Abstract
Low density lipoprotein receptor-related protein (LRP1) is an endocytic receptor for diverse proteases, protease inhibitors, and other plasma membrane proteins, including the urokinase receptor (uPAR). LRP1 also functions in cell-signaling and regulates gene expression. The goal of this study was to determine whether LRP1 regulates remodeling of provisional extracellular matrix (ECM) by fibroblasts. To address this problem, we utilized an in vitro model in which type I collagen was reconstituted and overlaid with fibronectin. Either the collagen or fibronectin was fluorescently-labeled. ECM remodeling by fibroblasts deficient in LRP1, uPAR, or MT1-MMP was studied. MT1-MMP was required for efficient remodeling of the deep collagen layer but not involved in fibronectin remodeling. Instead, fibronectin was remodeled by a system that required urokinase-type plasminogen activator (uPA), uPAR, and exogenously-added plasminogen. LRP1 markedly inhibited fibronectin remodeling by regulating cell-surface uPAR and plasminogen activation. LRP1 also regulated remodeling of the deep collagen layer but not by controlling MT1-MMP. Instead, LRP1 deficiency or inhibition de-repressed a secondary pathway for collagen remodeling, which was active in MT1-MMP-deficient cells but not in uPAR-deficient cells. These results demonstrate that LRP1 regulates ECM remodeling principally by repressing pathways that require plasminogen activation by uPA in association with uPAR.
1. Introduction
Remodeling of the extracellular matrix (ECM) is a highly regulated process, integral to wound healing (Singer and Clark, 1999). ECM remodeling is controlled by growth factors, cytokines, proteases, and cell adhesion receptors, including integrins and CD44, expressed by cells that enter the injury site (Davis and Senger, 2005; Okamoto et al., 1999). The same gene predicts function in pathologic processes that involve ECM remodeling, including infection, atherogenesis and cancer invasion (Elkington et al., 2005; Galis and Khatri, 2002; Polette et al., 2004). Understanding factors that regulate ECM remodeling remains an important problem.
Low density lipoprotein receptor-related protein (LRP1) is a member of the LDL receptor family (Strickland et al., 2002), which localizes in lipid rafts and clathrin-coated pits, where it undergoes constitutive endocytosis and recycling (Boucher et al., 2002; Weaver et al., 1996; Wu et al., 2004). Over 40 ligands bind to LRP1, including proteases such as urokinase-type plasminogen activator (uPA), tissue-type plasminogen activator (tPA), and matrix metalloprotease-9 (MMP-9) (Bu et al., 1992; Hahn-Dantona et al., 2001; Kounnas et al., 1993). MMP-2 and MMP-13 undergo endocytosis by LRP1-dependent pathways (Barmina et al., 1999; Yang et al., 2001). Additional proteases are internalized by LRP1 after binding Serpins or α2-macroglobulin (Strickland et al., 2002). The function of LRP1 as an endocytic protease receptor represents one mechanism by which LRP1 may regulate ECM remodeling. LRP1 also may regulate ECM remodeling by controlling expression and/or catabolism of ECM proteins. LRP1 functions as an endocytic receptor for fibronectin and decorin and controls expression of type III collagen (Brandan et al., 2006; Gaultier et al., 2006; Salicioni et al., 2002).
By binding uPA-Serpin complex that is already urokinase receptor (uPAR)-associated, LRP1 mediates uPAR endocytosis and down-regulates the cell-surface abundance of this receptor (Conese et al., 1995; Weaver, 2006). By this mechanism, LRP1 controls uPAR-dependent cell-signaling (Ma et al., 2002; Webb et al., 2000) and plasminogen activation at the cell surface (Weaver et al., 1997). Plasmin directly cleaves non-collagen glycoprotein components of the ECM and serves as a potential activator of pro-MMP-2 and pro-MMP-9 (Mignatti and Rifkin, 1993). MMP-2 and MMP-9 cleave type IV collagen (Brinckerhoff and Matrisian, 2002); MMP-2 also demonstrates type I collagenolytic activity (Seandel et al., 2001; Tam et al., 2004). Together, plasmin, MMP-2, and MMP-9 constitute a system capable of targeting diverse ECM components. However, more recent studies suggest that ECM remodeling involves multiple proteases assembled into partially redundant pathways, many of which are plasmin-independent.
pro-MMP-2 is activated by a mechanism that requires membrane type-1 matrix metalloprotease (MT1-MMP) and tissue inhibitor of metalloprotease-2, but not plasmin (Seiki and Yana, 2003). An alternative pathway for pro-MMP-2 activation utilizes plasmin but also requires MT1-MMP (Monea et al., 2002). Similarly, pro-MMP-9 may be activated by plasmin-dependent and -independent pathways (Fridman et al., 2003; Ramos-DeSimone et al., 1999). MT1-MMP demonstrates robust type I collagenolytic activity, independently of plasmin and other MMPs (Sabeh et al., 2004; Sabeh et al., 2009).
The goal of this study was to assess the function of LRP1 as a regulator of ECM remodeling using an in vitro model of provisional ECM in which type I collagen is reconstituted and then overlaid with fibronectin. In fibroblasts, LRP1 played a major role as an inhibitor of remodeling of fibronectin by regulating cell-surface uPAR and plasminogen activation by uPA. LRP1 also regulated remodeling of the deep collagen layer, but not by controlling the activity or cell-surface abundance of MT1-MMP, which was principally responsible for collagen remodeling. Instead, LRP1 deficiency or inhibition de-repressed a secondary collagen-remodeling pathway that was uPAR-dependent and operational in the absence of MT1-MMP.
2. Results
2.1. MT1-MMP regulates remodeling of collagen but not fibronectin
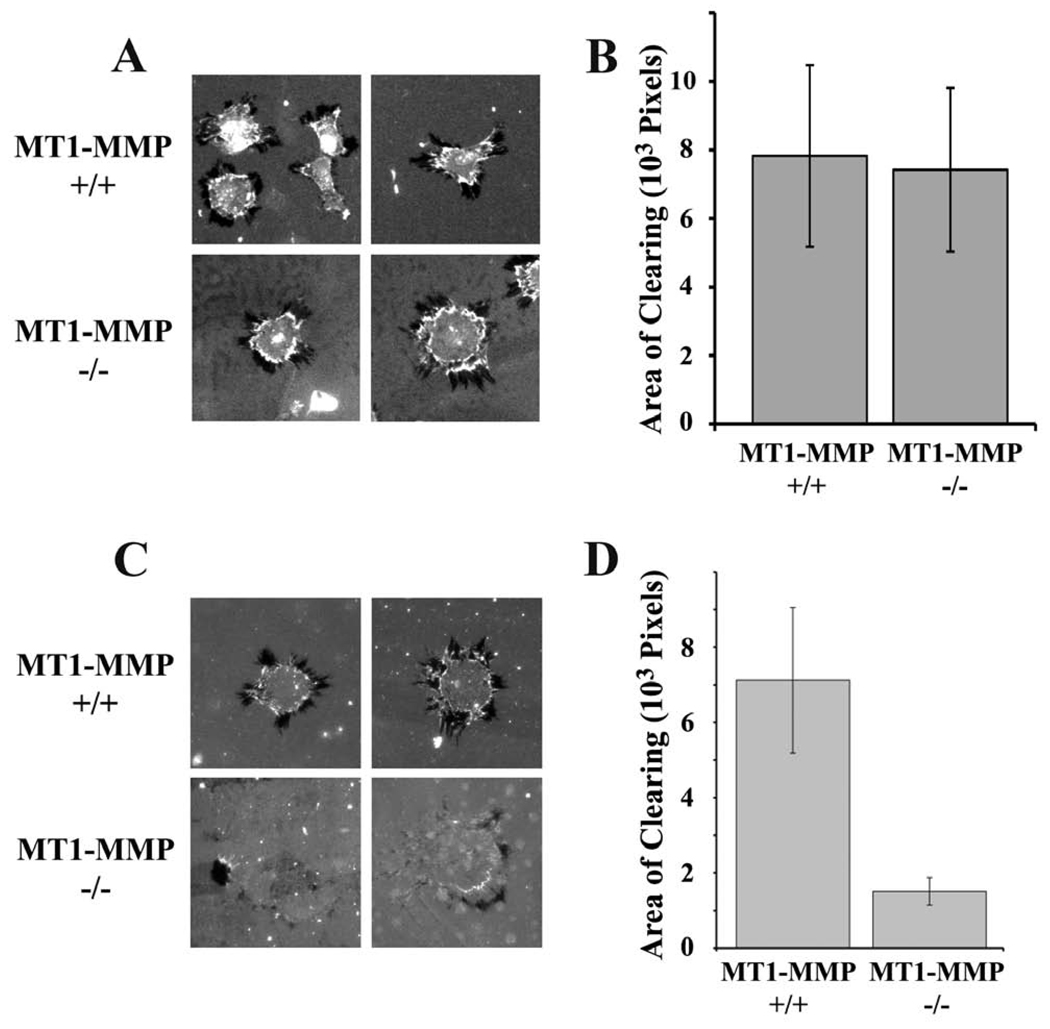
MT1-MMP is a robust type I collagenolytic protease (Sabeh et al., 2004), which also cleaves LRP1 and thereby regulates its activity (Rozanov et al., 2004). To begin, we studied ECM remodeling by MT1-MMP-positive and -negative mouse skin fibroblasts (Sabeh et al., 2004). Cells were plated on type 1 collagen, overlaid with fluorescently-labeled fibronectin. As shown in Fig. 1A, MT1-MMP-positive and -negative cells remodeled the fibronectin, forming areas that were cleared of fluorescent label (areas of clearing or AOCs) in at least one and usually multiple foci, surrounding the nuclei. Intensification of fluorescence near AOC margins suggested contraction of the fibronectin matrix.
