Abstract
The use of alcohol by adolescents is a growing problem and has become an important research topic in the etiology of the alcohol use disorders. A key component of this research has been the development of animal models of adolescent alcohol consumption and alcohol response. Due to their extended period of adolescence, rhesus macaques are especially well-suited for modeling alcohol-related phenotypes that contribute to the adolescent propensity for alcohol consumption. In this review, we discuss studies from our laboratory that have investigated both the initial response to acute alcohol administration and the consumption of alcohol in voluntary self-administration paradigms in adolescent rhesus macaques. These studies confirm that adolescence is a time of dynamic change both behaviorally and physiologically, and that alcohol response and alcohol consumption are influenced by life history variables such as age, sex, and adverse early experience in the form of peer-rearing. Furthermore, genetic variants that alter functioning of the serotonin, endogenous opioid, and corticotropin releasing hormone systems are shown to influence both physiological and behavioral outcomes, in some cases interacting with early experience to indicate gene by environment interactions. These findings highlight several of the pathways involved in alcohol response and consumption, namely reward, behavioral dyscontrol, and vulnerability to stress, and demonstrate a role for these pathways during the early stages of alcohol exposure in adolescence.
Keywords: Macaca mulatta, alcoholism, gene by environment interaction, serotonin, endogenous opioids, corticotropin releasing hormone
Introduction
Adolescence, the development stage between childhood and adulthood, is a time of dynamic change both behaviorally and physiologically. The changes that take place in the adolescent brain in particular are considerable, and these changes have been linked to the propensity to engage in risk-taking behaviors, including experimenting with alcohol (Clark et al., 2008; Spear, 2000b, 2002; Witt, 1994). This is reflected in the commonly cited statistics from the “Monitoring the Future” Study, which indicate that alcohol has been tried by 39% of current 8th graders, 62% of 10th graders, 72% of 12th graders, and 83% of college students. Even more significant are the findings that 10% of 8th graders, 22% of 10th graders, 26% of 12th graders, and 41% of college students surveyed in 2007 reported occasions of heavy drinking (five or more consecutive drinks at least once in the prior two-weeks) (Johnston et al., 2008), suggesting that not only are adolescents experimenting with alcohol, in some cases they are abusing alcohol.
As the impact of adolescent alcohol consumption in the etiology of the alcohol use disorders has grown, so has the need to develop animal models that permit investigators to examine the effects of alcohol exposure during adolescence (Witt, 1994). Fortunately, certain critical neurobiological and behavioral changes that occur during adolescence translate across species, including changes in key brain regions such as the prefrontal cortex and mesolimbic forebrain structures, and novelty seeking, risk-taking, and peer interactions (Spear, 2000a). Most importantly, adolescent rodents and nonhuman primates will voluntarily consume alcohol in the laboratory (Flory et al., 2006; Grant and Bennett, 2003; Higley et al., 1996c; McKinzie et al., 1999; McKinzie et al., 1998). Rodent models have been effective for examining alcohol response during adolescence (pharmacokinetics, tolerance, motor impairment, etc.) (Silveri and Spear, 1998, 1999, 2000; White et al., 2002), for characterizing adolescent patterns of consumption (Brunell and Spear, 2005; Doremus et al., 2005; Vetter et al., 2007), and for examining the adverse effects of alcohol on brain development (Crews et al., 2000; Crews et al., 2006). However, rodent models are limited by several factors, including a short adolescent time course and differences in key brain systems (Spear, 2000a), some of which are involved in the neuroadaptive changes that drive the development of alcohol dependence (i.e., CRH) (Sanchez et al., 1999). Nonhuman primates have an extended period of adolescence and are similar to humans in neuroanatomy and neurobiology, as well behavior and social organization. Furthermore, the genetic similarities between humans and nonhuman primates, especially Old World species, make nonhuman primates a valuable model for genetic risk factors for alcohol dependence. As a consequence of these shared characteristics between nonhuman primates and humans, paradigms that use nonhuman primates to model aspects of human alcoholism provide a high degree of face validity (Tabakoff and Hoffman, 2000).
In this review, we discuss studies from our laboratory at the National Institutes of Health Animal Center (NIHAC) that have investigated two important intermediate phenotypes in the etiology of alcohol use disorders—the level of response to alcohol, and alcohol consumption behavior—in rhesus macaques (Macaca mulatta). Our studies have primarily involved adolescent subjects, with the goal of modeling early risk factors for excessive alcohol consumption. Rhesus macaques are well suited to the study of adolescent alcohol response and consumption due to the their extended period of adolescence (about 2 years, generally between 2 and 4 years of age) (Lewis, 1997; Watts and Gavan, 1982). Moreover, in addition to meeting all the criteria outlined above for nonhuman primate models, rhesus macaques offer several additional benefits: 1) their behavior has been extensively studied in both natural habitats and in the laboratory, 2) a number of genetic polymorphisms that are functionally equivalent to polymorphisms observed in humans have been identified, and 3) there is an established paradigm for modeling early adversity, in which animals are reared without a mother under standardized conditions in a nursery environment with access only to same-aged peers. This paradigm, referred to as nursery- or peer-rearing, has been repeatedly demonstrated to be useful for modeling gene x environment (GxE) interactions, which have become increasingly important in the study of psychopathology and addiction (Rutter, 2007; Rutter et al., 2006).
Over the years, our laboratory has employed several different alcohol testing paradigms, designed to access various traits and/or motivational factors that are known to moderate risk for developing alcohol problems in humans. In the first paradigm, naïve animals were administered a binge dose of alcohol and then observed in their home environment. With this paradigm, we investigated the behavioral and physiological consequences of acute alcohol exposure while simultaneously examining individual differences in alcohol response. Subjects were typically tested in this paradigm at about 3-4 years of age (roughly equivalent to 12-16 years of age in humans). At approximately 4-5 years of age (roughly equivalent to 16-20 years of age in humans), these subjects were then tested in one of several paradigms used to measure voluntary self-administration of alcohol under limited-access conditions. Similar to the acute administration paradigm, we were specifically interested in individual differences in voluntary alcohol consumption. For these studies, animals were tested under one of two conditions: 1) individually, in single cages, or 2) in a social group. While these two drinking conditions were initially developed in response to practical concerns, we believe that the different environments in which the monkeys were tested characterize different motivational components of alcohol consumption, as will be discussed in a later section. In all, our laboratory has tested numerous cohorts of monkeys in both the acute administration and voluntary self-administration paradigms, thus allowing for the investigation of factors contributing to individual differences in alcohol response and consumption in adolescents.
Acute Alcohol Exposure in Adolescent Rhesus Macaques
The Model
The acute alcohol exposure model was designed to investigate innate sensitivity to alcohol in alcohol-naïve animals (Barr et al., 2003a; Barr et al., 2003b). In these studies, monkeys were administered a fixed dose of alcohol intravenously, and then systematically observed for behaviors indicative of alcohol response and alcohol-induced aggression. For each dosing session, the subject was removed from its home cage and restrained on a flat surface while ethanol (16.8%[v/v] USP) was infused into the saphenous vein at a constant rate over a 15 minute period. Males were administered a dose of 2.2 g/kg while females were administered a dose of 2.0 g/kg, since males require a higher dose of ethanol to produce the same blood ethanol concentration (BEC), due to differences in alcohol metabolism (Baraona et al., 2001; Bennett and DePetrillo, 2004; Ely et al., 1999; Thomasson, 1995). Blood samples were collected at 5 and 10 minutes following initiation of the infusion, and then the subject was transferred back into their home cage for 30-minutes of behavioral testing. Following behavioral testing, another blood sample was collected at 60 minutes post-infusion, followed by the collection of a cerebrospinal fluid (CSF) sample under ketamine anesthesia. The blood samples were collected in order to supplement the behavioral observations with measurements of BEC, and plasma adrenocorticotropic hormone (ACTH) and cortisol. CSF samples were collected to measure the monoamine metabolites 5–hydroxyindole-3-acetic acid (5-HIAA), homovanillic acid (HVA), and 3–methoxy–4–hydroxy-phenylglycol (MHPG).
In the original study design, each subject received two separate injections of ethanol in order to collect repeated outcome measures for each individual, thus reducing the effect of random measurement error (Martin and Kraemer, 1987). However, the administration of two equivalent doses also provided the opportunity to investigate changes in response from one dose to the next. Consequently, in addition to innate sensitivity, we were able to investigate both rapid tolerance—tolerance that develops between just two doses of ethanol (Kalant, 1993; Khanna et al., 1996)—and sensitization to the acute effects of ethanol (Schwandt et al., 2008).
Effects of Age, Sex, and Early Experience
Adolescence is a time of dynamic change. It follows, then, that in addition to comparing alcohol-related phenotypes between adolescents and adults, it is also important to investigate changes that occur across this important developmental stage. Moreover, adolescence is characterized by the emergence of sex differences in many aspects of behavior, including alcohol consumption patterns (Higley, 2003; Lancaster et al., 1996; Lorenz et al., 2006; White et al., 1993), suggesting that sex differences in the effects of ethanol on the brain and body also develop during this time. In our laboratory, the monkeys that were tested for acute alcohol response, while typically in the early stages of adolescence, did vary somewhat in the actual age at which they were tested. We used this opportunity to investigate age-related differences in response to alcohol, as well as potential differences in the pattern of change across adolescence in males and females. Utilizing factors analysis, we first identified three factors that summarized the behavioral response to acute alcohol: ataxia, stimulation, and disinhibition (Barr et al., 2007; Schwandt et al., 2007; Schwandt et al., 2008). When we analyzed these factors in relation to age at testing, we found that in both males and females there was an age-related decline in behavioral signs of ataxia (e.g., stumbles, falls, and sways) across the age range of 28 to 48 months, a period essentially coinciding with rhesus macaque adolescence (Schwandt et al., 2007). This finding conflicted with previous research using rodents showing an age-related increase in alcohol-induced effects on motor behavior from early to late adolescence (Hollstedt et al., 1980; Lagerspetz, 1972), and suggests that the development of sensitivity to the motor-impairing effects of alcohol differs between nonhuman primates and rodents. Among females, we also observed an age-related increase in locomotor stimulation following alcohol administration, suggesting an increase in sensitivity to the reinforcing effects of alcohol (Schwandt et al., 2007). This finding, although limited to females, was consistent with previous studies showing a similar increase in sensitivity to the reinforcing effects of alcohol in rodents (Philpot et al., 2003). It also suggested that underlying mechanisms involved in the response to alcohol may develop differently in males and females, since we did not observe the same effect in both sexes.
