Abstract
Inflammation-induced activation of the tryptophan catabolizing enzyme indoleamine 2,3-dioxygenase (IDO) causes depressive-like behavior in mice following acute activation of the innate immune system by lipopolysaccharide (LPS). Here we investigated the mechanism of IDO expression induced by LPS in primary cultures of microglia derived from neonatal C57BL/6J mice. LPS (10 ng/ml) induced IDO transcripts that peaked at 8 h and enzymatic activity at 24 h, resulting in an increase in extracellular kynurenine, the catabolic product of IDO-induced tryptophan catabolism. This IDO induction by LPS was accompanied by synthesis and secretion of the proinflammatory cytokines TNFα and IL-6, but without detectable IFNγ expression. To explore the mechanism of LPS-induced IDO expression, microglia were pretreated with the c-Jun-N-terminal kinase (JNK) inhibitor SP600125 for 30 min before LPS treatment. We found that SP600125 blocked JNK phosphorylation and significantly decreased IDO expression induced by LPS, which was accompanied by a reduction of LPS-induced expression of TNFα and IL-6. Collectively, these data extend to microglia the property that LPS induces IDO expression via an IFNγ-independent mechanism that depends upon activation of JNK. Inhibition of the JNK pathway may provide a new therapy for inflammatory depression.
Keywords: Neuroinflammation; Indoleamine 2,3-dioxygenase; Lipopolysaccharide; c-Jun-N-terminal kinase; Primary microglia
Introduction
Toll-like receptors (TLRs) are important mediators of neuroinflammation and tissue damage during infectious and non-infectious diseases of the central nervous system (CNS) (Carpentier et al., 2008). Microglia are known to be key cellular mediators of neuroimmune responses, that constitutively express a wide complement of TLRs (Bsibsi et al., 2002). Microglia are activated in most pathological conditions of the CNS and play an important role in sensing and propagating inflammatory signals in response to activation of the peripheral innate immune system (Hanisch and Kettenmann., 2007). In the absence of inflammatory stimuli, microglia are quiescent even though they are actively involved in immune surveillance (Nimmerjahn et al., 2005; Soulet and Rivest, 2008). Once activated, microglial cells display macrophage-like capabilities including phagocytosis, antigen presentation and inflammatory cytokine production (Garden and Moller, 2006).
Chronic inflammation is often associated with clinical depression (Evans et al., 2005; Adler et al., 2008; Dantzer et al., 2008a,b). Recent studies have focused on potential mechanisms that might link inflammation-induced depression to tryptophan metabolism, particularly in the brain, where a reduction in the bioavailability of tryptophan could affect serotoninergic neurotransmission and play a synergistic role in the induction of depressive symptoms (Widner et al., 2002; Neumeister, 2003; Fitzgerald et al., 2008). A pivotal protein that has recently been shown to be required for development of inflammation-induced depressive-like behavior in mice is indoleamine 2,3-dioxygenase (IDO), the first rate-limiting tryptophan-degrading enzyme in the kynurenine pathway (Raison et al., 2006; O’Connor et al., 2009a,b). Activation of this enzyme by inflammatory signals leads to an increase in the kynurenine/tryptophan ratio in plasma and the generation of neuroactive mediators, including 3-hydroxykynurenine (3-HK) and quinolinic acid (QUIN) (Guillemin et al., 2005). High levels of 3-HK and QUIN induce neuronal damage via oxidative stress (Lehrmannn et al., 2008) and over stimulation of N-methyl-D-aspartate (NMDA) receptors (Guillemin et al., 2005; Spalletta et al., 2006). In animal studies, inhibition of IDO abrogates depressive-like behaviors induced by acute (O’Connor et al., 2009a) or chronic inflammation (O’Connor et al., 2009b). IFNγ is the predominant cytokine implicated in the induction of IDO (O’Connor et al., 2009c). However, subsequent studies have identified IFNγ-independent pathways, including TNFα and lipopolysaccharide (LPS), which are capable of inducing IDO activity (Fujigaki et al., 2006; Jung et al., 2007; Connor et al., 2008). The IFNγ-independent up-regulation of IDO was first described in the LPS model of immune activation in human acute monocyte leukemia cell line THP-1 (Fujigaki et al., 2001). However, it is not known if this property of IDO induction extends to primary microglia. Furthermore, the signal transduction mechanisms responsible for IFNγ-independent induction of IDO following exposure to LPS require further investigation.
In dendritic cells, Jung et al. (2007) recently reported that LPS induces IDO expression via an IFNγ-independent mechanism. In this system, c-Jun-N-terminal kinase (JNK) was required for LPS to induce IDO in the absence of IFNγ. Sickness behavior always precedes development of depressive-like behavior induced by an acute inflammatory stimulus, and we previously demonstrated that infusion of a specific JNK inhibitor i.c.v. completely blocks TNFα-induced sickness behavior (Palin et al., 2008). Since IDO is primarily expressed by activated microglia in the brain, we queried whether JNK is also involved in LPS-induced expression of IDO in these cells. These experiments establish that the JNK pathway plays a critical role in the microglial induction of IDO expression and activity following LPS stimulation via an IFNγ-independent mechanism.
Materials and methods
Reagents
Fetal bovine serum (FBS; <0.25 EU/ml endotoxin), 0.25% trypsin, Dulbecco’s modified Eagle’s medium/high glucose (DMEM) containing 0.584 g/l glutamine and 4.5 g/l glucose, sodium pyruvate and antibiotics (100 U/ml penicillin and 100 µg/ml streptomycin) were purchased from HyClone (Logan, UT). Nylon cell strainers (70 µm) were obtained from BD Falcon (Bedford, MA) and the CytoTox96 non-radioactive cytotoxicity kit (cat# G1781) was from Promega Corporation (Madison, WI). Enzyme-linked immunosorbent assay (ELISA) kits were from R&D Systems (Wiesbaden, DE). Rabbit polyclonal antibodies (IgG) specific for JNK (cat# 9252) and phosphorylated JNK (p-JNK, cat# 9251) were purchased from Cell Signaling Biotechnology (Danvers, MA), whereas the secondary horseradish peroxidase (HRP)-linked donkey anti-rabbit antibody (NA934V) was purchased from GE Healthcare Biosciences (Piscataway, NJ). The JNK inhibitor SP600125 (cat# 420119) was obtained from Calbiochem (USA). Purity of primary microglia was confirmed with a rat FITC-labeled anti-mouse CD11b (IgG2b, cat# 557396) using a FITC-labeled rat IgG2b isotype antibody as a control (cat# 553988; BD Biosciences, Pharmingen,USA). Protein was measured with a standard Bradford assay kit (cat# 500-0113, 0114, 0115) and Immun-Blot polyvinylidene difluoride (PVDF, cat# 162-0177) membranes were from Bio-Rad (Hercules, CA). ECL Western blotting detection reagents (cat# RPN2106V1 and RPN2106V2) were from GE Healthcare Little Chalfont (Bucks, UK). TRIzol reagent was purchased from Invitrogen Life Technologies (Carlsbad, CA). Ambion (cat# 1710) reverse transcriptase kit, Ambion’s DNA-free™ DNase treatment and removal reagents (cat# AM1906), RT-PCR primers for TNFα (cat# Mm00443258_m1), IL-6 (cat# Mm00446190_m1), IFNγ (cat# Mm00801778_m1), IDO (cat# Mm00492586_m1), KMO (cat#Mm00505511_m1), KYNU (cat# Mm0051012_m1), KAT (cat# Mm00496169.m1), HAO (cat# 005177945_m1), IL-1β (cat# Mm00434228_m1) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH; cat# Mm999999_g1) were all obtained from Applied Biosystems. The protease inhibitor cocktail (cat# P2714), lipopolysaccharide (LPS) from Escherichia coli 0127:B8 (cat# L-3137), poly-L-lysine (cat# P4832) and other reagents and chemicals were purchased from Sigma-Aldrich. (St. Louis, MO). L-cell conditioned medium used to culture primary microglia was obtained from L-929 cells obtained from American Type Culture Collection (ATCC, cat# CCL-1™, Manassas, USA).
Preparation of primary murine microglia
Primary mixed glial cultures were established from brains of <2-day-old neonatal C57BL/6J mice. After removal of the meninges, brains were mechanically minced and dissociated with 0.25% trypsin/0.5 mM EDTA. After inactivation of trypsin, the tissue suspension was passed through a 70 µm nylon cell strainer. This cell suspension was centrifuged at 100 × g for 15 min. Cell pellets were resuspended in DMEM supplemented with 10% heat-inactivated FBS and plated in poly-L-lysine pre-coated culture flasks. Cultures were maintained at 37°C in a humidified atmosphere containing 5% CO2, with the culture medium being changed twice weekly.
Cells became confluent by 15–20 days at which time microglial cells were separated from astrocytes by shaking the flasks for 1 h at 37°C on an Orbital platform shaker (Model Annova 2000; New Brunswick Scientific, Edison, NJ) at 150 rpm. Isolated microglia were collected and cultured in 20% (v:v) L-929-cell conditioned medium (LCCM) for 7–10 days. The LCCM provided a source of colony stimulating factors. Purity of microglia was confirmed as > 95% CD11b+ cells, as verified by flow cytometry using previously described techniques (Liu et al., 1999; Shen et al., 2002).
