Abstract
BK virus (BKV) can cause BKV nephritis in renal transplant patients and has become a significant reason of graft loss in this decade. BKV is latent in the urogenital tract and most likely is transported with the donor kidney to recipients. BKV replication occurs in the nucleus of human renal proximal tubular cells (HRPTEC) and daughter viruses are delivered to other cells to spread infection. A few in vitro studies have been reported about the mechanism and kinetics of BKV infection. However there are still a lot of unknown factors about BKV infection. This unit describes the handling of BKV, BKV propagation, determination of titer and ability to infect cells, as well as purification and labeling of BKV in order to analyze BKV cell entry.
Keywords: BK virus, human renal proximal tubular epithelial cells, infection, propagation, quantitation, purification, labeling
1. Introduction
BKV is a small DNA virus with a 40- to 44-nm-sized icosahedral capsid. This capsid contains a 5000-base-pair genome that encodes three capsid proteins, viral protein 1, 2, and 3 (VP-1, VP-2, and VP-3), and two nonstructural polypeptides, large tumor antigen (T-Ag) and small tumor antigen (t-Ag) (Ahsan and Shah, 2006). Antibody against T-Ag is useful to detect BKV infection. The other method to identify BKV infection is to detect viral DNA using PCR. Even though it is possible to detect BKV infection by Western blot analysis using antibodies against VP-1, these antibodies are not commercially available.
The other well studied viruses, which belong to polyomaviridae, are simian virus 40 (SV40), JC virus, and mouse polyoma virus (mPy). JCV has been reported to cause progressive multifocal leukoencephalopathy. BKV infection is relatively slow in comparison to infection with other polyoma viruses. BKV particles are most frequently found in caveolae at 4 hours after infection (Moriyama et al., 2007), transported along microtubules after caveolar endocytosis, and reach to the endoplasmic reticulum from 6 to 10 hours after infection (Moriyama and Sorokin, 2008). At least 36 hours are necessary to detect high levels of T-Ag and VP-1 expression by Western blots and 48 hours are necessary for detection of viral DNA by Southern blots (Low et al: 2004).
To elucidate precise mechanisms and kinetics of BKV’s invasive pathway, precipitating cause of BKV nephritis, factors and signaling related with BKV nephritis, and efficient therapeutic strategy, it is beneficial to use human renal proximal tubular epithelial cells (HRPTEC) because they are main natural targets of BKV infection and viral life cycles could be different in every cell type.
For that reason, this chapter describes the standard techniques suggested for the propagation, titration, purification, labeling, and infection of BKV in HRPTEC, to facilitate easy handling of BKV by any investigator.
2. Viral propagation
Before starting experiments, sufficient amount of BKV must be prepared to support the studies for the complete length of the project. The following method is a straightforward way to increase viral stock (Liu and Atwood, 2001; Eash et al., 2004; Eash and Atwood, 2005). Even though this method is rather time consuming, it generates large amount of viral stocks.
If the plan is to carry out weekly experiments which include incubation of BKV at a multiplicity of infection (MOI) of 0.5 FFU (fluorescence forming units) / cell with 5.0 × 105 cells in ten of 60 mm cell culture dish and 7.2 × 105 (24 wells × 3.0 × 104cells/well) in 24-well tissue culture plate, the required amount of BKV is at least 1.5× 108 FFU per year.
2.1 Materials
Passage 6 HRPTEC (Cambrex Bio Science Inc)
REBM containing 5 % FBS (See recipe)
BKV stock (ATCC). Gardner strain was used for all described procedures.
75 cm2 tissue culture flasks
2.5% deoxycholate
Sonicator (e.g., Fisher scientific, sonic dismembrator 550) with microtip
2.2 Propagation and harvesting of BKV
- Seed 2.0 ~ 2.5 × 106 HRPTEC in REBM with 5 % FBS on 75 cm2 tissue culture flasks.2.0 ~ 2.5 × 106 HRPTEC will result in about 70 ~ 80 % confluency in 75 cm2 tissue culture flasks. Knowing the exact number of cells in the flask is necessary, because the amount of BKV used for infection is determined based on the amount of cells available.
Prepare 3 ml of REBM with 5 % FBS containing BKV at a multiplicity of infection (MOI) of 0.5 FFU (fluorescence forming units) / cell. Aliquot of BKV containing the corresponding amount of BKV particles is added directly frozen stock to medium using barrier tips.
Incubate cells with 3 ml of REBM containing BKV for 1 hour with rocking the flasks by hand several times every 15 minutes to distribute the virus / medium inoculum to cells evenly.
- Add 7 ml of fresh REBM / 5%FBS.Add the fresh medium without aspiration of 3ml of virus / medium inoculum to end up with a total of 10 ml. To keep the appropriate amount of HRPTEC for 4 weeks {prevent over growing in REBM / 10 % FBS and severe reduction of cell numbers in REBM / 0.5 % FBS with cytopathic effect (CPE) of BKV}, REBM / 5 % FBS is recommended.
