in non-human primates, and seem to be partly due to changes in melatonin levels and its effects on the SCN (Aujard et al. 1998; Perret et al. 2001; Van Someren et al. 2002).
Summary and Conclusions
Temperature and caloric homeostasis are two components of energy homeostasis influencing each other. Aging can be slowed by lowering core body temperature and by calorie restriction that in homeotherms also cause lowered Tcore. The confluence of nutrient homeostasis and that of temperature homeostasis occurs anatomically in different nuclei of the hypothalamus. Temperature regulation relies on temperature sensitive neurons found in the preoptic area (POA) that can be distinguished in warm and cold sensitive. These cells are elements of the so called “central thermostat” that maintains a nearly constant Tcore at different environmental temperature. Changes in the hypothalamic temperature set-point occurring during fever or with dietary restriction are important survival mechanisms influencing metabolic rate and aging. Investigating the interaction between the POA/temperature sensitive neurons and nutrient sensing neurons in other hypothalamic regions including the ARC and the PVN may be key to understand the integration of energy metabolism and temperature homeostasis and their effects on aging. The neuroendocrine hypothalmus is also known to undergo changes with aging and these changes also affect its sensitivity to thermal signals.
Acknowledgements
Supported by The Ellison Medical Foundation and NIH AG028040.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alam MN, McGinty D, Szymusiak R. Preoptic/anterior hypothalamic neurons: thermosensitivity in rapid eye movement sleep. Am J Physiol. 1995;269:R1250–7. doi: 10.1152/ajpregu.1995.269.5.R1250. [DOI] [PubMed] [Google Scholar]
- Alcedo J, Kenyon C. Regulation of C. elegans longevity by specific gustatory and olfactory neurons. Neuron. 2004;41:45–55. doi: 10.1016/s0896-6273(03)00816-x. [DOI] [PubMed] [Google Scholar]
- Anderson RM, Weindruch R. Calorie restriction: progress during mid-2005-mid-2006. Exp Gerontol. 2006;41:1247–9. doi: 10.1016/j.exger.2006.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aujard F, Perret M, Vannier G. Thermoregulatory responses to variations of photoperiod and ambient temperature in the male lesser mouse lemur: a primitive or an advanced adaptive character? J Comp Physiol [B] 1998;168:540–8. doi: 10.1007/s003600050175. [DOI] [PubMed] [Google Scholar]
- Barbour HG. Effect of direct warming and cooling of the heat centers on body temperature. Naunyn-Schmiedeberg's Archiv. 1912;70:1–26. [Google Scholar]
- Barker JL, Carpenter DO. Thermosensitivity of neurons in the sensorimotor cortex of the cat. Science. 1970;169:597–8. doi: 10.1126/science.169.3945.597. [DOI] [PubMed] [Google Scholar]
- Bartke A, Coschigano K, Kopchick J, Chandrashekar V, Mattison J, Kinney B, Hauck S. Genes that prolong life: relationships of growth hormone and growth to aging and life span. J Gerontol A Biol Sci Med Sci. 2001;56:B340–9. doi: 10.1093/gerona/56.8.b340. [DOI] [PubMed] [Google Scholar]
- Bernardis LL, Davis PJ. Aging and the hypothalamus: research perspectives. Physiol Behav. 1996;59:523–36. doi: 10.1016/0031-9384(95)02101-9. [DOI] [PubMed] [Google Scholar]
- Bishop NA, Guarente L. Genetic links between diet and lifespan: shared mechanisms from yeast to humans. Nat Rev Genet. 2007;8:835–44. doi: 10.1038/nrg2188. [DOI] [PubMed] [Google Scholar]
