Introduction
Cochlear implant outcomes vary widely among patients. This variability has been attributed to factors such as differences in patient age at implantation (1–3), duration of deafness (4–6), and coding strategies (7). Recently, greater attention has been paid to the effect of surgical technique on outcomes. Patients with electrodes in the scala tympani enjoy better hearing outcomes (8,9) and less postoperative vertigo (10) than those with electrodes in the scala vestibuli. Placement of the implant in the scala tympani may also facilitate preservation of residual hearing following surgery (11,12).
Accurate placement of the cochleostomy is critical in ensuring a scala tympani insertion. While even experienced cochlear implant surgeons vary widely in their choice of preferred cochleostomy sites, several anatomic studies have shown that a position adjacent to the inferior or anteroinferior annulus of the round window membrane is most favorable for successful insertion into the scala tympani (13–15). Insertions anterior or superior to the round window are more likely to enter the scala vestibuli directly or initially enter scala tympani but later traverse into the scala vestibuli (9,11,13,15).
Once inserted, the implant should remain in the scala tympani as it is advanced into the cochlea. The optimal surgical insertion vector to maintain this position is coaxial with the centerline of the scala tympani. If this direction is not maintained, the implant may either penetrate the basilar membrane directly, or after glancing off the wall of the cochlea further along the course of the basal turn (16–18). The surgeon may attempt to ensure a coaxial insertion by estimating the course of the basal turn by observing its lumen through the cochleostomy, but this view is necessarily limited by the size of the cochleostomy and the presence of perilymphatic fluid. Contemporary techniques that emphasize minimizing the size of the cochleostomy and avoiding suctioning of the perilymph make these limitations more significant.