- Incubate cells for 4 weeks, changing medium and collecting the supernatant every week. Centrifuge the collected supernatant at 5,000 rpm for 15 min at 4 °C and resuspend in 1 / 10 volume of the collected supernatant (Discard the rest of supernatant). Store the resuspended supernatant at −80°C.Collected supernatant contains detached cells by CPE caused by BKV.
At 4 weeks after incubation, collect the supernatant and harvest cells by scraping. Centrifuge at 10,000 rpm for 15 min at 4 °C and re-suspend in 1 / 10 volume of the collected supernatant.
- Combine with the previously harvested cells and supernatant collected when changing medium. Freeze at −80 °C and thaw at 37 °C three times.About 4 to 5 ml of stock will be obtained from one T-75 flask at this step.
- Sonicate three times for 30 sec (Fisher scientific, sonic dismembrator 550 with microtip, output setting of 4, continuous operation, approximate power output of 20 %)This process is very important to release BKV from cytoplasm and nucleus in HRPTEC.
Add 2.5 % deoxycholate to the cell suspension to achieve the final concentration of 0.25 % and incubate in 37 °C water bath for 15 min.
Centrifuge the lysate and medium mixture at 10,000 rpm for 30 minutes at 4 °C.
- Collect supernatant and after aliquoting store it at −80 °C.Repeat freezing and thawing of the viral samples should be avoided virus can be stored for more than one year at −80 °C.
3. Fluorescent Focus Assay ~Viral titration~
It is necessary to determine the titer of the virus stock so as carry out each experiment in identical conditions. Fluorescent focus assay (FFA) is an improved method of the standard plaque assay for quantification of BKV. What distinguishes FFA from the plaque assay is the detection of a number of infected cells by antibody against BKV large T-Antigen (T-Ag) in indirect immunofluorescent analysis (Low et al., 2004; Abend et al., 2007). Description of FFA for other viruses can be found in Lonardo et al., 2002; Payne et al., 2006)
3.1 Materials
Passage 6 HRPTEC (Cambrex Bio Science Inc)
REBM containing 0.5 % fetal bovine serum (See recipe)
BKV stock prepared for titration
24-well tissue culture plate
Cover glass (a diameter: 13 mm)
70 % Ethanol
Phosphate-buffered saline (PBS)
Methanol
Tris-buffered saline with 0.1 % Tween (TTBS) (see recipe)
TTBS with 3 % FBS
TTBS with 1 % FBS
Primary antibody (SV40 T-antigen antibody (PAb 416) can be used to recognize BKV T-Ag)
Secondary antibody (Invitorogen, Alexa Fluor 350 (detect blue color as nuclei in BKV infected cells), Alexa Fluor 488 (green color), and Alexa Fluor 680 (red color).
Mounting medium
Varnish (Nail-polish)
3.2 Infect cells with BKV
-
1
Flame the cover glasses after dipping in 70 % ethanol to sterilize. Transfer them to a 24-well tissue culture plate.
-
2
Seed cells on 24-well tissue culture plate with cover glasses and cultivate until cells reach about 70 % confluence.
-
3Incubate with 10 -fold serial dilutions of prepared BKV stock solution in a final volume of 200 µl of REBM / 0.5 % FBS for 72 hours.Triplicates should be used for incubation with each dose of 10 –fold serial dilutions of BKV to determine the precise titer.
-
3Add 300 µl of REBM / 0.5 % FBS and incubate for another 48 hours.Do not aspirate 200 µl of virus / medium inoculum, just add the fresh medium to a final volume 500 µl.
3.3 Indirect immunofluorescent assay
Wash the cover glasses once for 1 min with PBS
Incubate with 100 % methanol for 20 min at − 20 °C to fix the cells.
Wash the cover glasses three times (5 min each) with 500 µl of TTBS and incubate with TTBS with 3 % FBS for 30 min at room temperature for blocking.
Incubate with primary antibody (PA416) diluted in TTBS with 1 % FBS for 1 hour at room temperature, and then wash three times (5 min each) with TTBS at room temperature.
- Incubate with second antibody diluted in TTBS with 1 % FBS for 40 min at room temperature, and then wash three times (5 min each) with 500 µl of TTBS.This procedure should be done with protection from the direct light.
- Wash the cover glasses once with 500 µl of distilled water and mount the cover glasses with one drop of mounting medium on the slide.Mounting medium can be purchased (SlowFade® Antifade Kit, Molecular Probes), but it also can be prepared by mixing glycerol and PBS (final concentration: 70 % glycerol and 30 % PBS) and be kept at least for one year at 4 °C.
Shield with varnish (nail-polish) along the edge of cover glasses to prevent from dry-up.
