Abstract
Interferon-alpha (IFNα) is used for the treatment of various disorders, most notable chronic hepatitis C virus (HCV) infection. One of the commonest side effects of IFNα therapy is thyroiditis, with up to 40% of HCV patients on IFNα developing clinical or subclinical disease. In some cases interferon induced thyroiditis (IIT) may result in severe symptomatology necessitating discontinuation of therapy. IIT can manifest as clinical autoimmune thyroiditis, presenting with symptoms of classical Hashimoto’s thyroiditis or Graves’ disease, or as non-autoimmune thyroiditis. Non-autoimmune thyroiditis can manifest as destructive thyroiditis, with early thyrotoxicosis and later hypothyroidism, or as non-autoimmune hypothyroidism. While the epidemiology and clinical presentation of IIT have been well characterized the mechanisms causing IIT are still poorly understood. It is likely that the hepatitis C virus (HCV) itself plays a role in the disease, as the association between HCV infection and thyroiditis is well established. It is believed that IFNα induces thyroiditis by both immune stimulatory effects and by direct effects on the thyroid. Early detection and therapy of this condition are important in order to avoid complications of thyroid disease such as cardiac arrhythmias.
Keywords: Interferon, thyroiditis, autoimmunity
INTRODUCTION
Interferon alpha (IFNα) is a type I interferon that has been widely used as a therapeutic agent mostly, but not exclusively, for infectious and malignant diseases (1). IFNα binds to interferon receptors, and activates various signaling pathways, including the JAK-STAT pathway, the Crk-pathway, the IRS signaling pathway, and the MAP kinase pathway thereby stimulating transcription of target proteins which mediate its immune anti-tumor effects (2–4). One of the major indications for IFNα treatment is chronic hepatitis C. Approximately 50% of patients with chronic hepatitis C, who are treated with IFNα plus ribavirin, will achieve a sustained virologic response (5).
IFNα therapy is associated with many side effects ranging from influenza-like symptoms to hematologic and neuropsychiatric side effects (6). One of the commonest side-effects of IFNα therapy is thyroiditis. The association between IFNα and thyroid disease was first reported more than two decades ago (7), and since the original description of interferon induced thyroiditis (IIT) numerous studies have reported a high incidence of thyroid disease in patients treated with IFNα (8;9). IIT is a major complication of IFNα therapy and can interfere with effective management of chronic hepatitis C. Indeed, some of the manifestations of IIT, especially thyrotoxicosis, can be severe and may result in stopping of interferon therapy (10;11). Moreover, since the symptoms of hypothyroidism such as fatigue, and weight gain might be attributable to chronic hepatitis C or to IFNα therapy (6), the diagnosis of hypothyroidism in these patients might be delayed leading to development of further complications. In this chapter we will review the epidemiology, etiology, manifestations, and clinical approach to IIT.
EPIDEMIOLOGY
Based on the epidemiological characteristics of IIT we have recently suggested a new classification of IIT into autoimmune IIT and non-autoimmune IIT (12). Autoimmune IIT can manifest as Graves’ disease (GD), Hashimoto’s thyroiditis (HT), or the production of thyroid autoantibodies (TAb’s) without clinical disease, while non-autoimmune IIT can manifest as destructive thyroiditis, or non-autoimmune hypothyroidism.
Autoimmune IIT
Hashimoto’s thyroiditis (HT) is the commonest clinical manifestation of autoimmune IIT (13–15). The presence of TAb’s prior to the initiation of IFNα therapy is a risk factor for the development of autoimmune IIT manifesting as Hashimoto’s thyroiditis (9;16–18). Roti et al. calculated that elevated TPO antibodies before IFNα therapy had a positive predictive value of 67% for the development of clinical autoimmune IIT (10). The fact that pre-IFNα treatment TAb’s are a risk factor for IIT suggests a genetic predisposition to the development of IIT (12).
The development of Graves’ disease secondary to IFNα therapy is less common (10;19). A retrospective review of 321 patients with hepatitis B or C treated with IFNα found 10 patients who developed clinical thyrotoxicosis and of them six had classical signs of GD (19). In all cases of GD the thyrotoxicosis did not resolve after discontinuation of IFNα therapy (19). In most reported cases of autoimmune IIT manifesting as GD the disease did not go into remission when IFNα therapy was completed or stopped (16;19;20). One case report of Graves’ ophthalmopathy secondary to IFNα treatment for hepatitis C was also reported (21).
The most common form of thyroid autoimmunity is the presence of thyroid antibodies [including thyroid peroxidase antibodies (TPO-Ab), and thyroglobulin antibodies (Tg-Ab)], without clinical disease (22). The presence of TAb’s is usually a pre-clinical phase of AITD (23), and is genetically determined (24). TAb’s without clinical disease commonly develop during or following IFNα therapy. TAb’s can develop de novo during IFNα therapy, or their levels can increase during IFNα therapy if they were positive prior to treatment. Thus, it seems that IFNα can induce thyroid autoimmunity de novo, as well as exacerbate pre-existing thyroid autoimmunity (16;18). The incidence of de novo development of TAb’s is about 10–40% (10;16–18;25). TAb’s develop more frequently in women than in men treated with IFNα, and they are also associated with increasing age (26). The majority of individuals who develop “de novo” TAb’s on IFNα therapy remain TAb positive after the end of treatment (15).
Non-Autoimmune IIT
Non-autoimmune IIT is also common and is seen in up to 50% of IIT cases. Non-autoimmune IIT usually manifests as destructive thyroiditis. Destructive thyroiditis is a self-limited inflammatory disorder of the thyroid gland characterized by an early thyrotoxic phase, caused by the release of preformed thyroid hormones, and a late hypothyroid phase, with complete resolution in most cases (12). Since many cases of destructive thyroiditis secondary to IFNα are mild or subclinical, it is possible that destructive thyroiditis occurs more frequently than reported. Permanent hypothyroidism is unusual and develops in less than 5% of cases (27). Destructive thyroiditis in patients receiving IFNα therapy is diagnosed based on negative TSH-receptor antibodies (TRAb) and low thyroid radioactive iodine uptake (12). On re-treatment with IFNα patients frequently develop recurrent thyroiditis, and therefore, thyroid functions should be carefully monitored if treatment with IFNα is resumed (28). While most cases of destructive thyroiditis due to IFNα resolve spontaneously, some patients may develop complications, such as rapid atrial fibrillation, requiring thyroid ablation prior to re-treatment with IFNα. Moreover, a subset of patients with destructive thyroiditis may progress to permanent hypothyroidism (11). The presence of TAb’s prior to the initiation of IFNα is a significant risk factor for developing permanent hypothyroidism due to autoimmune thyroiditis. Interestingly, one group from France reported 3 patients that developed destructive thyroiditis followed by hypothyroidism, and a few months later by recurrent thyrotoxicosis due to Graves’ disease (29). Of note all 3 patients had positive TAb’s during the DT episode again demonstrating that IFNα triggers autoimmune thyroiditis in genetically predisposed individuals.