When we examined the change in response from the first dose to the second dose of acutely administered alcohol, we found that ataxic behavior decreased from dose one to dose two, while locomotor stimulation increased between doses. This occurred in the absence of any difference in blood ethanol concentrations obtained after each dose. Furthermore, the magnitude of change in response in both behavioral domains was not associated with the amount of time between doses, which ranged from 5 to 30 days (Schwandt et al., 2008). This suggested that, not only do adolescent rhesus macaques develop rapid tolerance to the motor impairing effects of alcohol while at the same time developing locomotor sensitization, but that the effects of a single dose of ethanol in naïve subjects on these behavioral responses are not transient, but rather, persist for some time. Similar evidence has been found in rodent studies, where locomotor sensitization has been shown to last as long as 30 to 60 days (Fish et al., 2002; Lessov and Phillips, 1998) and rapid tolerance has been shown to persist as long as 10 days (Gatto et al., 1987). Both rapid tolerance and sensitization have been linked to persistent changes in neural pathways related to alcohol response, and our results for adolescent nonhuman primates add to the already existing evidence that such changes can occur even in response to a single binge dose of alcohol.
Acutely, ethanol acts as a physiological stressor to increase corticotropin releasing hormone (CRH) and vasopressin (AVP) release from the PVN, resulting in activation of the hypothalamic-pituitary-adrenal (HPA) axis (Laszlo et al., 2001; Ogilvie et al., 1998; Schuckit et al., 1987). Studies in both humans and animal models have shown that early adverse experience and sex influence HPA-axis function, both under baseline conditions and in response to stress (Heim and Nemeroff, 2001; Kudielka and Kirschbaum, 2005; Rhodes and Rubin, 1999). Consequently, we investigated the effects of early experience and sex on the HPA-axis response to acute alcohol administration in the rhesus macaques. As noted previously, peer-rearing is an established model of early adversity that has been linked to a number of behavioral and physiological aberrations both during development and later in life, including altered HPA axis function (Clarke, 1993; Fahlke et al., 2000; Higley et al., 1996c; Shannon et al., 1998; Shannon et al., 2005; Suomi, 1997). In the context of alcohol-induced HPA-axis activity, we found that female rhesus macaques that are peer-reared exhibit higher levels of ACTH compared to females reared by their mothers under normal conditions and males of either rearing condition (Barr et al., 2004a). The fact that we observed these differences in adolescent, alcohol-naïve subjects, suggests that both sex and early experience are factors that should be considered when studying HPA-axis dysregulation. These findings are particularly relevant, in that individual differences in HPA-axis function may actually predict ethanol sensitivity and drinking behavior (Morrow et al., 2006). Furthermore, dysregulation of the HPA-axis has been associated with both alcohol abuse and with a family history of alcohol dependence (Clarke et al., 2008; Dai et al., 2007; Schuckit et al., 1987; Sorocco et al., 2006; Zimmermann et al., 2004).
Neurobiological and Genetic Effects
Innate sensitivity to alcohol, in addition to being influenced by the life history variables discussed above, is undoubtedly under the influence of neurobiological and genetic factors as well. Of the systems thought to be involved in level of response to alcohol, the serotonin system is perhaps the most well-studied. Serotonin dysfunction has been associated with a low level of response to alcohol, impaired impulse control, and early-onset, Type II alcoholism (Heinz et al., 2001; Heinz et al., 2003; Linnoila et al., 1994; Virkkunen and Linnoila, 1997). Moreover, studies of human alcoholics have identified candidate genes involved in serotonergic neurotransmission that are associated with a low level of response to alcohol and alcohol tolerance (Hinckers et al., 2006; Schuckit et al., 1999; Turker et al., 1998). In rhesus macaques, associations between serotonin dysfunction, as reflected by low CSF concentrations of 5-HIAA, and related phenotypes, such as impulsivity and escalated aggression, have also been observed (Higley et al., 1996a; Higley et al., 1996b; Mehlman et al., 1994), as they have in humans. Using the acute alcohol administration paradigm, we investigated the relationship between serotonin dysfunction, the level of response to alcohol, and alcohol-related aggression (Barr et al., 2003a). While a direct correlation between CSF concentrations of 5-HIAA and level of response to alcohol was not reported, decreased sensitivity to alcohol, alcohol-induced increases in CSF MHPG, and baseline CSF levels of 5–HIAA were associated independently with aggression during intoxication. Furthermore, aggression during intoxication –proposed to be a marker of enhanced alcohol-induced stimulation- was found to be positively correlated with voluntary alcohol consumption measured two months later, via the voluntary self-administration paradigm discussed below. These relationships among low level of response to alcohol, CSF monoamine function, and alcohol-induced aggression in rhesus macaques known to have had no prior exposure to alcohol provide important evidence that alcohol-induced stimulation and neurotransmitter-linked predisposition to impulsive aggression independently contribute to aggression during intoxication (Barr et al., 2003a).
Subsequent investigation of individual differences in alcohol response revealed a relationship between genetic variation affecting serotonergic neurotransmission and the level of response. In humans, a common polymorphism in the promoter region of the serotonin transporter gene (5-HTTLPR) alters in vitro gene transcription (Lesch et al., 1996), in vitro transporter activity (Stoltenberg et al., 2002), and in vivo serotonin transporter density (Heinz et al., 2001). In the rhesus macaque, a similar variant (rH5-HTTLPR) has been identified in the same transcriptional control region (Lesch et al., 1997), resulting in allelic variation that alters serotonin transporter gene expression and CNS functioning (Bennett et al., 2002; Heinz et al., 2000; Little et al., 1998). The evidence linking 5-HTTLPR variation with level of response to alcohol in humans has been mixed, with some studies suggesting an association between the long (l) allele and a low level of response to alcohol (Hinckers et al., 2006; Schuckit et al., 1999) and another suggesting that the short (s) allele is associated with a low level of response (Turker et al., 1998). In our population of adolescent rhesus macaques, we found not only that individuals homozygous for the long (l) allele exhibited decreased sensitivity to the intoxicating effects of alcohol, but that early experience played a moderating role—the effect of genotype was only seen among peer-reared monkeys (Barr et al., 2003b). This gene by environment (GxE) interaction, one of several involving the serotonin transporter that we have observed to influence phenotypic outcomes in the rhesus macaque, highlights the importance of considering early adverse experience on genetic effects when studying phenotypes implicated in the etiology of alcohol use disorders. It also suggests a possible explanation for the inconsistencies in findings concerning the relationships among serotonin transporter genotype, alcohol sensitivity, and alcohol intake in human studies, namely, the lack of control over variables such as prior exposure to alcohol and early-life experiences that typically exist in studies of human subjects.
Another system that plays an important role in the alcohol response is the endogenous opioid system. The endogenous opioids are a family of neuropeptides that are involved in a number of physiological processes, including nociception, appetite, stress, and the experience of reward (Akil et al., 1998; Howlett and Rees, 1986). More importantly, endogenous opioids have been linked to addiction vulnerability, including alcohol dependence (Gianoulakis, 2004; Koob, 2009; Oswald and Wand, 2004). In humans, a variant in the μ-opioid receptor gene (OPRM1) has been identified in the N-terminal arm of the receptor, which is bound by β-endorphin. This single nucleotide polymorphism (SNP) in the gene (A118G) results in an amino acid substitution and has been demonstrated to have functional consequences (Bond et al., 1998). Of note, the A118G allele has been associated with increased subjective euphoria and stimulation following intravenous alcohol administration (Ray and Hutchison, 2004). It is suggested that the increased alcohol-induced positive reinforcement that is mediated by the OPRM1 A118G allele could be a heritable factor that increases susceptibility to both initiation and maintenance of alcohol seeking behavior (Ray and Hutchison, 2004; van den Wildenberg et al., 2007). In rhesus macaques there is a nonsynonymous SNP in the OPRM1 gene (OPRM1 C77G), which also results in an amino acid substitution in the N-terminal arm of the μ-opioid receptor (Miller et al., 2004). The existence of this polymorphism in rhesus macaques presents a unique opportunity to model the effects of OPRM1 gene variation on alcohol-related phenotypes. Along those lines, we investigated the effects of the OPRM1 C77G allele on the response to acute alcohol administration in the rhesus macaques. The results indicated that males carrying the G allele displayed increased alcohol-induced stimulation (Barr et al., 2007). These results were consistent with the increased feeling of euphoria following intravenous alcohol administration observed in humans carrying the A118G allele (Ray and Hutchison, 2004). The fact that the effect was more pronounced among males may relate to the fact that, at high blood ethanol concentrations, alcohol's sedative effects may predominate over stimulation, especially among females. These findings are also in agreement with the observation that men generally exhibit more alcohol-induced stimulation and that alcohol-dependent human subjects who are carriers of the OPRM1 A118G allele are more responsive to μ-opioid receptor blockade (Anton et al., 2008; Garbutt et al., 2005; Kim et al., 2009; Oslin et al., 2003).