- Analyze the cells with the fluorescent microscope immediately.If immediate analysis is not possible, slides can be kept in the dark box at 4 °C. However analysis should be carried out within a few days.
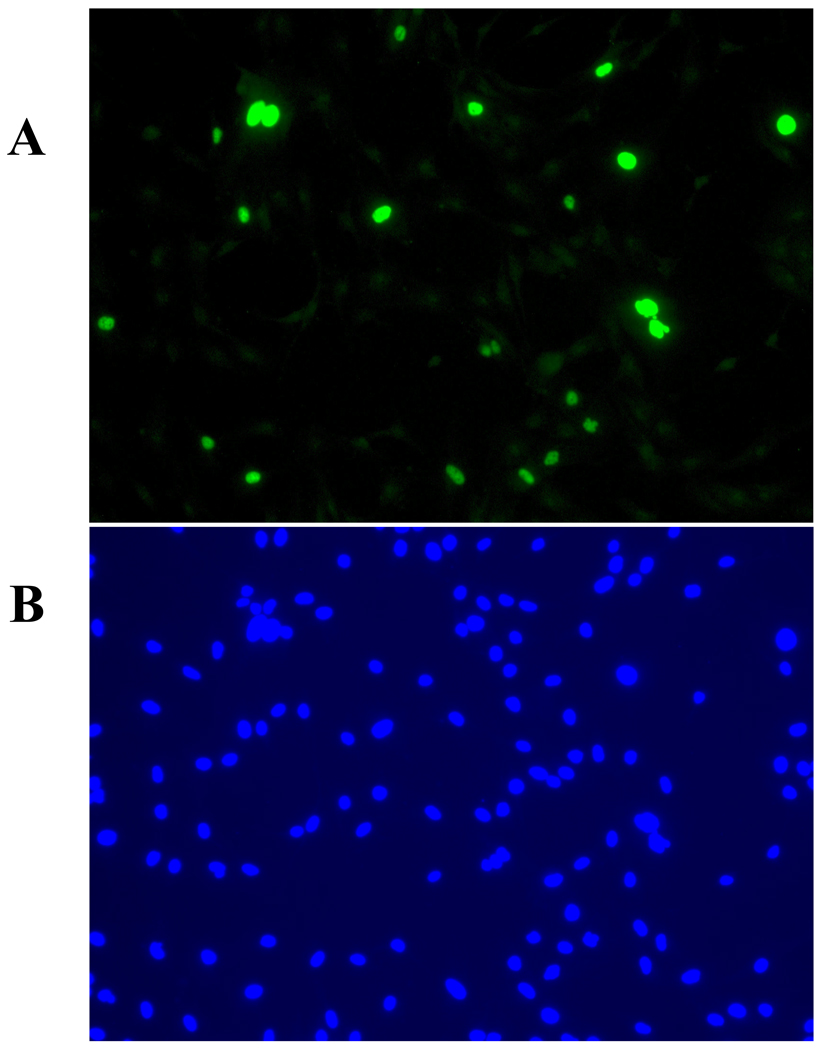
Figure 1 illustrates analysis of BKV infection using indirect immunofluorescent assay with anti-T-Ag antibodies.
Fig. 1. Detection of HRPTEC BKV infection by immunofluorescence. HRPTEC are either stained with anti-T-Ag antibodies (A), or stained with 4’,6-diamidino-2-phenylindole, dilactate (DAPI) (B).
HRPTEC were incubated with BKV (MOI 0.5 FFU/cell). After 72 hours fresh medium was added and incubated for another 48 hours. After incubation, cells were fixed and blocked. Then cells were incubated with primary antibody {5 µl of PA 416 (Calbiochem, San Diego, CA) against 1 ml of TTBS with 1 % FBS} and second antibody{1:200 dilutied Alexa flour™ 488 goat anti-mouse IgG (H+L) (Molecular Probes, Eugene, OR) against TTBS with 1 % FBS}. To stain nuclei, cells were incubated with DAPI (300 nM) (Molecular Probes, Eugene, OR) for 5 minutes and washed three times with PBS. Cells were observed by fluorescent microscope (Nikon Eclipse E600) with 20× objective lens and images were captured by SPOT® version 4.0.9 (Diagnostic instruments, Scotland, UK).
3.4 Calculation of Fluorescence-Forming Unit (FFU)
Count the number of infected cells from five different fields on each cover glass and calculate the average.