Non-autoimmune IIT can also manifest by hypothyroidism without TAb’s (20). Frequently the hypothyroidism is transient and resolves spontaneously upon completion of IFNα therapy (10;14–16).
ETIOLOGY
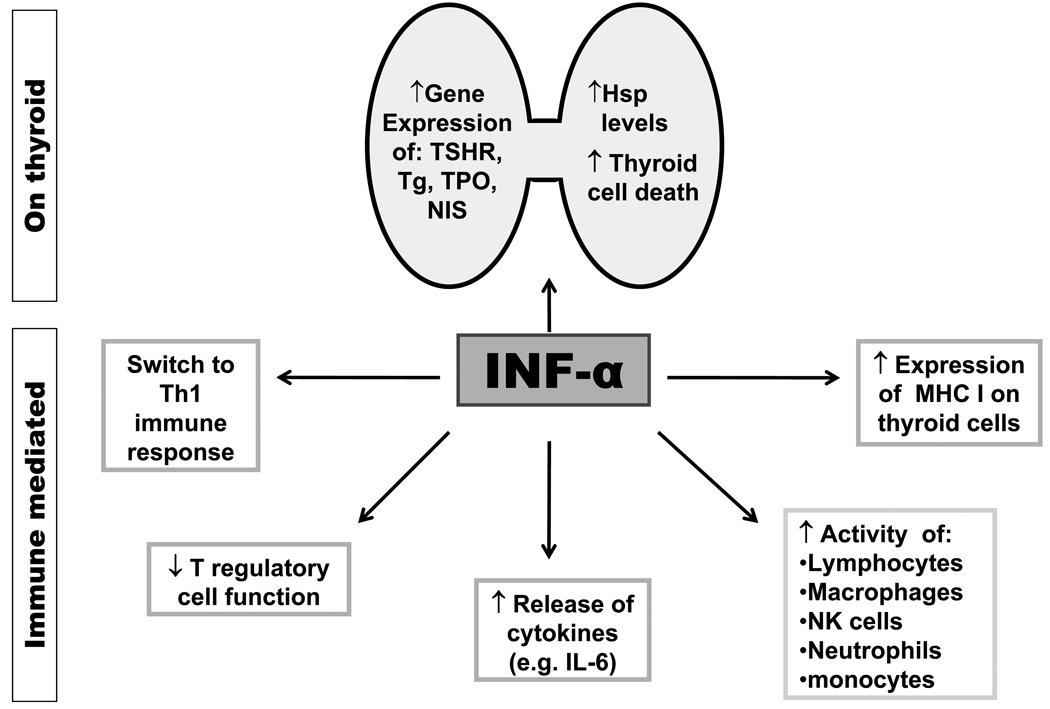
Most of the beneficial effects of IFNα in infectious and malignant diseases are due to its effects on the immune system. Therefore, it was assumed for many years that the side effects associated with IFNα therapy including thyroiditis are due to immune dysregulation. However, while, immune mechanisms must play a role in the development of IIT, the predilection to the thyroid, and the non-autoimmune manifestations of IIT suggested that direct thyroid effects of IFNα play a major role in the etiology of IIT (Figure 1).
Figure 1.
Postulated mechanism for the development of IIT. Both autoimmune and non-autoimmune effects of IFNα are involved. Immune effects of IFNα include activation of immune cells, switching the immune response to Th1 pathways, downregulation of Treg cells, and induction of cytokine release and MHC I expression. Direct thyroid toxic effects include upregulation of thyroid-specific proteins (TSHR, Tg, TPO, NIS) expression, as well as induction of heat shock proteins (Hsp) expression, and thyroid cell death. The combination of direct thyroid toxicity and immune stimulation can cause release of thyroid auto-antigens and their presentation to resident T-cells. This will initiate an autoimmune response by a bystander mechanism.
Immune mechanism
IFNα receptor binding results in activation of the JAK-STAT pathway (30), leading to activation of a large number of interferon-stimulated genes (ISGs) including cytokine and adhesion molecule genes (31;32). These combined effects can contribute to the development of thyroid autoimmunity. IFNα increases the expression of MHC class I antigens on cells. As expected, IFNα was shown to increase the expression of MHC class I antigens on thyroid epithelial cells (10). Over-expression of class I antigens can activate cytotoxic T cells, thereby leading to tissue damage and inflammatory response (31).
Another effect of IFNα that could trigger autoimmune IIT is switching of the immune response to a Th1 mediated pattern (33). This causes production of IFN-γ and IL-2, two potent proinflammatory cytokines (34). Indeed, hepatitis C patients, treated with IFNα, that developed IIT showed Th1 polarization of their immune response (35). However, Graves’ disease, which is generally believed to be a Th2 mediated disease, is one of the manifestations of autoimmune IIT (36;37). Since INFα is associated with Th1 switching it is unclear how IFNα can induce GD in some patients. One explanation of this paradox comes from recent studies by Rapoport and colleagues suggesting that the initiation phase of GD may be Th1 mediated (38).
IFNα enhances the activity of lymphocytes, macrophages, and NK cells (1;31;39), and it also activates neutrophils and monocytes (31). IFNα can induce the release of potent cytokines, such as IL-6 (31), a cytokine that has been associated with autoimmune thyroiditis (40). Additional effects of IFNα include alteration of immunoglobulin production and decrease in T regulatory cell function, which can contribute to the development of autoimmune responses (41;42).
Direct effects of interferon alpha on the Thyroid
The predilection of the autoimmune effects of IFNα to the thyroid and the occurrence of non-autoimmune IIT in up to 50% of patients strongly suggest that IFNα exerts direct effects on the thyroid. Few studies to date examined the direct effects of IFNα on thyroid cells. IFNα was shown to inhibit TSH-induced gene expression of thyroglobulin (Tg), TPO, and sodium iodide symporter (NIS) in cultured human thyrocytes (43). We have recently tested the expression levels of the TSHR, Tg, TPO, and NIS genes in a rat thyroid cell line. Our results showed an early (24 hours) increase in the levels of TSHR, Tg, TPO, and NIS with a later decrease (at 48 hrs) in the levels of TPO and NIS, but not TSHR (44). Moreover, we have shown increased thyroid cell death induced by IFNα (44). Increased thyroid cell death was also recently reported by another group (45). Combined, these data demonstrate a direct toxic effect of IFNα on thyroid follicular cells. Such effects can explain the non-autoimmune manifestations of IIT.
