Abstract
Cachexia is a devastating syndrome of body wasting that is associated with multiple common chronic diseases including cancer, chronic kidney disease, and chronic heart failure. These underlying diseases are associated with increased levels of inflammatory cytokines and result in anorexia, increased resting energy expenditure and loss of fat and lean body mass. Prior experiments have implicated the central melanocortin system in the hypothalamus with the propagation of these symptoms of cachexia. Pharmacologic blockade of this system using melanocortin antagonists causes attenuation of the signs of cachexia in laboratory models. Recent advances in our knowledge of this disease process have involved further elucidation of the pathophysiology of melanocortin activation and demonstration of the efficacy of melanocortin antagonists in new models of cachexia, including cardiac cachexia. Additionally, small molecule antagonists of the melanocortin-4 receptor (MC4R) continue to be introduced, including ones with oral bioavailability. These developments generate optimism that melanocortin antagonism will be used to treat humans with disease-associated cachexia. However, to date human application has remained elusive and it is unclear when we will know whether humans with cachexia would benefit from treatment with these compounds.
Introduction
The past decade has provided an enlightening glimpse into the neural networks responsible for the suppression of appetite seen in disease-associated cachexia [1]. Disease processes as diverse as cancer, renal failure, cardiac failure and AIDS can result in this pathologic process that involves increased resting metabolic rate, loss of muscle and fat stores, and anorexia at a time when energy requirements are elevated. The similarity of this process across such diverse underlying disease states is partly explained by a unifying characteristic of these diseases—their increase in systemic cytokines [2]—and partly explained by the involvement of a vital appetite-regulating center in the hypothalamus: the central melanocortin system.
Since the initial description of this neural center's role in the process of cachexia in 2001 [3], insights continue to be elucidated regarding 1) the mechanisms through which the melanocortin system propagates features of cachexia 2) new disease indications that respond to melanocortin antagonism and 3) novel means of inhibiting the system. This review will focus on both our current understanding of the mechanism by which the melanocortin system produces cachexia of chronic disease and on updates regarding pharamacologic blockade of this system as a promising treatment for cachexia. We will close by considering outstanding issues needed to advance the field.
Melanocortin physiology
The central melanocortin system is located principally in the arcuate nucleus of the hypothalamus, in an area adjacent to the 3rd ventricle [4]. This is an area of relative permeability of the blood-brain barrier, giving the arcuate nucleus exposure to circulating indicators of disease activity, including inflammatory cytokines [5]. The melanocortin system is comprised of two types of neurons with opposing actions regarding appetite.
The first of these classes of neurons are anorexigenic in nature and express pro-opiomelanocortin (POMC), a large peptide that is cleaved to yield a-melanocyte stimulating hormone (α-MSH)(Figure 1). POMC-expressing neurons send processes that synapse with second order neurons in multiple areas around the brain and brainstem, including the paraventricular nucleus of the hypothalamus (also involved in appetite regulation), the lateral hypothalamus and the nucleus of the solitary tract in the brainstem [4]. Once α-MSH is released in synapses with these second order neurons, it binds to melanocortin 3 receptors and melanocortin 4 receptors (MC4R), leading to widespread downstream effects, including a decrease in food-seeking behavior, an increase in basal metabolic rate and a decrease in lean body mass [6-9]. As such, activation of these POMC neurons is a major source of the symptoms that are seen consistently in cachexia.
Figure 1. Model for activation of the central melanocortin system in the hypothalamus during cachexia.
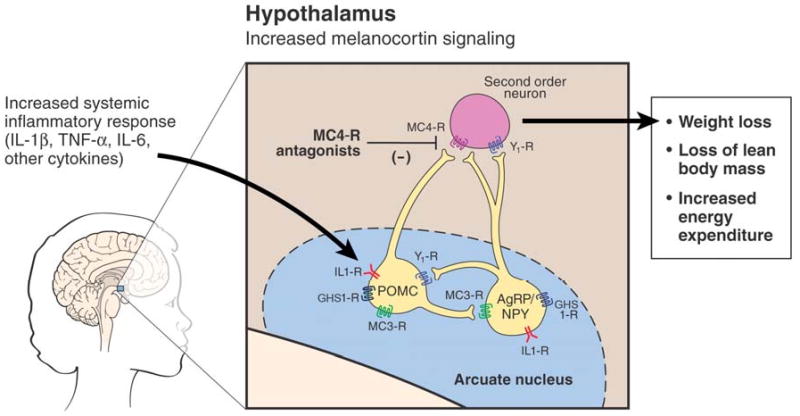
The melanocortin system is comprised of neurons expressing either POMC or AgRP, each or which express receptors to IL-1β. During cachexia inflammatory cytokines are released; IL-1β acts on the IL1-RI to increase release of a-MSH from POMC neurons and decrease release of AgRP. This causes an increase in activity at the melanocortin-4 receptors (MC4R) at second order neurons and downstream events characteristic of cachexia. Blockade of this signal by melanocortin antagonists attenuates these downstream events. Additionally, increased production of AgRP, as is caused by the effect of ghrelin at the GHS-1 receptor, also blocks melanocortin output. (Figure adapted from reference [50], used by permission.)