The facial nerve defines the posterior perimeter of the facial recess, allowing access to the middle ear and cochlea during implantation. We hypothesized that the position of the nerve could help determine the best vector of insertion to optimize the implant’s position within the cochlea.
Materials and Methods
We obtained 8 cadaveric human temporal bones from adult donors with no evidence of ear disease and imaged them using a clinical scanner (Siemens Volume Zoom, Siemens, Forchheim, Germany) producing isotropic voxels with an edge length of 100 microns. The vestibule, semicircular canals, facial nerve, chorda tympani, and promontory were segmented from these scans using AMIRA imaging software (Visage Imaging, Carlsbad, CA). A cylinder of tissue 36 mm in diameter, including the labyrinth, facial nerve, cochlea, and surrounding structures, was then removed from each temporal bone and imaged in a microCT scanner (Scanco u40, Bassersdorf, Switzerland) producing isotropic voxels with an edge length of 36 microns to allow more refined visualization of the intracochlear structures. The anatomy of the basilar membrane was preserved adequately to allow segmentation of the scala tympani, but it was necessary to segment the scala media as a unit together with the scala vestibuli because Reissner’s membrane could not be visualized reliably. These procedures have been described previously (19). These high-resolution scans were fused with the low-resolution scans obtained using the clinical scanner into one volume using Analyze imaging software (AnalyzeDirect, Overland, KS) to allow analysis of microscopic and macroscopic detail in the same volume. For ease of comparison, all images were adjusted to represent the right ear (Fig. 1).
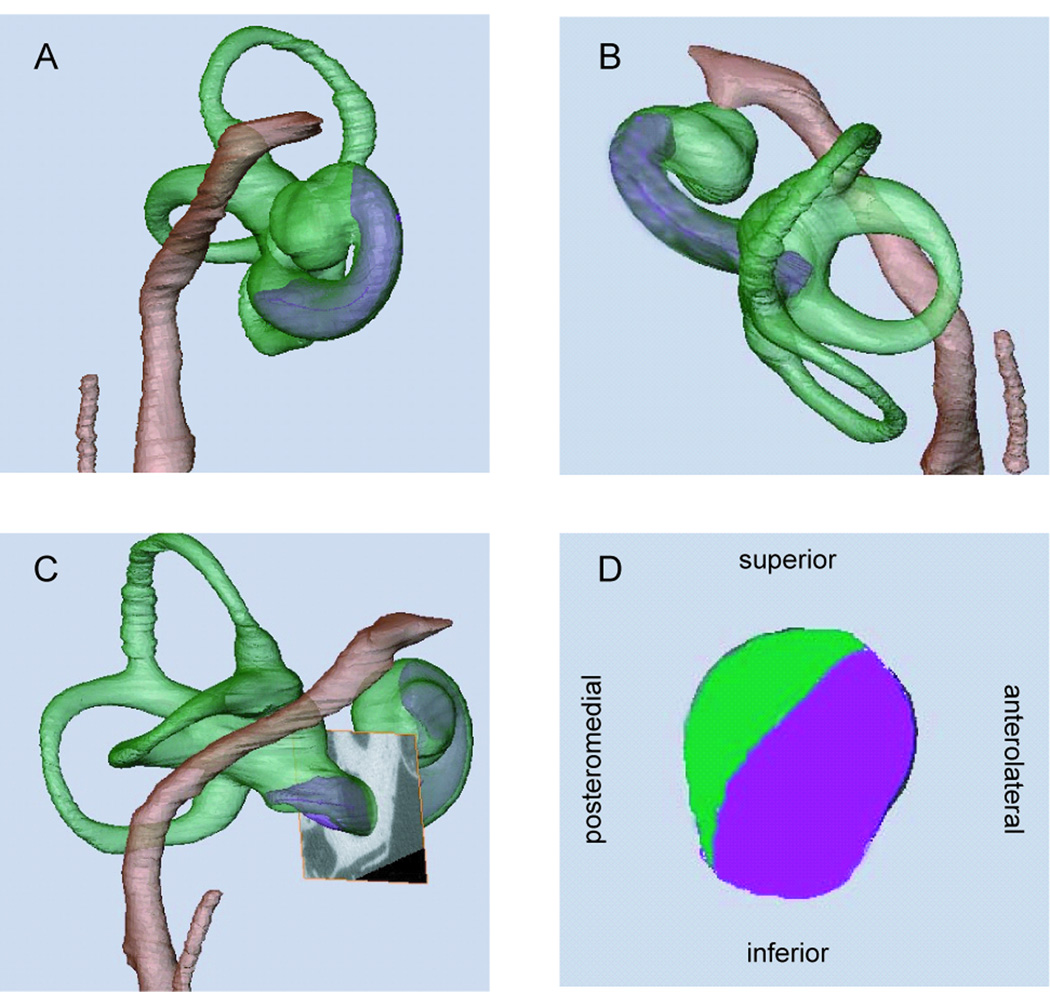
Figure 1.
View of segmented structures of right ear from anterior (A), superior (B), and lateral (C). A cross-section of the cochlea is shown in D, with the scala tympani shown in purple and the scala vestibuli shown in green. This cross-section corresponds to the plane shown in C, just anterior to the round window.