DIAGNOSIS AND TREATMENT (12)
The diagnosis of IIT is not always simple and straightforward. Since the patients have a plethora of symptoms associated with chronic hepatitis C and interferon alpha treatment, symptoms of hypo- or hyper-thyroidism may be attributed to their underlying disease and not to a thyroid abnormality. For example, classical symptoms of hypothyroidism such as weight gain, lack of energy, depression, or hair loss could be caused by the liver disease or interferon alpha.
Diagnosis of IIT
Since IIT can cause severe hyper-or hypo-thyroidism (10;11;21;26;46;47), it is now recommended that all patients receiving interferon alpha therapy for hepatitis C have a baseline TSH level measured and repeat TSH measured at regular intervals during therapy (12). Clinicians should routinely examine for signs of thyroid dysfunction such as tachycardia or bradycardia, heat or cold intolerance, and unexpected weight change.
In addition, we recommend screening all hepatitis C patients for thyroid disease prior to starting IFNα therapy. Baseline screening should include at least TSH levels but we recommend screening also for thyroid antibodies (TAb’s) (TPO-Ab, Tg-Ab) since positive TAb’s are a significant risk factor for IIT (9). If the TSH level is normal and TAb’s negative, TSH levels should be monitored every three months until completion of IFNα course. If TSH levels are normal, but TAb’s are positive we recommend monitoring TSH levels every two months since clinical thyroid disease is much more likely to develop. Currently there are not tests to predict the development of IIT in patients starting IFNα course. However, a recent study from Italy showing a significant association between lower serum CXCL10 levels and the development of IIT, suggested that this might be a useful predictive test (48). Since pre-treatment TAb’s also have a high predictive value for the development of autoimmune IIT (10), it would be interesting to test in the future the predictive value both of these tests combined.
If the patient develops abnormal thyroid functions while on IFNα a full workup needs to be completed. For thyrotoxicosis, in order to discern between GD and destructive thyroiditis, workup should also include TSH receptor antibody (TRAb), and TPO-Ab, and Tg-Ab levels. If antibodies are negative I-123 uptake and scan may be performed. For hypothyroidism workup should include full thyroid functions and antibodies (TPO-Ab, and Tg-Ab).
Treatment of IIT
Thyrotoxicosis
In most cases thyrotoxicosis is caused by destructive thyroiditis (DT) or by Graves’ disease (GD). If the TRAb and TAb’s are negative and the radio-iodine uptake is low the patient most likely has DT, and should be treated with a beta-blocker, if symptomatic. Patients with DT should be monitored for the development of hypothyroidism, which usually follows the hyperthyroid phase within a few weeks. Corticosteroids, while helpful in subacute thyroiditis, are generally contraindicated in hepatitis C patients. In most cases IFNα therapy can be continued. However, if severe symptomatic thyrotoxicosis develops withholding IFNα therapy should be considered in consultation with the hepatologist (19). In addition, re-challenge with IFNα may result in a recurrence of DT (28), and, therefore, patients need close monitoring of thyroid functions if they are re-treated with IFNα. In cases of IIT that manifest as Graves’ disease (GD), thyroid ablation with radioactive iodine and/or surgery is preferred (49). We do not recommend treating patients with interferon-induced GD with anti-thyroid medications since they can worsen the liver dysfunction.
Hypothyroidism
Patients are treated with thyroid hormone replacement, with no need to stop IFNα therapy. However, close monitoring of thyroid functions, at least every two months, should be performed since the disease may progress leading to increased T4 requirements. Moreover, T4 replacement requirements may increase if patients are treated with a second course of interferon, or may decrease or end altogether after completion of IFNα course (14;26).
IS THERE A GENETIC SUSCETPIBILITY TO IIT?
GD and HT have a strong genetic predisposition (reviewed in (50;51)). Therefore, one hypothesis on the pathogenesis of IIT is that IFNα triggers thyroiditis in genetically predisposed individuals (12). Support for a genetic predisposition for IIT comes from epidemiological observations showing variations in the prevalence of IIT in different ethnic populations. In one study Asian origin was an independent predictor of thyroid dysfunction in patients receiving IFNα (52). Like in AITD there is a female preponderance in IIT (8). The female predisposition to IIT could be due to an X-chromosome susceptibility locus, or due to the effects of estrogens (53).
The presence of TAb’s before initiation of IFNα therapy is a strong risk factor for the development of clinical IIT (9;10;16). Since the presence of TAb’s is a pre-clinical stage of AITD and represents a marker for genetic predisposition to AITD (23), this finding also supports the notion that IFNα may trigger thyroiditis in genetically predisposed individuals. We have studied the genetic predisposition to IIT in the thyroiditis-prone NOD-H2h4 mouse (54). When we treated NOD-H2h4 mice with IFNα for eight weeks the interferon-injected group showed increased frequency thyroiditis and/or thyroid antibodies, compared to the saline–injected group, (46.2% vs. 30.8%); however, this difference was not statistically significant and more studies are needed to examine the effects of IFNα on thyroiditis in NOD-H2h4 mice (54;54).
In recent years several susceptibility genes for thyroid autoimmunity have been identified (55), including HLA-DR (56;57), CTLA-4 (58–60), PTPN22 (61;62), thyroglobulin (63) and TSHR (64). It is likely that some of these genes also contribute to the genetic susceptibility to IIT. Indeed, two small studies showed HLA associations of IIT (46;65). We recently tested several candidate genes for association with IIT. Our preliminary data, in a small cohort, showed evidence for association of IIT with polymorphisms in the CTLA-4 and CD40 genes (66). Taken together, this preliminary evidence supports a genetic role in the etiology of IIT.
DOES CHRONIC HEPATITIS C VIRUS INFECTION CONTRIBUTE TO THE PATHOGENESIS OF IIT?
IIT has been reported in patients receiving IFNα for various diseases (67). However, by far IIT is most common in patients with chronic HCV infection. Could HCV infection play a role in the etiology of IIT? In fact, there is strong evidence that infectious agents may trigger thyroid autoimmunity (68). Among the possible infectious triggers of AITD, HCV has received much attention (69).