Voluntary Self-Administration of Alcohol in Adolescent Rhesus Macaques
The Model
The limited access self-administration paradigm was developed in order to assess individual differences in alcohol consumption in subjects first exposed to alcohol during adolescence. In this paradigm, consumption of the alcohol solution was completely voluntary and did not involve any special manipulations or inducements, nor was the alcohol available on a 24-hour, continuous access schedule. On average, testing under a limited access schedule (1 h/day) results in lower volumes of intake (average consumption rates are about 1.24 g/kg/h in the single cage condition and approximately 0.3 g/kg/h in the social group condition) compared to some other models of nonhuman primate alcohol consumption, such as schedule-induced polydipsia (Grant and Johanson, 1988; Grant et al., 2008; Vivian et al., 2001). Unlike these other models, which are useful for assessing consequences of chronic, heavy alcohol consumption in nonhuman primates, our model of adolescent alcohol consumption is focused on investigating the factors contributing to individual differences in early alcohol intake and preference. By studying these factors, we hope to gain a better understanding of potential risk factors that may lead to the development of excessive alcohol consumption, a necessary condition for the development of dependence. One important advantage of the limited-access paradigm has been the ability to test relatively large numbers of monkeys both at the same time (e.g., testing in social groups), and systematically across a number of cohorts. As a result, we have generated a large database of subjects, which has proved quite valuable in evaluating individual differences in alcohol intake levels and patterns.
The limited-access self-administration paradigm began with an initial oral ethanol exposure training phase to ensure that all animals experienced the pharmacological effects of ethanol before beginning the experimental phase. During the training phase, animals were first given access to an aspartame-sweetened vehicle until they freely consumed the vehicle. Ethanol was then added to the vehicle to produce an 8.4% ethanol water-aspartame solution, and the subjects were given free access to the solution for one hour a day until each of the animals fulfilled a pre-established criterion of consuming more than 0.67 g of ethanol per kg of body weight on two or more occasions (subjects typically met this criterion and established stable consumption patterns within two weeks). A 0.67 g/kg dose of alcohol has been shown to produce BACs that are on average 0.10% (Higley et al., 1991), or above the legal limit of intoxication in most states. Once all animals met the criterion for entering the experimental phase, both the sweetened vehicle and the sweetened 8.4% ethanol solution were available in addition to normal drinking water, for one hour a day, five days a week, between 1:00 PM and 2:00 PM, while the animals were in their home cage environment. No special methods, such as deprivation of food or water, were used to induce drinking of the vehicle or the ethanol solution (Higley et al., 1991; Higley et al., 1996c).
Under this paradigm, two drinking conditions have been employed: 1) individual testing in single cages, and 2) testing in a social group setting. Single cage subjects were pair-housed in quad-cages but then separated into individual quadrants for the training and experimental phases, where they had visual and auditory contact with other monkeys but no physical contact. These subjects had access to two drinking spouts, one that dispensed vehicle and one that dispensed the ethanol solution. The presentations of alcohol solution and vehicle were regularly switched from one side to the other to control for preference for one of the spouts. For animals tested in the social group, there were Plexiglas-enclosed “drinking stations” equipped with two drinking spouts, similar to those employed in the single cage condition (i.e., one dispensing the ethanol solution and one dispensing the vehicle) (Flory et al., 2006). Several drinking stations were available to each social group. Subjects were fitted with collars implanted with an identifier chip in order to assess individual levels of consumption. In order to drink from the dispensers, a subject would climb up and place its head in the chamber so that the identifier chip could be read and the alcohol/vehicle dispenser activated. As with the single cage testing condition, the presentations of ethanol solution and vehicle were regularly switched from one side to the other. In addition to activating the drinking station, the chip allowed direct recording by computer of the volume of each solution consumed by a subject. These two paradigms were designed largely based on practical considerations. Single cage testing was easier to execute and, therefore, was the first testing paradigm that was employed by the lab. As the technology for subject identification was developed, social group testing was instigated so that the macaques could remain in their social groups, which meant that more animals could be tested at a time and that continuous access paradigms could potentially be used without having to resort to single-cage housing of the animals. Social group testing was conducted in sex-limited social groups, however, in order to prevent any pregnancies among female subjects during the course of the alcohol testing. It is important to note that these sex-limited social groups were not formed solely for the alcohol consumption testing. Rhesus macaques at the NIHAC ultimately are part of a controlled breeding program, and as such the males and females from a common birth cohort are separated into sex-limited social groups at about 2-3 years of age. Thus, subjects have been housed in the sex-limited social groups for about least 1 year prior to undergoing the alcohol consumption testing.
One limiting aspect of these paradigms is the lack of opportunity to continually monitor BEC values. The collection of regular blood samples from these monkeys, especially those tested in social groups, is problematic because the monkeys must be anesthetized (granted, you can train some singly caged animals to present their legs for blood samples, but this is extremely time consuming and in any event cannot be done for socially-housed animals). Anesthetizing monkeys on a daily basis disrupts their normal behavior and very likely would have an effect on alcohol intake levels. Consequently, blood samples for analysis of BEC were only collected on the final day of testing. In the social setting, BECs from this sample collection ranged from 0 to 163 mg/dL; in the single cage setting, BECs ranged from 6 to 222 mg/dL.
Although the two different testing conditions (single cage vs. social group setting) we employed arose primarily due to practical considerations, we have come to view the two conditions as accessing different motivational components of alcohol consumption. Monkeys who were given access to alcohol in single cages were tested in manner comparable to the two-bottle choice paradigms commonly used in rodents, and were able to freely consume alcohol without interference from other animals. Indeed, subjects tested in single cages on average consume about four times as much alcohol as those tested in the social group setting. Monkeys tested in the social group, on the other hand, are subject to competition for the alcohol dispensers as well as social pressures that likely influence how much they will drink (Barr et al., 2007). For example, just the act of drinking theoretically puts an animal at risk for injury from other group members. In order to acquire ethanol or vehicle in the social group setting, animals must climb up and place their heads into Plexiglas chambers, during which time they have their backs turned toward other group members and have restricted opportunity for egress if surprised or cornered. Among those that consume alcohol in this testing paradigm, two patterns of behavior are typically observed: 1) animals will remain at a station and continually interrupt their drinking to pull their heads out of the chamber and visually scan the run, or 2) they will exhibit frequent cycles of rapidly approaching, drinking, and leaving the stations. We believe these patterns of behavior relate to the fact that animals perceive themselves to be vulnerable to unexpected aggressive encounters while drinking at the stations. The behaviors exhibited suggest that the monkeys could be anxious, but it is important to note that this anxiety appears to be a consequence of the act of drinking, and not the other way around. In other words, the anxiety is not driving the drinking of the alcohol, but rather is a factor in reducing the amount of alcohol consumption that would otherwise occur in the absence of threat of injury from other group members.
In addition to the potential risks in utilizing the drinking stations, monkeys that become intoxicated and impaired in the social context are less able to defend themselves and thus may also be vulnerable to injury during aggressive encounters. Taken together, these potential negative consequences of alcohol consumption in the social group setting suggests to us that this paradigm may be a model for accessing not only reward-based drinking, but high-risk drinking behavior. In humans, one of the defining elements of high-risk drinking is that it is associated with negative consequences and harms (e.g., alcohol-related injuries, driving under the influence, academic and career consequences (Schaus et al., 2009). Some humans, despite the risk of these negative consequences, will continue to consume alcohol under such conditions while others will not. Obviously, the specific negative consequences that characterize human high-risk drinking cannot be modeled in animals; however, we can study alcohol consumption under conditions in which other, more species-typical negative consequences of that consumption are a factor. In this respect, the social group testing paradigm offers the opportunity to investigate individual differences in high risk drinking behavior.
Effects of Age, Sex, and Early Experience
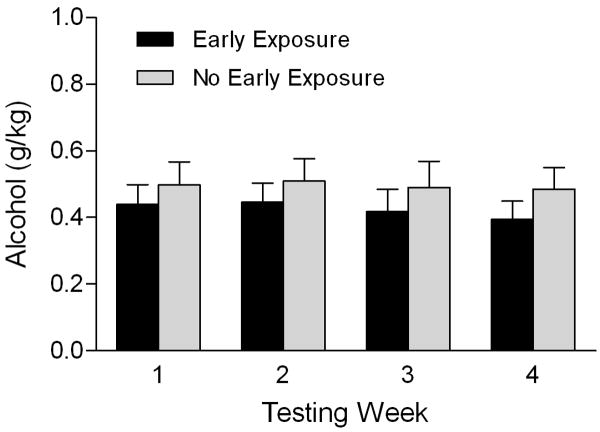
An early age of onset of alcohol use has been shown to be an important factor for developing alcohol dependence later in life (DeWit et al., 2000; Grant, 1998; Grant et al., 2001). Just how much this factor contributes to subsequent alcohol-related problems is difficult to determine, however, due to the multitude of environmental factors that influence not only age of onset of alcohol use, but later consumption patterns and the development of dependence. In this context, animal models of adolescent alcohol consumption are particularly useful because such environmental factors can be minimized or experimentally controlled. Attempts to model whether exposure to alcohol in adolescence leads to increased alcohol intake in adulthood have met with inconsistent results in rodent studies (Hayashi and Tadokoro, 1985; Ho et al., 1989; Rodd-Henricks et al., 2002; Tolliver and Samson, 1991). In the rhesus macaques, we have found that early adolescent subjects will consume more alcohol than individuals given their first access to alcohol in late adolescence (Barr et al., 2004d). Recently, we conducted a small, systematic study of the effects of early exposure to alcohol on subsequent alcohol consumption later in life. Using the single cage paradigm described above, we tested half of the monkeys from the same birth cohort (n = 30) during early adolescence (∼2.5 years of age, roughly equivalent to 10-12 years of age in humans). When the entire birth cohort was tested is the social group setting during late adolescence/early adulthood (4-5 years of age), there were no differences in the amount of alcohol consumed by subjects who received early exposure and those who did not (Figure 1). This suggests that early exposure by itself may not necessarily lead to elevated alcohol consumption later in life, and that other factors are certainly involved. These findings are in agreement with those that demonstrate the association between an early age of onset of alcohol use and alcohol dependence in humans is noncausal and in fact reflects shared genetic and environmental factors (Prescott and Kendler, 1999).