Primary microglia were treated with 10 ng/ml LPS, the optimal concentration to stimulate expression of IDO, in DMEM supplemented with 2% FBS. At various times following addition of LPS, supernatants were collected and stored at −80°C for measurement of kynurenine and cytokines. Cells were washed twice with cold PBS and stored at −80°C for isolation of mRNA, Western blot and IDO enzymatic activity. For inhibition of JNK, cells were pre-incubated with SP600125 for 30 m and then treated with 10 ng/ml LPS for 0, 15, 30 and 60 m. Cell viability was evaluated using both trypan blue staining and the amount of lactate dehydrogenase (LDH) released into the culture medium by CytoTox96 non-radioactive cytotoxicity kit.
RNA extraction and reverse transcription
Total RNA was extracted from cultured microglial using TRIzol reagent as previously described (O’Connor et al., 2009b). Total mRNA (1–2 µg) was reverse transcribed using reverse transcriptase kits from Ambion according to the manufacturer’s description. Briefly, RNA (1–2µg) was pretreated with DNA-free™ DNase treatment at 37°C for 20–30 min. RNA samples were incubated with a mixture containing a mixture of dNTPs, random primers, 1× first-strand buffer, a rRNase inhibitor, MMLV reverse transcriptase and water to a final volume of 20 µl at 44°C for 1 h, followed by 10 min at 92°C to inactivate the reverse transcription reagents.
Real-time RT-PCR
Real-time RT-PCR was used to quantify mRNA as the number of target gene cycle amplifications, as described previously (O’Connor et al., 2009a).
Enzyme-linked immunosorbent assays (ELISAs)
IL-6 and TNFα were measured with validated specific ELISA assays according to the manufacturer’s instructions. Briefly, adding 100 µl of each sample in duplicate to ELISA plates pre-coated with an IL-6 or a TNFα capture antibody. Recombinant murine IL-6 and TNFα standards ranged from 0 to 1000 pg/ml, and the lower assay limit of detection is 16 pg/ml. Absorbance was measured on an OPTImax ELISA plate reader. IL-6 and TNFα concentrations were expressed as picograms per milliliter.
Determination of IDO activity
Cultured microglial cells were stimulated with 10 ng/ml LPS for 24 h in DMEM supplied with 2% FBS. Thereafter, cells were washed and homogenized with ice-cold lysing buffer (140 mM KCl, 20 mM potassium phosphate buffer, pH 7.0) with a cocktail of protease inhibitors compound as previously described (Lestage et al., 2002). IDO enzymatic activity was measured in cell lysates that were extracted with lysing buffer described above. The amount of protein in all samples was adjusted to 30 µg/ml. IDO activity was determined as described previously, with minor modifications (Sono, 1989; Lestage et al., 2002). Briefly, microglial cell lysates (50 µl) were incubated with 200 µl of assay buffer (400 µM L-tryptophan, 20 mM ascorbate, 10 µM methylene blue, 100 µg catalase, in 50 mM potassium phosphate buffer pH 6.5) at 37° C for 1 h. The reaction was stopped by adding 50 µl of 10% sulfosalicylic acid solution (SSA) and then incubated for an additional 30 min at 50 °C to hydrolyze N-formylkynurenine to L-kynurenine. The reaction mixture was then centrifuged at 13,000 × g for 10 min at 4°C and filtered through 0.2 µM filter tubes at 13,000 × g for 5 min. Kynurenine was analyzed by high-pressure liquid chromatography (HPLC), as previously described (O’Connor et al., 2009a).
Measurement of kynurenine by HPLC
Kynurenine in the microglial conditioned medium or formed in the enzymatic activity reaction was determined by HPLC, as described previously (O’Connor et al., 2009a). Briefly, cell culture medium supernatant (50 µl) was mixed with a solution of 10% sulfosalicylic acid solution (10 µl) and allowed to precipitate proteins on ice for 30 min. Samples were centrifuged at 12,000 × g for 10 min at 4°C, diluted, then analyzed for kynurenine content using HPLC.
Western blot analysis
Western blotting experiments were conducted as per our previously described technique (Strle et al., 2006). Primary antibodies specific to phosphorylated JNK or JNK (1:1000 dilution) were incubated overnight. Membranes were then incubated for 1 h at room temperature with a secondary antibody coupled to horseradish peroxidase (HRP)-conjugated donkey anti-rabbit IgG antibodies at a dilution of 1:2000. Finally, membranes were developed with an enhanced chemiluminescence ECL Western Blot Detection Reagent. Blots were covered with transparency film and then were inserted into a Fujifilm LAS-4000 System configured for multifunctional analysis (Fujifilm, Life Science, Stamford, CT). Densitometric analysis of autoradiographs was performed using publically available IMAGE-J software from the National Institutes of Health (Bethesda, MD). Densitometric summaries were expressed as ratios of phosphorylated JNK to total JNK.
Statistical analysis
Data were analyzed using a one-way (treatment) or two-way (pretreatment × treatment) ANOVA, followed by a post-hoc pairwise multiple comparison using Fisher’s least significant difference test if the interaction was significant. All data are presented as means ± SEM.
Results
LPS induces expression of IDO in the absence of IFNγ transcripts in primary microglia
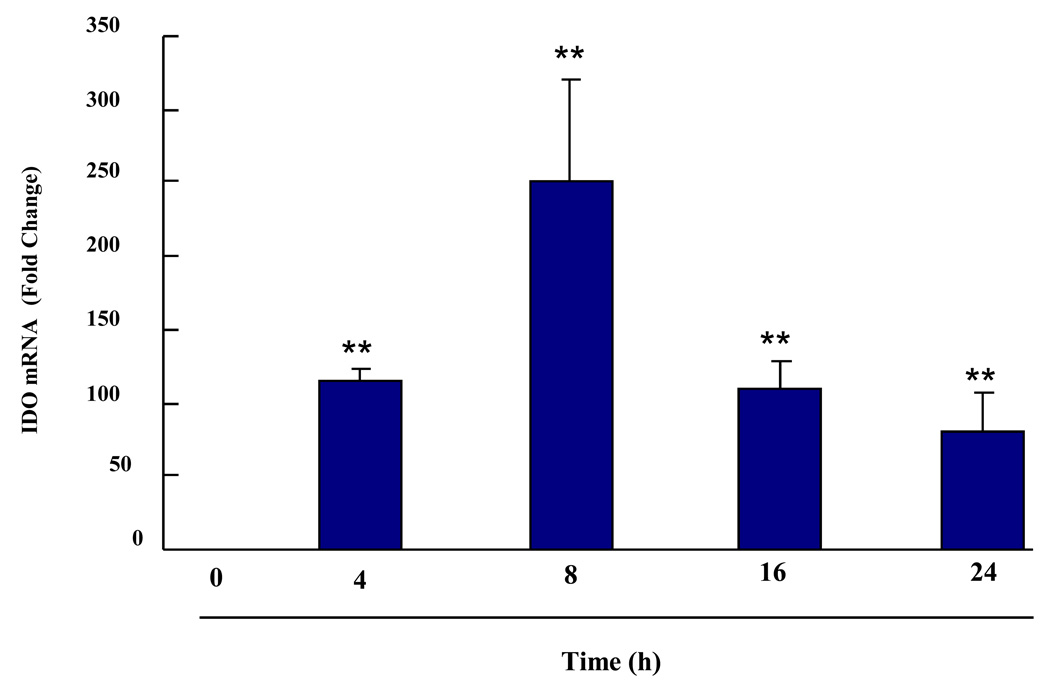
We previously established that LPS given i.p. induces expression of IDO in the brain (O’Connor et al., 2009b), but the type of cell in the CNS that produces IDO was not identified. Here we used real-time RT-PCR to determine whether LPS induces IDO steady-state transcripts in primary murine microglial cells. Preliminary experiments established that 10 ng/ml LPS reliably induced IDO as well as proinflammatory cytokine mRNAs in these cells. As shown in Fig. 1, IDO mRNA could not be detected in microglia prior to addition of LPS (40 amplification cycles). However, within 4 h of addition of LPS, IDO expression was strongly enhanced (p < 0.01) in a time-dependent manner. LPS-induced IDO mRNA peaked at 8 h (p < 0.01) and remained elevated (p < 0.01) 24 h later. Importantly, this LPS-induced expression of IDO did not require synthesis of IFNγ because no IFNγ mRNA could be detected in LPS-stimulated microglia at any time point (40 amplification cycles). This finding is consistent with the recent report of Hoshi et al. (2009) who reported that neuronal IDO is clearly up-regulated in IFNγ KO mice. IDO mRNA was also measured in primary microglia isolated from IDO KO mice. In either the absence or presence of LPS, no IDO mRNA could be detected (40 amplification cycles). These data establish an in vitro model using primary mouse microglia in which LPS is able to induce IDO mRNA through an IFNγ-independent mechanism.
Fig. 1. Induction of IDO Transcripts by LPS in a Time-Dependent Manner in Primary Murine Microglia.