The second class of neurons in the melanocortin system are orexigenic in nature and express neuropeptide-Y (NPY) and Agouti related protein (AgRP)(Figure 1). AgRP is a natural inverse agonist of the MC4R, producing a decrease in the constant tone that POMC neurons place on restraining appetite. Thus, administration of AgRP or a synthetic MC4R antagonist into the intraventricular space result in an increase in food-seeking behavior [3,10]. Similarly, genetic mutation of the MC4R results in a decrease in melanocortin tone and is the most common monogenic cause of obesity [11,12]. The potential to reverse the anorexia, loss of lean body mass and increased basal energy expenditure that result from melanocortin activation has made antagonism of this system a major focus in the treatment for cachexia syndromes [3,13].
Recent insights into melanocortin physiology
Role of inflammation in POMC/AgRP neuronal activity
The past 3 years have further clarified multiple aspects of our understanding of the regulation of the central melanocortin system—particularly in the setting of cachexia. POMC and AgRP neurons have been known to express receptors to circulating factors such as leptin [14,15], which allows for appetite regulation based on a signal of long-term energy reserves. Administration of leptin had been shown to increase expression of POMC accompanied by a decrease in feeding behavior [16]. In an analogous manner, POMC and AgRP neurons have recently also been shown to express the receptor for IL-1β (Figure 1) [17,18]. This is significant with respect to cachexia, given the presence of inflammatory mediators that are seen in the various underlying diseases associated with cachexia [2], Recent work has demonstrated that when hypothalamic explants are exposed to IL-1β, POMC neurons respond by increasing release of α-MSH [17], while AgRP/NPY neurons respond by decreasing release of AgRP [18]. These responses thus likely affect activity at MC4-receptors on second order neurons during worsening cachexia, causing a decrease feeding response, an increase in BMR and an increase in catabolism of lean mass and fat mass.
Role of the brainstem in cachexia
Recent investigations also suggest a role for the brainstem in cachexia-associated anorexia. This point is particularly pertinent because the only location of POMC-expressing neurons outside of the hypothalamus is the nucleus of the solitary tract (NTS), a neural center in close proximity to the dorsal motor nucleus of the vagus, influencing gut motility and cardiovascular function [19,20]. Neurons in the NTS (though, interestingly, not POMC neurons) are transcriptionally-activated during cancer cachexia [21] and during injection of inflammatory cytokines into the NTS [17,22,23]. Moreover, administration of AgRP or other melanocortin antagonist into the region of the NTS increases food intake [24] and ameliorates inflammation-associated anorexia during a 12-hour period after injection of IL-1β into the 4th ventricle [22]. Thus, in addition to decreasing melanocortin output from the hypothalamus, treatment with MC4R antagonists may also act at brainstem centers in addition to hypothalamic centers to influence food intake.
Role of ghrelin in melanocortin inhibition
POMC and AgRP neurons also respond to circulating factors that are orexigenic in nature, such as the stomach hormone ghrelin. Both POMC and AgRP neurons express Growth Hormone Secretegogue receptor-1, the receptor to ghrelin (Figure 1), and treatment of cachexia with ghrelin has been shown to increase expression of AgRP, associated with an improvement in food intake and lean mass retention [25,26]. This serves as a reminder that additional treatments such as ghrelin or ghrelin agonists may provide a further way to manipulate melanocortin output toward improving cachexia-associated indices [27].