Fig. 1.
ECM remodeling by MT1-MMP-deficient (−/−) and expressing (+/+) fibroblasts. The substratum consisted of fluorescently-labeled fibronectin layered on top of type I collagen. (A) Representative cells are shown. Darkened or black areas represent “areas of clearing” or “AOCs”, which are measured as evidence of ECM remodeling. (B) Quantification of the total AOCs for individual MT1-MMP(−/−) or (+/+) cells. AOCs were measured using image J software (mean ± SEM). (C) MT1-MMP-deficient and wild type skin fibroblasts were plated on fibronectin layered over fluorescein-labeled type 1 collagen and allowed to remodel the ECM for 3 h prior to fixation. Representative cells are shown. Many MT1-MMP-defiicent cells showed no evidence of remodeling. (D) Quantification of the total AOCs for wild type and MT1-MMP-deficient cells (mean ± SEM).
To compare ECM remodeling by the two cell types, we integrated the collective area occupied by AOCs surrounding each cell. Fig. 1B shows that fibronectin remodeling by MT1-MMP-positive and -negative fibroblasts was equivalent. Next, we compared ECM remodeling using type I collagen that was fluorescein-labeled and over-coated with fibronectin. To assure that fluorescent-labeling did not denature the collagen, we incubated the labeled protein with 0.1 µM trypsin for 30 min. No degradation was observed by SDS-PAGE (results not shown). Figs. 1C and 1D show that MT1-MMP deficiency decreased AOCs in labeled collagen by 80±5% (Fig. 1C, D). The AOCs generated by MT1-MMP-deficient fibroblasts were frequently incomplete; residual fluorophore was apparent in cleared areas. The activity of MT1-MMP in remodeling type I collagen was anticipated (Sabeh et al., 2004) and interpreted as evidence that this model system accurately reports ECM remodeling (Sabeh et al., 2009).
2.2. LRP1-deficient fibroblasts demonstrate increased fibronectin remodeling
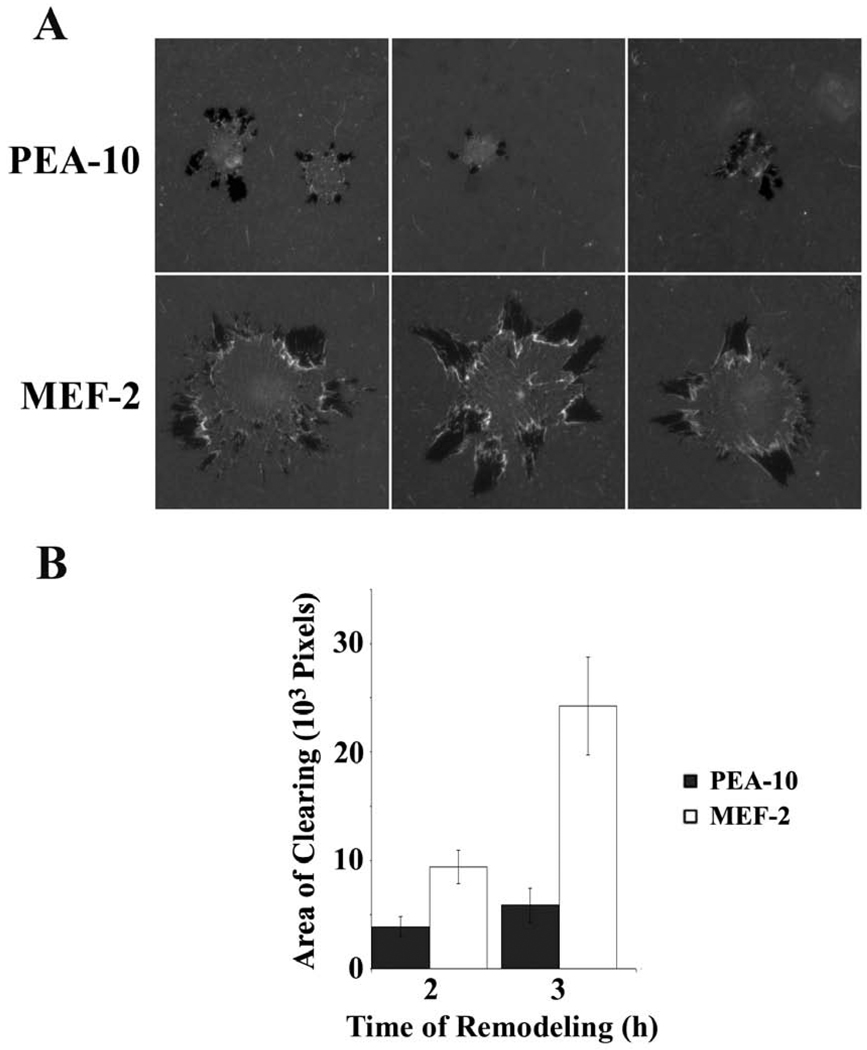
Next, we examined ECM remodeling by murine embryonic fibroblasts (MEFs) that express LRP1 (PEA-10 cells) or do not (MEF-2 cells). Cells were plated on type 1 collagen, overlaid with fluorescently-labeled fibronectin. The cells were allowed to remodel the fibronectin for 2 or 3 h. Fig. 2A shows that AOCs formed by MEF-2 cells were more peripherally located relative to the nuclei, possibly reflecting the propensity of these cells to spread more rapidly (Ma et al., 2002). The collective area of AOCs generated by MEF-2 cells was signficiantly greater than that generated by PEA-10 cells (p<0.05) (FIG. 1B), suggesting that LRP1 inhibits fibronectin remodeling. Candidate mechanisms include regulation of proteases, integrins that remodel fibronectin (Davis and Senger, 2005), and/or fibronectin endocytosis (Salicioni et al., 2002).
Fig. 2.
ECM remodeling by LRP1-deficient MEF-2 cells and LRP1-expressing PEA-10 cells. The substratum consisted of fluorescently-labeled fibronectin layered on top of type I collagen. (A) Representative cells are shown. Darkened or black areas represent AOCs. (B) Quantification of AOCs for individual PEA-10 and MEF-2 cells 2h and 3h after plating. AOCs were measured using image J software (mean ± SEM).