- Bishop NA, Guarente L. Two neurons mediate diet-restriction-induced longevity in C. elegans. Nature. 2007;447:545–9. doi: 10.1038/nature05904. [DOI] [PubMed] [Google Scholar]
- Bligh J. North-Holland Publishing Company; Amsterdam: 1973. Temperature Regulation in Mammals and other Vertebrates. [Google Scholar]
- Bluher M, Kahn BB, Kahn CR. Extended longevity in mice lacking the insulin receptor in adipose tissue. Science. 2003;299:572–4. doi: 10.1126/science.1078223. [DOI] [PubMed] [Google Scholar]
- Boulant JA. Hypothalamic mechanisms in thermoregulation. Fed Proc. 1981;40:2843–50. [PubMed] [Google Scholar]
- Boulant JA. Cellular mechanisms of temperature sensitivity in hypothalamic neurons. Prog Brain Res. 1998;115:3–8. doi: 10.1016/s0079-6123(08)62026-9. [DOI] [PubMed] [Google Scholar]
- Boulant JA, Hardy JD. The effect of spinal and skin temperatures on the firing rate and thermosensitivity of preoptic neurones. J Physiol. 1974;240:639–60. doi: 10.1113/jphysiol.1974.sp010627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulant JA, Silva NL. Interactions of reproductive steroids, osmotic pressure, and glucose on thermosensitive neurons in preoptic tissue slices. Can J Physiol Pharmacol. 1987;65:1267–73. doi: 10.1139/y87-202. [DOI] [PubMed] [Google Scholar]
- Broughton S, Partridge L. Insulin/IGF-like signalling, the central nervous system and aging. Biochem J. 2009;418:1–12. doi: 10.1042/BJ20082102. [DOI] [PubMed] [Google Scholar]
- Broughton SJ, Piper MD, Ikeya T, Bass TM, Jacobson J, Driege Y, Martinez P, Hafen E, Withers DJ, Leevers SJ, Partridge L. Longer lifespan, altered metabolism, and stress resistance in Drosophila from ablation of cells making insulin-like ligands. Proc Natl Acad Sci U S A. 2005;102:3105–10. doi: 10.1073/pnas.0405775102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen XM, Hosono T, Yoda T, Fukuda Y, Kanosue K. Efferent projection from the preoptic area for the control of non-shivering thermogenesis in rats. J Physiol. 1998;512(Pt 3):883–92. doi: 10.1111/j.1469-7793.1998.883bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark G, Magoun HW, Ranson SW. Hypothalamic regulation of body temperature. Journal of Neurophysiology. 1939;2:61–80. [Google Scholar]
- Conti B, Sanchez-Alavez M, Winsky-Sommerer R, Morale MC, Lucero J, Brownell S, Fabre V, Huitron-Resendiz S, Henriksen S, Zorrilla EP, de Lecea L, Bartfai T. Transgenic mice with a reduced core body temperature have an increased life span. Science. 2006;314:825–8. doi: 10.1126/science.1132191. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci. 2002;3:655–66. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Curras MC, Kelso SR, Boulant JA. Intracellular analysis of inherent and synaptic activity in hypothalamic thermosensitive neurones in the rat. J Physiol. 1991;440:257–71. doi: 10.1113/jphysiol.1991.sp018707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis CN, Tabarean I, Gaidarova S, Behrens MM, Bartfai T. IL-1beta induces a MyD88-dependent and ceramide-mediated activation of Src in anterior hypothalamic neurons. J Neurochem. 2006;98:1379–89. doi: 10.1111/j.1471-4159.2006.03951.x. [DOI] [PubMed] [Google Scholar]