Figure 1.
Lack of an effect of early exposure to alcohol on later alcohol consumption. Subjects (n = 53) were tested in the social group condition for 1 hour a day, five days a week, for 4 weeks when they were 4-5 years of age. A subset of subjects (n = 30) had been similarly tested in the single cage condition when they were about 2.5 years of age. Analysis was performed using repeated measures ANOVA, with testing week as the within subjects variable and sex and early exposure (yes/no) as the between subjects variables. Precise age at the time of testing in the social group was also included as a covariate. Data are presented as mean (± SEM) alcohol consumption for each testing week. There was no main effect of sex F(1,48) = 3.91; P = 0.06 and no main effect of early exposure F(1,48) = 0.72; P = 0.40, nor any interactive effects of early exposure with sex F(1,48) = 0.04; P = 0.84 or with week of testing F(3, 144) = 0.11; P = 0.95.
Sex differences in alcohol consumption and in the risk for alcoholism have long been noted in humans. Cross-culturally, men tend to consume alcohol more frequently and in greater amounts than women (Wilsnack et al., 2000). Moreover, men tend to be more at risk for developing Type II alcoholism, which is early in onset, involves impaired impulse control, and is thought to have a large genetic component (Cloninger, 1987). The sex differences in alcohol consumption that are observed in humans are likely based to some extent on differences between males and females in the pharmacokinetics of alcohol (Baraona et al., 2001; Thomasson, 1995); however, the influence of culturally-based gender roles is certainly also a factor. Once again, animal models offer a means to investigate sex differences in alcohol consumption under experimentally controlled conditions. In this context, however, rodent models have proven problematic in that among many rat strains, females consume more ethanol (g/kg) than males, in clear contrast to what is observed in humans (Juarez and Barrios de Tomasi, 1999; Li and Lumeng, 1984; Piano et al., 2005). Among nonhuman primates, the pattern appears to be more similar to humans, with males typically consuming more alcohol than females (Grant and Bennett, 2003; Vivian et al., 2001), although exceptions have been noted (e.g., vervet monkeys) (Ervin et al., 1990). In our population of rhesus macaques, the finding of sex differences in consumption has varied depending on the subset of subjects tested and the conditions under which they were studied. Early studies of the initial 2-3 cohorts found no significant differences in consumption between males and females, under either standard testing conditions or when alcohol was presented during a social separation challenge (Higley et al., 1991; Higley et al., 1996c). When further data collection using the self-administration model was completed, allowing for larger samples of subjects to be analyzed, males in general were found to consume more alcohol than females (Fahlke et al., 2000). However, as more subjects have been added to the overall dataset, we have noted that this sex difference in consumption is more pronounced among monkeys tested in the single cage condition versus those tested in social groups When the two conditions are considered separately, males drink more than females in the single cage condition while there is no difference between the sexes in the social group condition (Barr et al., 2007; Schwandt et al., 2008). This is interesting in light of the fact that the social group testing condition may model high-risk drinking, in which case our findings suggest that even though rhesus macaques tested in the social group condition tend to drink less alcohol overall, males and females seem to be equal risk-takers in this context. However, among rhesus macaques adolescent males are more likely to be aggressive towards one another than are adolescent females (Bernstein and Ehardt, 1985; Higley, 2003), so it may be that females tested in social groups are actually at less risk for attack and thus will consume alcohol at the same level as males.
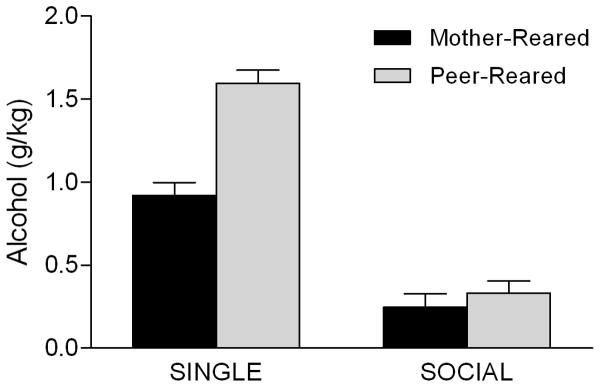
As mentioned above, early studies of rhesus macaques in our laboratory did not find significant sex differences in alcohol consumption during the voluntary self-administration paradigm. These studies were noteworthy for a different reason, though, namely the finding of significant early rearing effects on alcohol consumption. Adverse early experience, such as abuse or loss of a parent, has been shown to increase risk for anxiety and depression in humans (Heim and Nemeroff, 2001; Sanchez et al., 2001), conditions which are known risk factors for alcoholism. Moreover, there is evidence linking parental loss early in life directly to excessive alcohol consumption in adulthood (Hope et al., 1998; Kendler et al., 1996). The early macaque studies by Higley et al. were landmark in that they demonstrated similar effects among nonhuman primates reared without parental input, i.e., peer-reared monkeys consumed significantly more alcohol that mother-reared monkeys (Higley et al., 1991; Higley et al., 1996c). Subsequent studies have replicated this finding in larger samples of subjects (Barr et al., 2004b; Fahlke et al., 2000; Lorenz et al., 2006). More recent studies, though, have indicated that this effect may in fact be moderated by testing condition, similar to what was seen for differences in consumption between males and females. More specifically, among monkeys tested in single cages there is an effect of rearing condition such that peer-reared monkeys drink more alcohol than mother-reared monkeys (Higley et al., 1991; Higley et al., 1996c); however, this effect is absent among monkeys tested in social groups (Barr et al., 2007). Thus, when unfettered access to alcohol is present, peer-reared monkeys will drink substantially more than mother-reared monkeys, but when access to alcohol is given in a social context, there are no differences in intake between the two rearing groups. Interestingly, both groups consume less alcohol in the social group condition, but the decrease is much more marked among peer-reared subjects (Figure 2). It's possible that peer-reared monkeys are more sensitive to the social forces that may inhibit alcohol consumption when tested with other members of the group. As with the sex differences discussed above, it may be that the potential risk involved in consuming alcohol in the social group overrides other mediating factors
Figure 2.
Rearing condition influences alcohol intake in the single cage but not the social group setting. Data on 211 monkeys from the limited-access, voluntary self-administration paradigm were analyzed using 2-way ANOVA, with rearing history, sex, and testing condition as independent variables and mean alcohol consumption (g/kg) across testing weeks as the dependent variable. Data are presented as means ± SEM. There was a main effect of rearing history F(1,202) = 23.47; P = 0.00003, a main effect of testing condition F(1,202) = 158.85; P = 0.00000, and an interaction between rearing history and testing condition F(1,202) = 14.66; P = 0.0002. Post-hoc tests revealed significant differences between peer-reared and mother-reared subjects in the single cage condition, as well as differences between the single cage and social setting conditions within each rearing group (Neuman-Keuls tests, all p < 0.05).
Neurobiological and Genetic Effects
Alcohol consumption is a complex behavior that is regulated by a number of neurobiological systems. These systems play into the different processes that are involved in alcohol seeking and self-administration, such as consummatory behavior and reinforcement. Dysregulations of these systems are believed to be important factors in the development of alcohol dependence and, as with alcohol consumption, there are several pathways that influence addiction vulnerability. Included in these pathways are reward, behavioral dyscontrol, and vulnerability to stress and anxiety (Barr and Goldman, 2006; Goldman et al., 2005). Several of the genetic variants that have been identified in the rhesus macaque are either orthologous or functionally equivalent to candidate genes that influence these risk factors for alcohol dependence in humans, including the 5-HTTLPR and OPRM1 polymorphisms already discussed. Using the voluntary self-administration paradigm, we have been able to investigate the effects of these genetic variants on individual differences in adolescent alcohol consumption.
Reward
As discussed previously, the endogenous opioids mediate natural rewards as well as alcohol-induced positive reinforcement. Evidence further suggests that the endogenous opioids regulate alcohol consumption behavior and craving. In rodents, μ-opioid knock-out mice do not self-administer alcohol (Roberts et al., 2000), and blockade of μ-opioid receptors decreases ethanol drinking in both non-selected rats and rats selectively bred for high alcohol consumption (Coonfield et al., 2004; Krishnan-Sarin et al., 1998; Parkes and Sinclair, 2000; Stromberg et al., 2002; Stromberg et al., 1998). Human males carrying the OPRM1 118G allele exhibit higher levels of alcohol craving (van den Wildenberg et al., 2007). These findings, along with the aforementioned effects of OPRM1 variation on alcohol-induced stimulation in the rhesus macaques, led us to investigate whether this variation might also influence alcohol consumption and preference in monkeys. The 77G allele did, in fact, predict higher levels of alcohol consumption, and this effect was particularly pronounced among males (Barr et al., 2007). Males carrying the 77G allele also consumed doses of alcohol sufficient to produce intoxication (at least 0.67 g/kg of body weight per hour) more frequently, and exhibited increased preference for the alcohol solution over the sweetened vehicle, compared to all other groups. Interestingly, these findings were based on alcohol consumption in the social setting, in which intake levels are relatively low, and in the overall sample there are no sex differences in consumption. Male carriers of the 77G allele consumed twice as much alcohol in this setting compared to all other groups, and consumed alcohol to intoxication nearly 4 times as often. These findings suggest the possibility of a more prominent role of μ-opioid transmission in susceptibility for alcohol use and potential dependence in males. It could be that in a subset of men, in particular those with an early onset of alcohol problems and a positive family history, alcohol intake is more likely to be driven by the positively reinforcing effects of alcohol (reward craving), while in late-onset men and a majority of women, alcohol intake may be more often affected by negative reinforcement of alcohol (relief craving) (Barr et al., 2007; Heilig and Koob, 2007).