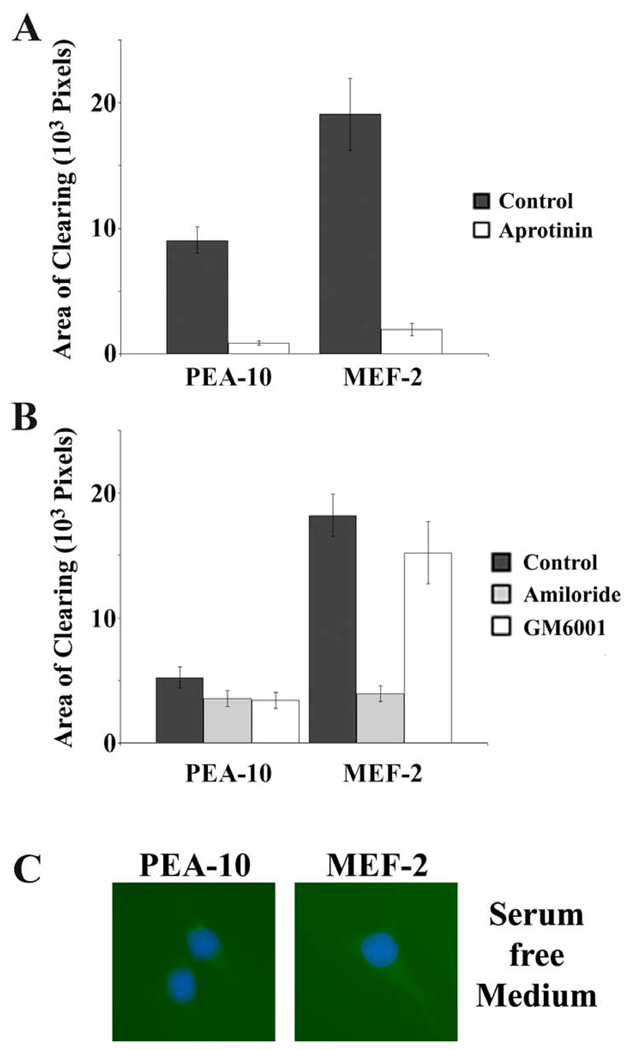
To test the role of proteases in AOC formation by MEFs, specific inhibitors were added. Aprotinin is a serine protease inhibitor that readily inhibits plasmin but not uPA (Violand and Castellino, 1976). As shown in Fig. 3A, aprotinin almost completely blocked fibronectin remodeling by LRP1-positive and -negative MEFs. The uPA-specific inhibitor, amiloride, which does not react with plasmin or tPA (Weaver et al., 1997), inhibited AOC formation mainly by LRP1-deficient cells (Fig. 3B), consistent with the known activity of LRP1 as a catabolic receptor for uPA (Kounnas et al., 1993) and inhibitor of cell-surface plasminogen activation (Weaver et al., 1997). The general MMP inhibitor, GM6001, did not significantly regulate AOC formation in fibronectin, despite previous reports suggesting that MMPs contribute to fibronectin matrix remodeling (Nezi et al., 2002). When PEA-10 and MEF-2 cells were allowed to remodel fibronectin in serum-free medium, eliminating the external source of plasminogen, AOCs were abolished (Fig. 3C). These results support the hypothesis that fibronectin remodeling, in this model system, depends on uPA-mediated plasminogen activation, which is accelerated when LRP1 is absent.
Fig. 3.
Effects of protease inhibitors on ECM remodeling. The experimental substratum for these studies consisted of fluorescently-labeled fibronectin layered over type I collagen. (A) PEA-10 and MEF-2 were treated with 25 µM aprotinin or with vehicle. Remodeling was allowed to occur for 3 h. (B) PEA-10 and MEF-2 cells were treated with 0.5 mM amiloride, 10 µM GM6001 or with vehicle (DMSO) and then allowed to remodel ECM for 3 h. (C) PEA-10 and MEF-2 cells were cultured on the ECM surface for 3 h in serum-free medium. Cells were permeabilized and treated with the nuclear stain, DAPI. Note that neither cell type remodeled fibronectin in the absence of serum.
2.3. LRP1-deficient fibroblasts demonstrate increased collagen remodeling
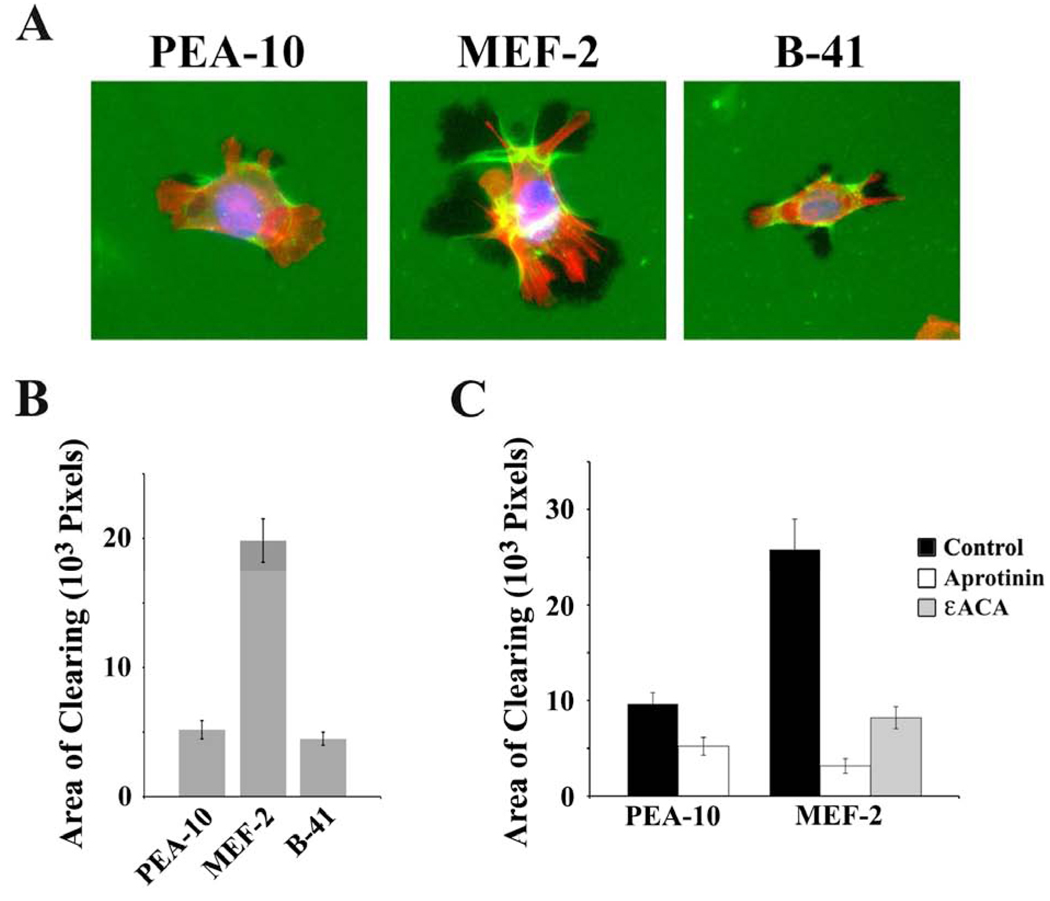
Next, we compared ECM remodeling by LRP1-expressing and -deficient MEFs using fluorescein-labeled collagen over-coated with fibronectin. Fig. 4A shows that LRP1-deficient MEF-2 cells generated larger AOCs in the collagen, compared with LRP1-expressing PEA-10 cells. B-41 cells, which are MEF-2 cells that are stably transfected to express human LRP1 (Salicioni et al., 2002), rescued the phenotype, reducing AOCs to the level observed with PEA-10 cells (p<0.05, Fig. 4B).
Fig. 4.
Remodeling of ECM in which the deep layer of type 1 collagen is fluorescently labeled. The experimental substratum for these studies consisted of fibronectin layered over fluorescently-labeled type I collagen. (A) Representative PEA-10, MEF-2, and B-41 cells are shown. These cells were allowed to remodel the ECM for 3 h prior to fixation. Cells were permeabilized and treated with the nuclear stain, DAPI and Phalloidin conjugated to Alexa 598. AOCs are black or darkened areas devoid of fluorophore. (B) Quantification of the total AOCs for individual PEA-10, MEF-2 and B-41 cells. AOCs were measured using image J software (mean ± SEM). (C) PEA-10 and MEF-2 cells were treated with 25 µM aprotinin, 20 mM εACA, or with vehicle. MEFs were allowed to remodel ECM for 3 h. The bar graph shows that the total AOC associated with individual cells was decreased by aprotinin and εACA (mean ± SEM).