Genetic variation in MC4R
As the field of cachexia research moves closer to the use of melanocortin antagonists to treat humans with cachexia, an additional issue of interest has been in underlying variability in the MC4-receptor itself. Exploration into common polymorphisms of the MC4R have revealed a differential of cachexia responses during cancer [28]. Individuals who have were homozygotes for a Val103Ile polymorphism (approximately 2% of the population) appeared to be more resistant to cancer cachexia caused by solid tumors than were homozygotes for the more common allele. Though these finding need to be verified in a separate population, they are an intriguing extension of research into amino acid sequences of the MC4R that play a role in ligand binding or receptor function [29,30]. This underscores the possibility that not all individuals will respond identically to new melanocortin antagonists, and that some interventions may need to be tailored to a given patient's genotype [31].
Role of the central melanocortin system in blood pressure regulation
One final development in our understanding of the physiology of the melacortin system pertains to additional systemic effects mediated by the melocortin system. Recent evaluation of individuals who are deficient in the MC4R revealed that individuals that lack functional MC4R (leading to early-onset obesity) have lower blood pressure than weight-matched controls [32]. Futhermore, treatment of obese individuals with an agonist to MC4R (which acts like a-MSH and was developed as a potential treatment for obesity) were found to have an increase in blood pressure. Given the baseline hypertension commonly seen in obese individuals, this revelation of increased blood pressure during therapy to activate the melanocortin system raises questions regarding the safety of melanocortin agonists as a treatment for obesity. These findings also give one pause in wondering whether treatment with melanocortin antagonists may result in hypotension that would limit its therapeutic potential as well. Clearly a significant amount of further investigation is needed to begin to start answering some of these questions.
New animal models of cachexia demonstrating efficacy of melanocortin blockade
In addition to an expanded understanding of melanocortin physiology and output effects, efficacy of melanocortin inhibition continues to be demonstrated in an expanding number of cachexia-associated diseases. Having been previously shown to improve food intake and lean body mass in the settings of cancer cachexia and uremia, efficacy of melanocortin treatment has been recently demonstrated in the setting of chronic heart failure (CHF) [33] and radiation injury [34]. Confirmation of benefit in the setting of CHF represents a significant advance given that CHF is estimated to affect 5 million individuals in the U.S. [35]. Of these, approximately 15% suffer from cachexia due to their cardiac condition [36] and those who do have a worsening in survival [37].
In their experiments, Scarlett et al. examined the effect of melanocortin inhibition using two different models of cardiac failure: 1) surgical ligation of a branch of the left main coronary artery, producing myocardial infarction (MI), and 2) banding of the ascending aorta using titanium clips, resulting in congestive heart failure. Both models resulted in heart failure and cachexia with a >75% decrease in lean body mass accrual 6 weeks after surgery in wild-type animals receiving either of the procedures. In both cases, however, melanocortin antagonism completely ameliorated the loss of lean body mass.
When coronary artery ligation was performed in MC4R-KO mice, the mice did not display any decrease in lean body mass relative to sham-operated MC4R-KO mice and did not exhibit the increase in basal oxygen consumption seen in wild type mice with surgical MI. When aortic-banded rats received AgRP via ICV catheter as a means of blocking melanocortin output, they actually exhibited an increase in lean body mass accrual relative to sham-treated rats receiving artificial saline ICV. In this case the melanocortin inhibition more than compensated for lean mass losses due to heart failure.
These results, while not unexpected considering the efficacy of melanocortin antagonists in other settings of cachexia, confirm potential for melanocortin treatment among the large population of individuals with heart failure. An intriguing concept related to the lower blood pressure seen in humans with MC4R deletions is that afterload reduction—an effect not uncommonly sought in the treatment of heart failure [38]—may prove a further benefit to melanocortin antagonists in this setting, though clearly a vast amount of research is needed before this is clear.
Emergence of bioavailable small-molecule inhibitors
Initial demonstrations of efficacy of melanocortin antagonism—including the CHF study just described—relied on either ICV treatment with AgRP or animals with genetic deletions of MC4R. These experiments were then followed by treatment with small molecules inhibitors which themselves were administered via ICV catheters [3,39-41]. Perhaps the biggest advance in the field of melanocortin antagonism from a clinical perspective has been the introduction of peripherally-administrated compounds with efficacy in cachexia treatment (Table 1) [8,42-48]. Over the past 5 years, the field has rapidly evolved to the point were at least 7 small molecule inhibitors have been reported in the scientific literature to have efficacy following peripheral administration in a variety of animal models of disease-associated cachexia. Table 1 lists these small molecule inhibitors, including 2 compounds with oral bioavailability [42,46]—which are more likely to be preferred in clinical use if equally efficacious as compounds requiring subcutaneous injection.
Table 1.