- Calculate the number of infected cells in the whole well from the average of the number of infected cells in each field.For example, the average of number of infected cells in the field of 10−4 diluted BKV is 10, and the area of the field is 1.5386 mm2. The number of infected cells in the well is 1000 (=10× 153.86 / 1.5386).{The area of one well of 24-well plate is 153.86 mm2 (=7mm × 7mm × 3.14)}
- Multiply the number of infected cells in each well by the reciprocal of the volume of sample added to the well, and multiply by the reciprocal of the dilution factor.For example, the number of infected cells in the well of 10−4 diluted BKV is 1000. The volume of BKV diluted sample is 0.2 ml. 1000 × 5 × 104 = 5.0 × 107 FFU/ml {1000 = the number of infected cells, 5 = 1 / 0.2 (reciprocal of the volume of BKV diluted sample), and 104 = 1 / 10−4 (reciprocal of the dilution factor)}This result has to show the same titer in the each diluted point, because 10-fold serial dilution of BKV should cause 1 / 10 fold bacteriopharge.For example, the number of infected cells in the well of 10−3 diluted BKV has to be 10000. 10000 × 5 × 103 = 5.0 × 107 FFU/mlThe number of infected cells in the well of 10−5 diluted BKV has to be 100. 100 × 5 × 105 = 5.0 × 107 FFU/mlIf the results do not meet this condition, it may be an error and the titration should be repeated.
4. Purification of BKV
Observation of viral particle itself is valid for the investigation of the kinetics and traffic of virus. However, viral stocks may contain a lot of other proteins besides BK viral proteins, after propagation of BKV. Therefore, purifying BKV from viral stocks is necessary for the labeling BKV particles. Moreover, viral purification is also capable of preventing contaminations with other viruses and bacterium (Eash et al., 2004; Sawa and Komagome, 2005).
4.1 Materials
20% sucrose in re-association buffer (See recipe)
Buffer A (See recipe)
Cesium Chloride (CsCl) solutions (1.40 g/ml, 1.20 g/ml; CsCl dissolved by DH2O)
Slide-A-Lyzer® dialysis cassette (Pierce)
Ultra centrifuge
Ultra centrifuge tube (10ml, 20ml)
5ml syringe
18 gauge needle
4.2 Viral Purification
10–20 ml of virus-containing supernatant is gently layered at the top of 2 ml of 20 % sucrose / re-association buffer and centrifuged at 100,000 × g for 2 hours at 4 °C.
Pellets are dissolved with 3 ml of buffer A and sonicated three times for 30 sec each.
3 ml of 1.40 g/ml CsCl solution is added and then 3 ml of 1.20 g/ml CsCl solution is gently overlaid at 1.40 g/ml CsCl solution.
3 ml of viral sample is gently overlaid on the 1.20 g/ml CsCl solution and centrifuged at 120,000 × g at 16 °C overnight.
- The viral fraction between low and high density of CsCl is extracted carefully by 5ml syringe with 18 gauge needle and diluted with buffer A to the final volume 3 ml.Insert the needle through the side of the tube at 5mm below the viral band.
The viral sample is centrifuged on CsCl gradient again.
The viral fraction is extracted and dialyzed against 500 ml of buffer A at 4 °C overnight.
- Transfer the viral sample into smaller aliquots and store at −80 °C.Repeat freezing and thawing of the viral sample should be avoided. It can be stored for more than one year.
5. Labeling purified BKV
Icosahedral capsid of BKV contains a 5000 base-pair genome that encodes three capsid proteins, viral protein 1 (VP1), viral proteins 2 and 3 (VP2 and VP3), and two nonstructural polypeptides: the large tumor antigen (T-Ag) and small tumor antigen (t-Ag). The major capsid protein, VP1 is likely to be a target of the labeling procedure (Molecular weight (MW): 42 KD). Protein labeling kits (Invitrogen) are simple and handy tools to label proteins, and different kits are available depending on the amount of purified protein for labeling and desired fluorochrome for detection. The proper kit should be selected according to the manufacture’s suggestion. The general information about Alexa Fluor Dye Series can be found at the following link: http://probes.invitrogen.com/media/publications/150.pdf.
Comparison of Invitrogen Kits for labeling proteins can be found at this link: http://probes.invitrogen.com/handbook/sections/0102.html
Description of Alexa Fluor Dyes Spanning the Visible and Infrared Spectrum can be found at this link: http://probes.invitrogen.com/handbook/sections/0103.html
In this unit we describe Alexa Fluor® 488 Microscale protein labeling kit for 20–100 µg protein which we used to label BKV particles: http://probes.invitrogen.com/media/pis/mp30006.pdf
5.1 Materials
Alexa Fluor® 488 Microscale Protein Labeling Kit (Invitrogen)
Kit contents
Alexa Fluor® 488 tetrafluorophenyl (TFP) ester (Component A) 3 vials
Sodium bicarbonate (Component B) 84 mg
Reaction tubes (Component C) 3 tubes
Spin filters (Component D) Nanosep MF 0.2 µm centrifugation devices, 3 tubes
Purification resin (Component E) Bio-Gel P-6 fine resin suspended in PBS, 3ml DH2O
5.2 Labeling BKV
- Calculate the amount of reactive dye (Component A) to add to labeled BKV according to the formula shown below.