Custom-written software using MATLAB (Natick, MA) was used to find the centerline of each scala tympani volume, which was then merged with the AMIRA-generated surfaces. Five vectors were aligned tangent to the centerline of the scala tympani and intersecting it at points evenly spaced between the round window and the inferiormost curve of the basal turn, with the uppermost vector passing through the center of the round window membrane (Fig. 2). These represented five possible insertion vectors likely to optimize the chance of achieving an insertion along the scala tympani. We then compared these tangents for the 8 temporal bones to determine their relationships to the facial canal and the round window membrane.
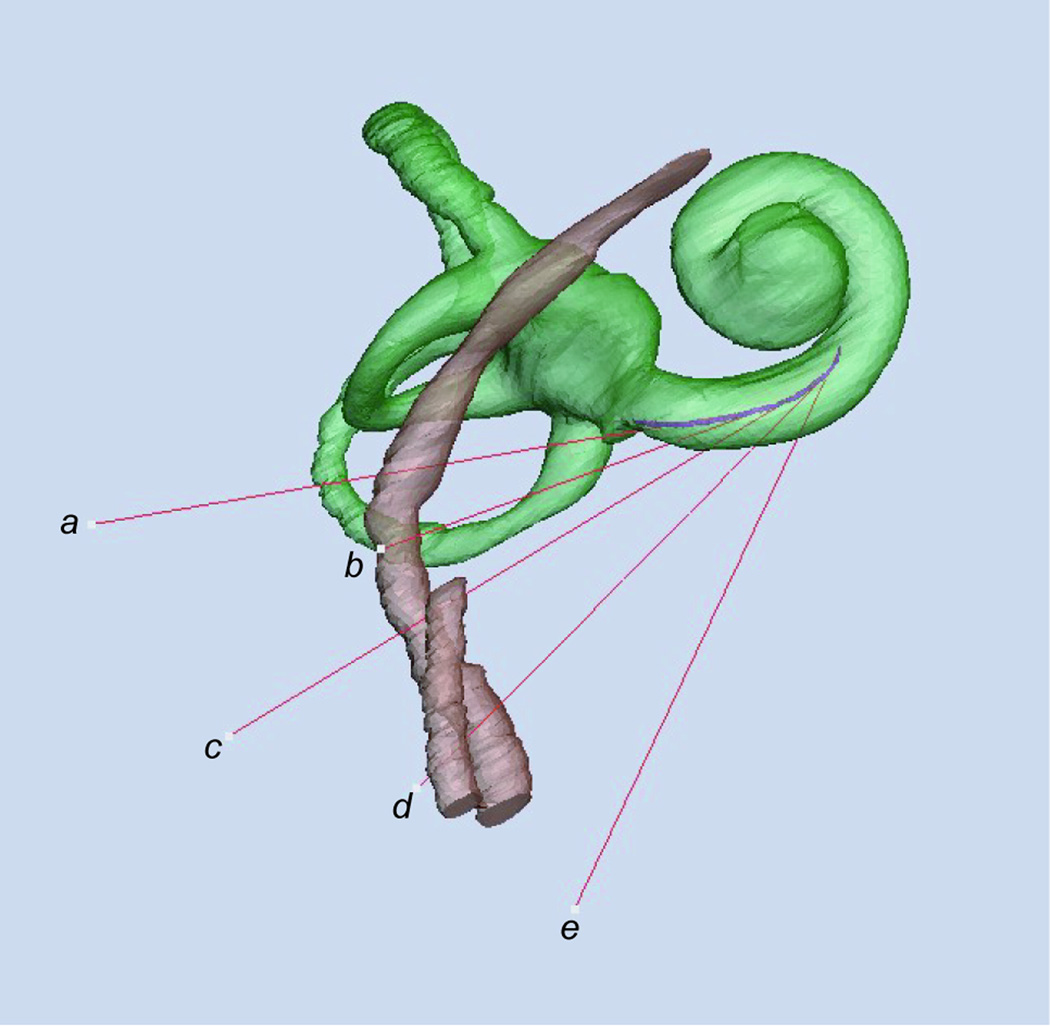
Figure 2.
Anterolateral view of right labyrinth. Five vectors tangent to the centerline of the scala tympani are labeled a to e in order from the round window superiorly to the inferiormost extent of the basal turn. Vector a passes through the center of the round window. The purple line represents the centerline of the scala tympani, which changes its orientation as the scala tympani curves along the basal turn. The changing curvature of the centerline causes the angles among the vectors to appear unequal.
Results
Images of the eight temporal bones are shown in Fig. 3. The straight red lines indicate five representative insertion vectors, each tangent to the centerline of the scala tympani in the basal turn of the cochlea. In most cases, the vector entering the center of the round window membrane passes through the tectulum (“round window overhang”) before reaching the membrane. None of the vectors enters the promontory anterior or anterosuperior to the round window. In all cases, at least one vector enters the cochlea inferior to or slightly anterior to the inferior border of the round window.
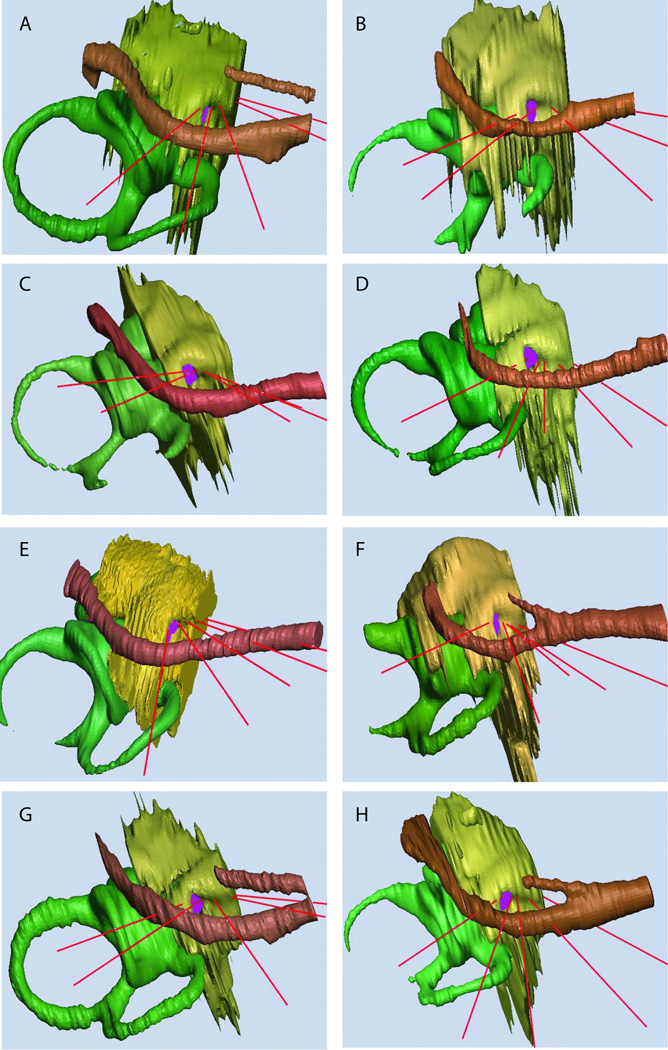
Figure 3.