Earlier studies of patients with hepatitis C that never received IFNα showed mixed results. Some studies did not show a correlation between hepatitis C infection and the presence of thyroid antibodies (70–72). On the other hand, other studies have shown a significant association between hepatitis C infection and thyroid disorders (17;73–76). Some of the weaknesses of earlier studies included the use of less sensitive TAb assays and the lack of control for factors, which may affect the development of thyroid autoimmunity, mainly iodine intake. However, a recent well-controlled study demonstrated that both hypothyroidism and thyroid autoimmunity were significantly more common in patients with hepatitis C compared to controls (69;76). Similarly, Indolfi et al found that the prevalence of non autoimmune hypothyroidism, as well as the presence of Tg-Ab, were higher in untreated children with HCV compared to healthy non-HCV infected controls. This increased prevalence was not associated with other parameters (family history of autoimmune diseases, duration of HCV infection, viral genotype, viral load or liver function) except active HCV infection (77). Moreover, pooling of data from all studies on HCV infection and thyroid autoimmunity demonstrated a significant increase in the risk of thyroiditis in HCV patients (78). Therefore, we conclude that HCV infection is most likely a risk factor for thyroiditis both autoimmune and non-autoimmune.
Could HCV trigger thyroiditis by infecting thyroid cells? A recent study demonstrated HCV virions inside thyroid follicular cells (79) suggesting that this could be a potential mechanism. However, even if HCV cannot infect thyroid cells, viral proteins that are shed from virions may also have important physiological consequences. For example, it was shown that HCV E2 proteins can induce apoptosis (80;81), and upregulate the pro-inflammatory cytokine interleukin 8 (IL-8) (82). These data suggested that HCV envelope proteins themselves could significantly impact the thyroid environment and contribute to thyroid dysfunction. Indeed, we have recently shown that HCV E2 protein can bind to CD81 molecules on thyroid cells and trigger IL-8 secretion (83). Thus, a potential mechanism exist whereby HCV itself infects thyroid cells, or its envelope proteins bind to thyroid cells, triggering cytokine secretion that can activate resident T-cells and, in genetically susceptible individuals, result in thyroid autoimmunity through a bystander mechanism.
CONCLUSIONS
Interferon induced thyroiditis (IIT) is a common complication of IFNα therapy of HCV patients (84). IFNα can induce thyroiditis by at least two mechanisms: (1) Immune dysregulation resulting in activation of T-cells which, in genetically predisposed individuals, will trigger AITD; (2) Direct thyroid toxic effects resulting in thyroid inflammation which, in genetically predisposed individuals, can cause autoimmune thyroiditis by bystander mechanisms. HCV infection most likely has a synergistic effect in the triggering of thyroiditis by IFNα. IIT has very high prevalence and all patients with chronic hepatitis C receiving IFNα therapy should undergo routine thyroid screening. In most cases IIT can be treated without discontinuing IFNα therapy, but occasionally the manifestations are severe requiring halting IFNα therapy.
CLINICAL PRACTICE POINTS.
Interferon induced thyroiditis (IIT) is a common complication of interferon alpha (IFNα) treatment of chronic hepatitis C with up to 10% of patients developing clinical thyroiditis and up to 30% developing subclinical autoimmune thyroiditis.
The main clinical manifestations of IIT can be divided into: (1) autoimmune IIT, which can manifest as Hashimoto’s thyroiditis, Graves’ disease, or the development of thyroid antibodies; and (2) non-autoimmune IIT which can manifest as destructive thyroiditis or non-autoimmune hypothyroidism.
While most cases of IIT are mild, in some cases the thyrotoxicosis can be severe and require discontinuation of IFNα therapy.
The etiology of IIT is unknown, but both immune effects and direct thyroid toxic effects of IFNα have been shown to play a role. Genetic factors also play an important role.
The hepatitis C virus infection itself contributes to the development of IIT.
All patients with hepatitis C starting an IFNα course should have a baseline level of TSH and thyroid antibodies, and then TSH levels should be monitored every 2–3 months.
If clinical IIT develops in a patient on IFNα therapy it should be treated based on the clinical presentation. IFNα therapy can be continued in most cases, but if thyrotoxicosis is severe clinicians should consider stopping IFNα therapy.
RESEARCH AGENDA.
Genetic studies to identify the genetic factors contributing to the development of IIT in order to develop prediction algorithms and prevention strategies.
In vitro and in vivo basic studies to identify the mechanisms by which IFNα causes thyroiditis, focusing on the immune effects and direct thyroid toxic effects of IFNα. These mechanistic studies will enable development of preventive therapies.
Prospective studies to develop risk stratification algorithms, and guidelines for follow-up and therapy, as well as to evaluate the usefulness of T4 therapy in patients starting IFNα therapy.
Acknowledgments
This work was supported in part by: DK61659 from NIDDK and a VA Merit Award (to YT).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Yaron Tomer, James J. Peters VA Medical New York, NY.
Francesca Menconi, Department of Medicine, Division of Endocrinology, Mount Sinai School of Medicine.