IDO transcripts in microglia are induced by LPS in a time-dependent manner. Primary murine microglia were treated with LPS (10 ng/ml) for 4, 8, 16 and 24 h. Data were normalized to mRNA expression of GAPDH. No IDO mRNA was detected at time zero (>40 amplification cycles). Each bar represents the mean ± SEM of results from 3 independent experiments. ** p < 0.01 compared to time zero (Ct cycles for LPS treatment were 32.0 ± 0.3, 32.3 ± 0.5, 31.5 ± 0.4, 33.1 ± 0.60, respectively, for different time points from 4 to 24 h)
LPS increases IDO enzymatic activity in primary murine microglia
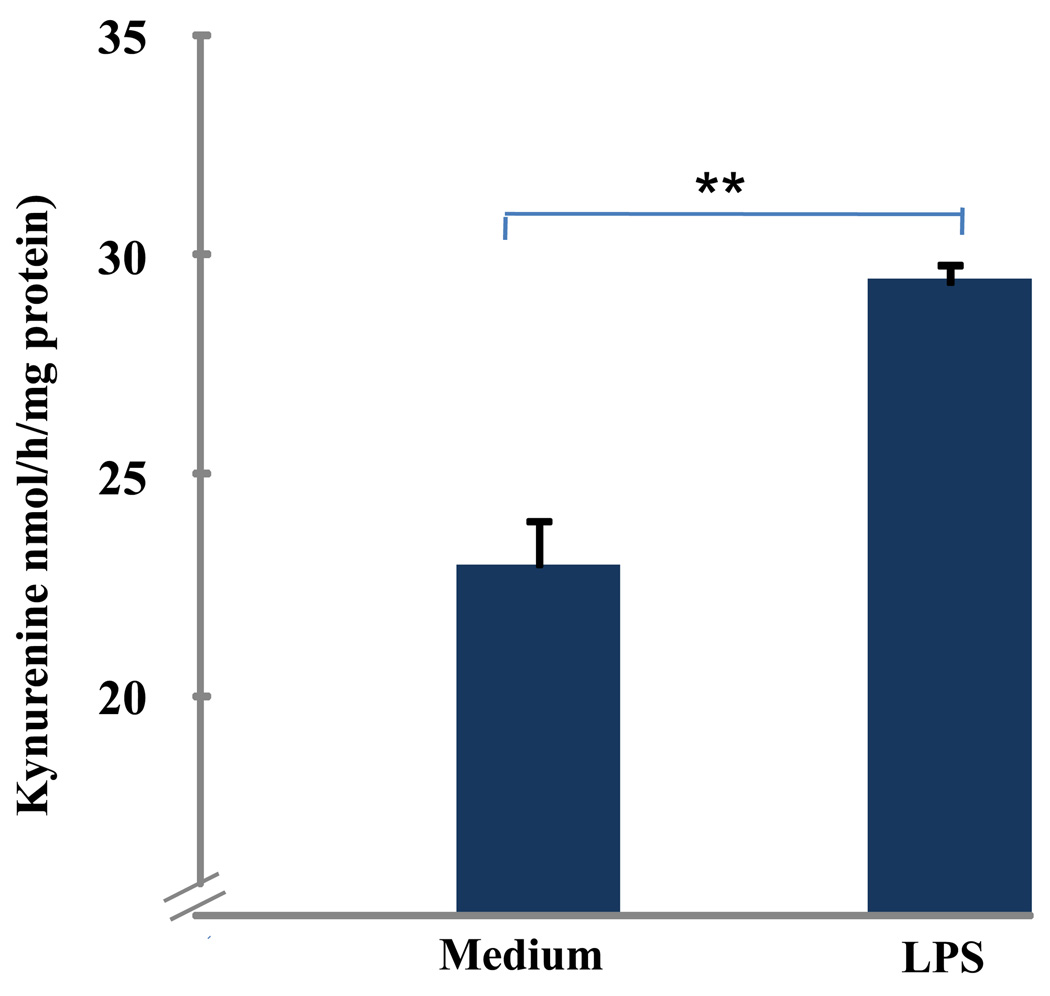
To evaluate whether primary murine microglia produce functional IDO, we determined IDO enzymatic activity by measuring the amount of kynurenine formed following incubation of microglial lysates with exogenous L-tryptophan (Fig. 2). These experiments showed that IDO enzymatic activity was augmented, as assessed by an increase in (p < 0.01) the amount of kynurenine produced from tryptophan following exposure to LPS for 24 h.
Fig. 2. LPS Increases IDO Activity in Microglia.
LPS increases IDO enzymatic activity. Primary mouse microglia were treated with LPS (10 ng/ml) for 24 h. IDO enzymatic activity was determined by measuring the amount of KYN formed upon incubation of microglial lysates with exogenous L-tryptophan. IDO enzymatic activity was expressed as kynurenine formed per hour for per miligram of protein. Data were expressed as mean ± SEM of 3 separate experiments. ** p < 0.01 compared to control medium
Extracellular kynurenine is increased by LPS
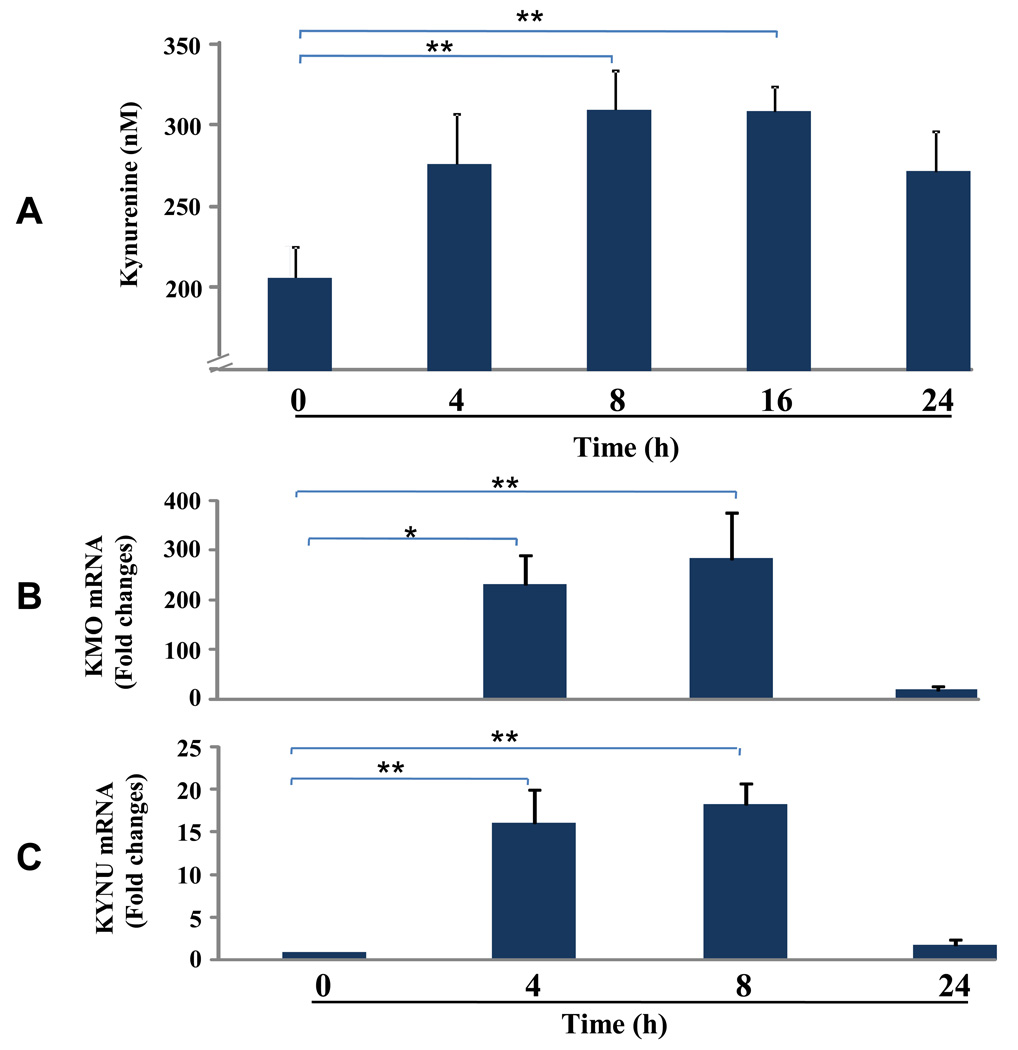
Intracelluar kynurenine is actively transported from cells via membrane bound L-type amino acid transporters (LAT) which mediates tryptophan influx/kynurenine efflux exchange (Ruddick et al., 2006; Kaper, 2007). To determine if IDO activation in microglia could metabolize tryptophan to kynurenine in microglia, we measured the accumulation of extracellular kynurenine from LPS-treated microglia since tryptophan is in excess in the culture medium. As shown in Fig. 3A, the amount of kynurenine in the culture medium increased with time following exposure to LPS (main effect, p = 0.054). By 8 and 16 h, kynurenine levels were enhanced (p < 0.01) compared to medium control. By 24 h, however, extracellular kynurenine no longer accumulated and began to decline. Three enzymes are known to catabolize kynurenine: kynurenine 3-monooxygenase (KMO), kynureninase (KYNU), and kynurenine aminotransferase (KAT). One of the metabolic products of kynurenine, 3-hydroxyanthranilic acid, can be degraded further by 3-hydroxyanthrailic acid oxygenase (HAO). To explore the possibility that kynurenine was further degraded by these downstream enzymes in the kynurenine pathway, we determined if LPS increased their expression in a time-dependent manner. As shown in Fig. 3B, within the first 4 h following addition of LPS, expression of KMO (P<0.05) and KYNU (P<0.01) were strongly enhanced, and these transcripts disappeared by 24 h. Neither KAT nor HAO was inducible by LPS (data not shown). These experiments demonstrate that LPS activation of IDO not only leads to a fairly rapid increase in extracellular kynurenine but also to increased expression of two enzymes, KMO and KYNU, that convert kynurenine to downstream active metabolites.