Approximate surgical view of the 8 temporal bones. The five vectors tangent to the scala tympani centerline are shown in red. The semicircular canals, vestibule and cochlear duct are in green, the promontory is in yellow, the facial nerve and chorda tympani are in brown, and the scala tympani is in purple.
The relationships of the optimal insertion vectors to the facial nerve are demonstrated in Fig. 3. Variability in the direction of these vectors relative to the nerve may be understood as being determined by the rotation of the cochlea in the axial plane. In the case of Fig. 3C, the apex of the cochlea is rotated medially so that four of five vectors of insertion pass lateral to the nerve. In contrast, the apex of the cochlea shown in Fig. 3D is rotated laterally so that none of the vectors passes lateral to the nerve. The relationships between the insertion vectors and the facial nerve for all eight specimens are summarized graphically in Figure 4. The shape of the basal turn of the cochlea determines that only some of the superior vectors (a, b) and none of the inferior vectors (d, e) pass safely lateral to the facial nerve.
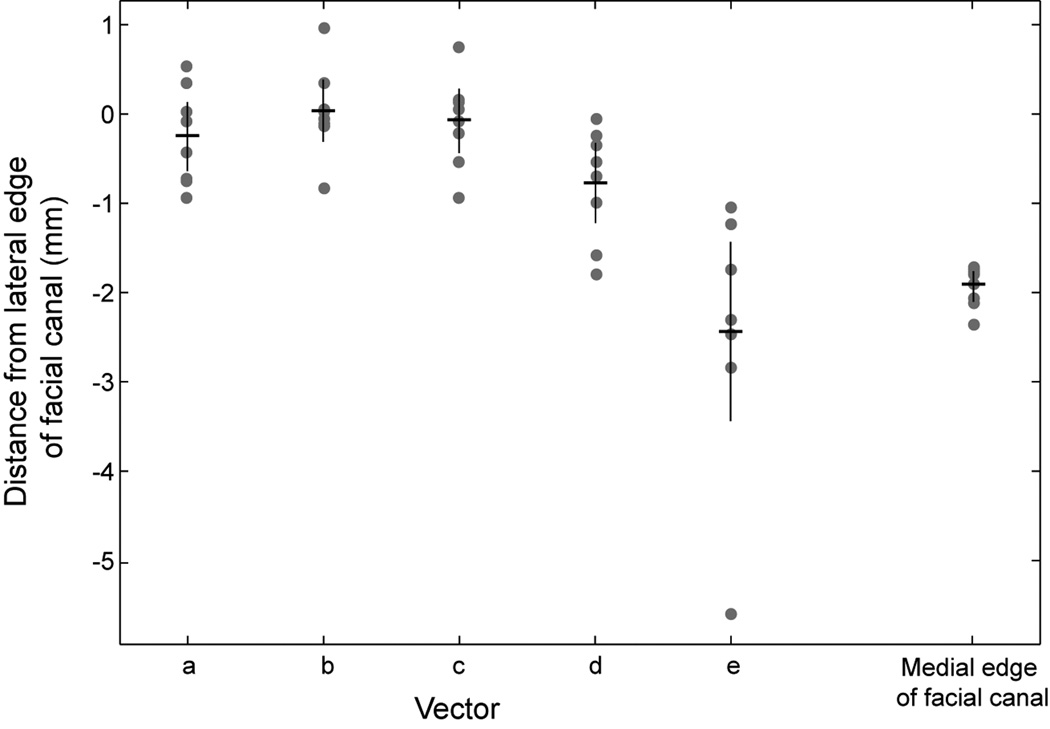
Figure 4.
Relationship of insertion vectors to the facial canal. Each column represents one vector, from superior (a) to inferior (e). Gray circles represent the location of this vector from each of eight temporal bones as the vector passes through the facial recess. The superimposed horizontal black line represents the mean and vertical black line indicates the 95% confidence interval of the distance of the vector from the lateral edge of the facial canal. Column at extreme right indicates the location of medial edge of facial canal at vector e.