REFERENCES
- 1.Pfeffer LM, Dinarello CA, Herberman RB, Williams BR, Borden EC, Bordens R, et al. Biological properties of recombinant alpha-interferons: 40th anniversary of the discovery of interferons. Cancer Res. 1998;58(12):2489–2499. [PubMed] [Google Scholar]
- 2.Parmar S, Platanias LC. Interferons: mechanisms of action and clinical applications. Curr Opin Oncol. 2003;15(6):431–439. doi: 10.1097/00001622-200311000-00005. [DOI] [PubMed] [Google Scholar]
- 3.Jonasch E, Haluska FG. Interferon in oncological practice: review of interferon biology, clinical applications, and toxicities. Oncologist. 2001;6(1):34–55. doi: 10.1634/theoncologist.6-1-34. [DOI] [PubMed] [Google Scholar]
- 4.Baron S, Tyring SK, Fleischmann WR, Jr, Coppenhaver DH, Niesel DW, Klimpel GR, et al. The interferons. Mechanisms of action and clinical applications. JAMA. 1991;266(10):1375–1383. doi: 10.1001/jama.266.10.1375. [DOI] [PubMed] [Google Scholar]
- 5.Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Goncales FL, Jr, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347(13):975–982. doi: 10.1056/NEJMoa020047. [DOI] [PubMed] [Google Scholar]
- 6.Russo MW, Fried MW. Side effects of therapy for chronic hepatitis C. Gastroenterology. 2003;124(6):1711–1719. doi: 10.1016/s0016-5085(03)00394-9. [DOI] [PubMed] [Google Scholar]
- 7.Fentiman IS, Thomas BS, Balkwill FR, Rubens RD, Hayward JL. Primary hypothyroidism associated with interferon therapy of breast cancer. Lancet. 1985;1(8438):1166. doi: 10.1016/s0140-6736(85)92475-4. [DOI] [PubMed] [Google Scholar]
- 8.Prummel MF, Laurberg P. Interferon-alpha and autoimmune thyroid disease. Thyroid. 2003;13(6):547–551. doi: 10.1089/105072503322238809. [DOI] [PubMed] [Google Scholar]
- 9.Koh LK, Greenspan FS, Yeo PP. Interferon-alpha induced thyroid dysfunction: three clinical presentations and a review of the literature. Thyroid. 1997;7(6):891–896. doi: 10.1089/thy.1997.7.891. [DOI] [PubMed] [Google Scholar]
- 10.Roti E, Minelli R, Giuberti T, Marchelli S, Schianchi C, Gardini E, et al. Multiple changes in thyroid function in patients with chronic active HCV hepatitis treated with recombinant interferon-alpha. Am J Med. 1996;101(5):482–487. doi: 10.1016/s0002-9343(96)00259-8. [DOI] [PubMed] [Google Scholar]
- 11.Mazziotti G, Sorvillo F, Stornaiuolo G, Rotondi M, Morisco F, Ruberto M, et al. Temporal relationship between the appearance of thyroid autoantibodies and development of destructive thyroiditis in patients undergoing treatment with two different type-1 interferons for HCV-related chronic hepatitis: a prospective study. J Endocrinol Invest. 2002;25(7):624–630. doi: 10.1007/BF03345087. [DOI] [PubMed] [Google Scholar]
- 12.Mandac JC, Chaudhry S, Sherman KE, Tomer Y. The clinical and physiological spectrum of interferon-alpha induced thyroiditis: Toward a new classification. Hepatology. 2006;43(4):661–672. doi: 10.1002/hep.21146. [DOI] [PubMed] [Google Scholar]
- 13.Martocchia A, Labbadia G, Paoletti V, Gargano S, Grossi A, Trabace S, et al. Hashimoto's disease during interferon-alpha therapy in a patient with pre-treatment negative anti-thyroid autoantibodies and with the specific genetic susceptibility to the thyroid disease. Neuro Endocrinol Lett. 2001;22(1):49–52. [PubMed] [Google Scholar]
- 14.Baudin E, Marcellin P, Pouteau M, Colas-Linhart N, Le Floch JP, Lemmonier C, et al. Reversibility of thyroid dysfunction induced by recombinant alpha interferon in chronic hepatitis C. Clin Endocrinol (Oxf) 1993;39(6):657–661. doi: 10.1111/j.1365-2265.1993.tb02423.x. [DOI] [PubMed] [Google Scholar]
- 15.Carella C, Mazziotti G, Morisco F, Manganella G, Rotondi M, Tuccillo C, et al. Long-term outcome of interferon-alpha-induced thyroid autoimmunity and prognostic influence of thyroid autoantibody pattern at the end of treatment. J Clin Endocrinol Metab. 2001;86(5):1925–1929. doi: 10.1210/jcem.86.5.7459. [DOI] [PubMed] [Google Scholar]
- 16.Watanabe U, Hashimoto E, Hisamitsu T, Obata H, Hayashi N. The risk factor for development of thyroid disease during interferon-alpha therapy for chronic hepatitis C. Am J Gastroenterol. 1994;89(3):399–403. [PubMed] [Google Scholar]
- 17.Preziati D, La Rosa L, Covini G, Marcelli R, Rescalli S, Persani L, et al. Autoimmunity and thyroid function in patients with chronic active hepatitis treated with recombinant interferon alpha-2a. Eur J Endocrinol. 1995;132(5):587–593. doi: 10.1530/eje.0.1320587. [DOI] [PubMed] [Google Scholar]
- 18.Imagawa A, Itoh N, Hanafusa T, Oda Y, Waguri M, Miyagawa J, et al. Autoimmune endocrine disease induced by recombinant interferon-alpha therapy for chronic active type C hepatitis. J Clin Endocrinol Metab. 1995;80(3):922–926. doi: 10.1210/jcem.80.3.7883851. [DOI] [PubMed] [Google Scholar]
- 19.Wong V, Fu AX, George J, Cheung NW. Thyrotoxicosis induced by alpha-interferon therapy in chronic viral hepatitis. Clin Endocrinol (Oxf) 2002;56(6):793–798. doi: 10.1046/j.1365-2265.2002.01553.x. [DOI] [PubMed] [Google Scholar]
- 20.Lisker-Melman M, Di Bisceglie AM, Usala SJ, Weintraub B, Murray LM, Hoofnagle JH. Development of thyroid disease during therapy of chronic viral hepatitis with interferon alfa. Gastroenterology. 1992;102(6):2155–2160. doi: 10.1016/0016-5085(92)90348-3. [DOI] [PubMed] [Google Scholar]
- 21.Villanueva RB, Brau N. Graves' ophthalmopathy associated with interferon-alpha treatment for hepatitis C. Thyroid. 2002;12(8):737–738. doi: 10.1089/105072502760258730. [DOI] [PubMed] [Google Scholar]