Several recent studies have suggested that alcohol-dependent humans who carry the 118G allele may be more responsive to treatment with μ-opioid receptor antagnoists (Anton et al., 2008; Garbutt et al., 2005; Kim et al., 2009; Oslin et al., 2003). Although we did not have alcohol-dependent monkeys in our colony, we were able to test whether or not OPRM1 genotype would mediate the effects of μ-opioid receptor blockade on alcohol preference in the rhesus macaques. When treated with naltrexone, 77G carriers significantly decreased their preference while 77C homozygous subjects were unaffected and, in fact, showed a trend-level increase of preference following naltrexone (Barr et al., in press). Furthermore, these findings applied to subjects tested in both the single cage and the social group conditions, suggesting a role for the positive reinforcing effects of alcohol in both contexts that is susceptible to μ-opioid receptor blockade in some individuals. Our results lend additional support to the notion that OPRM1 genotype is important in alcohol-related phenotypes and that naltrexone treatment for alcohol dependence may only be effective in certain individuals matched to the treatment through genotype.
Behavioral Dyscontrol
The ability to inhibit the pursuit of immediate reward is a valuable trait, especially during adolescent development. Behavioral dyscontrol, or impairments in behavioral control, increase risk for the development of substance use disorders (including alcoholism) and other externalizing disorders such as attention-deficit/hyperactivity disorder, conduct disorder, and antisocial personality disorder (ASPD) (Wills et al., 2006; Young et al., 2009). One gene at which functional variation has been implicated in several of these disorders is the monoamine oxidase A (MAOA) gene, which is located on the X chromosome and as a result may have differential effects in males and females. A variable number of tandem repeats (VNTR) in the transcriptional control region for MAOA (MAOA-LPR) results in altered transcriptional activity (Sabol et al., 1998) that in turn influences synaptic levels of serotonin. In human males, the low activity variant of MAOA-LPR has been associated with early antisocial traits in individuals with a history of early childhood maltreatment (Caspi et al., 2002) and with antisocial behavior in alcohol dependent individuals (Samochowiec et al., 1999). Recently, an association between low activity variants of MAOA-LPR and alcoholism and ASPD in women with a history of abuse was also reported (Ducci et al., 2008). A length polymorphism in the orthologous region of the MAOA gene of rhesus macaques has been identified, and low activity alleles in rhesus macaques have been shown to predict aggressive behavior and impulsivity in males (Newman et al., 2005; Wendland et al., 2006). Among adolescent male macaques, variation in the MAOA length polymorphism also accounts for approximately 10% of the variance in consumption (Barr et al., 2004d). These results from rhesus macaques, coupled with the findings in humans for antisocial behavior suggest that variation in the MAOA-LPR may increase risk specifically for Type II alcoholism (Barr and Goldman, 2006).
Behavioral dyscontrol may also be involved in how individuals respond to novelty and socially threatening situations. Individuals who readily seek out and investigate novel stimuli are considered “exploratory” or “bold”, while those more likely to show fear or withdrawal when confronted with new objects or unfamiliar conspecifics are described as more “inhibited” or “shy” (Korte et al., 2005). The CRH system, which is critical to behavioral and endocrine adaptation to stress, is a key neurobiological system involved in this variation in coping style. Interestingly, individuals on both ends of the spectrum (impulsive/bold versus anxious/inhibited temperaments) are more likely to regularly consume alcohol (Barr and Goldman, 2006; Goldman et al., 2005), suggesting that those who have either significantly high or significantly low levels of CRH system function could be at increased risk for developing alcohol problems. It has been well demonstrated that the CRH system becomes dysregulated in alcohol dependent rodent and human subjects (Adinoff et al., 1996; Adinoff et al., 1990; Funk and Koob, 2007; Funk et al., 2006; Funk et al., 2007; Hansson et al., 2007; Heilig and Koob, 2007; Sillaber et al., 2002; Sommer et al., 2008). However, whether there are effects of genetic variation at the CRH locus on the risk for developing alcohol use disorders in humans is unknown. We screened the rhesus macaque CRH gene and identified a functional variant (−2232C>G) in the CRH regulatory region that disrupts a glucocorticoid response element (Barr et al., 2008). G allele carriers, in addition to exhibiting lower levels of CSF CRH and higher plasma levels of ACTH, were found to be more exploratory/bold in both infancy and adolescence, suggesting that differences in temperament related to CRH gene variation appear early in development. Moreover, adolescents with the G allele consumed more alcohol in the social group condition, which we have argued is a model for high-risk alcohol consumption. These findings demonstrate that genetic variation in the CRH locus influences alcohol consumption behavior and suggest that functionally similar human CRH haplotypes may increase risk for developing externalizing disorders in human subjects (Barr et al., 2008).
Stress Vulnerability
5-HTTLPR genotype has been linked to a wide variety of behavioral phenotypes, most notably anxiety (Mazzanti et al., 1998; Serretti et al., 2006). Several lines of evidence link differences in serotonin transporter activity to stress-related physiology and anxiety in animal models as well. In mice, targeted disruption of the serotonin transporter gene results in increased ACTH and corticosterone responses to immobilization stress as well as increased anxiety-related behavior (Holmes et al., 2003; Kalueff et al., 2007; Lanfumey et al., 2000; Li et al., 1999). In rhesus macaques, individuals who are more stress reactive have lower gene expression levels for the serotonin transporter (Bethea et al., 2005), and we have shown that s allele carriers show increased ACTH responses to a social stressor (Barr et al., 2004c). Interestingly, in both of these studies the effects were more marked among female monkeys. When we tested for effects of rh5-HTTLPR variation on voluntary alcohol consumption in the rhesus macaques, we found that female s allele carriers exhibited higher levels of alcohol preference and, among peer-reared individuals, the s allele carriers progressively increased their levels of consumption across the 6-week course of the study (Barr et al., 2004b). These parallel associations between altered serotonin transporter activity and both anxiety-related phenotypes and alcohol consumption during adolescence suggests that females may be more prone to consume alcohol, at least initially, for its anxiolytic effects. The progressive increase in alcohol consumption observed in adolescent PR females carrying the s allele may reflect sensitization to the negative reinforcing effects of alcohol, and as such suggests that in human populations, women with variation in the serotonin transporter gene promoter who are also exposed to early-life stress may be particularly vulnerable to developing alcohol dependence.
Conclusions
Rhesus macaques, like humans, exhibit complex behaviors that are influenced by life history factors as well as genetic makeup. Due to their extended period of adolescence, rhesus macaques are especially well-suited for modeling alcohol-related phenotypes that either contribute to or are a reflection of the adolescent propensity for alcohol consumption and the path to dependence. Our studies have shown that individual variation in both the initial response to acute alcohol and in voluntary self-administration of alcohol among adolescent rhesus macaques is influenced by age, sex, early experience, and genetic variation, and in some cases, the effects of these variables interact with one another. We aim to extend these findings through ongoing research involving an extended 22-hour exposure self-administration model that uses intermittent access to mimic the repeated cycles of intoxication and withdrawal believed to induce dependence. Future studies will investigate how genetic variation influences alcohol-induced neuroadaptation and pharmacological treatment response in the rhesus macaque and should further promote the translational value of nonhuman primate models in the study of human alcoholism.
Acknowledgments
All research discussed in this review was reviewed and approved by the animal care and use committees (ACUC) of NIAAA and NICHD and was conducted according to the NIH Guide for the Care and Use of Laboratory Animals.