Increased remodeling of collagen by LRP1-deficient cells may reflect effects on remodeling of the collagen itself and/or enhanced remodeling of the superficial fibronectin layer, allowing the MEFs more rapid access to the collagen. To test whether plasmin promotes remodeling of the deep collagen layer, aprotinin was added. Fig. 4C shows that aprotinin significantly decreased AOCs in collagen, especially in association with MEF-2 cells (p<0.05). We also studied the effects of εACA, which binds to plasmin(ogen) kringle domains and blocks the interaction of plasmin(ogen) with cell-surface receptors and other macromolecules that support plasminogen activation (Miles et al., 2005; Plow et al., 1986; Salonen et al., 1985). εACA significantly inhibited remodeling of the collagen layer by MEF-2 cells, decreasing AOCs to the level observed with PEA-10 cells (p<0.05). To determine whether the effects of LRP1 on collagen remodeling are dependent on fibronectin over-coating, we performed experiments in which fluorescently-labeled collagen was overlaid with Matrigel instead of fibronectin. Matrigel consists primarily of laminin and type IV collagen (Kleinman, 2001). Equivalent results were obtained; once again, LRP1-deficient MEFs demonstrated increased AOCs compared with LRP1-expressing cells (results not shown).
2.4. LRP1 regulates ECM remodeling only in uPAR-expressing cells
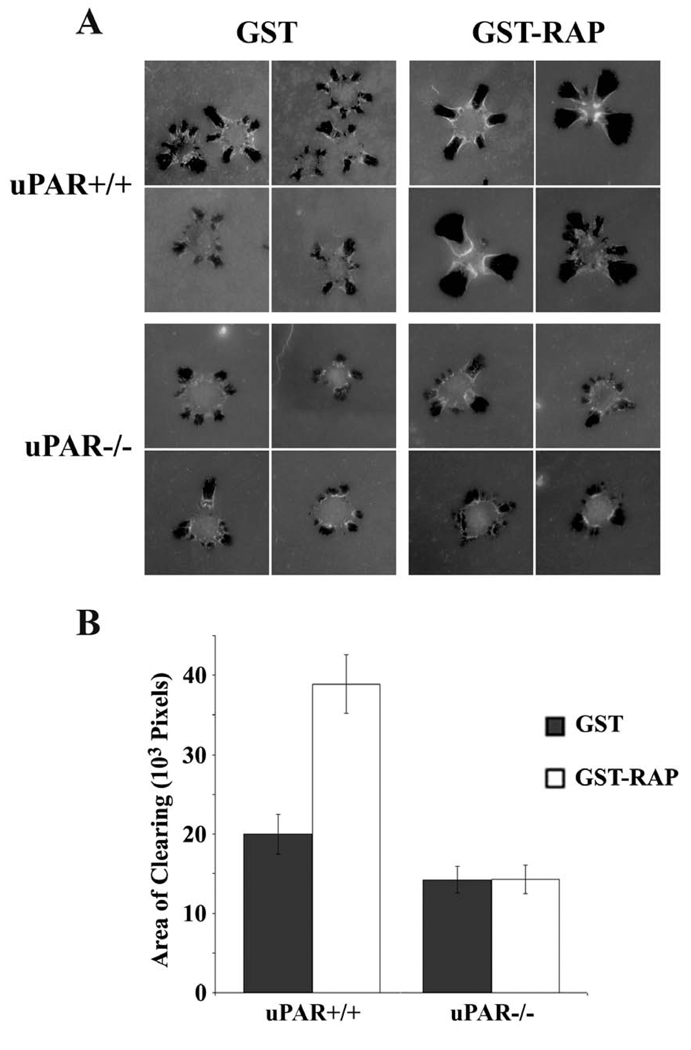
Our results with amiloride, aprotinin, and εACA suggested that LRP1 regulates ECM remodeling by regulating uPA-mediated plasminogen activation. To further test this hypothesis, we performed experiments with uPAR-expressing and -deficient MEFs (Ma et al., 2002). Cells were cultured for three days in the presence of the LRP1 antagonist, receptor-associate protein (RAP), which is known to increase the cell-surface abundance of uPAR (Webb et al., 2000; Webb et al., 1999). RAP blocks binding of soluble ligands to LRP1 as well, including uPA and other proteases (Herz et al., 1991; Williams et al., 1992). RAP-treated and control cells were plated on fluorescein-labeled collagen, over-coated with fibronectin. In the absence of RAP, uPAR-positive cells demonstrated modestly but significantly increased AOCs compared with uPAR-deficient cells (p<0.05) (Fig. 5A and B). RAP increased the AOCs generated by uPAR-positive cells two-fold, but had no effect on AOCs generated by uPAR-deficient cells. Thus, in the absence of uPAR, antagonizing the endocytic activity of LRP1 does not regulate ECM remodeling.
Fig. 5.
uPAR is essential for the effects of LRP1 on ECM remodeling. (A) uPAR(+/+) and uPAR(−/−) MEFs were cultured in the presence of GST-RAP (0.2 µM) or GST (0.2 µM), as a control, for 72 h. The cells were then allowed to remodel ECM, in which fluorescently-labeled type 1 collagen was over-coated with fibronectin, for 3 h. Representative cells are shown. (B) AOCs are shown for uPAR(+/+) and uPAR(−/−) cells that were treated with GST-RAP or GST (mean ± SEM).
2.5. LRP1 and MT1-MMP control collagen remodeling independently
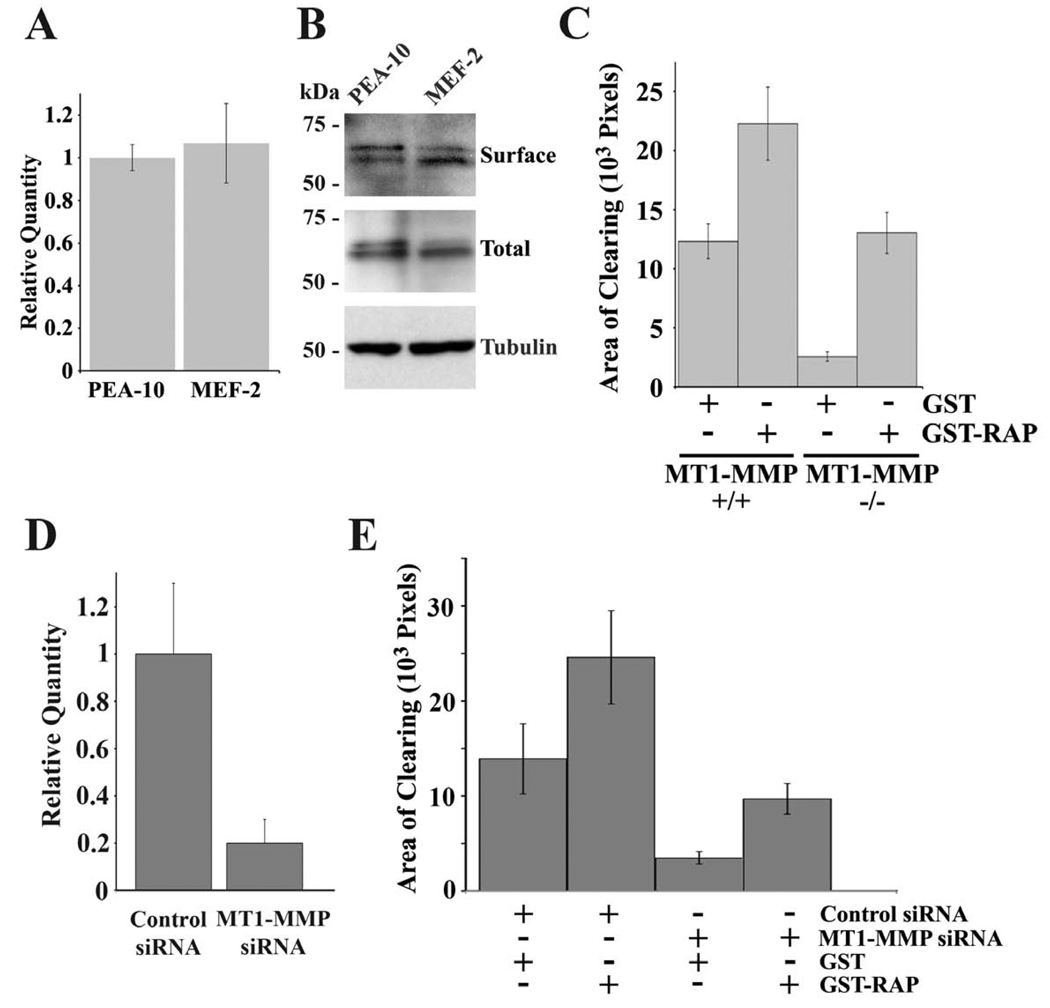
Although MT1-MMP and LRP1 both regulated ECM remodeling, we were unable to demonstrate regulation of MT1-MMP by LRP1 in MEFs. MT1-MMP mRNA levels were equivalent in PEA-10 and MEF-2 cells (Fig. 6A). Furthermore, the cell-surface abundance of MT-MMP1, as determined by cell-surface protein biotinylation, and total cellular MT1-MMP were equivalent in PEA-10 and MEF-2 cells (Fig. 6B).