- Diem R, Hobom M, Grotsch P, Kramer B, Bahr M. Interleukin-1 beta protects neurons via the interleukin-1 (IL-1) receptor-mediated Akt pathway and by IL-1 receptor-independent decrease of transmembrane currents in vivo. Mol Cell Neurosci. 2003;22:487–500. doi: 10.1016/s1044-7431(02)00042-8. [DOI] [PubMed] [Google Scholar]
- Dodson PD, Forsythe ID. Presynaptic K+ channels: electrifying regulators of synaptic terminal excitability. Trends Neurosci. 2004;27:210–7. doi: 10.1016/j.tins.2004.02.012. [DOI] [PubMed] [Google Scholar]
- Druzin M, Haage D, Malinina E, Johansson S. Dual and opposing roles of presynaptic Ca2+ influx for spontaneous GABA release from rat medial preoptic nerve terminals. J Physiol. 2002;542:131–46. doi: 10.1113/jphysiol.2001.015610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodrick CL, Ingram DK, Reynolds MA, Freeman JR, Cider N. Effects of intermittent feeding upon body weight and lifespan in inbred mice: interaction of genotype and age. Mech Ageing Dev. 1990;55:69–87. doi: 10.1016/0047-6374(90)90107-q. [DOI] [PubMed] [Google Scholar]
- Gordon CJ, Heath JE. Effects of prostaglandin E2 on the activity of thermosensitive and insensitive single units in the preoptic/anterior hypothalamus of unanesthetized rabbits. Brain Res. 1980;183:113–21. doi: 10.1016/0006-8993(80)90123-7. [DOI] [PubMed] [Google Scholar]
- Griffin JD, Saper CB, Boulant JA. Synaptic and morphological characteristics of temperature-sensitive and -insensitive rat hypothalamic neurones. J Physiol. 2001;537:521–35. doi: 10.1111/j.1469-7793.2001.00521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammel HT. Neurons and temperature regulation. In: Yamamoto WS, Brobeck JR, editors. Physiological controls and regulations. Saunders; Philadelphia: 1965. pp. 71–97. [Google Scholar]
- Hammel HT, Hardy JD, Fusco MM. Thermoregulatory responses to hypothalamic cooling in unanesthetized dogs. Am J Physiol. 1960;198:481–6. doi: 10.1152/ajplegacy.1960.198.3.481. [DOI] [PubMed] [Google Scholar]
- Hammel HT, Jackson DC, Stolwijk JA, Hardy JD, Stromme SB. Temperature Regulation by Hypothalamic Proportional Control with an Adjustable Set Point. J Appl Physiol. 1963;18:1146–54. doi: 10.1152/jappl.1963.18.6.1146. [DOI] [PubMed] [Google Scholar]
- Hauck SJ, Bartke A. Free radical defenses in the liver and kidney of human growth hormone transgenic mice: possible mechanisms of early mortality. J Gerontol A Biol Sci Med Sci. 2001;56:B153–62. doi: 10.1093/gerona/56.4.b153. [DOI] [PubMed] [Google Scholar]
- Hauck SJ, Hunter WS, Danilovich N, Kopchick JJ, Bartke A. Reduced levels of thyroid hormones, insulin, and glucose, and lower body core temperature in the growth hormone receptor/binding protein knockout mouse. Exp Biol Med (Maywood) 2001;226:552–8. doi: 10.1177/153537020122600607. [DOI] [PubMed] [Google Scholar]
- Hays T, Morairty S, Szymusiak R, McGinty D. The effects of temperature and synaptic blockade in vitro on neurons of the horizontal limb of the diagonal band of Broca in the rat basal forebrain. Brain Res. 1997;746:52–8. doi: 10.1016/s0006-8993(96)01138-9. [DOI] [PubMed] [Google Scholar]
- Heilbronn LK, de Jonge L, Frisard MI, DeLany JP, Larson-Meyer DE, Rood J, Nguyen T, Martin CK, Volaufova J, Most MM, Greenway FL, Smith SR, Deutsch WA, Williamson DA, Ravussin E. Effect of 6-month calorie restriction on biomarkers of longevity, metabolic adaptation, and oxidative stress in overweight individuals: a randomized controlled trial. JAMA. 2006;295:1539–48. doi: 10.1001/jama.295.13.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofman MA. Lifespan changes in the human hypothalamus. Exp Gerontol. 1997;32:559–75. doi: 10.1016/s0531-5565(96)00162-3. [DOI] [PubMed] [Google Scholar]