-
Amount of reactive dye (µl)= [(µg protein / protein MW) × 1,000] × dye: protein molar ratio (MR) / 11.3µg protein is the mass of protein you want to label
- protein MW is the molecular weight of VP-1 (= 42,000 D)
- MR is the optimal degree of labeling of VP-1 from manufacture’s manual (= 60)
- 11.3 is the concentration of the reactive dye stock solution (See step 4 below).For example, to the label of 24 µg of BKV (VP-1):[(24 / 42,000) × 1,000] × 60 / 11.3 = 3.0 µl of dye
-
- Add 1ml of DH2O to the vial of sodium bicarbonate (Component B) and vortex or pipet up and down until the reagent is fully dissoleved.This bicarbonate solution is 1 M, pH ~8.3.Store at 4 °C for 2 weeks and − 20 °C for long period.
Transfer 20 – 100 µl of labeled BKV to a reaction tube (Component C), add 1/10 volume (2 – 10 µl) of sodium bicarbonate, and mix by pipetting up and down several times.
- Add 10 µl of DH2O to Alexa Fluor® 488 TFP ester (Component A) and pipet up and down until the reagent is fully dissolvedThe concentration of this reactive dye stock solution is 11.3 mM.
Add the appropriate volume of reactive dye solution according to the formula shown above (See step 1) to a reaction tube (Component C), mix by pipetting up and down, and incubate for 15 minutes at room temperature.
-
During 15 minute incubation, resin bed should be prepared.
Add about 800 µl of gel resin (Component E) to the spin filter (Component D), and centrifuge the spin filter at 16,000 × g for 15 seconds.Fixed-angle rotor will make resin bed tilt and lower side should be 2 – 3 mm above the bottom of upper chamber.Swinging bucket rotor will make resin bed horizontal and 5 mm above the bottom of upper chamber. - Transfer 50 µl of purified BKV sample with reactive dye solution from reaction tube (Component C) to the spin filter (Component D) with resin bed, and centrifuge at 16,000 × g for 1 minute.Sample should be added on top of the center of the resin bed.If the amount of sample is over 50 µl, divide it and purify by separate spin filters with resin bed.
Transfer the purified and labeled viral sample into smaller aliquots and store at −80 °C. Before experiments, labeled virus must be titrated again.
6. BKV infection of human renal proximal tubular epithelial cells (HRPTEC)
In this chapter, the method of BKV infection in HRPTEC is described. BKV infection in HRPTEC is relatively slow, BKV is most frequently trapped in caveolae at 4 hours after incubation, reaches the endoplasmic reticulum from 6 to 10 hours after incubation, and high levels of T-Ag expression detected by western blots require at least 36 hours of infection (Low et al., 2004; Moriyama et al., 2007; Moriyama and Sorokin, 2008). It is important to prevent from contamination by bacterium and cell detachment from wells or dishes by CPE of BKV, because the incubation period is long.
6.1 Materials
HRPTEC
REBM containing 0.5 % FBS (see recipie)
BKV stocks
60-mm tissue culture dishes
24-well tissue culture plates with cover glasses
6.2 Infect cells with BKV
- Seed 5.0 × 105 HRPTEC on 60 mm tissue culture dish or 3.0 × 104 HRPTEC on 24-well tissue culture plate with cover glasses one day prior to incubation with BKV.To calculate MOI for BKV infection correctly it is important to know how many cells are incubated with virus.5.0 × 105 HRPTEC provides 70 % of confluence at 60 mm tissue culture dish and 3.0 × 104 HRPTEC provides 60 ~ 70 % confluence at one well of 24-well tissue culture plate on the next day after seeding.
- Calculate the required amount of BKVAccording to our experience, appropriate MOI of BKV against HRPTEC is 0.5 FFU/cell, because higher MOI and long incubation period sometimes cause the detachment of cells by CPE (Moriyama et al., 2007).If the titer of BKV is 5.0 × 107 FFU/ml, the required amount of BKV is 5 µl {= 2.5 × 105 FFU (= 0.5 × 5.0 × 105 = MOI × number of HRPTEC)} for 60 mm tissue culture dish and 0.3 µl {= 1.5 × 104 FFU (= 0.5 × 3.0 × 104 = MOI × number of HRPTEC)} for 24- well tissue culture plate.
- Dilute BKV stocks with REBM containing 0.5 % FBS.5 µl of BKV stock should be diluted with 1.5 ml of REBM containing 0.5 % FBS for 60 mm tissue culture plate and 0.3 µl of BKV stock with 200 µl of REBM containing 0.5 % FBS for 24- well tissue culture plate.
Incubate HRPTEC with medium containing BKV for 72 hours.