Fig. 6.
LRP1 regulates ECM remodeling in the presence and absence of MT1-MMP. (A) Total RNA was isolated from PEA-10 and MEF-2 cells and analyzed by qPCR using specific primers for MT1-MMP. (B) Equal amounts of cellular protein from detergentsoluble cell extracts of PEA-10 and MEF-2, which were labeled with biotin, were subjected to affinity precipitation. The precipitates were analyzed by immunoblot analysis to determine “surface” MT1-MMP. Whole cell extracts also were subjected to immunoblot analysis to detect “total” MT1-MMP and tubulin, as a loading control. (C) MT1-MMP-deficient and wild-type skin fibroblasts were cultured in the presence of GST-RAP (0.2 µM) or GST for 72 h. Cells were plated on fibronectin layered over fluoresceinlabeled type 1 collagen and allowed to remodel the ECM for 3 h prior to fixation. AOCs were measured using image J software (mean ± SEM). (D) PEA-10 cells were co-transfected to express RFP and with MT1-MMP-specific or non-targeting control siRNA. Total RNA was isolated from these cells and analyzed by qPCR using specific primers for MT1-MMP. (E) RFP-transfected PEA-10 cells in which MT1-MMP was silenced or not were cultured in the presence of GST-RAP (0.2 µM) or GST for 72 h. The cells were plated on fibronectin layered over fluorescein-labeled type 1 collagen. Transfected cells were allowed to remodel the ECM for 3 h prior to fixation. AOCs of RFP-positive cells were measured using image J software (mean ± SEM).
MT1-MMP-deficient and control fibroblasts were cultured in the presence of RAP for 3 days. The cells were then plated on fluorescently-labeled type I collagen, overlaid with fibronectin. RAP significantly increased AOCs generated by MT1-MMP-positive and -negative cells (Fig. 6C). In MT1-MMP-negative fibroblasts, the increase in remodeling induced by RAP was greater than 5-fold. The ability of RAP-treated MT1-MMPdeficient cells to form prominent AOCs in collagen demonstrates that collagen remodeling is not strictly dependent on MT1-MMP. Neutralizing LRP1 activates a secondary MT1-MMP-independent collagen-remodeling pathway.
To confirm the results obtained with MT1-MMP-deficient and -expressing skin fibroblasts, we silenced MT1-MMP in PEA-10 MEFs and treated these cells with RAP for 3 days. The extent of gene-silencing was 80% at the mRNA level (Fig. 6D). Control cells were transfected with non-targeting siRNA. Both cell populations were co-transfected to express red fluorescence protein (RFP) so that collagen remodeling by transfected cells could be studied selectively. MT1-MMP gene-silencing substantially decreased the AOCs generated by PEA-10 cells (Fig. 6E). RAP increased remodeling by control cells and by cells in which MT1-MMP was silenced. These studies confirm that collagen may be remodeled by an MT1-MMP-independent pathway, which is activated when LRP1 activity is neutralized.
3. Discussion
LRP1 is an endocytic and cell-signaling receptor for diverse ligands (Shi et al., 2009; Strickland et al., 2002). By binding and internalizing multiprotein ligands, which include other membrane proteins, LRP1 regulates the cell-surface abundance of other membrane proteins (Conese et al., 1995; Hamik et al., 1999). uPAR is amongst the membrane receptors that increase in abundance when LRP1 expression or endocytic activity is neutralized (Weaver et al., 1997; Webb et al., 2000). The VLDL receptor controls uPAR abundance by a similar mechanism (Webb et al., 1999). The increase in cell-surface uPAR activates uPAR-initiated cell-signaling leading to Rac1, Ras, and ERK/MAP kinase (Ma et al., 2002; Webb et al., 2000). In MEFs, uPAR-promoted Rac1 activation is largely responsible for the increase in cell migration observed when LRP1 is absent (Ma et al., 2002).
Cell migration in vivo requires ECM remodeling and penetration of tissue boundaries. Because of the ability of LRP1 to regulate cell-surface uPAR and catabolize proteases such as MMP-2 and MMP-9, we hypothesized that LRP1 may regulate ECM remodeling. Other proteases that have been implicated in ECM remodeling, including stromelysin-1/MMP-3 and MT1-MMP (Seiki and Yana, 2003; Wilhelm et al., 1993), have incompletely elucidated relationships with LRP1. MT1-MMP cleaves LRP1 and thereby regulates its activity in PDGF-BB-initiated cell-signaling (Lehti et al., 2009). However, pathways by which LRP1 may control MT1-MMP remain less clear. The goal of the present study was to uncover activities of LRP1 in ECM remodeling.
To address this problem, we developed a straightforward in vitro model system in which type I collagen is overlaid with fibronectin. Like fibrinogen and vitronectin, fibronectin is a provisional extracellular matrix protein that populates wound sites and facilitates essential interactions involving integrins expressed by cells that enter the wound (Singer and Clark, 1999). Our model system is equivalent to that frequently used to study invadopodia in cancer cells (Weaver, 2006). Invadopodia, like podosomes, are structures that penetrate ECM and clear fluorescently-labeled ECM proteins. Proteases that are associated with invadopodia include MMP-2, MMP-9, and MT1-MMP. However, ECM remodeling also may be mediated by integrins, especially in the superficial fibronectin layer (Davis and Senger, 2005) and by proteases that diffuse away from the cell (Polette et al., 2004).
In order to control expression of LRP1, uPAR, and MT1-MMP, we studied internally controlled sets of MEFs and skin fibroblasts from gene knock-out and control mice in the same genetic background. Gene-silencing was applied as an complementary approach. Our results demonstrated that, in our model system, MT1-MMP plays a major role in collagen remodeling as previously described (Sabeh et al., 2009); however, MT1-MMP had no discernable activity in fibronectin remodeling. Instead, fibronectin remodeling depended on uPA-mediated plasminogen activation. Inhibition of fibronectin remodeling was observed with aprotinin and amiloride. Furthermore, fibronectin remodeling was entirely blocked by eliminating serum from the medium, which is a source for plasminogen. Without plasmin generation, the cells appeared unable to penetrate the superficial fibronectin layer.
Plasmin-dependent pathways also accelerated remodeling of the deep collagen layer, primarily when LRP1 was absent and thus, unavailable to suppress uPA-dependent plasminogen activation. Two mechanisms were probably operational here. First, increased fibronectin remodeling probably allowed more rapid penetration of the superficial layer so that the collagen was accessed sooner. However, uPA/uPAR-promoted plasminogen activation also provided a secondary or “back-up” pathway for collagen remodeling in the absence of MT1-MMP, which was significant when LRP1 was inhibited or deficient. Clearly defined AOCs were formed in the collagen layer by MT1-MMP-deficient cells, which were treated with RAP. Although the plasmin-dependent pathway by which collagen is remodeled was not completely defined in this study, activation of MMP-2 may be involved. In MT1-MMP-expressing cells, plasmin also may support collagen remodeling by activating the pro-form of MT1-MMP if this protease trafficks to the cell surface without modification by furin-like proteases (Okumura et al., 1997).