- Holzenberger M, Dupont J, Ducos B, Leneuve P, Geloen A, Even PC, Cervera P, Bouc Y. Le. IGF-1 receptor regulates lifespan and resistance to oxidative stress in mice. Nature. 2003;421:182–7. doi: 10.1038/nature01298. [DOI] [PubMed] [Google Scholar]
- Hunter WS, Croson WB, Bartke A, Gentry MV, Meliska CJ. Low body temperature in long-lived Ames dwarf mice at rest and during stress. Physiol Behav. 1999;67:433–7. doi: 10.1016/s0031-9384(99)00098-0. [DOI] [PubMed] [Google Scholar]
- Ishikawa Y, Nakayama T, Kanosue K, Matsumura K. Activation of central warm-sensitive neurons and the tail vasomotor response in rats during brain and scrotal thermal stimulation. Pflugers Arch. 1984;400:222–7. doi: 10.1007/BF00581551. [DOI] [PubMed] [Google Scholar]
- Kanosue K, Yanase-Fujiwara M, Hosono T. Hypothalamic network for thermoregulatory vasomotor control. Am J Physiol. 1994;267:R283–8. doi: 10.1152/ajpregu.1994.267.1.R283. [DOI] [PubMed] [Google Scholar]
- Kanosue K, Zhang YH, Yanase-Fujiwara M, Hosono T. Hypothalamic network for thermoregulatory shivering. Am J Physiol. 1994;267:R275–82. doi: 10.1152/ajpregu.1994.267.1.R275. [DOI] [PubMed] [Google Scholar]
- Katz B, Miledi R. The effect of temperature on the synaptic delay at the neuromuscular junction. J Physiol. 1965;181:656–70. doi: 10.1113/jphysiol.1965.sp007790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller AD. Separation in the brain stem of the mechanisms of heat loss from those of heat production. Journal of Neurophysiology. 1938;1:543–557. [Google Scholar]
- Kelso SR, Boulant JA. Effect of synaptic blockade on thermosensitive neurons in hypothalamic tissue slices. Am J Physiol. 1982;243:R480–90. doi: 10.1152/ajpregu.1982.243.5.R480. [DOI] [PubMed] [Google Scholar]
- Kenney WL, Munce TA. Invited review: aging and human temperature regulation. J Appl Physiol. 2003;95:2598–603. doi: 10.1152/japplphysiol.00202.2003. [DOI] [PubMed] [Google Scholar]
- Kiyohara T, Hirata M, Hori T, Akaike N. Hypothalamic warm-sensitive neurons possess a tetrodotoxin-sensitive sodium channel with a high Q10. Neurosci Res. 1990;8:48–53. doi: 10.1016/0168-0102(90)90056-k. [DOI] [PubMed] [Google Scholar]
- Kobayashi S, Takahashi T. Whole-cell properties of temperature-sensitive neurons in rat hypothalamic slices. Proc Biol Sci. 1993;251:89–94. doi: 10.1098/rspb.1993.0013. [DOI] [PubMed] [Google Scholar]
- Koizumi A, Wada Y, Tuskada M, Kayo T, Naruse M, Horiuchi K, Mogi T, Yoshioka M, Sasaki M, Miyamaura Y, Abe T, Ohtomo K, Walford RL. A tumor preventive effect of dietary restriction is antagonized by a high housing temperature through deprivation of torpor. Mech Ageing Dev. 1996;92:67–82. doi: 10.1016/s0047-6374(96)01803-9. [DOI] [PubMed] [Google Scholar]
- Lane MA, Baer DJ, Rumpler WV, Weindruch R, Ingram DK, Tilmont EM, Cutler RG, Roth GS. Calorie restriction lowers body temperature in rhesus monkeys, consistent with a postulated anti-aging mechanism in rodents. Proc Natl Acad Sci U S A. 1996;93:4159–64. doi: 10.1073/pnas.93.9.4159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lans H, Jansen G. Multiple sensory G proteins in the olfactory, gustatory and nociceptive neurons modulate longevity in Caenorhabditis elegans. Dev Biol. 2007;303:474–82. doi: 10.1016/j.ydbio.2006.11.028. [DOI] [PubMed] [Google Scholar]