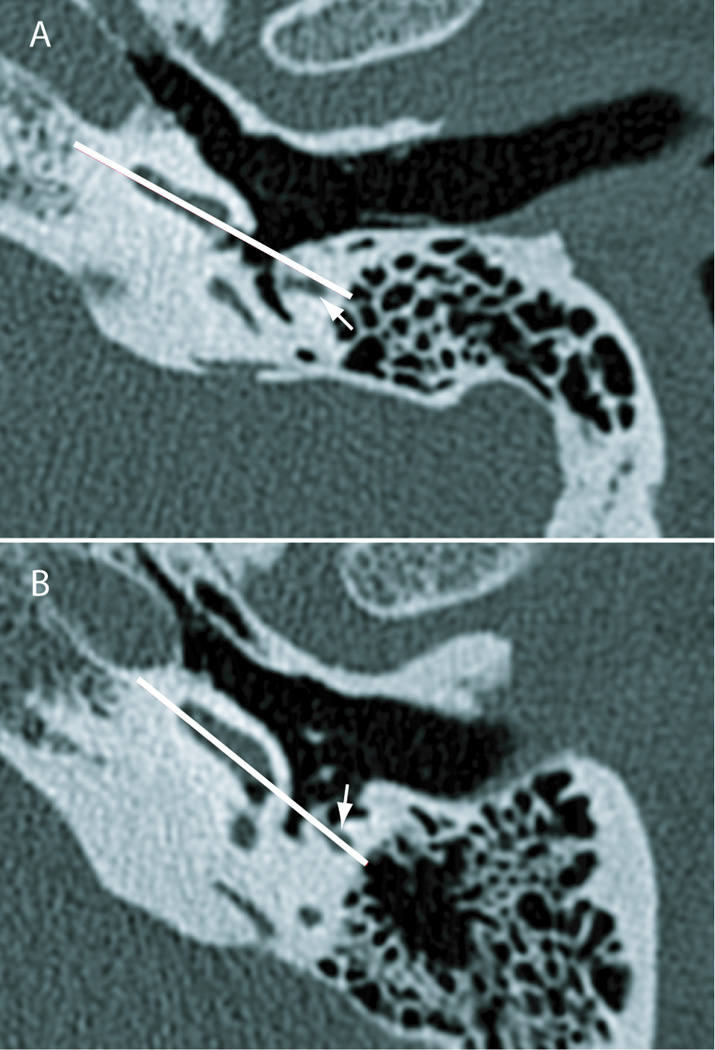
The variability of orientation of the cochlea can be seen on standard high-resolution axial preoperative scans (Fig. 5). Fig. 5A represents a cochlea with its apex rotated anteriorly in the axial plane, while Fig. 5B shows a cochlea with its apex rotated more laterally in the same plane. An appropriate insertion vector, shown in white on each specimen, was determined on each image by placing a line parallel to the posteromedial face of the basal turn of the cochlea (representing the approximate centerline of the scala tympani) and extending this line lateral into the mastoid. The relative rotation of the cochlea in the axial plane is responsible for the insertion vector intersecting the facial nerve in the scan shown in Fig. 5B but passing lateral to it in Fig. 5A.
Figure 5.
Relationship of the plane of the basal turn of the cochlea to the facial nerve. These axial CT scans (done at an isotropic resolution of 100 microns) indicate rotation of the cochlea in the axial plane, with the apex facing more anteriorly (specimen shown in panel A) and facing more laterally (specimen shown in panel B). The white line is determined by the posteromedial face of the basal half of the basal turn, approximately representing the scala tympani. This indicates the approximate optimal vector of insertion coaxial with the centerline of the basal turn. White arrows represent the facial nerve. The line of insertion passes lateral to the facial nerve in the specimen shown in panel A but directly through it in the specimen shown in panel B.
The orientations of the insertion vectors also depend on the rotation of the cochlea in the parasagittal plane. The angle of the round window overhang with respect to the surgical perspective may serve as a useful guide to the degree of parasagittal rotation. In some cases, the round window overhang may be oriented at a raking angle to the surgical perspective (Figs. 3E, 3F) consistent with a rotation of the anterior cochlea superiorly. In this case, the insertion vector nearest the round window faces relatively anteriorly. In other cases (Fig. 3C, 3G), the round window overhang is seen facing the surgeon and the anterior cochlea is rotated more inferiorly. Visualizing the entire round window in order to estimate its orientation relative to the observer requires removing the tectulum (“round window overhang”) in most cases such as shown in Figs. 3A, B, D ,F, and H.