In studies with LRP1-deficient MEFs and RAP-treated cells, LRP1 regulated remodeling of both the superficial fibronectin layer and the deep collagen layer. Because RAP was ineffective at promoting remodeling by uPAR−/− MEFs, we propose that the activity of LRP1 is related principally to its ability to regulate cell-surface uPAR and plasminogen activation. Our model in which LRP1 regulates ECM remodeling by regulating cell-surface uPAR is supported by results obtained with amiloride. In the absence of amiloride, remodeling by MEF-2 cells was 2–3-fold greater than that observed with PEA-10 cells; however, when uPA activity was inhibited by amiloride, remodeling by the two cell lines was equivalent.
Given the central role of MT1-MMP in collagen remodeling, we conducted experiments to test whether LRP1 regulates ECM remodeling by altering expression of MT1-MMP; however, regulation was not observed at the mRNA level. We also observed no difference in total or cell-surface MT1-MMP protein in LRP1-expressing and -deficient cells. Thus, LRP1 does not appear to regulate fibroblast MT1-MMP abundance, as it does other membrane proteins (Gonias et al., 2004). The possibility remains that under some conditions, MT1-MMP may proteolyze LRP1 and thereby de-repress uPA-mediated plasminogen activation; however, this pathway was not apparent in our studies. Overall, our results support a model in which LRP1 controls plasmin-dependent ECM remodeling by its effects on cell-surface uPAR. This pathway may be particularly important in remodeling of provisional ECM and play a secondary role in collagen remodeling when MT1-MMP is absent. The ability of LRP1 to regulate ECM remodeling may be significant in wound healing, atherosclerosis, and cancer.
4. Experimental Procedures
4.1. Reagents and proteins
Type I collagen was from BD Biosciences. Sulfo-NHS-LC-biotin and NHS-fluorescein were from Pierce (Rockford, IL). Gelatin type B, amiloride, aprotinin, mouse anti-tubulin-α and ε-amino caproic acid (εACA) were from Sigma-Aldrich (St Louis, MO). Fibronectin and GM6001 were from Chemicon (Temecula, CA). Glutathione-S-transferase (GST) and GST-RAP were expressed in bacteria and purified as previously described (Herz et al., 1991). RAP binds to LRP1 and blocks binding of other LRP1 ligands (Herz et al., 1991). RAP also blocks LRP1-mediated internalization of uPAR (Webb et al., 1999). Smart pool siRNA against mouse MT1-MMP and non-targeting pooled siRNA were from Dharmacon (Lafayette, CO). The plasmid encoding RFP (pDsRed-N1) was from Clontech (Mountain View, CA). Rabbit polyclonal anti-MT1-MMP was a kind gift from Dr. Strongin (Burnham Institute for Medical Research, La Jolla, CA) (Rozanov et al., 2006). Cell culture reagents were from Invitrogen (Carlsbad, CA).
4.2. Cell culture
MEFs that are genetically deficient in LRP1 (MEF-2) and control LRP1-expressing MEFs (PEA-10) were obtained from the ATCC (Willnow et al., 1995). B-41 cells are MEF-2 cells that were transfected for stable expression of full-length human LRP1 (Salicioni et al., 2002). uPAR+/+ and uPAR−/− MEFs were previously described (Ma et al., 2002). Wild type and MT1-MMP-deficient skin fibroblasts were a kind gift from Dr. Stephen Weiss (University of Michigan, Ann Harbor, MI) (Sabeh et al., 2004). All cells were cultured in DMEM supplemented with 10% FBS, penicillin (10 IU/ml), streptomycin (100 µg/ml), L-glutamine (2 mM) and sodium pyruvate (1 mM).
4.3. In vitro ECM remodeling assays
Type I collagen was reconstituted on glass coverslips (18-mm), as described by Sabeh et al. (Sabeh et al., 2004). In brief, 100 µg/ml of type I collagen was incubated on the coverslips in 20 mM sodium phosphate, 150 mM NaCl, pH 7.4 (PBS) overnight at 4° C. The collagen was over-coated with human fibronectin (5 µg/ml) in PBS for 2 h at 22° C. To monitor ECM remodeling, either the type I collagen or the fibronectin was labeled with NHS-fluorescein in 50 mM sodium carbonate buffer (pH 9) for 15 min at 22° C. The labeled protein was washed twice with 50 mM sodium carbonate buffer, pH 9, and once with PBS before adding the next protein or cells.
Cells were dissociated from monolayer culture using Cell Dissociation Buffer (Invitrogen), washed, and transferred to coverslips with reconstituted, fluorescently-labeled ECM proteins. 105 cells in 50 µl were added to each coverslip. In some experiments, cells were pretreated for 1 h at 37° C in suspension with 25 µM aprotinin, 0.5 mM amiloride, 10 µM GM6001, or 20 mM εACA. Control cells were maintained in suspension, in serum-free DMEM supplemented with vehicle. Treatment protocols were continued, using the equivalent concentration of each reagent, while cells were allowed to adhere to the coverslips and remodel ECM.
PEA-10 cells were co-transfected with siRNA targeting MT1-MMP or with non targeting siRNA (1 µg) and RFP (0.5 µg) using nucleofactor technology, according to manufacturer’s instructions (Amaxa, Germany). Silencing efficiency was determined by qPCR, as previously described (Gaultier et al., 2006). RFP expression was used as a marker of co-transfection (Nguyen et al., 1999). Cells in which MT1-MMP was silenced and control cells were cultured in complete medium supplemented with 200 nM GST-RAP or, as a control, 200 nM GST for 3 days, beginning immediately after transfection, to neutralize the endocytic activity of LRP1. The medium was changed daily and fresh GST or GST-RAP was added, as previously described (Webb et al., 2000; Webb et al., 1999). The cells were then allowed to adhere to coverslips and remodel ECM for 3 h. GST-RAP or GST was maintained in the medium during the ECM remodeling phase.
To terminate an ECM remodeling experiment, cells on coverslips were fixed in 4% formaldehyde for 20 min and then made permeable with 0.1% Triton X-100 in PBS. In some experiments, cells were stained with Phalloidin conjugated to Alexa 598. The coverslips were mounted in Prolong gold mounting medium containing the nuclear stain, 4',6-diamidino-2-phenylindole dihydrochloride (DAPI).
4.4. Fluorescence microscopy and analysis of remodeling
Preparations were examined using a Leica DMIRE2 inverted microscope, equipped with an ORCA digital camera (Hamamatsu, Japan) and Simple PCI imaging software (Compix Inc, Sewickley, PA). Areas that were cleared of fluorescently-labeled protein (AOCs) were determined using Image J software. Presented results were derived by analyzing 20 cells in at least two independent experiments (mean±SEM).
4.5. Surface-protein labeling and affinity precipitation
Cell surface proteins were biotinylated using sulfo-NHS-LC-Biotin (1 mg/ml), according to manufacturer instructions. Biotynilated proteins were purified as previously described (Gaultier et al., 2008).
4.6. SDS-PAGE and Immunoblotting
Cells were extracted in 1% Triton X-100, 0.5% deoxycholate, 0.1% SDS in PBS and complete protease inhibitor cocktail. Equal amounts of cellular protein were subjected to SDS-PAGE and electrotransferred to polyvinylidene fluoride membranes (Bio-Rad, Hercules, CA). Membranes were blocked with 5% nonfat dry milk and then incubated with primary antibodies and horseradish peroxidase-conjugated secondary antibodies (Amersham Bioscience, Piscataway, NJ). Detection was performed using Western Lightning horseradish peroxidase chemiluminescence (Perkin-Elmer, Boston, MA).