- Lazarus M, Yoshida K, Coppari R, Bass CE, Mochizuki T, Lowell BB, Saper CB. EP3 prostaglandin receptors in the median preoptic nucleus are critical for fever responses. Nat Neurosci. 2007;10:1131–3. doi: 10.1038/nn1949. [DOI] [PubMed] [Google Scholar]
- Leibowitz SF, Wortley KE. Hypothalamic control of energy balance: different peptides, different functions. Peptides. 2004;25:473–504. doi: 10.1016/j.peptides.2004.02.006. [DOI] [PubMed] [Google Scholar]
- Leto S, Kokkonen GC, Barrows CH., Jr. Dietary protein, life-span, and biochemical variables in female mice. J Gerontol. 1976;31:144–8. doi: 10.1093/geronj/31.2.144. [DOI] [PubMed] [Google Scholar]
- Libert S, Zwiener J, Chu X, Vanvoorhies W, Roman G, Pletcher SD. Regulation of Drosophila life span by olfaction and food-derived odors. Science. 2007;315:1133–7. doi: 10.1126/science.1136610. [DOI] [PubMed] [Google Scholar]
- Liu R, Walford RL. Increased growth and life-span with lowered ambient temperature in the annual fish Cynolebia adloffi. Nature. 1966:1277–1278. [Google Scholar]
- Liu RK, Walford RL. The effect of lowered body temperature on lifespan and immune and non-immune processes. Gerontologia. 1972;18:363–88. doi: 10.1159/000211944. [DOI] [PubMed] [Google Scholar]
- Llinas R, Yarom Y. Oscillatory properties of guinea-pig inferior olivary neurones and their pharmacological modulation: an in vitro study. J Physiol. 1986;376:163–82. doi: 10.1113/jphysiol.1986.sp016147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mair W, Dillin A. Aging and survival: the genetics of life span extension by dietary restriction. Annu Rev Biochem. 2008;77:727–54. doi: 10.1146/annurev.biochem.77.061206.171059. [DOI] [PubMed] [Google Scholar]
- Mair W, Goymer P, Pletcher SD, Partridge L. Demography of dietary restriction and death in Drosophila. Science. 2003;301:1731–3. doi: 10.1126/science.1086016. [DOI] [PubMed] [Google Scholar]
- Masoro EJ. Overview of caloric restriction and ageing. Mech Ageing Dev. 2005;126:913–22. doi: 10.1016/j.mad.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Matsuda T, Hori T, Nakashima T. Thermal and PGE2 sensitivity of the organum vasculosum lamina terminalis region and preoptic area in rat brain slices. J Physiol. 1992;454:197–212. doi: 10.1113/jphysiol.1992.sp019260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattison JA, Lane MA, Roth GS, Ingram DK. Calorie restriction in rhesus monkeys. Exp Gerontol. 2003;38:35–46. doi: 10.1016/s0531-5565(02)00146-8. [DOI] [PubMed] [Google Scholar]
- McCay CM, Crowell MF, Maynard LA. The Effect of Retarded Growth Upon the Length of Life Span and Upon the Ultimate Body Size: One Figure. J. Nutr. 1935:63–79. [PubMed] [Google Scholar]
- Morimoto A, Murakami N, Nakamori T, Watanabe T. Multiple control of fever production in the central nervous system of rabbits. J Physiol. 1988;397:269–80. doi: 10.1113/jphysiol.1988.sp017000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison CD, Berthoud HR. Neurobiology of nutrition and obesity. Nutr Rev. 2007;65:517–34. doi: 10.1301/nr.2007.dec.517-534. [DOI] [PubMed] [Google Scholar]