- 22.Hollowell JG, Staehling NW, Flanders WD, Hannon WH, Gunter EW, Spencer CA, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III) J Clin Endocrinol Metab. 2002;87(2):489–499. doi: 10.1210/jcem.87.2.8182. [DOI] [PubMed] [Google Scholar]
- 23.Vanderpump MPJ, Tunbridge WMG, French JM, Appleton D, Bates D, Clark F, et al. The incidence of thyroid disorders in the community: a twenty-year follow-up of the Whickham survey. Clin Endocrinol (Oxf) 1995;43:55–68. doi: 10.1111/j.1365-2265.1995.tb01894.x. [DOI] [PubMed] [Google Scholar]
- 24.Ban Y, Greenberg DA, Davies TF, Jacobson E, Concepcion E, Tomer Y. Linkage analysis of thyroid antibody production: evidence for shared susceptibility to clinical autoimmune thyroid disease. J Clin Endocrinol Metab. 2008;93(9):3589–3596. doi: 10.1210/jc.2008-0364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carella C, Amato G, Biondi B, Rotondi M, Morisco F, Tuccillo C, et al. Longitudinal study of antibodies against thyroid in patients undergoing interferon-alpha therapy for HCV chronic hepatitis. Horm Res. 1995;44(3):110–114. doi: 10.1159/000184606. [DOI] [PubMed] [Google Scholar]
- 26.Marazuela M, Garcia-Buey L, Gonzalez-Fernandez B, Garcia-Monzon C, Arranz A, Borque MJ, et al. Thyroid autoimmune disorders in patients with chronic hepatitis C before and during interferon-alpha therapy. Clin Endocrinol (Oxf) 1996;44(6):635–642. doi: 10.1046/j.1365-2265.1996.751768.x. [DOI] [PubMed] [Google Scholar]
- 27.Weetman AP, Smallridge RC, Nutman TB, Burman KD. Persistent thyroid autoimmunity after subacute thyroiditis. J Clin Lab Immunol. 1987;23:1–6. [PubMed] [Google Scholar]
- 28.Parana R, Cruz M, Lyra L, Cruz T. Subacute thyroiditis during treatment with combination therapy (interferon plus ribavirin) for hepatitis C virus. J Viral Hepat. 2000;7(5):393–395. doi: 10.1046/j.1365-2893.2000.00247.x. [DOI] [PubMed] [Google Scholar]
- 29.Bohbot NL, Young J, Orgiazzi J, Buffet C, Francois M, Bernard-Chabert B, et al. Interferon-alpha-induced hyperthyroidism: a three-stage evolution from silent thyroiditis towards Graves' disease. Eur J Endocrinol. 2006;154(3):367–372. doi: 10.1530/eje.1.02104. [DOI] [PubMed] [Google Scholar]
- 30.Nguyen KB, Watford WT, Salomon R, Hofmann SR, Pien GC, Morinobu A, et al. Critical role for STAT4 activation by type 1 interferons in the interferon-gamma response to viral infection. Science. 2002;297(5589):2063–2066. doi: 10.1126/science.1074900. [DOI] [PubMed] [Google Scholar]
- 31.Corssmit EP, de Metz J, Sauerwein HP, Romijn JA. Biologic responses to IFN-alpha administration in humans. J Interferon Cytokine Res. 2000;20(12):1039–1047. doi: 10.1089/107999000750053690. [DOI] [PubMed] [Google Scholar]
- 32.You X, Teng W, Shan Z. Expression of ICAM-1, B7.1 and TPO on human thyrocytes induced by IFN-alpha. Chin Med J (Engl) 1999;112(1):61–66. [PubMed] [Google Scholar]
- 33.Farrar JD, Murphy KM. Type I interferons and T helper development. Immunol Today. 2000;21(10):484–489. doi: 10.1016/s0167-5699(00)01710-2. [DOI] [PubMed] [Google Scholar]
- 34.Tilg H. New insights into the mechanisms of interferon alfa: an immunoregulatory and anti-inflammatory cytokine. Gastroenterology. 1997;112(3):1017–1021. doi: 10.1053/gast.1997.v112.pm9041265. [DOI] [PubMed] [Google Scholar]
- 35.Mazziotti G, Sorvillo F, Piscopo M, Morisco F, Cioffi M, Stornaiuolo G, et al. Innate and acquired immune system in patients developing interferon-alpha-related autoimmune thyroiditis: a prospective study. J Clin Endocrinol Metab. 2005;90(7):4138–4144. doi: 10.1210/jc.2005-0093. [DOI] [PubMed] [Google Scholar]
- 36.Land KJ, Moll JS, Kaplan MH, Seetharamaiah GS. Signal transducer and activator of transcription (Stat)-6-dependent, but not Stat4-dependent, immunity is required for the development of autoimmunity in Graves' hyperthyroidism. Endocrinology. 2004;145(8):3724–3730. doi: 10.1210/en.2004-0352. [DOI] [PubMed] [Google Scholar]
- 37.Mazziotti G, Sorvillo F, Carbone A, Cioffi M, Morisco F, Carella C. Is the IFN-alpha-related thyroid autoimmunity an immunologically heterogeneous disease? J Intern Med. 2002;252(4):377–378. doi: 10.1046/j.1365-2796.2002.01045.x. [DOI] [PubMed] [Google Scholar]
- 38.Nagayama Y, Mizuguchi H, Hayakawa T, Niwa M, McLachlan SM, Rapoport B. Prevention of autoantibody-mediated Graves'-like hyperthyroidism in mice with IL-4, a Th2 cytokine. J Immunol. 2003;170(7):3522–3527. doi: 10.4049/jimmunol.170.7.3522. [DOI] [PubMed] [Google Scholar]
- 39.Corssmit EP, Heijligenberg R, Hack CE, Endert E, Sauerwein HP, Romijn JA. Effects of interferon-alpha (IFN-alpha) administration on leucocytes in healthy humans. Clin Exp Immunol. 1997;107(2):359–363. doi: 10.1111/j.1365-2249.1997.269-ce1161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ajjan RA, Watson PF, McIntosh RS, Weetman AP. Intrathyroidal cytokine gene expression in Hashimoto's thyroiditis. Clin Exp Immunol. 1996;105(3):523–528. doi: 10.1046/j.1365-2249.1996.d01-784.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Krause I, Valesini G, Scrivo R, Shoenfeld Y. Autoimmune aspects of cytokine and anticytokine therapies. Am J Med. 2003;115(5):390–397. doi: 10.1016/s0002-9343(03)00390-5. [DOI] [PubMed] [Google Scholar]
- 42.Lindahl P, Leary P, Gresser I. Enhancement by interferon of the expression of surface antigens on murine leukemia L 1210 cells. Proc Natl Acad Sci U S A. 1973;70(10):2785–2788. doi: 10.1073/pnas.70.10.2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Caraccio N, Giannini R, Cuccato S, Faviana P, Berti P, Galleri D, et al. Type I interferons modulate the expression of thyroid peroxidase, sodium/iodide symporter, and thyroglobulin genes in primary human thyrocyte cultures. J Clin Endocrinol Metab. 2005;90(2):1156–1162. doi: 10.1210/jc.2004-1173. [DOI] [PubMed] [Google Scholar]