Fig. 3. Extracellular kynurenine, KMO and KYNU mRNA Induced by LPS.
LPS increases extracellular kynurenine and induces kynurenine 3-monooxygenase (KMO) and kynureninase (KYNU) expression. Microglial cells were treated with LPS (10 ng/ml), and both culture medium and cells were collected 4, 8, 16 and 24 h later. Kynurenine was measured in the conditioned medium using HPLC and KMO and KYNU mRNA expression in the cells were analyzed using real time RT-PCR. (A) Accumulation of kynurenine in conditioned medium. (B) Time course of KMO and KYNU transcripts. Data were normalized to mRNA expression of GAPDH. No KMO mRNA was detected at time zero (>40 amplification cycles). Constitutive KYNU mRNA was expressed in microglial cells at baseline (Ct cycle was 32.1 ± 0.1 at time zero). Both KMO and KYNU were inducible with LPS treatment. Ct cycle for LPS treatment for KMO was 33.9 ± 0.4, 33.1 ± 0.4, 35.8 ± 0.7, respectively for different time points from 4 to 24 h) and Ct cycle for LPS treatment for KYNU was 29.8 ± 0.1, 29.0 ±0.4, 31.2 ± 0.4, respectively for different time points from 4 to 24 h). Data were expressed as mean ± SEM of 3 separate experiments. * p < 0.05, ** p < 0.01 compared to time zero.
LPS-induced IDO expression is abrogated by a JNK inhibitor in primary microglia
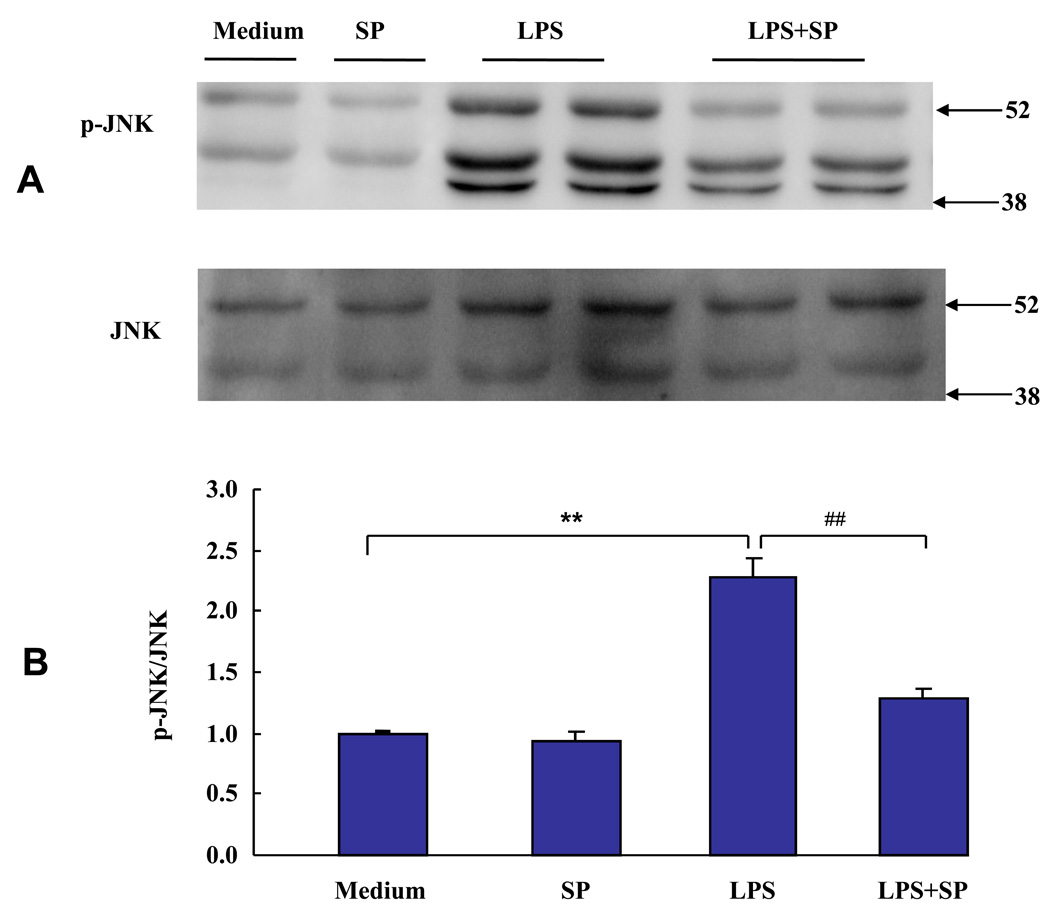
A variety of signaling pathways are activated in response to LPS, including JNK (Lee and Lim, 2009). We have already established JNK to be critical for the development of sickness behavior (Palin et al., 2008). Here we examined whether JNK is also involved in LPS-induced expression of IDO in primary mouse microglia. We first confirmed that JNK is activated by LPS by performing time course experiments on LPS-induced JNK activation. Primary microglia were incubated with LPS (10 ng/ml) for 15, 30 and 60 min respectively. Phosphorylation of JNK was determined by probing membranes with a polyclonal antibody against phospho-JNK, as determined by phosphorylation of residues Thr183/Tyr185. Membranes were then stripped and reprobed with antibodies specific for total JNK to ensure that an equal amount of JNK protein was loaded into each lane of the SDS-PAGE gels and subsequently transferred to PVDF membranes. We found that LPS increased Thr183/Tyr185 phosphorylation of JNK in a time-dependent manner, with maximal phosphorylation of JNK after 60 min (data not shown). Phosphorylation of JNK by LPS stimulation often peaks within 60 min in several cellular system (Gong et al., 2008; Pocivavsek et al., 2009, Tanaka et al., 2008), so a timeline of 60 min was selected for future experiments with JNK phosphorylation.
Next, we tested whether the well-known JNK inhibitor, SP600125, would affect LPS-induced IDO expression. In these experiments, microglial cells were pretreated with SP600125 (12 µM) for 30 min prior to treatment with LPS for another 60 min. JNK is expressed as three isoforms (JNK1, JNK2 and JNK3), and all three JNK isoforms have been reported in microglia (Hidding et al., 2002). Consistent with these earlier results, we detected the three isoforms of phosphorylated JNK with a molecular mass ranging from 46 and 54 kDa. We found that suppression of all three isoforms of phospho-JNK, but not the JNK proteins, was reduced (p < 0.01) by SP600125. The data and statistical analysis therefore summarizes the results from all three JNK isoforms. These data confirmed that SP600125 significantly inhibits phosphorylation of JNK following exposure to LPS in primary murine microglia (p < 0.01; Figs. 4A and B).
Fig. 4.
The SP600125 JNK inhibitor abrogates Thr183/Tyr185 phosphorylation of JNK caused by LPS. Primary mouse microglia were treated with LPS (10 ng/ml) in the presence or absence of SP600125 (12 µM) for 30 m, and cell lysates were collected for JNK phosphorylation analysis by Western blotting. (A) A representative Western blot showing results with microglial cells after pretreatment with SP600125 for 30 min and then incubated with or without LPS for 60 min. (B) Densitometric summary of Western blots from four independent experiments. Densitometric summaries were calculated as the ratio of phosphorylated JNK to total JNK. Figs. 4A and 4B demonstrate that SP600125 significantly abrogated Thr183/Tyr185 phonsphorylation of JNK activated by LPS in primary murine microglia. ** p < 0.01 compared with control medium; ## p < 0.01 compared with LPS treatment.