- Morrison SF, Nakamura K, Madden CJ. Central control of thermogenesis in mammals. Exp Physiol. 2008;93:773–97. doi: 10.1113/expphysiol.2007.041848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagashima K, Nakai S, Tanaka M, Kanosue K. Neuronal circuitries involved in thermoregulation. Auton Neurosci. 2000;85:18–25. doi: 10.1016/S1566-0702(00)00216-2. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Matsumura K, Kaneko T, Kobayashi S, Katoh H, Negishi M. The rostral raphe pallidus nucleus mediates pyrogenic transmission from the preoptic area. J Neurosci. 2002;22:4600–10. doi: 10.1523/JNEUROSCI.22-11-04600.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Morrison SF. Central efferent pathways mediating skin cooling-evoked sympathetic thermogenesis in brown adipose tissue. Am J Physiol Regul Integr Comp Physiol. 2007;292:R127–36. doi: 10.1152/ajpregu.00427.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Morrison SF. Identification of lateral parabrachial neurons mediating cutaneous thermoregulatory afferent signals to the preoptic area. Soc Neurosci Abstr. 2007;33:731.713. [Google Scholar]
- Nakamura K, Morrison SF. A thermosensory pathway that controls body temperature. Nat Neurosci. 2008;11:62–71. doi: 10.1038/nn2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y, Nakamura K, Matsumura K, Kobayashi S, Kaneko T, Morrison SF. Direct pyrogenic input from prostaglandin EP3 receptor-expressing preoptic neurons to the dorsomedial hypothalamus. Eur J Neurosci. 2005;22:3137–46. doi: 10.1111/j.1460-9568.2005.04515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama T, Eisenman JS, Hardy JD. Single unit activity of anterior hypothalamus during local heating. Science. 1961;134:560–1. doi: 10.1126/science.134.3478.560. [DOI] [PubMed] [Google Scholar]
- Nakayama T, Hammel HT, Hardy JD, Eisenman JS. Thermal stimulation of electrical activity of single units of preoptic region. American Journal of Physiology. 1963;204:1122. &. [Google Scholar]
- Norman DC. Fever in the elderly. Clin Infect Dis. 2000;31:148–51. doi: 10.1086/313896. [DOI] [PubMed] [Google Scholar]
- Osaka T. Thermogenesis elicited by skin cooling in anaesthetized rats: lack of contribution of the cerebral cortex. J Physiol. 2004;555:503–13. doi: 10.1113/jphysiol.2003.053215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osaka T. LPS-induced thermogenesismediated by GABA in the preoptic area of anesthetized rats. J Thermal Biol. 2006;31:229–34. [Google Scholar]
- Osborne PG, Onoe H, Watanabe Y. GABAergic system inducing hyperthermia in the rat preoptic area: its independence of prostaglandin E2 system. Brain Res. 1994;661:237–42. doi: 10.1016/0006-8993(94)91200-9. [DOI] [PubMed] [Google Scholar]
- Paglia DE, Walford RL. Atypical hematological response to combined calorie restriction and chronic hypoxia in Biosphere 2 crew: a possible link to latent features of hibernation capacity. Habitation (Elmsford) 2005;10:79–85. doi: 10.3727/154296605774791223. [DOI] [PubMed] [Google Scholar]
- Perret M, Aujard F. Daily hypothermia and torpor in a tropical primate: synchronization by 24-h light-dark cycle. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1925–33. doi: 10.1152/ajpregu.2001.281.6.R1925. [DOI] [PubMed] [Google Scholar]
- Promislow DE, Tatar M, Pletcher SD, Carey JR. Below-threshold mortality and its impact on studies in evolutionary ecology. Journal of Evolutionary Biology. 1999;12:314–328. [Google Scholar]