- 44.Akeno N, Tomer Y. Dissecting the mechanisms of interferon induced thyroiditis (IIT): Direct effects of interferon alpha on thyroid epithelial cells. The 89th Meeting of the Endocrine Society; June 2007; Toronto, Canda. 2007. [Google Scholar]
- 45.Caraccio N, Cuccato S, Pratesi F, Dardano A, Ursino S, Chimenti D, et al. Effect of type I interferon(s) on cell viability and apoptosis in primary human thyrocyte cultures. Thyroid. 2009;19(2):149–155. doi: 10.1089/thy.2008.0290. [DOI] [PubMed] [Google Scholar]
- 46.Kryczka W, Brojer E, Kowalska A, Zarebska-Michaluk D. Thyroid gland dysfunctions during antiviral therapy of chronic hepatitis C. Med Sci Monit. 2001;7 Suppl 1:221–225. [PubMed] [Google Scholar]
- 47.Deutsch M, Koskinas J, Tzannos K, Vassilopoulos D, Mailis A, Tolis G, et al. Hashimoto encephalopathy with pegylated interferon alfa-2b and ribavirin. Ann Pharmacother. 2005;39(10):1745–1747. doi: 10.1345/aph.1G144. [DOI] [PubMed] [Google Scholar]
- 48.Rotondi M, Minelli R, Magri F, Leporati P, Romagnani P, Baroni MC, et al. Serum CXCL10 levels and occurrence of thyroid dysfunction in patients treated with interferon-{alpha} therapy for hepatitis C virus-related hepatitis. Eur J Endocrinol. 2007;156(4):409–414. doi: 10.1530/EJE-06-0735. [DOI] [PubMed] [Google Scholar]
- 49.Weetman AP. Graves' disease. N Engl J Med. 2000;343(17):1236–1248. doi: 10.1056/NEJM200010263431707. [DOI] [PubMed] [Google Scholar]
- 50.Tomer Y, Davies TF. Searching for the autoimmune thyroid disease susceptibility genes: From gene mapping to gene function. Endocr Rev. 2003;24:694–717. doi: 10.1210/er.2002-0030. [DOI] [PubMed] [Google Scholar]
- 51.Huber A, Menconi F, Corathers S, Jacobson EM, Tomer Y. Joint genetic susceptibility to type 1 diabetes and autoimmune thyroiditis: from epidemiology to mechanisms. Endocr Rev. 2008;29(6):697–725. doi: 10.1210/er.2008-0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dalgard O, Bjoro K, Hellum K, Myrvang B, Bjoro T, Haug E, et al. Thyroid dysfunction during treatment of chronic hepatitis C with interferon alpha: no association with either interferon dosage or efficacy of therapy. J Intern Med. 2002;251(5):400–406. doi: 10.1046/j.1365-2796.2002.00974.x. [DOI] [PubMed] [Google Scholar]
- 53.Grossman CJ, Roselle GA, Mendenhall CL. Sex steroid regulation of autoimmunity. J Steroid Biochem Mol Biol. 1991;40(4–6):649–659. doi: 10.1016/0960-0760(91)90287-f. [DOI] [PubMed] [Google Scholar]
- 54.Oppenheim Y, Kim G, Ban Y, Unger P, Concepcion E, Ando T, et al. The effects of alpha interferon on the development of autoimmune thyroiditis in the NOD H2h4 mouse. Clin Dev Immunol. 2003;10(2–4):161–165. doi: 10.1080/10446670310001642177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jacobson EM, Tomer Y. The CD40, CTLA-4, thyroglobulin, TSH receptor, and PTPN22 gene quintet and its contribution to thyroid autoimmunity: Back to the future. J Autoimmun. 2007;28:85–98. doi: 10.1016/j.jaut.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stenszky V, Kozma L, Balazs C, Rochkitz S, Bear JC, Farid NR. The genetics of Graves' disease: HLA and disease susceptibility. J Clin Endocrinol Metab. 1985;61:735–740. doi: 10.1210/jcem-61-4-735. [DOI] [PubMed] [Google Scholar]
- 57.Ban Y, Davies TF, Greenberg DA, Concepcion ES, Osman R, Oashi T, et al. Arginine at position 74 of the HLA-DRb1 chain is associated with Graves' disease. Genes Immun. 2004;5:203–208. doi: 10.1038/sj.gene.6364059. [DOI] [PubMed] [Google Scholar]
- 58.Yanagawa T, Hidaka Y, Guimaraes V, Soliman M, DeGroot LJ. CTLA-4 gene polymorphism associated with Graves' disease in a Caucasian population. J Clin Endocrinol Metab. 1995;80:41–45. doi: 10.1210/jcem.80.1.7829637. [DOI] [PubMed] [Google Scholar]
- 59.Vaidya B, Imrie H, Perros P, Young ET, Kelly WF, Carr D, et al. The cytotoxic T lymphocyte antigen-4 is a major Graves' disease locus. Hum Mol Genet. 1999;8(7):1195–1199. doi: 10.1093/hmg/8.7.1195. [DOI] [PubMed] [Google Scholar]
- 60.Tomer Y, Greenberg DA, Barbesino G, Concepcion ES, Davies TF. CTLA-4 and not CD28 is a susceptibility gene for thyroid autoantibody production. J Clin Endocrinol Metab. 2001;86:1687–1693. doi: 10.1210/jcem.86.4.7372. [DOI] [PubMed] [Google Scholar]
- 61.Smyth D, Cooper JD, Collins JE, Heward JM, Franklyn JA, Howson JM, et al. Replication of an association between the lymphoid tyrosine phosphatase locus (LYP/PTPN22) with type 1 diabetes, and evidence for its role as a general autoimmunity locus. Diabetes. 2004;53(11):3020–3023. doi: 10.2337/diabetes.53.11.3020. [DOI] [PubMed] [Google Scholar]
- 62.Velaga MR, Wilson V, Jennings CE, Owen CJ, Herington S, Donaldson PT, et al. The codon 620 tryptophan allele of the lymphoid tyrosine phosphatase (LYP) gene is a major determinant of Graves' disease. J Clin Endocrinol Metab. 2004;89(11):5862–5865. doi: 10.1210/jc.2004-1108. [DOI] [PubMed] [Google Scholar]
- 63.Ban Y, Greenberg DA, Concepcion E, Skrabanek L, Villanueva R, Tomer Y. Amino acid substitutions in the thyroglobulin gene are associated with susceptibility to human and murine autoimmune thyroid disease. Proc Natl Acad Sci USA. 2003;100:15119–15124. doi: 10.1073/pnas.2434175100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hiratani H, Bowden DW, Ikegami S, Shirasawa S, Shimizu A, Iwatani Y, et al. Multiple SNPs in intron 7 of thyrotropin receptor are associated with Graves' disease. J Clin Endocrinol Metab. 2005;90(5):2898–2903. doi: 10.1210/jc.2004-2148. [DOI] [PubMed] [Google Scholar]