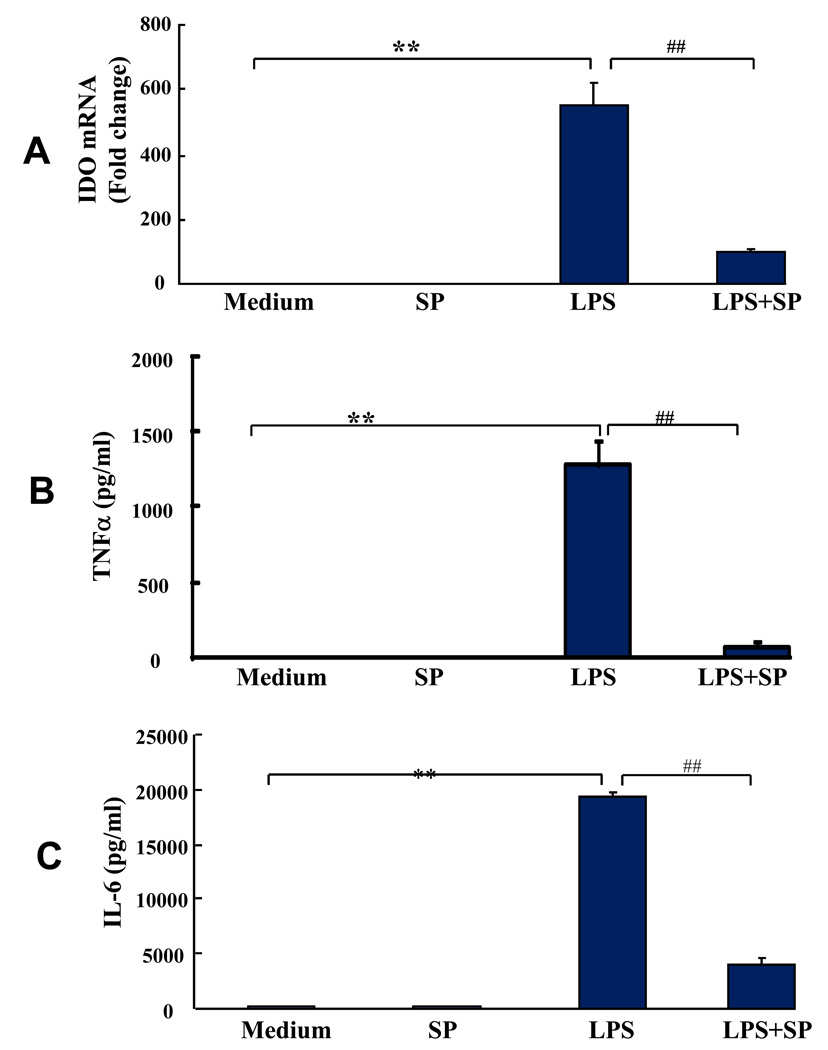
We hypothesized that inhibition of phospho-JNK would impair the ability of LPS to induce IDO. In order to do so, we first confirmed that SP600125 inhibits LPS-induction of proinflammatory cytokines, as has been recently reported in microglia (Jang et al., 2008). We found that the LPS-induced expression of both TNFα transcripts (data not shown) and protein (p < 0.01; Fig. 5B), as well as mRNA for IL-6 (data not shown) and IL-6 protein (p < 0.01; Fig. 5C), was significantly inhibited by SP600125. IL-1β mRNA was also induced by LPS and inhibited by SP600125 (data not shown). Because of the requirement to add ATP to induce processing and secretion of the mature IL-1β protein (Mingam et al., 2008), the effect of SP600125 on IL-1β protein was not measured. The critical question was whether IDO expression is also inhibited by SP600125. We found that LPS-induced IDO was abrogated by the JNK inhibitor (p < 0.01; Fig. 5A) indicating that JNK pathway is involved in expression of IDO in primary murine microglial cells. Importantly, neither SP600125 alone nor the diluent in which it was prepared (1% DMSO) affected viability of the cells, as determined by measuring both the proportion of trypan blue-positive cells and the release of lactate dehydrogenase into the culture medium. These data clearly demonstrate that the JNK signaling pathway is critical for the LPS-induced expression of IDO and proinflammatory cytokines.
Fig. 5. IDO, TNFα and IL-6 Expression by LPS.
IDO and inflammatory cytokine expression induced by LPS are blocked by pretreatment with the SP600125 JNK inhibitor. Primary murine microglial cells were pretreated with SP600125 (12 µM) for 30 min and then incubated with or without LPS (10 ng/ml) for 6 h. SP600125 inhibited LPS-induced (A) IDO, (B) TNFα and (C) IL-6 expression. Values are means ± S.E.M. obtained from three separate experiments. *p < 0.05 ** p < 0.01 compared with medium control; #P < 0.05 ## P < 0.01 compared with LPS treatment.
Discussion
These experiments establish that LPS induces IDO expression in the absence of detectable IFNγ in primary murine microglial cells. This IFNγ-independent pathway involves the MAPK JNK because blockade of LPS-induced JNK signaling abrogates LPS-induced IDO expression. Collectively, these new data extend the emerging concept that IDO can be induced by LPS through an IFNγ-independent mechanism and that microglia are important cellular constituents in this process.
The amino acid tryptophan is the precursor of serotonin and melatonin following activation of tryptophan hydroxylase, and tryptophan serves as the precursor of kynurenine following activation of IDO or hepatic tryptophan 2,3-dioxygenase (TDO; Ruddick et al., 2006). When tryptophan is diverted down the kynurenine pathway, there is less substrate available to form serotonin and melatonin. Although kynurenine lacks biological activity (Capuron et al., 2002; Ruddick et al., 2006), it serves as a substrate that yields several neuroactive metabolites, including kynurenic acid (KA) and quinolinic acid (QUIN; Guillemin et al., 2005). Inflammation-mediated dysregulation of the kynurenine pathway has been implicated as a contributor to a number of major brain disorders (Pérez-De La Cruz et al., 2007), including depression (Dantzer et al., 2008a).
IDO is the first rate-limiting enzyme in the synthesis of kynurenine and is required to mediate depressive-like behavior in response to infection with Bacille Calmette-Guérin (BCG; O’Connor et al., 2009b). IFNγ is a major inducer of IDO since IFNγ receptor KO mice do not develop depressive-like behavior following exposure to BCG (O’Connor et al., 2009c) However, IFNγ-independent pathways of IDO activation have recently been reported (Fujigaki et al., 2006; Jung et al., 2007; Connor et al., 2008). Several studies have suggested that a functional kynurenine metabolic pathway exists within the CNS (Gál and Sherman, 1980; Stone and Connick, 1985). Several cell types in the CNS express IDO, but only microglia maintain all the enzymes required to produce 3-hydroxykynurenine (3-HK) and QUIN (Guillemin et al., 2001, 2005). Our previous experiments demonstrated that LPS given i.p. induces expression of IDO in the brain (O’Connor et al., 2009a), but the type of cell that produces IDO in the brain was not identified. Here we focused on the modulation of IDO expression by LPS in primary mouse microglia.
Microglia are CNS tissue resident macrophages, and their activation by LPS is one of the first to be described in the literature (Hetier et al., 1988). LPS has been shown to induce IDO expression in primary rat glial cultures without an increase in detectible IFNγ (Agaugue et al., 2006; Connor et al., 2008). Microglia, as well as astrocytes, endothelial cells, and neurons, are all capable of producing IDO (Dantzer et al., 2008a). However, only microglia maintain all the enzymes required to produce 3-hydroxykynurenine (3-HK) and QUIN (Guillemin et al., 2001, 2005). IDO expression can be induced in primary murine microglia with stimuli such as TNFα, LPS, and IFNγ, but the receptor signaling mechanism for IDO induction has not been determined (Kwidzinski et al., 2005; Yadav et al., 2007). Here we report IDO gene expression, activity and regulatory mechanisms by LPS in activated primary murine microglia. Our results showed that LPS significantly induces expression of IDO mRNA and enzymatic activity. Accordingly, extracellular kynurenine levels in LPS-treated microglia conditioned medium increased at 8 h and 16 h following addition of LPS. This result is interpreted to mean that functional tryptophan catabolism via the kynurenine pathway exists in activated microglia in the absence of IFNγ. We expected that kynurenine in the extracellular fluid would continue to increase at 24 h because IDO activity was elevated at 24 h. However, extracellular kynurenine following incubation with LPS was not elevated at 24 h and began to decline. There are several downstream enzymes that catabolize kynurenine and its metabolites, which include KAT, KMO, KYNU and HAO. Because these enzymes further degrade kynurenine formed by IDO, kynurenine concentration can decline (Chiarugi et al., 2000; Kujundzic and Lowenthal, 2008). By measuring the expression of these downstream enzymes in the kynurenine pathway, we found that KMO and KYNU, but not KAT or HAO, were markedly enhanced by LPS in an identical time-dependent manner as IDO expression. Increased expression of the kynurenine-degrading enzymes KMO and KYNU, could contribute to the reduction in kynurenine in the extracellular fluid at 24 h.
It is widely accepted that IFNγ is an essential factor for IDO induction because IFNγ induces IDO in many types of cells (Hassanain et al., 1993; Chon et al., 1995). However, recent studies have demonstrated that IDO expression can be regulated by other inflammatory stimuli, including TNFα and LPS (Connor et al., 2008; Nisapakultorn et al., 2009). These data indicate that IDO can be up-regulated by both IFNγ–dependent and an independent pathway, but the mechanism for the latter has not been identified (Fujigaki et al., 2006; Jung et al., 2007). Some authors have reported that IFNγ can be induced in microglia (De Simone et al., 1998; Suzuki et al., 2005). However, in these reports, only IFNγ transcripts were detected with very little or no IFNγ protein. Our data demonstrate that IDO induction by LPS does not require synthesis of IFNγ because no IFNγ transcripts could be amplified in any treatment at any time point. These results are consistent with those of Connor et al. (2008) and Agaugue et al. (2006) who also reported that no IFNγ could be detected in rat glia or dendritic cells following LPS stimulation. Therefore, these data extend the developing concept that IDO can be induced by LPS in an IFNγ–independent mechanism in murine microglia (Fujigaki et al., 2001, 2006; Jung et al., 2007; Connor et al., 2008).