- Discard medium and wash three times with REBM containing 0.5 % FBS three times. Add fresh medium and incubate for another 48 hours.Add 3 ml fresh medium to 60 mm tissue culture plate and 500 µl to 24- well tissue culture plate.First 72 hours are to infect BKV infection and next 48 hours are to spread BKV infection to the other cells.
After total 5 days incubation, harvest cells for analysis.
7. Analysis of BKV entry and intracellular trafficking pathway in HRPTEC using confocal microscope and MetaVue™ Imaging System
To understand precise mechanisms and kinetics of BKV invasive pathway, investigation of co-localization of BKV particles with target organelles in HRPTEC is a useful method. Co-localization rate of purified and labeled BKV with target organelles indicates the intracellular trafficking pathway and time course of BKV invasion.
7.1. Materials
HRPTEC
Purified and Labeled BKV by Alexa Fluor® 488 Microscale Protein Labeling Kit 24-well tissue culture plates with cover glasses
Phosphate-buffered saline (PBS)
Methanol
Tris-buffered saline with 0.1 % Tween (TTBS) (see recipe)
TTBS with 3 % FBS
TTBS with 1 % FBS
Primary antibody (antibody against target organelle)
Second antibody {Different color (wavelength) from labeled BKV}
Mounting medium (70 % glycerol and 30 % PBS)
Varnish (Nail-polish)
Confocal microscope
MetaVue™ Imaging System.
7.2. Incubation of HRPTEC with purified and labeled BKV
Seed 3.0 × 104 HRPTEC on 24- well tissue culture plate with cover glasses one day prior to incubate with BKV.
- Calculate the required amount of BKVAccording to our experience, the appropriate MOI of purified and labeled BKV to use in HRPTEC to observe viral particles is 5 FFU/cell.If the titer of BKV is 1.0 × 107 FFU/ml, the required amount of BKV is 15 µl {=1.5 × 105 FFU (=5.0 × 3.0 × 104 = MOI × number of HRPTEC)} for 24- well tissue culture plate.
- Dilute BKV stocks with REBM containing 0.5 % FBS.15 µl of BKV stock should be diluted with 200 µl of REBM containing 0.5 % FBS for 24- well tissue culture plate.
- Incubate HRPTEC with medium containing BKV for the target period.According to our experience, BKV are most frequently trapped in caveolae at 4 hours after incubation, transported along microtubules, and reach to the endoplasmic reticulum from 6 to 10 hours after incubation (Moriyama et al., 2007; Moriyama and Sorokin, 2008).
Observe cells by indirect immunofluorescent assay (See protocol 3.3).
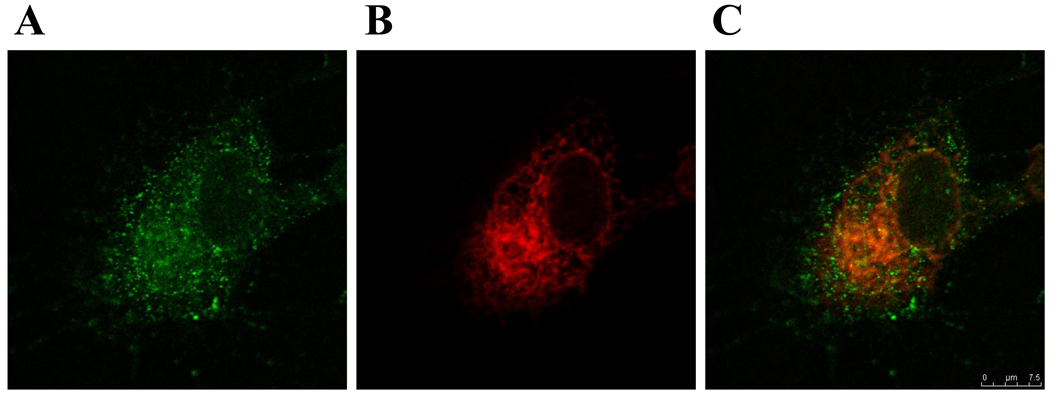
Co-localization of labeled BKV particles with endoplasmic reticulum markers is shown in Figure 2.
Fig. 2. Localization of labeled BKV particles in HRPTEC. (A) Fluorescence of Alexa Fluor 488 labeled BKV particles in HRPTEC, (B) Staining for Endoplasmic reticulum (ER) in HRPTEC, (C) Co-localization of purified and labeled BKV with ER marker.
HRPTEC were incubated with purified and labeled BKV for 6 hours. After incubation, cells were fixed and blocked. Then cells were incubated with primary antibody {1: 100 dilution of PDI (Abcam, Cambridge, MA) as ER marker against TTBS with 1 % FBS} and second antibody {1:200 dilutied Alexa flour™ 680 goat anti-mouse IgG (H+L) (Molecular Probes, Eugene, OR) in TTBS with 1 % FBS}. Cells were analyzed by confocal microscope (Leica TCS SP5) with 63× objective lens and images were captured by Leica application suite advanced fluorescence. Line is 10 µm.