References
- Adinoff B, Anton R, Linnoila M, Guidotti A, Nemeroff CB, Bissette G. Cerebrospinal fluid concentrations of corticotropin-releasing hormone (CRH) and diazepam-binding inhibitor (DBI) during alcohol withdrawal and abstinence. Neuropsychopharmacology. 1996;15:288–295. doi: 10.1016/0893-133X(95)00212-V. [DOI] [PubMed] [Google Scholar]
- Adinoff B, Martin PR, Bone GH, Eckardt MJ, Roehrich L, George DT, Moss HB, Eskay R, Linnoila M, Gold PW. Hypothalamic-pituitary-adrenal axis functioning and cerebrospinal fluid corticotropin releasing hormone and corticotropin levels in alcoholics after recent and long-term abstinence. Arch Gen Psychiatry. 1990;47:325–330. doi: 10.1001/archpsyc.1990.01810160025004. [DOI] [PubMed] [Google Scholar]
- Akil H, Owens C, Gutstein H, Taylor L, Curran E, Watson S. Endogenous opioids: overview and current issues. Drug Alcohol Depend. 1998;51:127–140. doi: 10.1016/s0376-8716(98)00071-4. [DOI] [PubMed] [Google Scholar]
- Anton RF, Oroszi G, O'Malley S, Couper D, Swift R, Pettinati H, Goldman D. An evaluation of mu-opioid receptor (OPRM1) as a predictor of naltrexone response in the treatment of alcohol dependence: results from the Combined Pharmacotherapies and Behavioral Interventions for Alcohol Dependence (COMBINE) study. Arch Gen Psychiatry. 2008;65:135–144. doi: 10.1001/archpsyc.65.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baraona E, Abittan CS, Dohmen K, Moretti M, Pozzato G, Chayes ZW, Schaefer C, Lieber CS. Gender differences in pharmacokinetics of alcohol. Alcohol Clin Exp Res. 2001;25:502–507. [PubMed] [Google Scholar]
- Barr CS, Becker ML, Suomi SJ, Higley JD. Relationships among CSF monoamine metabolite levels, alcohol sensitivity, and alcohol-related aggression in rhesus macaques. Aggressive Behavior. 2003a;29:288–301. [Google Scholar]
- Barr CS, Chen SC, Schwandt ML, Lindell SG, Sun H, Suomi SJ, Heilig M. Suppression of alcohol preference by naltrexone in the rhesus macaque: a critical role of genetic variation at the mu-opioid receptor gene (OPRM1) locus. Biological Psychiatry. doi: 10.1016/j.biopsych.2009.07.026. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr CS, Dvoskin RL, Yuan Q, Lipsky RH, Gupte M, Hu X, Zhou Z, Schwandt ML, Lindell SG, McKee M, et al. CRH haplotype as a factor influencing cerebrospinal fluid levels of corticotropin-releasing hormone, hypothalamic-pituitary-adrenal axis activity, temperament, and alcohol consumption in rhesus macaques. Arch Gen Psychiatry. 2008;65:934–944. doi: 10.1001/archpsyc.65.8.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr CS, Goldman D. Non-human primate models of inheritance vulnerability to alcohol use disorders. Addict Biol. 2006;11:374–385. doi: 10.1111/j.1369-1600.2005.00034.x. [DOI] [PubMed] [Google Scholar]
- Barr CS, Newman TK, Becker ML, Champoux M, Lesch KP, Suomi SJ, Goldman D, Higley JD. Serotonin transporter gene variation is associated with alcohol sensitivity in rhesus macaques exposed to early-life stress. Alcohol Clin Exp Res. 2003b;27:812–817. doi: 10.1097/01.ALC.0000067976.62827.ED. [DOI] [PubMed] [Google Scholar]
- Barr CS, Newman TK, Lindell S, Becker ML, Shannon C, Champoux M, Suomi SJ, Higley JD. Early experience and sex interact to influence limbic-hypothalamic-pituitary-adrenal-axis function after acute alcohol administration in rhesus macaques (Macaca mulatta) Alcohol Clin Exp Res. 2004a;28:1114–1119. doi: 10.1097/01.alc.0000130973.94350.8c. [DOI] [PubMed] [Google Scholar]
- Barr CS, Newman TK, Lindell S, Shannon C, Champoux M, Lesch KP, Suomi SJ, Goldman D, Higley JD. Interaction between serotonin transporter gene variation and rearing condition in alcohol preference and consumption in female primates. Arch Gen Psychiatry. 2004b;61:1146–1152. doi: 10.1001/archpsyc.61.11.1146. [DOI] [PubMed] [Google Scholar]
- Barr CS, Newman TK, Schwandt M, Shannon C, Dvoskin RL, Lindell SG, Taubman J, Thompson B, Champoux M, Lesch KP, et al. Sexual dichotomy of an interaction between early adversity and the serotonin transporter gene promoter variant in rhesus macaques. Proc Natl Acad Sci U S A. 2004c;101:12358–12363. doi: 10.1073/pnas.0403763101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr CS, Schwandt M, Lindell SG, Chen SA, Goldman D, Suomi SJ, Higley JD, Heilig M. Association of a Functional Polymorphism in the μ-Opioid Receptor Gene With Alcohol Response and Consumption in Male Rhesus Macaques. Arch Gen Psychiatry. 2007;64:369–376. doi: 10.1001/archpsyc.64.3.369. [DOI] [PubMed] [Google Scholar]
- Barr CS, Schwandt ML, Newman TK, Higley JD. The use of adolescent nonhuman primates to model human alcohol intake: neurobiological, genetic, and psychological variables. Ann N Y Acad Sci. 2004d;1021:221–233. doi: 10.1196/annals.1308.027. [DOI] [PubMed] [Google Scholar]
- Bennett AJ, DePetrillo PB. Sex differences in total body water in adolescent rhesus macaques estimated by ethanol dilution. Journal of Medical Primatolgy. 2004;33:163–166. doi: 10.1111/j.1600-0684.2004.00065.x. [DOI] [PubMed] [Google Scholar]
- Bennett AJ, Lesch KP, Heils A, Long JC, Lorenz JG, Shoaf SE, Champoux M, Suomi SJ, Linnoila MV, Higley JD. Early experience and serotonin transporter gene variation interaact to influence primate CNS function. Molecular Psychiatry. 2002;7:118–122. doi: 10.1038/sj.mp.4000949. [DOI] [PubMed] [Google Scholar]
- Bernstein IS, Ehardt CL. Age-sex differences in the expression of agonistic behavior in rhesus monkey (Macaca mulatta) groups. J Comp Psychol. 1985;99:115–132. [PubMed] [Google Scholar]
- Bethea CL, Streicher JM, Mirkes SJ, Sanchez RL, Reddy AP, Cameron JL. Serotonin-related gene expression in female monkeys with individual sensitivity to stress. Neuroscience. 2005;132:151–166. doi: 10.1016/j.neuroscience.2004.11.022. [DOI] [PubMed] [Google Scholar]
- Bond C, LaForge KS, Tian M, Melia D, Zhang S, Borg L, Gong J, Schluger J, Strong JA, Leal SM, et al. Single-nucleotide polymorphism in the human mu opioid receptor gene alters beta-endorphin binding and activity: possible implications for opiate addiction. Proc Natl Acad Sci U S A. 1998;95:9608–9613. doi: 10.1073/pnas.95.16.9608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunell SC, Spear LP. Effect of stress on the voluntary intake of a sweetened ethanol solution in pair-housed adolescent and adult rats. Alcoholism: Clinical and Experimental Research. 2005;29:1641–1653. doi: 10.1097/01.alc.0000179382.64752.13. [DOI] [PubMed] [Google Scholar]
- Caspi A, McClay J, Moffitt TE, Mill J, Martin J, Craig IW, Taylor A, Poulton R. Role of genotype in the cycle of violence in maltreated children. Science. 2002;297:851–854. doi: 10.1126/science.1072290. [DOI] [PubMed] [Google Scholar]
- Clark DB, Thatcher DL, Tapert SF. Alcohol, psychological dysregulation, and adolescent brain development. Alcohol Clin Exp Res. 2008;32:375–385. doi: 10.1111/j.1530-0277.2007.00601.x. [DOI] [PubMed] [Google Scholar]
- Clarke AS. Social rearing effects on HPA axis activity over early development and in response to stress in rhesus monkeys. Dev Psychobiol. 1993;26:433–446. doi: 10.1002/dev.420260802. [DOI] [PubMed] [Google Scholar]
- Clarke TK, Treutlein J, Zimmermann US, Kiefer F, Skowronek MH, Rietschel M, Mann K, Schumann G. HPA-axis activity in alcoholism: examples for a gene-environment interaction. Addict Biol. 2008;13:1–14. doi: 10.1111/j.1369-1600.2007.00084.x. [DOI] [PubMed] [Google Scholar]
- Cloninger CR. Neurogenetic adaptive mechanisms in alcoholism. Science. 1987;236:410–416. doi: 10.1126/science.2882604. [DOI] [PubMed] [Google Scholar]
- Coonfield DL, Kiefer SW, Ferraro FM, 3rd, Sinclair JD. Ethanol palatability and consumption by high ethanol-drinking rats: manipulation of the opioid system with naltrexone. Behav Neurosci. 2004;118:1089–1096. doi: 10.1037/0735-7044.118.5.1089. [DOI] [PubMed] [Google Scholar]
- Crews FT, Braun CJ, Hoplight B, Switzer RC, 3rd, Knapp DJ. Binge ethanol consumption causes differential brain damage in young adolescent rats compared with adult rats. Alcohol Clin Exp Res. 2000;24:1712–1723. [PubMed] [Google Scholar]
- Crews FT, Mdzinarishvili A, Kim D, He J, Nixon K. Neurogenesis in adolescent brain is potently inhibited by ethanol. Neuroscience. 2006;137:437–445. doi: 10.1016/j.neuroscience.2005.08.090. [DOI] [PubMed] [Google Scholar]
- Dai X, Thavundayil J, Santella S, Gianoulakis C. Response of the HPA-axis to alcohol and stress as a function of alcohol dependence and family history of alcoholism. Psychoneuroendocrinology. 2007;32:293–305. doi: 10.1016/j.psyneuen.2007.01.004. [DOI] [PubMed] [Google Scholar]
- DeWit DJ, Adlaf EM, Offord DR, Ogborne AC. Age at first alcohol use: a risk factor for the development of alcohol disorders. Am J Psychiatry. 2000;157:745–750. doi: 10.1176/appi.ajp.157.5.745. [DOI] [PubMed] [Google Scholar]