- Ranels HJ, Griffin JD. The effects of prostaglandin E2 on the firing rate activity of thermosensitive and temperature insensitive neurons in the ventromedial preoptic area of the rat hypothalamus. Brain Res. 2003;964:42–50. doi: 10.1016/s0006-8993(02)04063-5. [DOI] [PubMed] [Google Scholar]
- Ranson SW, Clark G, Magoun HW. The effect of hypothalamic lesions on fever induced by intravenous injection of typhoid-paratyphoid vaccine. Journal of Laboratory and Clinical Medicine. 1939;25:0160–0168. [Google Scholar]
- Rikke BA, Yerg JE, 3rd, Battaglia ME, Nagy TR, Allison DB, Johnson TE. Strain variation in the response of body temperature to dietary restriction. Mech Ageing Dev. 2003;124:663–78. doi: 10.1016/s0047-6374(03)00003-4. [DOI] [PubMed] [Google Scholar]
- Romanovsky AA. Thermoregulation: some concepts have changed. Functional architecture of the thermoregulatory system. Am J Physiol Regul Integr Comp Physiol. 2007;292:R37–46. doi: 10.1152/ajpregu.00668.2006. [DOI] [PubMed] [Google Scholar]
- Sanchez-Alavez M, Tabarean IV, Behrens MM, Bartfai T. Ceramide mediates the rapid phase of febrile response to IL-1{beta} Proc Natl Acad Sci U S A. 2006 doi: 10.1073/pnas.0510960103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva NL, Boulant JA. Effects of osmotic pressure, glucose, and temperature on neurons in preoptic tissue slices. Am J Physiol. 1984;247:R335–45. doi: 10.1152/ajpregu.1984.247.2.R335. [DOI] [PubMed] [Google Scholar]
- Silva NL, Boulant JA. Effects of testosterone, estradiol, and temperature on neurons in preoptic tissue slices. Am J Physiol. 1986;250:R625–32. doi: 10.1152/ajpregu.1986.250.4.R625. [DOI] [PubMed] [Google Scholar]
- Simon E, Pierau FK, Taylor DC. Central and peripheral thermal control of effectors in homeothermic temperature regulation. Physiol Rev. 1986;66:235–300. doi: 10.1152/physrev.1986.66.2.235. [DOI] [PubMed] [Google Scholar]
- Sun JR, Ma YC, Xu ZH, Zhao WJ, Cai YP. [Effect of norepinephrine on the thermosensitive neurons in preoptic area of hypothalamus tissue slices in cold acclimatized rats] Sheng Li Xue Bao. 1997;49:666–70. [PubMed] [Google Scholar]
- Sutarmo Setiadji V, Shibuya I, Kabashima N, Ibrahim N, Harayama N, Ueta Y, Yamashita H. Actions of prostaglandin E2 on rat supraoptic neurones. J Neuroendocrinol. 1998;10:927–36. doi: 10.1046/j.1365-2826.1998.00282.x. [DOI] [PubMed] [Google Scholar]
- Tabarean IV, Behrens MM, Bartfai T, Korn H. Prostaglandin E2-increased thermosensitivity of anterior hypothalamic neurons is associated with depressed inhibition. Proc Natl Acad Sci U S A. 2004;101:2590–5. doi: 10.1073/pnas.0308718101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabarean IV, Conti B, Behrens M, Korn H, Bartfai T. Electrophysiological properties and thermosensitivity of mouse preoptic and anterior hypothalamic neurons in culture. Neuroscience. 2005;135:433–49. doi: 10.1016/j.neuroscience.2005.06.053. [DOI] [PubMed] [Google Scholar]
- Tabarean IV, Korn H, Bartfai T. Interleukin-1beta induces hyperpolarization and modulates synaptic inhibition in preoptic and anterior hypothalamic neurons. Neuroscience. 2006;141:1685–95. doi: 10.1016/j.neuroscience.2006.05.007. [DOI] [PubMed] [Google Scholar]
- Taguchi A, Wartschow LM, White MF. Brain IRS2 signaling coordinates life span and nutrient homeostasis. Science. 2007;317:369–72. doi: 10.1126/science.1142179. [DOI] [PubMed] [Google Scholar]