Peripheral melanocortin antagonists with efficacy in rodent models of cachexia.
| Reference | Cachexia model | Melanocortin antagonist | Route, duration of treatment | Effects of treatment with melanocortin antagonist |
|---|---|---|---|---|
| Cancer Cachexia | ||||
| Oral administration | ||||
| Weyermann 200942 | C26 adenocarcinoma (colorectal tumor) |
SNT207707 | Oral once daily × 15 d | Body weight: gain 1% vs. -1.1% tumor control*** Fat mass change: +0.6 g vs. -0.6 g control* |
| SNT209858 | Oral once daily × 15 d | Body weight: gain 1% vs. -3% tumor control*** Fat mass change: +0.02 g vs. -1.15 g control* |
||
| Chen 200846 | Lewis Lung Carcinoma | Piperazine (name not yet attributed) |
Oral twice daily × 4 d | Body weight: +1.9% vs. -2.8% control* |
| IP/SC administration | ||||
| Chen 200846 | Lewis Lung Carcinoma | Piperazine (name not yet attributed) |
IP bid × 4 d | Food intake (day 13 of tumor burden): 2-fold increase above controls* |
| Tran 200744 | Lewis Lung Carcinoma | Phenyl piperazine (name not yet attributed) |
IP bid × 4 d | Food intake (day 14 of tumor burden): increased 86% above control* Lean body mass accrual: 7-fold above control* |
| Jiang 200745 | Lewis Lung Carcinoma | Pyrrolidinone (name not yet attributed) |
SC bid × 4 d | Food intake (day 13 of tumor burden): increased 82% above control* Lean body mass: -0.5% vs -9.5% control* |
| Vos 200448 | C26 adenocarcinoma (colorectal tumor) |
ML00253764 | SC bid × 11 d | Body weight: +8.3% vs. -7.2% control** |
| Markinson 20058 | Lewis Lung Carcinoma | NBI-12i | IP bid × 4 d | Food intake (day 13 of tumor burden): increased 90% above control* Lean body mass accrual: increased 4-fold above control* |
| Nicholson 200647 | Lewis Lung Carcinoma | ML00253764 | SC bid × 13 d | Lean body mass: unchanged vs. -4.8% for control** |
| Uremia | ||||
| Cheung 200743 | 5/6 nephrectomy | NBI-12i | IP bid × 14d | Weight gain: increased 2.7 fold above nephrectomy control* Lean body mass: 2% gain vs. -0.5% loss for nephrectomy control* |
Future needs
Despite recent advances in our knowledge of the melanocortin physiology and our ability to inhibit the system in laboratory models of cachexia, multiple questions remain, some of which give cause for optimism and some give cause for concern. Many of these questions relate to limitations of the rodent models of cachexia that have provided all of our information regarding the effects of melanocortin antagonism. Because of ethical concerns about the morbidity of the mice and rats used in these models, we are unable to test the long-term prognosis for animals receiving cachexia treatment. It has long been felt that improving the symptoms of cachexia may in turn improve the underlying disease process as well, and this remains an untested hope in the field of melanocortin antagonism.
Also unknown is whether long-term treatment with these compounds will lead to increased tolerance and diminished efficacy on a long-term basis, since redundant pathways may lead to similar systemic effects. The CHF model discussed previously [33] employed the longest melanocortin treatment to date (6 weeks) with continued efficacy; melanocortin treatments of this duration have thus far not been tested in models of cancer cachexia.
Finally, rodent models also make it difficult to adequately follow the sequelae of other systemic effects of melanocortin antagonism, such as possible changes in blood pressure and potentially other unanticipated effects. Unfortunately, the majority of these important issues may only be answered when melanocortin antagonism is tested in humans. To date there have been no published trials in humans, nor are such trials listed in the NIH clinical trials registry [49].
Thus, the success of melanocortin antagonists in animal models continues to buoy hopes regarding application of these compounds in humans suffering from disease associated cachexia. Still, many questions regarding safety and long-term efficacy remain and are unlikely to be answered by animal models alone. Many of these questions will require testing in humans, a step we await with cautious optimism.