- Doremus TL, Brunell SC, Rajendran P, Spear LP. Factors influencing elevated ethanol consumption in adolescent relative to adult rats. Alcoholism: Clinical and Experimental Research. 2005;29:1796–1808. doi: 10.1097/01.alc.0000183007.65998.aa. [DOI] [PubMed] [Google Scholar]
- Ducci F, Enoch MA, Hodgkinson C, Xu K, Catena M, Robin RW, Goldman D. Interaction between a functional MAOA locus and childhood sexual abuse predicts alcoholism and antisocial personality disorder in adult women. Mol Psychiatry. 2008;13:334–347. doi: 10.1038/sj.mp.4002034. [DOI] [PubMed] [Google Scholar]
- Ely M, Hardy R, Longford NT, Wadsworth MEJ. Gender differences in the relationship between alcohol consumption and drink problems are largely accounted for by body water. Alcohol and Alcoholism. 1999;34:894–902. doi: 10.1093/alcalc/34.6.894. [DOI] [PubMed] [Google Scholar]
- Ervin FR, Palmour RM, Young SN, Guzman-Flores C, Juarez J. Voluntary consumption of beverage alcohol by vervet monkeys: population screening, descriptive behavior and biochemical measures. Pharmacol Biochem Behav. 1990;36:367–373. doi: 10.1016/0091-3057(90)90417-g. [DOI] [PubMed] [Google Scholar]
- Fahlke C, Lorenz JG, Long J, Champoux M, Suomi SJ, Higley JD. Rearing experiences and stress-induced plasma cortisol as early risk factors for excessive alcohol consumption in nonhuman primates. Alcohol Clin Exp Res. 2000;24:644–650. [PubMed] [Google Scholar]
- Fish EW, DeBold JF, Miczek KA. Repeated alcohol: behavioral sensitization and alcohol-heightened aggression in mice. Psychopharmacology (Berl) 2002;160:39–48. doi: 10.1007/s00213-001-0934-9. [DOI] [PubMed] [Google Scholar]
- Flory GS, Chen SA, Woltz LA, Magleby S, Higley JD. A computerized apparatus designed to automatically dispense, measure, and record alcohol consumption by individual members of a rhesus macaque social group: trait-like drinking across social- and single-cage conditions. Methods. 2006;38:178–184. doi: 10.1016/j.ymeth.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Funk CK, Koob GF. A CRF(2) agonist administered into the central nucleus of the amygdala decreases ethanol self-administration in ethanol-dependent rats. Brain Res. 2007;1155:172–178. doi: 10.1016/j.brainres.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk CK, O'Dell LE, Crawford EF, Koob GF. Corticotropin-releasing factor within the central nucleus of the amygdala mediates enhanced ethanol self-administration in withdrawn, ethanol-dependent rats. J Neurosci. 2006;26:11324–11332. doi: 10.1523/JNEUROSCI.3096-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk CK, Zorrilla EP, Lee MJ, Rice KC, Koob GF. Corticotropin-releasing factor 1 antagonists selectively reduce ethanol self-administration in ethanol-dependent rats. Biol Psychiatry. 2007;61:78–86. doi: 10.1016/j.biopsych.2006.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbutt JC, Kranzler HR, O'Malley SS, Gastfriend DR, Pettinati HM, Silverman BL, Loewy JW, Ehrich EW. Efficacy and tolerability of long-acting injectable naltrexone for alcohol dependence: a randomized controlled trial. JAMA. 2005;293:1617–1625. doi: 10.1001/jama.293.13.1617. [DOI] [PubMed] [Google Scholar]
- Gatto GJ, Murphy JM, Waller MB, McBride WJ, Lumeng L, Li TK. Persistence of tolerance to a single dose of ethanol in the selectively-bred alcohol-preferring P rat. Pharmacol Biochem Behav. 1987;28:105–110. doi: 10.1016/0091-3057(87)90020-7. [DOI] [PubMed] [Google Scholar]
- Gianoulakis C. Endogenous opioids and addiction to alcohol and other drugs of abuse. Curr Top Med Chem. 2004;4:39–50. doi: 10.2174/1568026043451573. [DOI] [PubMed] [Google Scholar]
- Goldman D, Oroszi G, Ducci F. The genetics of addictions: uncovering the genes. Nat Rev Genet. 2005;6:521–532. doi: 10.1038/nrg1635. [DOI] [PubMed] [Google Scholar]
- Grant BF. The impact of a family history of alcoholism on the relationship between age at onset of alcohol use and DSM-IV alcohol dependence: results from the National Longitudinal Alcohol Epidemiologic Survey. Alcohol Health Res World. 1998;22:144–147. [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Stinson FS, Harford TC. Age at onset of alcohol use and DSM-IV alcohol abuse and dependence: a 12-year follow-up. J Subst Abuse. 2001;13:493–504. doi: 10.1016/s0899-3289(01)00096-7. [DOI] [PubMed] [Google Scholar]
- Grant KA, Bennett AJ. Advances in nonhuman primate alcohol abuse and alcoholism research. Pharmacol Ther. 2003;100:235–255. doi: 10.1016/j.pharmthera.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Grant KA, Johanson CE. Oral ethanol self-administration in free-feeding rhesus monkeys. Alcohol Clin Exp Res. 1988;12:780–784. doi: 10.1111/j.1530-0277.1988.tb01345.x. [DOI] [PubMed] [Google Scholar]
- Grant KA, Leng X, Green HL, Szeliga KT, Rogers LS, Gonzales SW. Drinking typography established by scheduled induction predicts chronic heavy drinking in a monkey model of ethanol self-administration. Alcohol Clin Exp Res. 2008;32:1824–1838. doi: 10.1111/j.1530-0277.2008.00765.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson AC, Cippitelli A, Sommer WH, Ciccocioppo R, Heilig M. Region-specific down-regulation of Crhr1 gene expression in alcohol-preferring msP rats following ad lib access to alcohol. Addict Biol. 2007;12:30–34. doi: 10.1111/j.1369-1600.2007.00050.x. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Tadokoro S. Learning retardation and enhanced ethanol preference produced by postnatal pretreatments with ethanol in adult rats. Jpn J Pharmacol. 1985;37:269–276. doi: 10.1254/jjp.37.269. [DOI] [PubMed] [Google Scholar]
- Heilig M, Koob GF. A key role for corticotropin-releasing factor in alcohol dependence. Trends Neurosci. 2007;30:399–406. doi: 10.1016/j.tins.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C, Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biological Psychiatry. 2001;49:1023–1039. doi: 10.1016/s0006-3223(01)01157-x. [DOI] [PubMed] [Google Scholar]
- Heinz A, Jones DW, Mazzanti C, Goldman D, Ragan P, Hommer D, Linnoila M, Weinberger DR. A relationship between serotonin transporter genotype and in vivo protein expression and alcohol neurotoxicity. Biol Psychiatry. 2000;47:643–649. doi: 10.1016/s0006-3223(99)00171-7. [DOI] [PubMed] [Google Scholar]
- Heinz A, Mann K, Weinberger DR, Goldman D. Serotonergic dysfunction, negative mood states, and response to alcohol. Alcohol Clin Exp Res. 2001;25:487–495. [PubMed] [Google Scholar]
- Heinz A, Schafer M, Higley JD, Krystal JH, Goldman D. Neurobiological correlates of the disposition and maintenance of alcoholism. Pharmacopsychiatry. 2003;36 3:S255–258. doi: 10.1055/s-2003-45139. [DOI] [PubMed] [Google Scholar]
- Higley JD. Aggression. In: Maestripieri D, editor. Primate Psychology. Cambridge: Harvard University Press; 2003. pp. 17–40. [Google Scholar]
- Higley JD, Hasert MF, Suomi SJ, Linnoila M. Nonhuman primate model of alcohol abuse: effects of early experience, personality, and stress on alcohol consumption. Proc Natl Acad Sci U S A. 1991;88:7261–7265. doi: 10.1073/pnas.88.16.7261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higley JD, Mehlman PT, Poland RE, Taub DM, Vickers J, Suomi SJ, Linnoila M. CSF testosterone and 5-HIAA correlate with different types of aggressive behaviors. Biol Psychiatry. 1996a;40:1067–1082. doi: 10.1016/S0006-3223(95)00675-3. [DOI] [PubMed] [Google Scholar]
- Higley JD, Suomi SJ, Linnoila M. A nonhuman primate model of type II alcoholism? Part 2. Diminished social competence and excessive aggression correlates with low cerebrospinal fluid 5-hydroxyindoleacetic acid concentrations. Alcohol Clin Exp Res. 1996b;20:643–650. doi: 10.1111/j.1530-0277.1996.tb01666.x. [DOI] [PubMed] [Google Scholar]
- Higley JD, Suomi SJ, Linnoila M. A nonhuman primate model of type II excessive alcohol consumption? Part 1. Low cerebrospinal fluid 5-hydroxyindoleacetic acid concentrations and diminished social competence correlate with excessive alcohol consumption. Alcohol Clin Exp Res. 1996c;20:629–642. doi: 10.1111/j.1530-0277.1996.tb01665.x. [DOI] [PubMed] [Google Scholar]
- Hinckers AS, Laucht M, Schmidt MH, Mann KF, Schumann G, Schuckit MA, Heinz A. Low level of response to alcohol as associated with serotonin transporter genotype and high alcohol intake in adolescents. Biol Psychiatry. 2006;60:282–287. doi: 10.1016/j.biopsych.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Ho A, Chin AJ, Dole VP. Early experience and the consumption of alcohol by adult C57BL/6J mice. Alcohol. 1989;6:511–515. doi: 10.1016/0741-8329(89)90060-8. [DOI] [PubMed] [Google Scholar]
- Hollstedt C, Olsson O, Rydberg U. Effects of ethanol on the developing rat. II. Coordination as measured by the tilting-plane test. Med Biol. 1980;58:164–168. [PubMed] [Google Scholar]
- Holmes A, Lit Q, Murphy DL, Gold E, Crawley JN. Abnormal anxiety-related behavior in serotonin transporter null mutant mice: the influence of genetic background. Genes Brain Behav. 2003;2:365–380. doi: 10.1046/j.1601-1848.2003.00050.x. [DOI] [PubMed] [Google Scholar]