7.3. Usage of MetaVue™ Imaging System to analyze interaction of BKV with target organelles
- After observing HRPTEC by confocal microscope and capturing images, MetaVue™ Imaging System is useful for analysis of co-localization.If purified BKV is labeled with green color (Wavelength around 500 nM), second antibody against target organelle should be used red (Wavelength around 650 nM) or blue color (Wavelength around 350 nM).
- Fix the boundary between BKV particles or target organelle and background according to the intensity of pixels and measure the area of BKV particles and co-localization area of BKV particles with target organelles.According to the BKV uninfected cells, the intensity of background will be calculated. Once threshold is fixed, MetaVue™ Imaging System recognizes and measures area from all pictures regularly and automatically.
- Calculate the co-localization rate by using formula shown below and the average of all the cells.Co-localization rate= Area of BKV particles co-localized with target organelle (Mixed color of green with red or blue) / Area of total BKV particles (Green color).To prevent bias, a lot of cells randomly selected from different wells in several independent experiments must be calculated to obtain the average value. We calculated the average of at least 100 cells from three independent experiments.
Reagents and solutions
Buffer A
1 M Tris (hydroxymethyl) aminomethane (pH 8)
5 M Sodium Chloride
0.1 M Calcium Chloride
Re-association buffer
1 mM Calcium Chloride in TTBS
REBM / 10% (5, 0.5%) FBS medium
Add renal epithelial growth media SingleQuots (human epidermal growth factor, insulin, hydrocortisone, gentamicin/amphotericin-B-1000, epinephrine, triiodothyronine, transferring) (Cambrex Bio Science Inc.), 5ml of 100 units/ml penicillin-G (Invitrogen, Carlsbad, CA) and 50 ml (25, 2.5 ml) fetal bovine serum (FBS) to 500 ml of renal epithelial basal medium (REBM) (Cambrex Bio Science Inc.).
Tris-buffered saline (TBS) 10 × stock solution
1.5 M (87.66 g) Sodium Chloride
0.2 M (24.22 g) Tris (hydroxymethyl) aminomethane
Add distilled H2O (DH2O) to final volume 1000ml and adjust pH to 7.5.
Store at room temperature.
Tris-buffered saline with Tween (TTBS)
Dilute TBS 10× stock slution to 1× working solution with DH2O and add 0.1 % Tween 20 (100 ml TBS 10×, 899 ml DH2O, and 1 ml Tween 20).
COMMENTARY
Background Information
BK Virus is a non-enveloped double-strand DNA virus which belongs to family polyoma viridae and it was initially isolated from the urine of renal transplant patients with ureteric obstruction in 1971 and named after the initials of this patient (Gardner and Field: 1971).
Primary BKV infection occurs by the upper respiratory route until age 10 without any obvious symptoms. More than 80 % of the population is infected by BKV, and approximately 50 % of healthy native kidneys contain latent BKV. BKV mainly persists in the urogenital tract and enters latent phase in the renal tubular epithelial and urothelial cells. In this decade, BKV has become a severe problem in allograft failure among renal transplant patients, because latent BKV likely transported with donor kidney progresses BKV nephritis in immunocompromised hosts, particularly in renal transplant patients. After renal transplantation, 35–60% of patients have BKV viruria, 5–30% of patients have BKV viremia, the prevalence of BKV nephropathy is about 10 % of renal transplant patients, and about half of them have been reported to progress irreversible allograft failure within one year after diagnosis (Nickleit et al., 2003).
Rapid progress is required to analyze BKV infection against HRPTEC, because the precise mechanism and kinetics of BKV infection have not been clearly understood and the efficient antiviral therapies have not been established yet. This unit will be useful for the in vitro analysis of BKV infection in HRPTEC.
Critical Parameters and Troubleshooting
The most important factor for BKV experiments is to determine appropriate dose of BKV and incubation period to cause the efficient BKV infection in HRPTEC. The higher titer and longer period may not always cause the most efficient BKV infection. If the percentage of BKV infection is below 30 % by indirect immunofluorescent analysis or T-Ag expression is extremely low, several titer of BKV and several incubation periods should be examined to determine appropriate dose and period. A point to notice is that another 48 hour incubation period to spread BKV infection to the other cells is required after initial BKV infection and the appropriate dose is different between purified and labeled BKV and untreated BKV. After purification and labeling, it is necessary to measure the viral titration and to determine the appropriate dose and period for infection. According to author’s observation, the appropriate titer of BKV without purification and labeling is MOI 0.5 FFU/cell and incubation period is 5 days (72 hours for primary infection and 48 hours for spreading) for the most efficient BKV infection, and the appropriate dose of purified and labeled BKV is MOI 5 FFU/ml within 24 hours incubation.