Acknowledgments
Funding Sources: NIH HD060739-01
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tisdale MJ. Biology of cachexia. J Natl Cancer Inst. 1997;89:1763–1773. doi: 10.1093/jnci/89.23.1763. [DOI] [PubMed] [Google Scholar]
- 2.DeBoer MD, Marks DL. Therapy insight: Use of melanocortin antagonists in the treatment of cachexia in chronic disease. Nat Clin Pract Endocrinol Metab. 2006;2:459–466. doi: 10.1038/ncpendmet0221. [DOI] [PubMed] [Google Scholar]
- 3.Marks DL, Ling N, Cone RD. Role of the Central Melanocortin System in Cachexia. Cancer Res. 2001;61:1432–1438. [PubMed] [Google Scholar]
- 4.Cone RD. Anatomy and regulation of the central melanocortin system. Nat Neurosci. 2005;8:571–578. doi: 10.1038/nn1455. [DOI] [PubMed] [Google Scholar]
- 5.Cheunsuang O, Morris R. Astrocytes in the arcuate nucleus and median eminence that take up a fluorescent dye from the circulation express leptin receptors and neuropeptide Y Y1 receptors. Glia. 2005;52:228–233. doi: 10.1002/glia.20239. [DOI] [PubMed] [Google Scholar]
- 6.Cowley MA, Pronchuk N, Fan W, Dinulescu DM, Colmers WF, Cone RD. Integration of NPY, AGRP, and melanocortin signals in the hypothalamic paraventricular nucleus: evidence of a cellular basis for the adipostat. Neuron. 1999;24:155–163. doi: 10.1016/s0896-6273(00)80829-6. [DOI] [PubMed] [Google Scholar]
- 7.Whitaker KW, Reyes TM. Central blockade of melanocortin receptors attenuates the metabolic and locomotor responses to peripheral interleukin-1beta administration. Neuropharmacology. 2008;54:509–520. doi: 10.1016/j.neuropharm.2007.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Markison S, Foster AC, Chen C, Brookhart GB, Hesse A, Hoare SR, Fleck BA, Brown BT, Marks DL. The regulation of feeding and metabolic rate and the prevention of murine cancer cachexia with a small-molecule melanocortin-4 receptor antagonist. Endocrinology. 2005;146:2766–2773. doi: 10.1210/en.2005-0142. [DOI] [PubMed] [Google Scholar]
- 9.Krakoff J, Ma L, Kobes S, Knowler WC, Hanson RL, Bogardus C, Baier LJ. Lower metabolic rate in individuals heterozygous for either a frameshift or a functional missense MC4R variant. Diabetes. 2008;57:3267–3272. doi: 10.2337/db08-0577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fan W, Boston BA, Kesterson RA, Hruby VJ, Cone RD. Role of melanocortinergic neurons in feeding and the agouti obesity syndrome. Nature. 1997;385:165–168. doi: 10.1038/385165a0. [DOI] [PubMed] [Google Scholar]
- 11.Farooqi IS, Keogh JM, Yeo GS, Lank EJ, Cheetham T, O'Rahilly S. Clinical spectrum of obesity and mutations in the melanocortin 4 receptor gene. N Engl J Med. 2003;348:1085–1095. doi: 10.1056/NEJMoa022050. [DOI] [PubMed] [Google Scholar]
- 12.Farooqi IS, Yeo GS, Keogh JM, Aminian S, Jebb SA, Butler G, Cheetham T, O'Rahilly S. Dominant and recessive inheritance of morbid obesity associated with melanocortin 4 receptor deficiency. J Clin Invest. 2000;106:271–279. doi: 10.1172/JCI9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DeBoer MD. Melanocortin interventions in cachexia: how soon from bench to bedside? Curr Opin Clin Nutr Metab Care. 2007;10:457–462. doi: 10.1097/MCO.0b013e328108f441. [DOI] [PubMed] [Google Scholar]
- 14.Thornton JE, Cheung CC, Clifton DK, Steiner RA. Regulation of hypothalamic proopiomelanocortin mRNA by leptin in ob/ob mice. Endocrinology. 1997;138:5063–5066. doi: 10.1210/endo.138.11.5651. [DOI] [PubMed] [Google Scholar]
- 15.Hakansson ML, Hulting AL, Meister B. Expression of leptin receptor mRNA in the hypothalamic arcuate nucleus--relationship with NPY neurones. Neuroreport. 1996;7:3087–3092. doi: 10.1097/00001756-199611250-00059. [DOI] [PubMed] [Google Scholar]