Although we have repeatedly demonstrated that LPS increases IFNγ in plasma and IFNγ mRNA in the brain (André et al., 2008; O’Connor et al., 2009a), Roche et al. (2006) reported that mature IFNγ protein could not be detected in the brains of LPS-treated rats. The most likely mediators of IFNγ-independent IDO expression in microglia are TNFα and IL-1β. Expression of mRNA transcripts for both TNFα and IL-1β appear earlier than those for IDO in the hippocampus of LPS-treated mice (André et al., 2008). The anti-depressant bupropion significantly reduces LPS-induced elevations in plasma TNFα and IL-1β (Brustolim et al., 2006). Mice that lack systemic and central expression of either type of TNF receptor develop an anti-depressant phenotype even in the absence of an exogenous inflammatory stimulus (Simen et al., 2006). Similarly, in a different model of inflammation-induced depressive-like behavior using Mycobacterium bovis, we recently reported that pretreatment of mice with a TNFα antagonist inhibits both IDO activation and depressive-like behavior (O’Connor et al., 2009c). Stronger evidence exists for a role of IL-1. Chronic stress exposure, which induces IL-1β in the hippocampus, induces depressive-like behaviors that are blocked by overexpression of the IL-1 receptor antagonist in the brain and in mice lacking the type I IL-1 receptor (Koo and Duman, 2008; Goshen et al., 2008).
Similar to all myeloid cells, microglia respond strongly to an LPS challenge with the release of a multitude of cytokines (Rivest, 2003). These effects are mediated by TLR4, a member of the TLR family (Takeda et al., 2003). Downstream of TLR4 activation is a complex signaling cascade involving MyD88, IRAK, ERK, p38, and JNK kinase activation (Laflamme and Rivest, 1999; Takeda et al., 2003). JNK plays a critical role in neurodegeneration and apoptosis (Hidding, et al., 2002; Gao and Ji, 2008). Inhibition of JNK activity diminishes excitotoxic neuronal loss associated with ischemic brain insults (Borsello et al., 2003; Repici et al., 2007). We previously demonstrated that a JNK inhibitor blocks TNFα-induced sickness behavior (Palin, et al., 2008). Because JNK has been shown to be required for IDO expression in CD11c+ dendritic cells following LPS stimulation (Jung et al., 2007), we tested the possibility that LPS-induced JNK promotes expression of IDO in primary microglia. We used SP600125 because it is a potent, cell-permeable, selective and reversible inhibitor of JNK. It competitively targets the ATP binding site of JNK1, JNK2, and JNK3, exhibiting over 300-fold greater selectivity for JNK as compared to ERK1 and p38. Our previous experiments (Strle et al., 2006) have demonstrated that SP600125 (10 µM) acts just like a novel I-JNK transduction protein to block the JNK activator anisomycin. The results showed that SP600125 significantly inhibits the phosphorylation of JNK residues Thr183/Tyr185 induced by LPS in primary murine microglia, indicating the inhibitor was very effective. Since SP600125 also blocked LPS-induction of IDO in microglia, it is likely that JNK is implicated in the development of inflammation-induced depressive-like behavior. As a positive control, we also queried whether JNK is needed for LPS-induced expression of proinflammatory cytokines. As expected, inhibition of JNK reduced the amount of TNFα and IL-6 synthesized in response to LPS. A report by Fujigaki et al. (2006) demonstrated that JNK signals can directly activate the transcription factor AP-1 or indirectly activate NFκB through downstream proinflammatory cytokines. These investigators established that there are multiple AP-1 and NFκB sites in the human IDO promoter. Preliminary sequencing and analysis (data not shown) in our laboratory indicates that most of these sites are conserved in the murine IDO promoter. Therefore, both AP-1 and NFκB, acting separately or synergistically, are potential regulatory elements for murine IDO. It is important to note that neither SP600125 alone nor the diluent in which it was prepared (1% DMSO) impaired cell viability. These data clearly demonstrate that JNK is involved in the up-regulation of LPS-induced IDO transcripts in primary murine microglia.
In conclusion, these data provide new insights into the IFNγ-independent mechanism of IDO gene expression. These results are the first to demonstrate that JNK is potently involved in LPS-induced expression of IDO in primary mouse microglia, thereby defining a TLR4 receptor signaling pathway that activates IDO in the complete absence of IFNγ. Inhibition of the JNK pathway may even provide a new approach for managing inflammation-induced depression (Manning and Davis, 2003). Indeed, these results are likely to extend to other important biological events in the brain such as ischemia. For example, recent results have established that mice deficient in TLR4 receptors (C3H/HeJ) have reduced CNS inflammatory responses, less brain damage and improved behavioral responses compared to normal (C3H/HeN) mice following restraint stress prior to permanent middle cerebral artery occlusion (Caso et al., 2007, 2008). A better understanding of the role of key signaling mediators like JNK in sickness behavior, depression, and a variety of neurological disorders could aid the development of novel pharmacological agents that might be used to treat inflammation-associated mental health disorders.
Acknowledgments
The authors have no conflicting financial interests. Supported by NIH to K.W.K. (AG029573) and R.D. (MH079829 and MH71349).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adler UC, Marques AH, Calil HM. Inflammatory aspects of depression. Inflamm Allergy Drug Targets. 2008;7:19–23. doi: 10.2174/187152808784165216. [DOI] [PubMed] [Google Scholar]
- André C, O’Connor JC, Kelley KW, Lestage J, Dantzer R, Castanon N. Spatiotemporal differences in the profile of murine brain expression of proinflammatory cytokines and indoleamine 2,3-dioxygenase in response to peripheral lipopolysaccharide administration. J. Neuroimmunology. 2008;200:90–99. doi: 10.1016/j.jneuroim.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agaugué S, Perrin-Cocon L, Coutant F, André P, Lotteau V. 1-Methyl-tryptophan can interfere with TLR signaling in dendritic cells independently of IDO activity. J. Immunol. 2006;177:2061–2071. doi: 10.4049/jimmunol.177.4.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsello T, Clarke PG, Hirt L, Vercelli A, Repici M, Schorderet DF, Bogousslavsky J, Bonny C. A peptide inhibitor of c-Jun N-terminal kinase protects against excitotoxicity and cerebral ischemia. Nat. Med. 2003;9:1180–1186. doi: 10.1038/nm911. [DOI] [PubMed] [Google Scholar]
- Brustolim D, Riberiro-dos-Santos R, Kast RE, Altschuler EL, Soares MB. A new chapter opens in anti-inflammatory treatments: the antidepressant bupropion lowers production of tumor necrosis factor-alpha and interferon-gamma in mice. Int. Immunopharmacol. 2006;6:903–907. doi: 10.1016/j.intimp.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Bsibsi M, Ravid R, Gveric D, van Noort JM. Broad expression of Toll-like receptors in the human central nervous system. J. Neuropathol. Exp. Neurol. 2002;61:1013–1021. doi: 10.1093/jnen/61.11.1013. [DOI] [PubMed] [Google Scholar]
- Capuron L, Ravaud A, Neveu PJ, Miller AH, Maes M, Dantzer R. Association between decreased serum tryptophan concentrations and depressive symptoms in cancer patients undergoin cytokine therapy. Mol. Psychiat. 2002;7:468–473. doi: 10.1038/sj.mp.4000995. [DOI] [PubMed] [Google Scholar]
- Carpentier PA, Duncan DS, Miller SD. Glial toll-like receptor signaling in central nervous system infection and autoimmunity. Brain. Behav. Immun. 2008;22:140–147. doi: 10.1016/j.bbi.2007.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caso JR, Pradillo JM, Hurtado O, Leza JC, Moro MA, Lizasoain I. Toll-like receptor 4 is involved in subacute stress-induced neuroinflammation and in the worsening of experimental stroke. Stroke. 2008;39:1314–1320. doi: 10.1161/STROKEAHA.107.498212. [DOI] [PubMed] [Google Scholar]