Acknowledgments
We are grateful to Dr. Strongin (Burnham Institute for Medical Research, La Jolla, CA) for the MT1-MMP antibody and Dr. Weiss (University of Michigan, Ann Harbor, MI) for the MT1-MMP deficient skin fibroblasts. This work was supported by grants HL-60551 and CA-94900 from the National Institutes of Health, USA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barmina OY, Walling HW, Fiacco GJ, Freije JM, Lopez-Otin C, Jeffrey JJ, Partridge NC. Collagenase-3 binds to a specific receptor and requires the low density lipoprotein receptor-related protein for internalization. J Biol Chem. 1999;274:30087–30093. doi: 10.1074/jbc.274.42.30087. [DOI] [PubMed] [Google Scholar]
- Boucher P, Liu P, Gotthardt M, Hiesberger T, Anderson RG, Herz J. Platelet-derived growth factor mediates tyrosine phosphorylation of the cytoplasmic domain of the low Density lipoprotein receptor-related protein in caveolae. J Biol Chem. 2002;277:15507–15513. doi: 10.1074/jbc.M200428200. [DOI] [PubMed] [Google Scholar]
- Brandan E, Retamal C, Cabello-Verrugio C, Marzolo MP. The low density lipoprotein receptor-related protein functions as an endocytic receptor for decorin. J Biol Chem. 2006;281:31562–31571. doi: 10.1074/jbc.M602919200. [DOI] [PubMed] [Google Scholar]
- Brinckerhoff CE, Matrisian LM. Matrix metalloproteinases: a tail of a frog that became a prince. Nat Rev Mol Cell Biol. 2002;3:207–214. doi: 10.1038/nrm763. [DOI] [PubMed] [Google Scholar]
- Bu G, Williams S, Strickland DK, Schwartz AL. Low density lipoprotein receptor-related protein/alpha 2-macroglobulin receptor is an hepatic receptor for tissue-type plasminogen activator. Proc Natl Acad Sci U S A. 1992;89:7427–7431. doi: 10.1073/pnas.89.16.7427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conese M, Nykjaer A, Petersen CM, Cremona O, Pardi R, Andreasen PA, Gliemann J, Christensen EI, Blasi F. alpha-2 Macroglobulin receptor/Ldl receptor-related protein(Lrp)-dependent internalization of the urokinase receptor. J Cell Biol. 1995;131:1609–1622. doi: 10.1083/jcb.131.6.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis GE, Senger DR. Endothelial extracellular matrix: biosynthesis, remodeling, and functions during vascular morphogenesis and neovessel stabilization. Circ Res. 2005;97:1093–1107. doi: 10.1161/01.RES.0000191547.64391.e3. [DOI] [PubMed] [Google Scholar]
- Elkington PT, O'Kane CM, Friedland JS. The paradox of matrix metalloproteinases in infectious disease. Clin Exp Immunol. 2005;142:12–20. doi: 10.1111/j.1365-2249.2005.02840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridman R, Toth M, Chvyrkova I, Meroueh SO, Mobashery S. Cell surface association of matrix metalloproteinase-9 (gelatinase B) Cancer Metastasis Rev. 2003;22:153–166. doi: 10.1023/a:1023091214123. [DOI] [PubMed] [Google Scholar]
- Galis ZS, Khatri JJ. Matrix metalloproteinases in vascular remodeling and atherogenesis: the good, the bad, and the ugly. Circ Res. 2002;90:251–262. [PubMed] [Google Scholar]
- Gaultier A, Arandjelovic S, Niessen S, Overton CD, Linton MF, Fazio S, Campana WM, Cravatt BF, 3rd, Gonias SL. Regulation of tumor necrosis factor receptor-1 and the IKK-NF-kappaB pathway by LDL receptor-related protein explains the antiinflammatory activity of this receptor. Blood. 2008;111:5316–5325. doi: 10.1182/blood-2007-12-127613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaultier A, Salicioni AM, Arandjelovic S, Gonias SL. Regulation of the composition of the extracellular matrix by low density lipoprotein receptor-related protein-1: activities based on regulation of mRNA expression. J Biol Chem. 2006;281:7332–7340. doi: 10.1074/jbc.M511857200. [DOI] [PubMed] [Google Scholar]
- Gonias SL, Wu L, Salicioni AM. Low density lipoprotein receptor-related protein: regulation of the plasma membrane proteome. Thromb Haemost. 2004;91:1056–1064. doi: 10.1160/TH04-01-0023. [DOI] [PubMed] [Google Scholar]
- Hahn-Dantona E, Ruiz JF, Bornstein P, Strickland DK. The low density lipoprotein receptor-related protein modulates levels of matrix metalloproteinase 9 (MMP-9) by mediating its cellular catabolism. J Biol Chem. 2001;276:15498–15503. doi: 10.1074/jbc.M100121200. [DOI] [PubMed] [Google Scholar]
- Hamik A, Setiadi H, Bu G, McEver RP, Morrissey JH. Down-regulation of monocyte tissue factor mediated by tissue factor pathway inhibitor and the low density lipoprotein receptor-related protein. J Biol Chem. 1999;274:4962–4969. doi: 10.1074/jbc.274.8.4962. [DOI] [PubMed] [Google Scholar]
- Herz J, Goldstein JL, Strickland DK, Ho YK, Brown MS. 39-kDa protein modulates binding of ligands to low density lipoprotein receptor-related protein/alpha 2-macroglobulin receptor. J Biol Chem. 1991;266:21232–21238. [PubMed] [Google Scholar]
- Kleinman HK. Preparation of basement membrane components from EHS tumors. Curr Protoc Cell Biol. 2001 doi: 10.1002/0471143030.cb1002s00. Chapter 10 Unit 10 2. [DOI] [PubMed] [Google Scholar]
- Kounnas MZ, Henkin J, Argraves WS, Strickland DK. Low density lipoprotein receptor-related protein/alpha 2-macroglobulin receptor mediates cellular uptake of pro-urokinase. J Biol Chem. 1993;268:21862–21867. [PubMed] [Google Scholar]
- Lehti K, Rose NF, Valavaara S, Weiss SJ, Keski-Oja J. MT1-MMP promotes vascular smooth muscle dedifferentiation through LRP1 processing. J Cell Sci. 2009;122:126–135. doi: 10.1242/jcs.035279. [DOI] [PubMed] [Google Scholar]
- Ma Z, Thomas KS, Webb DJ, Moravec R, Salicioni AM, Mars WM, Gonias SL. Regulation of Rac1 activation by the low density lipoprotein receptor-related protein. J Cell Biol. 2002;159:1061–1070. doi: 10.1083/jcb.200207070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mignatti P, Rifkin DB. Biology and biochemistry of proteinases in tumor invasion. Physiol Rev. 1993;73:161–195. doi: 10.1152/physrev.1993.73.1.161. [DOI] [PubMed] [Google Scholar]
- Miles LA, Hawley SB, Baik N, Andronicos NM, Castellino FJ, Parmer RJ. Plasminogen receptors: the sine qua non of cell surface plasminogen activation. Front Biosci. 2005;10:1754–1762. doi: 10.2741/1658. [DOI] [PubMed] [Google Scholar]
- Monea S, Lehti K, Keski-Oja J, Mignatti P. Plasmin activates promatrix metalloproteinase-2 with a membrane-type 1 matrix metalloproteinase-dependent mechanism. J Cell Physiol. 2002;192:160–170. doi: 10.1002/jcp.10126. [DOI] [PubMed] [Google Scholar]
- Nezi L, Greco D, Nitsch L, Garbi C. The role of proteases in fibronectin matrix remodeling in thyroid epithelial cell monolayer cultures. Biol Chem. 2002;383:167–176. doi: 10.1515/BC.2002.017. [DOI] [PubMed] [Google Scholar]
- Nguyen DH, Catling AD, Webb DJ, Sankovic M, Walker LA, Somlyo AV, Weber MJ, Gonias SL. Myosin light chain kinase functions downstream of Ras/ERK to promote migration of urokinase-type plasminogen activator-stimulated cells in an integrin-selective manner. J Cell Biol. 1999;146:149–164. doi: 10.1083/jcb.146.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto I, Kawano Y, Tsuiki H, Sasaki J, Nakao M, Matsumoto M, Suga M, Ando M, Nakajima M, Saya H. CD44 cleavage induced by a membrane-associated metalloprotease plays a critical role in tumor cell migration. Oncogene. 1999;18:1435–1446. doi: 10.1038/sj.onc.1202447. [DOI] [PubMed] [Google Scholar]