Discussion
Optimal cochlear implant performance depends on careful placement of the electrode array into the scala tympani of the cochlea. Several anatomic studies have described in detail the proper location of the cochleostomy to ensure a scala tympani insertion, but the direction of insertion has not been as thoroughly analyzed. Our results document the variability of optimal insertion vectors and suggest particular surgical techniques that may facilitate proper cochlear implant placement.
The anteroinferior edge of the round window has been advocated to be the best cochleostomy site to ensure entry into the scala tympani due to its relationship relative to the basilar membrane (11,20–22). Recent histologic data have shown that an inferior cochleostomy may be even better than an anteroinferior cochleostomy in avoiding intracochlear trauma (14). Our data show that optimal insertion vectors tend to pass near the inferior border of the round window, adding an independent line of evidence that this is a favorable location for the cochleostomy.
Rotation of the cochlea in the axial plane determines the relationship of the optimal insertion vector to the facial nerve. The data shown in Fig. 3 and Fig 4 indicate that vector b, which generally coincides with a favorable cochleostomy site at the inferior border of the round window, often touches the lateral surface of the mastoid segment of the facial nerve or even intersects the nerve. This observation emphasizes that the facial recess must be adequately enlarged with removal of all but a thin shell of bone anterior and lateral to the facial nerve in order to assure an implantation trajectory as close to the centerline of the scala tympani as possible (23). In some cases, such as shown in Figs. 3B, 3D, and 3H, even thinning this bone does not allow an optimal insertion because no vector passes lateral to the facial nerve. Thus the facial nerve serves as a critical landmark and should be well skeletonized within the Fallopian canal to assure the straightest vector of insertion.
The same data indicate that a cochleostomy site adjacent to the round window, rather than further along the basal turn, is preferable. The optimal insertion vectors for cochleostomies further along the basal turn drop below the facial nerve, assuring that insertions through them can never be coaxial with the scala tympani. Rotation of the cochlea in the parasagittal plane determines whether the favorable insertion vectors passing near the round window are directed more from posterior to anterior, as in Figs. 3E and 3F, or more from superior to inferior, as in Figs. 3C and 3G. Estimating the rotation of the cochlea in this plane requires removing the tectulum to visualize the entire round window membrane and allowing its angle relative to the surgeon to be estimated. This is particularly important in individuals with gross malformations of the middle and inner ear whose anatomy is less constant than in the normal specimens reported here.
Demonstrating the close relationship of optimal insertion vectors to the facial nerve may have important implications for electrode design and use of devices and tools meant to assist insertion. Particularly flexible electrodes may be able to enter the cochlea at more favorable angles because of their ability to bend around the Fallopian canal and facial nerve (24). For the same reason, pre-curving an electrode may affect its ability to follow an optimal insertion vector. Stiff insertion tools that force the electrode away from the facial nerve as it passes through the facial recess may prevent insertion along the most favorable vector.
A recent survey showed that approximately 16% of experienced surgeons insert implants through the round window membrane (23). This approach has the theoretic advantage of avoiding cochlear trauma due to drilling a cochleostomy (24), but was rejected during the initial development of hearing preservation surgical techniques due to the perceived risk of intracochlear damage (25). Since then, some studies have supported the use of the round window approach (24,26–28) and some have suggested it may not be as favorable as a more conventional cochleostomy (22,29–31). The line labeled a in Fig. 2 passes through the center of the round window membrane and tangent to the centerline of the scala tympani, thus representing the optimal angle of insertion through the round window to follow the scala tympani. The direction of this vector is quite different, however, than the likely direction of insertion of an implant array directly through the round window membrane from below. Such an insertion would require an abrupt turn around the crista fenestra to follow the lumen of the scala tympani along the basal turn (31,32). Increased mechanical resistance (and presumably intracochlear damage) has been documented during implant insertion using this approach (31). The data here indicate that advancing a standard cochlear implant array directly through the round window membrane is unlikely to achieve an optimal direction of insertion, although future development of a particularly flexible experimental electrode array may address this concern (24).
Some approaches to the cochlea that do not rely on performing a facial recess have been described (33). While these may have advantages in specific situations, the data presented here indicate that the posterior approach to the cochlea afforded by a facial recess exposure may facilitate achieving an optimal insertion trajectory.
Acknowledgments
This work was supported in part by Howard Hughes Medical Institute Summer Undergraduate Research Fellowship provided by Washington University in St. Louis (Meshik) and grants NIH R01 DC000581 (Holden); NIH R01 DC00263 (Chole) and NIH KO8 DC006869 (Hullar). Thanks to Dr. Alec Salt for his assistance with imaging software and to Drs. Keiko Hirose and J. Gail Neely for helpful comments on the manuscript.