- Caso JR, Pradillo JM, Hurtado O, Lorenzo P, Moro MA, Lizasoain I. Toll-like receptor 4 is involved in brain damage and inflammation after experimental stroke. Circulation. 2007;115:1599–1608. doi: 10.1161/CIRCULATIONAHA.106.603431. [DOI] [PubMed] [Google Scholar]
- Chiarugi A, Sbarba PD, Paccagnini A, Donnini S, Filippi S, Moroni F. Combined inhibition of indoleamine 2,3-dioxygenase and nitric oxide synthase modulates neurotoxin release by interferon-g-activated macrophages. J. Leukoc. Biol. 2000;68:260–266. [PubMed] [Google Scholar]
- Chon SY, Hassanain HH, Pine R, Gupta SL. Involvement of two regulatory elements in interferon-gamma-regulated expression of human indoleamine 2,3-dioxygenase gene. J. Interferon Cytokine Res. 1995;15:517–526. doi: 10.1089/jir.1995.15.517. [DOI] [PubMed] [Google Scholar]
- Connor TJ, Starr N, O’Sullivan JB, Harkin A. Induction of indolamine 2,3-dioxygenase and kynurenine 3-monooxygenase in rat brain following a systemic inflammatory challenge: A role for IFNγ? Neuroscience Letters. 2008;441:29–34. doi: 10.1016/j.neulet.2008.06.007. [DOI] [PubMed] [Google Scholar]
- Dantzer R, Capuron L, Irwin MR, Miller AH, Ollat H, Perry VH, Rousey S, Yirmiya R. Identification and treatment of symptoms associated with inflammation in medically ill patients. Psychoneuroendocrinology. 2008b;33:18–29. doi: 10.1016/j.psyneuen.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat. Rev. Neurosci. 2008a;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Simone R, Levi G, Aloisi F. Interferon gamma gene expression in rat central nervous system glial cells. Cytokine. 1998;10:418–422. doi: 10.1006/cyto.1997.0314. [DOI] [PubMed] [Google Scholar]
- Evans DL, Charney DS, Lewis L, Golden RN, Gorman JM, Krishnan KR, Nemeroff CB, Bremner JD, Carney RM, Coyne JC, Delong MR, Frasure Smith N, Glassman AH, Gold PW, Grant I, Gwyther L, Ironson G, Johnson RL, Kanner AM, Katon WJ, Kaufmann PG, Keefe FJ, Ketter T, Laughren TP, Leserman J, Lyketsos CG, McDonald WM, McEwen BS, Miller AH, Musselman D, O'Connor C, Petitto JM, Pollock BG, Robinson RG, Roose SP, Rowland J, Sheline Y, Sheps DS, Simon G, Spiegel D, Stunkard A, Sunderland T, Tibbits P, Jr, Valvo WJ. Mood disorders in the medically ill: scientific review and recommendations. Biol. Psychiatry. 2005;58:175–189. doi: 10.1016/j.biopsych.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Fitzgerald P, Cassidy EM, Clarke G, Scully P, Barry S, Quigley Eamonn MM, Shanahan F, Cryan J, Dinan Timothy G. Tryptophan catabolism in females with irritable bowel syndrome: relationship to interferon-gamma, severity of symptoms and psychiatric co-morbidity. Neurogastroenterol Motil. 2008;20:1291–1297. doi: 10.1111/j.1365-2982.2008.01195.x. [DOI] [PubMed] [Google Scholar]
- Fujigaki H, Saito K, Fujigaki S, Takemura M, Sudo K, Ishiguro H, Seishima M. The signal transducer and activator of transcription 1alpha and interferon regulatory factor 1 are not essential for the induction of indoleamine 2,3-dioxygenase by lipopolysaccharide: involvement of p38 mitogen-activated protein kinase and nuclear factor-kappaB pathways, and synergistic effect of several proinflammatory cytokines. J. Biochem. 2006;139:655–662. doi: 10.1093/jb/mvj072. [DOI] [PubMed] [Google Scholar]
- Fujigaki S, Saito J, Sekikawa K, Tone S, Takikawa O, Fujii H, Wada H, Noma A, Seishima M. Lipopolysaccharide induction of indoleamine 2,3-dioxygenase is mediated dominantly by an IFN-γ-independent mechanism. Eur. J. Immunol. 2001;31:2313–2318. doi: 10.1002/1521-4141(200108)31:8<2313::aid-immu2313>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Gál EM, Sherman AD. L-kynurenine: its synthesis and possible regulatory function in brain. Neurochem Res. 1980;5:223–239. doi: 10.1007/BF00964611. [DOI] [PubMed] [Google Scholar]
- Gao YJ, Ji RR. Activation of JNK pathway in persistent pain. Neurosci. Lett. 2008;437:180–183. doi: 10.1016/j.neulet.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garden GA, Möller T. Microglia biology in health and disease. J. Neuroimmune Pharmacol. 2006;1:127–137. doi: 10.1007/s11481-006-9015-5. [DOI] [PubMed] [Google Scholar]
- Gong Y, Xue B, Jiao J, Jing L, Wang X. Triptolide inhibits COX-2 expression and PGE2 release by suppressing the activity of NF-kappaB and JNK in LPS-treated microglia. J. Neurochem. 2008;107:779–788. doi: 10.1111/j.1471-4159.2008.05653.x. [DOI] [PubMed] [Google Scholar]
- Goshen I, Kreisel T, Ben-Menachem-Zidon O, Licht T, Weidenfeld J, Ben-Hur T, Yirmiya R. Brain interleukin-1 medicates chronic stress-induced depression in mice via adrenocortical activation and hippocampal neurogenesis suppression. Mol. Psychiatry. 2008;13:717–728. doi: 10.1038/sj.mp.4002055. [DOI] [PubMed] [Google Scholar]
- Guillemin GJ, Kerr SJ, Smythe GA, Smith DG, Kapoor V, Armati PJ, Croitoru J, Brew BJ. Kynurenine pathway metabolism in human astrocytes: A paradox for neuronal protection. J. Neurochem. 2001;78:842–853. doi: 10.1046/j.1471-4159.2001.00498.x. [DOI] [PubMed] [Google Scholar]
- Guillemin GJ, Smythe G, Takikawa O, Brew BJ. Expression of indoleamine 2,3-dioxygenase and production of quinolinic acid by human microglia, astrocytes, and neurons. Glia. 2005;49:15–23. doi: 10.1002/glia.20090. [DOI] [PubMed] [Google Scholar]
- Hanisch U, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathological brain. Nat. Neurosci. 2007;10:1387–1394. doi: 10.1038/nn1997. [DOI] [PubMed] [Google Scholar]
- Hassanain HH, Chon SY, Gupta SL. Differential regulation of human indoleamine 2,3-dioxygenase gene expression by interferons-gamma and -alpha. Analysis of the regulatory region of the gene and identification of an interferon-gamma-inducible DNA-binding factor. J. Biol. Chem. 1993;268:5077–5084. [PubMed] [Google Scholar]
- Hetier E, Ayala J, Denèfle P, Bousseau A, Rouget P, Mallat M, Prochiantz A. Brain macrophages synthesize interleukin-1 and interleukin-1 mRNAs in vitro. J Neurosci Res. 1988;21:391–397. doi: 10.1002/jnr.490210230. [DOI] [PubMed] [Google Scholar]
- Hidding U, Mielke K, Waetzig V, Brecht S, Hanisch U, Behrens A, Wagner E, Herdegen T. The c-Jun N-terminal kinases in cerebral microglia: immunological functions in the brain. Biochem Pharmacol. 2002;64:781–788. doi: 10.1016/s0006-2952(02)01139-5. [DOI] [PubMed] [Google Scholar]
- Hoshi M, Saito K, Murakami Y, Taguchi A, Fujigaki H, Tanaka R, Takemura M, Ito H, Hara A, Seishima M. Marked increases in hippocampal neuron indoleamine 2, 3-dioxygenase via IFN-gamma-independent pathway following transient global ischemia in mouse. Neurosci Res. 2009;63:194–198. doi: 10.1016/j.neures.2008.12.003. [DOI] [PubMed] [Google Scholar]
- Jang S, Kelley KW, Johnson RW. Luteolin reduces IL-6 production in microglia by inhibiting JNK phosphorylation and activation of AP-1. Proc. Natl. Acad. Sci. USA. 2008;105:7534–7539. doi: 10.1073/pnas.0802865105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung ID, Lee CM, Jeong YI1, Lee JS, Park WS, Han J, Park YM. Different regulation of indoleamine 2,3-dioxygenase by lipopolysaccharide and interferon gamma in murine bone marrow derived dendritic cells. FEBS Letter. 2007;581:1449–1456. doi: 10.1016/j.febslet.2007.02.073. [DOI] [PubMed] [Google Scholar]
- Kaper T, Looger LL, Takanaga H, Platten M, Steinman L, Frommer WB. Nanosensor detection of an immunoregulatory tryptophan influx/kynurenine efflux cycle. PLoS. Biol. 2007;5:2201–2210. doi: 10.1371/journal.pbio.0050257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo JW, Duman RS. IL-1β is an essential mediator of the antineurogenic and anhedonic effects of stress. Proc. Natl. Acad. Sci. USA. 2008;105:751–756. doi: 10.1073/pnas.0708092105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujundzic RN, Lowenthal JW. The role of tryptophan metabolism in iNOS transcription and nitric oxide production by chicken macrophage cells upon treatment with interferon gamma. Immunology Letters. 2008;115:153–159. doi: 10.1016/j.imlet.2007.11.003. [DOI] [PubMed] [Google Scholar]
- Kwidzinski E, Bunse J, Aktas O, Richter D, Mutlu L, Zipp F, Nitsch R, Bechmann I. Indolamine 2,3-dioxygenase is expressed in the CNS and down-regulates autoimmune inflammation. FASEB J. 2005;19:1347–1349. doi: 10.1096/fj.04-3228fje. [DOI] [PubMed] [Google Scholar]