- Okumura Y, Sato H, Seiki M, Kido H. Proteolytic activation of the precursor of membrane type 1 matrix metalloproteinase by human plasmin. A possible cell surface activator. FEBS Lett. 1997;402:181–184. doi: 10.1016/s0014-5793(96)01523-2. [DOI] [PubMed] [Google Scholar]
- Plow EF, Freaney DE, Plescia J, Miles LA. The plasminogen system and cell surfaces: evidence for plasminogen and urokinase receptors on the same cell type. J Cell Biol. 1986;103:2411–2420. doi: 10.1083/jcb.103.6.2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polette M, Nawrocki-Raby B, Gilles C, Clavel C, Birembaut P. Tumour invasion and matrix metalloproteinases. Crit Rev Oncol Hematol. 2004;49:179–186. doi: 10.1016/j.critrevonc.2003.10.008. [DOI] [PubMed] [Google Scholar]
- Ramos-DeSimone N, Hahn-Dantona E, Sipley J, Nagase H, French DL, Quigley JP. Activation of matrix metalloproteinase-9 (MMP-9) via a converging plasmin/stromelysin-1 cascade enhances tumor cell invasion. J Biol Chem. 1999;274:13066–13076. doi: 10.1074/jbc.274.19.13066. [DOI] [PubMed] [Google Scholar]
- Rozanov DV, Hahn-Dantona E, Strickland DK, Strongin AY. The low density lipoprotein receptor-related protein LRP is regulated by membrane type-1 matrix metalloproteinase (MT1-MMP) proteolysis in malignant cells. J Biol Chem. 2004;279:4260–4268. doi: 10.1074/jbc.M311569200. [DOI] [PubMed] [Google Scholar]
- Rozanov DV, Savinov AY, Golubkov VS, Tomlinson S, Strongin AY. Interference with the complement system by tumor cell membrane type-1 matrix metalloproteinase plays a significant role in promoting metastasis in mice. Cancer Res. 2006;66:6258–6263. doi: 10.1158/0008-5472.CAN-06-0539. [DOI] [PubMed] [Google Scholar]
- Sabeh F, Ota I, Holmbeck K, Birkedal-Hansen H, Soloway P, Balbin M, Lopez-Otin C, Shapiro S, Inada M, Krane S, et al. Tumor cell traffic through the extracellular matrix is controlled by the membrane-anchored collagenase MT1-MMP. J Cell Biol. 2004;167:769–781. doi: 10.1083/jcb.200408028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabeh F, Shimizu-Hirota R, Weiss SJ. Protease-dependent versus - independent cancer cell invasion programs: three-dimensional amoeboid movement revisited. J Cell Biol. 2009;185:11–19. doi: 10.1083/jcb.200807195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salicioni AM, Mizelle KS, Loukinova E, Mikhailenko I, Strickland DK, Gonias SL. The low density lipoprotein receptor-related protein mediates fibronectin catabolism and inhibits fibronectin accumulation on cell surfaces. J Biol Chem. 2002;277:16160–16166. doi: 10.1074/jbc.M201401200. [DOI] [PubMed] [Google Scholar]
- Salonen EM, Saksela O, Vartio T, Vaheri A, Nielsen LS, Zeuthen J. Plasminogen and tissue-type plasminogen activator bind to immobilized fibronectin. J Biol Chem. 1985;260:12302–12307. [PubMed] [Google Scholar]
- Seandel M, Noack-Kunnmann K, Zhu D, Aimes RT, Quigley JP. Growth factor-induced angiogenesis in vivo requires specific cleavage of fibrillar type I collagen. Blood. 2001;97:2323–2332. doi: 10.1182/blood.v97.8.2323. [DOI] [PubMed] [Google Scholar]
- Seiki M, Yana I. Roles of pericellular proteolysis by membrane type-1 matrix metalloproteinase in cancer invasion and angiogenesis. Cancer Sci. 2003;94:569–574. doi: 10.1111/j.1349-7006.2003.tb01484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Mantuano E, Inoue G, Campana WM, Gonias SL. Ligand binding to LRP1 transactivates Trk receptors by a Src family kinase-dependent pathway. Sci Signal. 2009;2:ra18. doi: 10.1126/scisignal.2000188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med. 1999;341:738–746. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- Strickland DK, Gonias SL, Argraves WS. Diverse roles for the LDL receptor family. Trends Endocrinol Metab. 2002;13:66–74. doi: 10.1016/s1043-2760(01)00526-4. [DOI] [PubMed] [Google Scholar]
- Tam EM, Moore TR, Butler GS, Overall CM. Characterization of the distinct collagen binding, helicase and cleavage mechanisms of matrix metalloproteinase 2 and 14 (gelatinase A and MT1-MMP): the differential roles of the MMP hemopexin c domains and the MMP-2 fibronectin type II modules in collagen triple helicase activities. J Biol Chem. 2004;279:43336–43344. doi: 10.1074/jbc.M407186200. [DOI] [PubMed] [Google Scholar]
- Violand BN, Castellino FJ. Mechanism of the urokinase-catalyzed activation of human plasminogen. J Biol Chem. 1976;251:3906–3912. [PubMed] [Google Scholar]
- Weaver AM. Invadopodia: specialized cell structures for cancer invasion. Clin Exp Metastasis. 2006;23:97–105. doi: 10.1007/s10585-006-9014-1. [DOI] [PubMed] [Google Scholar]
- Weaver AM, Hussaini IM, Mazar A, Henkin J, Gonias SL. Embryonic fibroblasts that are genetically deficient in low density lipoprotein receptor-related protein demonstrate increased activity of the urokinase receptor system and accelerated migration on vitronectin. J Biol Chem. 1997;272:14372–14379. doi: 10.1074/jbc.272.22.14372. [DOI] [PubMed] [Google Scholar]
- Weaver AM, McCabe M, Kim I, Allietta MM, Gonias SL. Epidermal growth factor and platelet-derived growth factor-BB induce a stable increase in the activity of low density lipoprotein receptor-related protein in vascular smooth muscle cells by altering receptor distribution and recycling. J Biol Chem. 1996;271:24894–24900. doi: 10.1074/jbc.271.40.24894. [DOI] [PubMed] [Google Scholar]
- Webb DJ, Nguyen DH, Gonias SL. Extracellular signal-regulated kinase functions in the urokinase receptor-dependent pathway by which neutralization of low density lipoprotein receptor-related protein promotes fibrosarcoma cell migration and matrigel invasion. J Cell Sci. 2000;113(Pt 1):123–134. doi: 10.1242/jcs.113.1.123. [DOI] [PubMed] [Google Scholar]
- Webb DJ, Nguyen DH, Sankovic M, Gonias SL. The very low density lipoprotein receptor regulates urokinase receptor catabolism and breast cancer cell motility in vitro. J Biol Chem. 1999;274:7412–7420. doi: 10.1074/jbc.274.11.7412. [DOI] [PubMed] [Google Scholar]
- Wilhelm SM, Shao ZH, Housley TJ, Seperack PK, Baumann AP, Gunja-Smith Z, Woessner JF., Jr Matrix metalloproteinase-3 (stromelysin-1). Identification as the cartilage acid metalloprotease and effect of pH on catalytic properties and calcium affinity. J Biol Chem. 1993;268:21906–21913. [PubMed] [Google Scholar]
- Williams SE, Ashcom JD, Argraves WS, Strickland DK. A novel mechanism for controlling the activity of alpha 2-macroglobulin receptor/low density lipoprotein receptor-related protein. Multiple regulatory sites for 39-kDa receptor-associated protein. J Biol Chem. 1992;267:9035–9040. [PubMed] [Google Scholar]
- Willnow TE, Armstrong SA, Hammer RE, Herz J. Functional expression of low density lipoprotein receptor-related protein is controlled by receptor-associated protein in vivo. Proc Natl Acad Sci U S A. 1995;92:4537–4541. doi: 10.1073/pnas.92.10.4537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, Arandjelovic S, Gonias SL. Effects of low density lipoprotein receptor-related protein-1 on the expression of platelet-derived growth factor beta-receptor in vitro. J Cell Biochem. 2004;93:1169–1177. doi: 10.1002/jcb.20288. [DOI] [PubMed] [Google Scholar]
- Yang Z, Strickland DK, Bornstein P. Extracellular matrix metalloproteinase 2 levels are regulated by the low density lipoprotein-related scavenger receptor and thrombospondin 2. J Biol Chem. 2001;276:8403–8408. doi: 10.1074/jbc.M008925200. [DOI] [PubMed] [Google Scholar]