- 16.Schwartz MW, Seeley RJ, Woods SC, Weigle DS, Campfield LA, Burn P, Baskin DG. Leptin increases hypothalamic pro-opiomelanocortin mRNA expression in the rostral arcuate nucleus. Diabetes. 1997;46:2119–2123. doi: 10.2337/diab.46.12.2119. [DOI] [PubMed] [Google Scholar]
- 17.Scarlett JM, Jobst EE, Enriori PJ, Bowe DD, Batra AK, Grant WF, Cowley MA, Marks DL. Regulation of Central Melanocortin Signaling by Interleukin-1{beta} Endocrinology. 2007;148:4217–4225. doi: 10.1210/en.2007-0017. [DOI] [PubMed] [Google Scholar]
- 18.Scarlett JM, Zhu X, Enriori PJ, Bowe DD, Batra AK, Levasseur PR, Grant WF, Meguid MM, Cowley MA, Marks DL. Regulation of agouti-related protein messenger ribonucleic acid transcription and peptide secretion by acute and chronic inflammation. Endocrinology. 2008;149:4837–4845. doi: 10.1210/en.2007-1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hayakawa T, Takanaga A, Tanaka K, Maeda S, Seki M. Ultrastructure of the central subnucleus of the nucleus tractus solitarii and the esophageal afferent terminals in the rat. Anat Embryol (Berl) 2003;206:273–281. doi: 10.1007/s00429-002-0288-z. [DOI] [PubMed] [Google Scholar]
- 20.Ritter RC. Gastrointestinal mechanisms of satiation for food. Physiol Behav. 2004;81:249–273. doi: 10.1016/j.physbeh.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 21.Ruud J, Blomqvist A. Identification of rat brainstem neuronal structures activated during cancer-induced anorexia. J Comp Neurol. 2007;504:275–286. doi: 10.1002/cne.21407. [DOI] [PubMed] [Google Scholar]
- 22.DeBoer MD, Scarlett JM, Levasseur PR, Grant WF, Marks DL. Administration of IL-1beta to the 4th ventricle causes anorexia that is blocked by agouti-related peptide and that coincides with activation of tyrosine-hydroxylase neurons in the nucleus of the solitary tract. Peptides. 2009;30:210–218. doi: 10.1016/j.peptides.2008.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marty V, El Hachmane M, Amedee T. Dual modulation of synaptic transmission in the nucleus tractus solitarius by prostaglandin E2 synthesized downstream of IL-1beta. Eur J Neurosci. 2008;27:3132–3150. doi: 10.1111/j.1460-9568.2008.06296.x. [DOI] [PubMed] [Google Scholar]
- 24.Zheng H, Patterson LM, Phifer CB, Berthoud HR. Brain stem melanocortinergic modulation of meal size and identification of hypothalamic POMC projections. Am J Physiol Regul Integr Comp Physiol. 2005;289:R247–258. doi: 10.1152/ajpregu.00869.2004. [DOI] [PubMed] [Google Scholar]
- 25.DeBoer MD, Zhu X, Levasseur PR, Inui A, Hu Z, Han G, Mitch WE, Taylor JE, Halem HA, Dong JZ, Datta R, Culler MD, Marks DL. Ghrelin treatment of chronic kidney disease: improvements in lean body mass and cytokine profile. Endocrinology. 2008;149:827–835. doi: 10.1210/en.2007-1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DeBoer MD, Zhu XX, Levasseur P, Meguid MM, Suzuki S, Inui A, Taylor JE, Halem HA, Dong JZ, Datta R, Culler MD, Marks DL. Ghrelin Treatment Causes Increased Food Intake and Retention of Lean Body Mass in a Rat Model of Cancer Cachexia. Endocrinology. 2007 doi: 10.1210/en.2007-0016. [DOI] [PubMed] [Google Scholar]
- 27.DeBoer MD. Emergence of ghrelin as a treatment for cachexia syndromes. Nutrition. 2008;24:806–814. doi: 10.1016/j.nut.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 28.Knoll S, Zimmer S, Hinney A, Scherag A, Neubauer A, Hebebrand J. Val103Ile polymorphism of the melanocortin-4 receptor gene (MC4R) in cancer cachexia. BMC Cancer. 2008;8:85. doi: 10.1186/1471-2407-8-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang YK, Fong TM, Dickinson CJ, Mao C, Li JY, Tota MR, Mosley R, Van Der Ploeg LH, Gantz I. Molecular determinants of ligand binding to the human melanocortin-4 receptor. Biochemistry. 2000;39:14900–14911. doi: 10.1021/bi001684q. [DOI] [PubMed] [Google Scholar]