- Laflamme N, Rivest S. Effects of systemic immunogenic insults and circulating proinflammatory cytokines on the transcription of the inhibitory factor kappaB alpha within specific cellular populations of the rat brain. J. Neurochem. 1999;73:309–321. doi: 10.1046/j.1471-4159.1999.0730309.x. [DOI] [PubMed] [Google Scholar]
- Lehrmann E, Guidetti P, Löve A, Williamson J, Bertram EH, Schwarcz R. Glial activation precedes seizures and hippocampal neurodegeneration in measles virus-infected mice. Epilepsia. 2008;49 Suppl 2:13–23. doi: 10.1111/j.1528-1167.2008.01489.x. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Lim KT. Inhibitory effect of ZPDC glycoprotein on the expression of inflammation-related cytokines through p38 MAP kinase and JNK in lipopolysaccharide-stimulated RAW 264.7 cells. Inflamm. Res. 2009;58:184–191. doi: 10.1007/s00011-008-8118-2. [DOI] [PubMed] [Google Scholar]
- Lestage J, Verrier D, Palin K, Dantzer R. The enzyme indoleamine 2,3-dioxygenase is induced in the mouse brain in response to peripheral administration of lipopolysaccharide and superantigen. Brain, Behavior, and Immunity. 2002;16:596–601. doi: 10.1016/s0889-1591(02)00014-4. [DOI] [PubMed] [Google Scholar]
- Liu Q, VanHoy RW, Zhou JH, Dantzer R, Freund GG, Kelley KW. Elevated cyclin E levels, inactive retinoblastoma protein, and suppression of the p27(KIP1) inhibitor characterize early development of promyeloid cells into macrophages. Mol Cell Biol. 1999;19:6229–6239. doi: 10.1128/mcb.19.9.6229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning AM, Davis RJ. Targeting JNK for therapeutic benefit: from junk to gold? Nat. Rev. Drug. Discov. 2003;2:554–565. doi: 10.1038/nrd1132. [DOI] [PubMed] [Google Scholar]
- Mingam R, De Smedt V, Amédée T, Bluthé RM, Kelley KW, Dantzer R, Layé S. In vitro and in vivo evidence for a role of the P2X7 receptor in the release of IL-1 beta in the murine brain. Brain. Behav. Immun. 2008;22:234–244. doi: 10.1016/j.bbi.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumeister A. Tryptophan depletion, serotonin, and depression: where do we stand? Psychopharmacol. Bull. 2003;37:99–115. [PubMed] [Google Scholar]
- Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- Nisapakultorn K, Makrudthong J, Sa-Ard-Iam N, Rerkyen P, Mahanonda R, Takikawa O. Indoleamine 2,3-dioxygenase expression and regulation in chronic periodontitis. J. Periodontol. 2009;80:114–121. doi: 10.1902/jop.2009.080315. [DOI] [PubMed] [Google Scholar]
- O'Connor JC, Lawson MA, André C, Moreau M, Lestage J, Castanon N, Kelley KW, Dantzer R. Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. Mol. Psychiatry. 2009a;14:511–522. doi: 10.1038/sj.mp.4002148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor JC, Lawson MA, André C, Briley EM, Szegedi SS, Lestage J, Castanon N, Herkenham M, Dantzer R, Kelley KW. Induction of IDO by bacille Calmette-Guérin is responsible for development of murine depressive-like behavior. J. Immunol. 2009b;182:3202–3212. doi: 10.4049/jimmunol.0802722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor JC, Andre C, Wang YX, Lawson MA, Szegedi SS, Lestage J, Castanon N, Kelley KW, Dantzer R. Interferon-γ and tumor necrosis factor-α mediate the upregulation of indoleamine 2,3-dioxygenase and the induction of depressive-like behavior in mice in response to bacillus Calmette-Guérin. J. Neuroscience. 2009c;29:4200–4209. doi: 10.1523/JNEUROSCI.5032-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palin K, McCusker RH, Strle K, Moos F, Dantzer R, Kelley KW. Tumor necrosis factor-α-induced sickness behavior is impaired by central administration of an inhibitor of c-Jun N-terminal kinase. Psychopharmacology. 2008;197:629–635. doi: 10.1007/s00213-008-1086-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-De La Cruz V, Königsberg M, Santamaría A. Kynurenine pathway and disease: an overview. CNS Neurol Disord Drug Targets. 2007;6:398–410. doi: 10.2174/187152707783399229. [DOI] [PubMed] [Google Scholar]
- Pocivavsek A, Burns MP, Rebeck GW. Low-density lipoprotein receptors regulate microglial inflammation through c-Jun N-terminal kinase. Glia. 2009;57:444–453. doi: 10.1002/glia.20772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. 2006;27:24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repici M, Centeno C, Tomasi S, Forloni G, Bonny C, Vercelli A, Borsello T. Time-course of c-Jun N-terminal kinase activation after cerebral ischemia and effect of D-JNKI1 on c-Jun and caspase-3 activation. Neuroscience. 2007;150:40–49. doi: 10.1016/j.neuroscience.2007.08.021. [DOI] [PubMed] [Google Scholar]
- Rivest S. Molecular insights on the cerebral innate immune system. Brain. Behav. Immun. 2003;17:13–19. doi: 10.1016/s0889-1591(02)00055-7. [DOI] [PubMed] [Google Scholar]
- Roche M, Diamond M, Kelly JP, Finn DP. In vivo modulation of LPS-induced alterations in brain and peripheral cytokines and HPA axis activity by cannabinoids. J. Neuroimmunol. 2006;181:57–67. doi: 10.1016/j.jneuroim.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Ruddick JP, Evans AK, Nutt DJ, Lightman SL, Rook GAW, Lowry CA. Tryptophan metabolism in the central nervous system: medical implications. Expert Rev. Mol. Med. 2006;8:1–27. doi: 10.1017/S1462399406000068. [DOI] [PubMed] [Google Scholar]
- Shen WH, Zhou JH, Broussard SR, Freund GG, Dantzer R, Kelley KW. Proinflammatory cytokines block growth of breast cancer cells by impairing signals from a growth factor receptor. Cancer Res. 2002;62:4746–4756. [PubMed] [Google Scholar]
- Simen BB, Duman CH, Simen AA, Duman RS. TNFα signaling in depression and anxiety: Behavioral consequences of individual receptor targeting. Biol. Psychiatry. 2006;59:775–785. doi: 10.1016/j.biopsych.2005.10.013. [DOI] [PubMed] [Google Scholar]
- Sono M. The roles of superoxide anion and methylene blue in the reductive activation of indoleamine 2,3-dioxygenase by ascorbic acid or by xanthine oxidase-hypoxanthine. J. Biol. Chem. 1989;264:1616–1622. [PubMed] [Google Scholar]
- Soulet D, Rivest S. Microglia. Curr Biol. 2008;18:R506–R508. doi: 10.1016/j.cub.2008.04.047. [DOI] [PubMed] [Google Scholar]
- Spalletta G, Bossù P, Ciaramella A, Bria P, Caltagirone C, Robinson RG. The etiology of poststroke depression: a review of the literature and a new hypothesis involving inflammatory cytokines. Mol. Psychiatry. 2006;11:984–991. doi: 10.1038/sj.mp.4001879. [DOI] [PubMed] [Google Scholar]
- Stone TW, Connick JH. Quinolinic acid and other kynurenines in the central nervous system. Neuroscience. 1985;15:597–617. doi: 10.1016/0306-4522(85)90063-6. [DOI] [PubMed] [Google Scholar]
- Strle K, Broussard SR, McCusker RH, Shen WH, LeCleir JM, Johnson RW, Freund GG, Dantzer R, Kelley KW. C-jun N-terminal kinase mediates tumor necrosis factor-alpha suppression of differentiation in myoblasts. Endocrinology. 2006;147:4363–4373. doi: 10.1210/en.2005-1541. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Claflin J, Wang X, Lengi A, Kikuchi T. Microglia and macrophages as innate producers of interferon-gamma in the brain following infection with Toxoplasma gondii. Int. J. Parasitol. 2005;35:83–90. doi: 10.1016/j.ijpara.2004.10.020. [DOI] [PubMed] [Google Scholar]
- Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu. Rev. Immunol. 2003;21:335–376. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Oh-Hashi K, Shitara H, Hirata Y, Kiuchi K. NF-kappaB independent signaling pathway is responsible for LPS-induced GDNF gene expression in primary rat glial cultures. Neurosci Lett. 2008;431:262–267. doi: 10.1016/j.neulet.2007.11.051. [DOI] [PubMed] [Google Scholar]
- Widner B, Laich A, Sperner-Unterweger B, Ledochowski M, Fuchs D. Neopterin production tryptophan degradation and mental depression: what is the link? Brain. Behav. Immun. 2002;16:590–595. doi: 10.1016/s0889-1591(02)00006-5. [DOI] [PubMed] [Google Scholar]
- Yadav MC, Burudi EM, Alirezaei M, Flynn CC, Watry DD, Lanigan CM, Fox HS. IFN-gamma-induced IDO and WRS expression in microglia is differentially regulated by IL-4. Glia. 2007;55:1385–1396. doi: 10.1002/glia.20544. [DOI] [PMC free article] [PubMed] [Google Scholar]