References
- 1.Vermeire K, Brokx JP, Wuyts FL, et al. Quality-of-life benefit from cochlear implantation in the elderly. Otol Neurotol. 2005;26:188–195. doi: 10.1097/00129492-200503000-00010. [DOI] [PubMed] [Google Scholar]
- 2.Tomblin JB, Barker BA, Spencer LJ, et al. The effect of age at cochlear implant initial stimulation on expressive language growth in infants and toddlers. J Speech Lang Hear Res. 2005;48:853–867. doi: 10.1044/1092-4388(2005/059). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nicholas JG, Geers AE. Will they catch up? The role of age at cochlear implantation in the spoken language development of children with severe to profound hearing loss. J Speech Lang Hear Res. 2007;50:1048–1062. doi: 10.1044/1092-4388(2007/073). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gantz BJ, Woodworth GG, Abbas PJ, et al. Multivariate predictors of audiological success with multichannel cochlear implants. Ann Otol Rhinol Laryngol. 1993;102:909–916. doi: 10.1177/000348949310201201. [DOI] [PubMed] [Google Scholar]
- 5.Green KM, Bhatt YM, Mawman DJ, et al. Predictors of audiological outcome following cochlear implantation in adults. Cochlear Implants Int. 2007;8:1–11. doi: 10.1179/cim.2007.8.1.1. [DOI] [PubMed] [Google Scholar]
- 6.Leung J, Wang NY, Yeagle JD, et al. Predictive models for cochlear implantation in elderly candidates. Arch Otolaryngol Head Neck Surg. 2005;131:1049–1054. doi: 10.1001/archotol.131.12.1049. [DOI] [PubMed] [Google Scholar]
- 7.Skinner MW, Holden LK, Whitford LA, et al. Speech recognition with the nucleus 24 SPEAK, ACE, and CIS speech coding strategies in newly implanted adults. Ear Hear. 2002;23:207–223. doi: 10.1097/00003446-200206000-00005. [DOI] [PubMed] [Google Scholar]
- 8.Aschendorff A, Kromeier J, Klenzner T, et al. Quality control after insertion of the nucleus contour and contour advance electrode in adults. Ear Hear. 2007;28:75S–79S. doi: 10.1097/AUD.0b013e318031542e. [DOI] [PubMed] [Google Scholar]
- 9.Finley CC, Holden TA, Holden LK, et al. Role of electrode placement as a contributor to variability in cochlear implant outcomes. Otol Neurotol. 2008;29:920–928. doi: 10.1097/MAO.0b013e318184f492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Todt I, Basta D, Ernst A. Does the surgical approach in cochlear implantation influence the occurrence of postoperative vertigo? Otolaryngol Head Neck Surg. 2008;138:8–12. doi: 10.1016/j.otohns.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 11.Briggs RJ, Tykocinski M, Stidham K, et al. Cochleostomy site: implications for electrode placement and hearing preservation. Acta Otolaryngol. 2005;125:870–876. doi: 10.1080/00016480510031489. [DOI] [PubMed] [Google Scholar]
- 12.Gstoettner W, Kiefer J, Baumgartner WD, et al. Hearing preservation in cochlear implantation for electric acoustic stimulation. Acta Otolaryngol. 2004;124:348–352. doi: 10.1080/00016480410016432. [DOI] [PubMed] [Google Scholar]
- 13.Roland PS, Wright CG, Isaacson B. Cochlear implant electrode insertion: the round window revisited. Laryngoscope. 2007;117:1397–1402. doi: 10.1097/MLG.0b013e318064e891. [DOI] [PubMed] [Google Scholar]
- 14.Adunka OF, Radeloff A, Gstoettner WK, et al. Scala tympani cochleostomy II: topography and histology. Laryngoscope. 2007;117:2195–2200. doi: 10.1097/MLG.0b013e3181453a53. [DOI] [PubMed] [Google Scholar]
- 15.Li PM, Wang H, Northrop C, et al. Anatomy of the round window and hook region of the cochlea with implications for cochlear implantation and other endocochlear surgical procedures. Otol Neurotol. 2007;28:641–648. doi: 10.1097/mao.0b013e3180577949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klenzner T, Richter B, Nagursky H, et al. Evaluation of the insertion-trauma of the Nucleus Contour Advance electrode-array in a human temporal bone model. Laryngorhinootologie. 2004;83:840–844. doi: 10.1055/s-2004-826067. [DOI] [PubMed] [Google Scholar]