- 65.Kakizaki S, Takagi H, Murakami M, Takayama H, Mori M. HLA antigens in patients with interferon-alpha-induced autoimmune thyroid disorders in chronic hepatitis C. J Hepatol. 1999;30(5):794–800. doi: 10.1016/s0168-8278(99)80131-7. [DOI] [PubMed] [Google Scholar]
- 66.Jacobson EM, Chaudhry S, Mandac JC, Concepcion E, Tomer Y. Immune-regulatory gene involvement in the etiology of interferon induced thyroiditis (IIT) Thyroid. 2006;16:926. [Google Scholar]
- 67.Oppenheim Y, Ban Y, Tomer Y. Interferon induced Autoimmune Thyroid Disease (AITD): a model for human autoimmunity. Autoimmun Rev. 2004;3(5):388–393. doi: 10.1016/j.autrev.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 68.Tomer Y, Davies TF. Infection, Thyroid Disease and Autoimmunity. Endocr Rev. 1993;14:107–120. doi: 10.1210/edrv-14-1-107. [DOI] [PubMed] [Google Scholar]
- 69.Tomer Y, Villanueva R. Hepatitis C and thyroid autoimmunity: is there a link? Am J Med. 2004;117(1):60–61. doi: 10.1016/j.amjmed.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 70.Loviselli A, Oppo A, Velluzzi F, Atzeni F, Mastinu GL, Farci P, et al. Independent expression of serological markers of thyroid autoimmunity and hepatitis virus C infection in the general population: results of a community-based study in north-western Sardinia. J Endocrinol Invest. 1999;22(9):660–665. doi: 10.1007/BF03343626. [DOI] [PubMed] [Google Scholar]
- 71.Metcalfe RA, Ball G, Kudesia G, Weetman AP. Failure to find an association between hepatitis C virus and thyroid autoimmunity. Thyroid. 1997;7(3):421–424. doi: 10.1089/thy.1997.7.421. [DOI] [PubMed] [Google Scholar]
- 72.Boadas J, Rodriguez-Espinosa J, Enriquez J, Miralles F, Martinez-Cerezo FJ, Gonzalez P, et al. Prevalence of thyroid autoantibodies is not increased in blood donors with hepatitis C virus infection. J Hepatol. 1995;22(6):611–615. doi: 10.1016/0168-8278(95)80216-9. [DOI] [PubMed] [Google Scholar]
- 73.Tran A, Quaranta JF, Benzaken S, Thiers V, Chau HT, Hastier P, et al. High prevalence of thyroid autoantibodies in a prospective series of patients with chronic hepatitis C before interferon therapy. Hepatology. 1993;18(3):253–257. [PubMed] [Google Scholar]
- 74.Ganne-Carrie N, Medini A, Coderc E, Seror O, Christidis C, Grimbert S, et al. Latent autoimmune thyroiditis in untreated patients with HCV chronic hepatitis: a case-control study. J Autoimmun. 2000;14(2):189–193. doi: 10.1006/jaut.1999.0360. [DOI] [PubMed] [Google Scholar]
- 75.Fernandez-Soto L, Gonzalez A, Escobar-Jimenez F, Vazquez R, Ocete E, Olea N, et al. Increased risk of autoimmune thyroid disease in hepatitis C vs hepatitis B before, during, and after discontinuing interferon therapy. Arch Intern Med. 1998;158(13):1445–1448. doi: 10.1001/archinte.158.13.1445. [DOI] [PubMed] [Google Scholar]
- 76.Antonelli A, Ferri C, Pampana A, Fallahi P, Nesti C, Pasquini M, et al. Thyroid disorders in chronic hepatitis C. Am J Med. 2004;117(1):10–13. doi: 10.1016/j.amjmed.2004.01.023. [DOI] [PubMed] [Google Scholar]
- 77.Indolfi G, Stagi S, Bartolini E, Salti R, De Martino M, Azzari C, et al. Thyroid function and anti-thyroid autoantibodies in untreated children with vertically acquired chronic hepatitis C virus infection. Clin Endocrinol (Oxf) 2008;68(1):117–121. doi: 10.1111/j.1365-2265.2007.03009.x. [DOI] [PubMed] [Google Scholar]
- 78.Antonelli A, Ferri C, Fallahi P, Ferrari SM, Ghinoi A, Rotondi M, et al. Thyroid disorders in chronic hepatitis C virus infection. Thyroid. 2006;16(6):563–572. doi: 10.1089/thy.2006.16.563. [DOI] [PubMed] [Google Scholar]
- 79.Bartolome J, Rodriguez-Inigo E, Quadros P, Vidal S, Pascual-Miguelanez I, Rodriguez-Montes JA, et al. Detection of hepatitis C virus in thyroid tissue from patients with chronic HCV infection. J Med Virol. 2008;80(9):1588–1594. doi: 10.1002/jmv.21269. [DOI] [PubMed] [Google Scholar]
- 80.Munshi N, Balasubramanian A, Koziel M, Ganju RK, Groopman JE. Hepatitis C and human immunodeficiency virus envelope proteins cooperatively induce hepatocytic apoptosis via an innocent bystander mechanism. J Infect Dis. 2003;188(8):1192–1204. doi: 10.1086/378643. [DOI] [PubMed] [Google Scholar]
- 81.Balasubramanian A, Ganju RK, Groopman JE. Signal transducer and activator of transcription factor 1 mediates apoptosis induced by hepatitis C virus and HIV envelope proteins in hepatocytes. J Infect Dis. 2006;194(5):670–681. doi: 10.1086/505708. [DOI] [PubMed] [Google Scholar]
- 82.Balasubramanian A, Ganju RK, Groopman JE. Hepatitis C virus and HIV envelope proteins collaboratively mediate interleukin-8 secretion through activation of p38 MAP kinase and SHP2 in hepatocytes. J Biol Chem. 2003;278(37):35755–35766. doi: 10.1074/jbc.M302889200. [DOI] [PubMed] [Google Scholar]
- 83.Akeno N, Blackard JT, Tomer Y. HCV E2 protein binds directly to thyroid cells and induces IL-8 production: a new mechanism for HCV induced thyroid autoimmunity. J Autoimmun. 2008;31(4):339–344. doi: 10.1016/j.jaut.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tomer Y, Blackard JT, Akeno N. Interferon alpha treatment and thyroid dysfunction. Endocrinol Metab Clin North Am. 2007;36(4):1051–1066. doi: 10.1016/j.ecl.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]