- Tanaka H, Kanosue K, Nakayama T, Shen Z. Grooming, body extension, and vasomotor responses induced by hypothalamic warming at different ambient temperatures in rats. Physiol Behav. 1986;38:145–51. doi: 10.1016/0031-9384(86)90145-9. [DOI] [PubMed] [Google Scholar]
- Tatar M. Senescence. Oxford University Press; Oxford: 2001. [Google Scholar]
- Tatar M, Carey JR, Vaupel JW. Long term cost of reproduction with and without accelerated senescence in Callosobruchus maculatus: analysis of age-specific mortality. Evolution. 1993:1302–1312. doi: 10.1111/j.1558-5646.1993.tb02156.x. [DOI] [PubMed] [Google Scholar]
- Travis KA, Johnson AK. In vitro sensitivity of median preoptic neurons to angiotensin II, osmotic pressure, and temperature. Am J Physiol. 1993;264:R1200–5. doi: 10.1152/ajpregu.1993.264.6.R1200. [DOI] [PubMed] [Google Scholar]
- Van Someren EJ, Raymann RJ, Scherder EJ, Daanen HA, Swaab DF. Circadian and age-related modulation of thermoreception and temperature regulation: mechanisms and functional implications. Ageing Res Rev. 2002;1:721–78. doi: 10.1016/s1568-1637(02)00030-2. [DOI] [PubMed] [Google Scholar]
- Vasilenko VY, Petruchuk TA, Gourine VN, Pierau FK. Interleukin-1beta reduces temperature sensitivity but elevates thermal thresholds in different populations of warm-sensitive hypothalamic neurons in rat brain slices. Neurosci Lett. 2000;292:207–10. doi: 10.1016/s0304-3940(00)01470-1. [DOI] [PubMed] [Google Scholar]
- Walford RL. Maximum Lifespan. WW Norton; New York, NY: 1983. [Google Scholar]
- Walford RL, Weber L, Panov S. Caloric restriction and aging as viewed from Biosphere 2. Receptor. 1995;5:29–33. [PubMed] [Google Scholar]
- Wang MC, Bohmann D, Jasper H. JNK extends life span and limits growth by antagonizing cellular and organism-wide responses to insulin signaling. Cell. 2005;121:115–25. doi: 10.1016/j.cell.2005.02.030. [DOI] [PubMed] [Google Scholar]
- Weinert D, Waterhouse J. The circadian rhythm of core temperature: effects of physical activity and aging. Physiol Behav. 2007;90:246–56. doi: 10.1016/j.physbeh.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Wolkow CA, Kimura KD, Lee MS, Ruvkun G. Regulation of C. elegans life-span by insulinlike signaling in the nervous system. Science. 2000;290:147–50. doi: 10.1126/science.290.5489.147. [DOI] [PubMed] [Google Scholar]
- Zaretskaia MV, Zaretsky DV, DiMicco JA. Role of the dorsomedial hypothalamus in thermogenesis and tachycardia caused by microinjection of prostaglandin E2 into the preoptic area in anesthetized rats. Neurosci Lett. 2003;340:1–4. doi: 10.1016/s0304-3940(03)00047-8. [DOI] [PubMed] [Google Scholar]
- Zhang YH, Hosono T, Yanase-Fujiwara M, Chen XM, Kanosue K. Effect of midbrain stimulations on thermoregulatory vasomotor responses in rats. J Physiol. 1997;503(Pt 1):177–86. doi: 10.1111/j.1469-7793.1997.177bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YH, Yanase-Fujiwara M, Hosono T, Kanosue K. Warm and cold signals from the preoptic area: which contribute more to the control of shivering in rats? J Physiol. 1995;485(Pt 1):195–202. doi: 10.1113/jphysiol.1995.sp020723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Boulant JA. Temperature effects on neuronal membrane potentials and inward currents in rat hypothalamic tissue slices. J Physiol. 2005;564:245–57. doi: 10.1113/jphysiol.2004.075473. [DOI] [PMC free article] [PubMed] [Google Scholar]