- 17.Wardrop P, Whinney D, Rebscher SJ, et al. A temporal bone study of insertion trauma and intracochlear position of cochlear implant electrodes. II: Comparison of Spiral Clarion and HiFocus II electrodes. Hear Res. 2005;203:68–79. doi: 10.1016/j.heares.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 18.Wardrop P, Whinney D, Rebscher SJ, et al. A temporal bone study of insertion trauma and intracochlear position of cochlear implant electrodes. I: Comparison of Nucleus banded and Nucleus Contour electrodes. Hear Res. 2005;203:54–67. doi: 10.1016/j.heares.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 19.Whiting BR, Holden TA, Brunsden BS, et al. Use of computed tomography scans for cochlear implants. J Digit Imaging. 2008;21:323–328. doi: 10.1007/s10278-007-9045-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Briggs RD, Vrabec JT, Cavey ML, et al. Virtual endoscopic evaluation of labyrinthine fistulae resulting from cholesteatoma. Laryngoscope. 2001;111:1828–1833. doi: 10.1097/00005537-200110000-00030. [DOI] [PubMed] [Google Scholar]
- 21.Gantz BJ, Turner C, Gfeller KE, et al. Preservation of hearing in cochlear implant surgery: advantages of combined electrical and acoustical speech processing. Laryngoscope. 2005;115:796–802. doi: 10.1097/01.MLG.0000157695.07536.D2. [DOI] [PubMed] [Google Scholar]
- 22.Berrettini S, Forli F, Passetti S. Preservation of residual hearing following cochlear implantation: comparison between three surgical techniques. J Laryngol Otol. 2008;122:246–252. doi: 10.1017/S0022215107000254. [DOI] [PubMed] [Google Scholar]
- 23.Adunka OF, Buchman CA. Scala tympani cochleostomy I: results of a survey. Laryngoscope. 2007;117:2187–2194. doi: 10.1097/MLG.0b013e3181453a6c. [DOI] [PubMed] [Google Scholar]
- 24.Briggs RJ, Tykocinski M, Xu J, et al. Comparison of round window and cochleostomy approaches with a prototype hearing preservation electrode. Audiol Neurootol. 2006;11 Suppl 1:42–48. doi: 10.1159/000095613. [DOI] [PubMed] [Google Scholar]
- 25.Lehnhardt E. Intracochlear placement of cochlear implant electrodes in soft surgery technique. HNO. 1993;41:356–359. [PubMed] [Google Scholar]
- 26.Lenarz T, Stover T, Buechner A, et al. Temporal bone results and hearing preservation with a new straight electrode. Audiol Neurootol. 2006;11 Suppl 1:34–41. doi: 10.1159/000095612. [DOI] [PubMed] [Google Scholar]
- 27.Adunka O, Gstoettner W, Hambek M, et al. Preservation of basal inner ear structures in cochlear implantation. ORL J Otorhinolaryngol Relat Spec. 2004;66:306–312. doi: 10.1159/000081887. [DOI] [PubMed] [Google Scholar]
- 28.Skarzynski H, Lorens A, Piotrowska A, et al. Preservation of low frequency hearing in partial deafness cochlear implantation (PDCI) using the round window surgical approach. Acta Otolaryngol. 2007;127:41–48. doi: 10.1080/00016480500488917. [DOI] [PubMed] [Google Scholar]
- 29.Adunka O, Kiefer J. Impact of electrode insertion depth on intracochlear trauma. Otolaryngol Head Neck Surg. 2006;135:374–382. doi: 10.1016/j.otohns.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 30.O'Leary MJ, Fayad J, House WF, et al. Electrode insertion trauma in cochlear implantation. Ann Otol Rhinol Laryngol. 1991;100:695–699. doi: 10.1177/000348949110000901. [DOI] [PubMed] [Google Scholar]
- 31.Webb RL, Clark GM, Shepherd RK, et al. The biologic safety of the Cochlear Corporation multiple-electrode intracochlear implant. Am J Otol. 1988;9:8–13. [PubMed] [Google Scholar]
- 32.Banfai P, Hortmann G, Kubik S, et al. Projection of the spiral cochlear canal on the medial wall of the tympanic cavity with regard to the cochlear implant. Scand Audiol Suppl. 1979;11:157–170. [PubMed] [Google Scholar]
- 33.Kiratzidis T. 'Veria operation': cochlear implantation without a mastoidectomy and a posterior tympanotomy. A new surgical technique. Adv Otorhinolaryngol. 2000;57:127–130. doi: 10.1159/000059218. [DOI] [PubMed] [Google Scholar]