Biosafety
BKV belongs to agents of moderate potential hazard to personnel and the environment. This class includes agents which may produce disease of varying degrees of severity from accidental inoculation or injection but which are contained by ordinary laboratory techniques (biosafety level 2 standards of practice and facility). The guidelines for handling this kind of agents can be found in Biosafety in Microbiological and Biomedical Laboratories (BMBL) (4th edition) available on line: http://www.cdc.gov/OD/ohs/biosfty/bmbl4/bmbl4toc.htm
Anticipated Results
Described protocols are generally straightforward, but require several days of procedures. However more than 1.0 × 107 FFU/ml BKV should be obtained after propagation and these viruses will cause infection of more than 30 % of cells as detected by indirect immunofluorescent analysis. Appearance of prominent T-Ag and VP-1 band in western blots analysis and viral DNA detected by PCR will provide additional evidence of BKV infection. Purified and labeled virus will be useful for experiments dealing with analysis of BKV infection of its natural target HRPTEC.
Time Considerations
As we described in this chapter, viral propagation takes about one month. However BKV, once propagated by this method, provides high amount of BKV. Titration of BKV using FFA takes 5 days, a time period similar to one required to cause the infection in HRPTEC for the western blots and indirect immunofluorescent analysis. Viral purification requires 3 days and labeling requires about 2 hours.
Footnotes
Internet Resources
http://probes.invitrogen.com/media/publications/150.pdf.
http://probes.invitrogen.com/handbook/sections/0102.html
http://probes.invitrogen.com/handbook/sections/0103.html
http://probes.invitrogen.com/media/pis/mp30006.pdf
Web site shows the product description of the labeling kit.
Literature Cited
- Abend JR, Low JA, Imperiale MJ. Inhibitory effect of gamma interferon on BK virus gene expression and replication. J. Virol. 2006;81:272–279. doi: 10.1128/JVI.01571-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahsan N, Shah KV. Polyomaviruses and human diseases. Adv Exp Med Biol. 2006;577:1–18. doi: 10.1007/0-387-32957-9_1. [DOI] [PubMed] [Google Scholar]
- Eash S, Querbes W, Atwood WJ. Infection of Vero cells by BK virus is dependent on caveolae. J. Virol. 2004;78:11583–11590. doi: 10.1128/JVI.78.21.11583-11590.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eash S, Atwood WJ. Imvolvement of cytoskeletal components in BK virus infectious entry. J. Virol. 2005;79:11734–11741. doi: 10.1128/JVI.79.18.11734-11741.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner SD, Field AM, Coleman DV, Hulme B. New human papovavirus (B.K.) isolated from urine after renal transplantation. Lancet. 1971;i:1253–1257. doi: 10.1016/s0140-6736(71)91776-4. [DOI] [PubMed] [Google Scholar]
- Liu CK, Atwood WJ. Propagation assay of the JC virus. Methods Mol Biol. 2001;165:9–17. doi: 10.1385/1-59259-117-5:9. [DOI] [PubMed] [Google Scholar]
- Lonardo AD, Buttinelli G, Amato C, Novello F, Ridolfi B, Fiore L. Rapid methods for identification of poliovirus isolates and determination of polio neutralizing antibody titers in human sera. J Virol Methods. 2006;101:189–196. doi: 10.1016/s0166-0934(01)00437-2. [DOI] [PubMed] [Google Scholar]
- Low J, Humes HD, Szczypka M, Imperiale M. BKV and SV40 infection of human kidney tubular epithelial cells in vitro. Virology. 2004;323:182–188. doi: 10.1016/j.virol.2004.03.027. [DOI] [PubMed] [Google Scholar]
- Moriyama T, Marquez JP, Wakatsuki T, Sorokin A. Caveolae endocytosis is critical for BK virus infection of human renal proximal tubular epithelial cells. J. Virol. 2007;81:8552–8562. doi: 10.1128/JVI.00924-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriyama T, Sorokin A. Intracellular trafficking pathway of BK virus in human renal proximal tubular epithelial cells. Virology. 2008;371:336–349. doi: 10.1016/j.virol.2007.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickeleit V, Singh HK, Mihatsh MJ. Polyomavirus nephropathy: morphology, pathophisiology, and clinical management. Curr Opin Nephrol Hypertens. 2003;12:599–605. doi: 10.1097/00041552-200311000-00005. [DOI] [PubMed] [Google Scholar]
- Payne AF, Binduga-Gajewska I, Kauffman EB, Kramer LD. Quantitation of flaviviruses by fluorescent focus assay. J Virol Methods. 2006;134:183–189. doi: 10.1016/j.jviromet.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Sawa H, Komagome R. The JC virus-like particle overlay assay. Methods Mol Biol. 2005;292:175–186. doi: 10.1385/1-59259-848-x:175. [DOI] [PubMed] [Google Scholar]