- 30.Chen M, Cai M, Aprahamian CJ, Georgeson KE, Hruby V, Harmon CM, Yang Y. Contribution of the conserved amino acids of the melanocortin-4 receptor in [corrected] [Nle4,D-Phe7]-alpha-melanocyte-stimulating [corrected] hormone binding and signaling. J Biol Chem. 2007;282:21712–21719. doi: 10.1074/jbc.M702285200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khoury MJ, Gwinn M, Yoon PW, Dowling N, Moore CA, Bradley L. The continuum of translation research in genomic medicine: how can we accelerate the appropriate integration of human genome discoveries into health care and disease prevention? Genet Med. 2007;9:665–674. doi: 10.1097/GIM.0b013e31815699d0. [DOI] [PubMed] [Google Scholar]
- 32.Greenfield JR, Miller JW, Keogh JM, Henning E, Satterwhite JH, Cameron GS, Astruc B, Mayer JP, Brage S, See TC, Lomas DJ, O'Rahilly S, Farooqi IS. Modulation of blood pressure by central melanocortinergic pathways. N Engl J Med. 2009;360:44–52. doi: 10.1056/NEJMoa0803085. [DOI] [PubMed] [Google Scholar]
- 33.Scarlett J, Bowe D, Zhu X, Batra A, Grant W, Marks D. Genetic and Pharmacologic Blockade of Central Melanocortin Signaling Attenuates Cardiac Cachexia in Rodent Models of Heart Failure. Am J Physiol-Heart C. 2009 doi: 10.1677/JOE-09-0397. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Joppa MA, Gogas KR, Foster AC, Markison S. Central infusion of the melanocortin receptor antagonist agouti-related peptide (AgRP(83-132)) prevents cachexia-related symptoms induced by radiation and colon-26 tumors in mice. Peptides. 2007;28:636–642. doi: 10.1016/j.peptides.2006.11.021. [DOI] [PubMed] [Google Scholar]
- 35.von Haehling S, Lainscak M, Springer J, Anker SD. Cardiac cachexia: a systematic overview. Pharmacol Ther. 2009;121:227–252. doi: 10.1016/j.pharmthera.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 36.Anker SD, Swan JW, Volterrani M, Chua TP, Clark AL, Poole-Wilson PA, Coats AJ. The influence of muscle mass, strength, fatigability and blood flow on exercise capacity in cachectic and non-cachectic patients with chronic heart failure. Eur Heart J. 1997;18:259–269. doi: 10.1093/oxfordjournals.eurheartj.a015229. [DOI] [PubMed] [Google Scholar]
- 37.Anker SD, Ponikowski P, Varney S, Chua TP, Clark AL, Webb-Peploe KM, Harrington D, Kox WJ, Poole-Wilson PA, Coats AJ. Wasting as independent risk factor for mortality in chronic heart failure. Lancet. 1997;349:1050–1053. doi: 10.1016/S0140-6736(96)07015-8. [DOI] [PubMed] [Google Scholar]
- 38.Antman EM, Hand M, Armstrong PW, Bates ER, Green LA, Halasyamani LK, Hochman JS, Krumholz HM, Lamas GA, Mullany CJ, Pearle DL, Sloan MA, Smith SC, Jr, Anbe DT, Kushner FG, Ornato JP, Jacobs AK, Adams CD, Anderson JL, Buller CE, Creager MA, Ettinger SM, Halperin JL, Hunt SA, Lytle BW, Nishimura R, Page RL, Riegel B, Tarkington LG, Yancy CW. 2007 Focused Update of the ACC/AHA 2004 Guidelines for the Management of Patients With ST-Elevation Myocardial Infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines: developed in collaboration With the Canadian Cardiovascular Society endorsed by the American Academy of Family Physicians: 2007 Writing Group to Review New Evidence and Update the ACC/AHA 2004 Guidelines for the Management of Patients With ST-Elevation Myocardial Infarction, Writing on Behalf of the 2004 Writing Committee. Circulation. 2008;117:296–329. doi: 10.1161/CIRCULATIONAHA.107.188209. [DOI] [PubMed] [Google Scholar]
- 39.Cheung W, Yu PX, Little BM, Cone RD, Marks DL, Mak RH. Role of leptin and melanocortin signaling in uremia-associated cachexia. J Clin Invest. 2005;115:1659–1665. doi: 10.1172/JCI22521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang QH, Hruby VJ, Tatro JB. Role of central melanocortins in endotoxin-induced anorexia. Am J Physiol. 1999;276:R864–R871. doi: 10.1152/ajpregu.1999.276.3.R864. [DOI] [PubMed] [Google Scholar]
- 41.Wisse BE, Frayo RS, Schwartz MW, Cummings DE. Reversal of cancer anorexia by blockade of central melanocortin receptors in rats. Endocrinology. 2001;142:3292–3301. doi: 10.1210/endo.142.8.8324. [DOI] [PubMed] [Google Scholar]
- 42.Weyermann P, Dallmann R, Magyar J, Anklin C, Hufschmid M, Dubach-Powell J, Courdier-Fruh I, Hennebohle M, Nordhoff S, Mondadori C. Orally available selective melanocortin-4 receptor antagonists stimulate food intake and reduce cancer-induced cachexia in mice. PLoS One. 2009;4:e4774. doi: 10.1371/journal.pone.0004774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cheung WW, Kuo HJ, Markison S, Chen C, Foster AC, Marks DL, Mak RH. Peripheral administration of the melanocortin-4 receptor antagonist NBI-12i ameliorates uremia-associated cachexia in mice. J Am Soc Nephrol. 2007;18:2517–2524. doi: 10.1681/ASN.2006091024. [DOI] [PubMed] [Google Scholar]
- 44.Tran JA, Jiang W, Tucci FC, Fleck BA, Wen J, Sai Y, Madan A, Chen TK, Markison S, Foster AC, Hoare SR, Marks D, Harman J, Chen CW, Arellano M, Marinkovic D, Bozigian H, Saunders J, Chen C. Design, synthesis, in vitro, and in vivo characterization of phenylpiperazines and pyridinylpiperazines as potent and selective antagonists of the melanocortin-4 receptor. J Med Chem. 2007;50:6356–6366. doi: 10.1021/jm701137s. [DOI] [PubMed] [Google Scholar]
- 45.Jiang W, Tucci FC, Tran JA, Fleck BA, Wen J, Markison S, Marinkovic D, Chen CW, Arellano M, Hoare SR, Johns M, Foster AC, Saunders J, Chen C. Pyrrolidinones as potent functional antagonists of the human melanocortin-4 receptor. Bioorg Med Chem Lett. 2007;17:5610–5613. doi: 10.1016/j.bmcl.2007.07.097. [DOI] [PubMed] [Google Scholar]
- 46.Chen C, Tucci FC, Jiang W, Tran JA, Fleck BA, Hoare SR, Wen J, Chen T, Johns M, Markison S, Foster AC, Marinkovic D, Chen CW, Arellano M, Harman J, Saunders J, Bozigian H, Marks D. Pharmacological and pharmacokinetic characterization of 2-piperazine-alpha-isopropyl benzylamine derivatives as melanocortin-4 receptor antagonists. Bioorg Med Chem. 2008;16:5606–5618. doi: 10.1016/j.bmc.2008.03.072. [DOI] [PubMed] [Google Scholar]
- 47.Nicholson JR, Kohler G, Schaerer F, Senn C, Weyermann P, Hofbauer KG. Peripheral administration of a melanocortin 4-receptor inverse agonist prevents loss of lean body mass in tumor-bearing mice. J Pharmacol Exp Ther. 2006;317:771–777. doi: 10.1124/jpet.105.097725. [DOI] [PubMed] [Google Scholar]
- 48.Vos TJ, Caracoti A, Che JL, Dai M, Farrer CA, Forsyth NE, Drabic SV, Horlick RA, Lamppu D, Yowe DL, Balani S, Li P, Zeng H, Joseph IB, Rodriguez LE, Maguire MP, Patane MA, Claiborne CF. Identification of 2-[2-[2-(5-bromo-2-methoxyphenyl)-ethyl]-3-fluorophenyl]-4,5-dihydro-1H-imidazole (ML00253764), a small molecule melanocortin 4 receptor antagonist that effectively reduces tumor-induced weight loss in a mouse model. J Med Chem. 2004;47:1602–1604. doi: 10.1021/jm034244g. [DOI] [PubMed] [Google Scholar]
- 49. [June 30, 2009];ClinicalTrials.gov: Trials for Cachexia Treatment. Available at: http://clinicaltrials.gov/ct2/results?term=cachexia&pg=4.
- 50.DeBoer MD, Marks DL. Cachexia: lessons from melanocortin antagonism. Trends Endocrinol Metab. 2006;17:199–204. doi: 10.1016/j.tem.2006.05.005. [DOI] [PubMed] [Google Scholar]