- Hope S, Power C, Rodgers B. The relationship between parental separation in childhood and problem drinking in adulthood. Addiction. 1998;93:505–514. doi: 10.1046/j.1360-0443.1998.9345056.x. [DOI] [PubMed] [Google Scholar]
- Howlett TA, Rees LH. Endogenous opioid peptides and hypothalamo-pituitary function. Annu Rev Physiol. 1986;48:527–536. doi: 10.1146/annurev.ph.48.030186.002523. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O'Malley PM, Bachman JG, Schulenberg JE. Monitoring the Future national survey results on drug use, 1975-2007: Volume 1, Secondary school students. Vol. 1. Bethesda, MD: National Institute on Drug Abuse; 2008. NIH Publication No 08-6418A. [Google Scholar]
- Juarez J, Barrios de Tomasi E. Sex differences in alcohol drinking patterns during forced and voluntary consumption in rats. Alcohol. 1999;19:15–22. doi: 10.1016/s0741-8329(99)00010-5. [DOI] [PubMed] [Google Scholar]
- Kalant H. Problems in the search for mechanisms of tolerance. Alcohol Alcohol Suppl. 1993;2:1–8. [PubMed] [Google Scholar]
- Kalueff AV, Fox MA, Gallagher PS, Murphy DL. Hypolocomotion, anxiety and serotonin syndrome-like behavior contribute to the complex phenotype of serotonin transporter knockout mice. Genes Brain Behav. 2007;6:389–400. doi: 10.1111/j.1601-183X.2006.00270.x. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Neale MC, Prescott CA, Kessler RC, Heath AC, Corey LA, Eaves LJ. Childhood parental loss and alcoholism in women: a causal analysis using a twin-family design. Psychol Med. 1996;26:79–95. doi: 10.1017/s0033291700033730. [DOI] [PubMed] [Google Scholar]
- Khanna JM, Chau A, Shah G. Characterization of the Phenomenon of rapid tolerance to ethanol. Alcohol. 1996;13:621–628. doi: 10.1016/s0741-8329(96)00083-3. [DOI] [PubMed] [Google Scholar]
- Kim SG, Kim CM, Choi SW, Jae YM, Lee HG, Son BK, Kim JG, Choi YS, Kim HO, Kim SY, et al. A mu-opioid receptor gene polymorphism (A118G) and naltrexone treatment response in adherent Korean alcohol-dependent patients. Psychopharmacology (Berl) 2009;201:611–618. doi: 10.1007/s00213-008-1330-5. [DOI] [PubMed] [Google Scholar]
- Koob GF. Neurobiological substrates for the dark side of compulsivity in addiction. Neuropharmacology. 2009;56 1:18–31. doi: 10.1016/j.neuropharm.2008.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korte SM, Koolhaas JM, Wingfield JC, McEwen BS. The Darwinian concept of stress: benefits of allostasis and costs of allostatic load and the trade-offs in health and disease. Neurosci Biobehav Rev. 2005;29:3–38. doi: 10.1016/j.neubiorev.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Krishnan-Sarin S, Wand GS, Li XW, Portoghese PS, Froehlich JC. Effect of mu opioid receptor blockade on alcohol intake in rats bred for high alcohol drinking. Pharmacol Biochem Behav. 1998;59:627–635. doi: 10.1016/s0091-3057(97)00474-7. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Kirschbaum C. Sex differences in HPA axis responses to stress: a review. Biological Psychology. 2005;69:113–132. doi: 10.1016/j.biopsycho.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Lagerspetz KY. Postnatal development of the effects of alcohol and of the induced tolerance to alcohol in mice. Acta Pharmacol Toxicol (Copenh) 1972;31:497–508. doi: 10.1111/j.1600-0773.1972.tb03613.x. [DOI] [PubMed] [Google Scholar]
- Lancaster FE, Brown TD, Coker KL, Elliott JA, Wren SB. Sex differences in alcohol preference and drinking patterns emerge during the early postpubertal period. Alcohol Clin Exp Res. 1996;20:1043–1049. doi: 10.1111/j.1530-0277.1996.tb01945.x. [DOI] [PubMed] [Google Scholar]
- Lanfumey L, Mannoury La Cour C, Froger N, Hamon M. 5-HT-HPA interactions in two models of transgenic mice relevant to major depression. Neurochem Res. 2000;25:1199–1206. doi: 10.1023/a:1007683810230. [DOI] [PubMed] [Google Scholar]
- Laszlo FA, Varga C, Pavo I, Gardi J, Vecsernyes M, Galfi M, Morschl E, Laszlo F, Makara GB. Vasopressin pressor receptor-mediated activation of HPA axis by acute ethanol stress in rats. Am J Physiol Regul Integr Comp Physiol. 2001;280:R458–465. doi: 10.1152/ajpregu.2001.280.2.R458. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, Benjamin J, Muller CR, Hamer DH, Murphy DL. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274:1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Meyer J, Glatz K, Flugge G, Hinney A, Hebebrand J, Klauck SM, Poustka A, Poustka F, Bengel D, et al. The 5-HT transporter gene-linked polymorphic region (5-HTTLPR) in evolutionary perspective: alternative biallelic variation in rhesus monkeys. Rapid communication. J Neural Transm. 1997;104:1259–1266. doi: 10.1007/BF01294726. [DOI] [PubMed] [Google Scholar]
- Lessov CN, Phillips TJ. Duration of sensitization to the locomotor stimulant effects of ethanol in mice. Psychopharmacology (Berl) 1998;135:374–382. doi: 10.1007/s002130050525. [DOI] [PubMed] [Google Scholar]
- Lewis DA. Development of the prefrontal cortex during adolescence: insights into vulnerable neural circuits in schizophrenia. Neuropsychopharmacology. 1997;16:385–398. doi: 10.1016/S0893-133X(96)00277-1. [DOI] [PubMed] [Google Scholar]
- Li Q, Wichems C, Heils A, Van De Kar LD, Lesch KP, Murphy DL. Reduction of 5-hydroxytryptamine (5-HT)(1A)-mediated temperature and neuroendocrine responses and 5-HT(1A) binding sites in 5-HT transporter knockout mice. J Pharmacol Exp Ther. 1999;291:999–1007. [PubMed] [Google Scholar]
- Li TK, Lumeng L. Alcohol preference and voluntary alcohol intakes of inbred rat strains and the National Institutes of Health heterogeneous stock of rats. Alcohol Clin Exp Res. 1984;8:485–486. doi: 10.1111/j.1530-0277.1984.tb05708.x. [DOI] [PubMed] [Google Scholar]
- Linnoila M, Virkkunen M, George T, Eckardt M, Higley JD, Nielsen D, Goldman D. Serotonin, violent behavior and alcohol. Exs. 1994;71:155–163. doi: 10.1007/978-3-0348-7330-7_16. [DOI] [PubMed] [Google Scholar]
- Little KY, McLaughlin DP, Zhang L, Livermore CS, Dalack GW, McFinton PR, DelProposto ZS, Hill E, Cassin BJ, Watson SJ, et al. Cocaine, ethanol, and genotype effects on human midbrain serotonin transporter binding sites and mRNA levels. Am J Psychiatry. 1998;155:207–213. doi: 10.1176/ajp.155.2.207. [DOI] [PubMed] [Google Scholar]
- Lorenz JG, Long JC, Linnoila M, Goldman D, Suomi SJ, Higley JD. Genetic and other contributions to alcohol intake in rhesus macaques (Macaca mulatta) Alcoholism: Clinical and Experimental Research. 2006;30:389–398. doi: 10.1111/j.1530-0277.2006.00044.x. [DOI] [PubMed] [Google Scholar]
- Martin P, Kraemer HC. Individual differences in behavior and their statistical consequences. Animal Behaviour. 1987;35:1366–1375. [Google Scholar]
- Mazzanti CM, Lappalainen J, Long JC, Bengel D, Naukkarinen H, Eggert M, Virkkunen M, Linnoila M, Goldman D. Role of the serotonin transporter promoter polymorphism in anxiety-related traits. Archives of General Psychiatry. 1998;55:936–940. doi: 10.1001/archpsyc.55.10.936. [DOI] [PubMed] [Google Scholar]
- McKinzie DL, McBride WJ, Murphy JM, Lumeng L, Li TK. Rat lines selectively bred for alcohol preference: a potential animal model of adolescent alcohol drinking. In: Hannigan JH, Spear LP, Spear NE, Goodlett CR, editors. Alcohol and Alcoholism: Effects on Brain and Development. Mahwah, NJ: Lawrence Erlbaum Associates; 1999. pp. 135–160. [Google Scholar]
- McKinzie DL, Nowak KL, Murphy JM, Li TK, Lumeng L, McBride WJ. Development of alcohol drinking behavior in rat lines selectively bred for divergent alcohol preference. Alcohol Clin Exp Res. 1998;22:1584–1590. [PubMed] [Google Scholar]
- Mehlman PT, Higley JD, Faucher I, Lilly AA, Taub DM, Vickers J, Suomi SJ, Linnoila M. Low CSF 5-HIAA concentrations and severe aggression and impaired impulse control in nonhuman primates. Am J Psychiatry. 1994;151:1485–1491. doi: 10.1176/ajp.151.10.1485. [DOI] [PubMed] [Google Scholar]
- Miller GM, Bendor J, Tiefenbacher S, Yang H, Novak MA, Madras BK. A mu-opioid receptor single nucleotide polymorphism in rhesus monkey: association with stress response and aggression. Mol Psychiatry. 2004;9:99–108. doi: 10.1038/sj.mp.4001378. [DOI] [PubMed] [Google Scholar]
- Morrow AL, Porcu P, Boyd KN, Grant KA. Hypothalamic-pituitary-adrenal axis modulation of GABAergic neuroactive steroids influences ethanol sensitivity and drinking behavior. Dialogues Clin Neurosci. 2006;8:463–477. doi: 10.31887/DCNS.2006.8.4/amorrow. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman TK, Syagailo YV, Barr CS, Wendland JR, Champoux M, Graessle M, Suomi SJ, Higley JD, Lesch KP. Monoamine oxidase A gene promoter variation and rearing experience influences aggressive behavior in rhesus monkeys. Biol Psychiatry. 2005;57:167–172. doi: 10.1016/j.biopsych.2004.10.012. [DOI] [PubMed] [Google Scholar]
- Ogilvie K, Lee S, Weiss B, Rivier C. Mechanisms mediating the influence of alcohol on the hypothalamic-pituitary-adrenal axis responses to immune and nonimmune signals. Alcohol Clin Exp Res. 1998;22:243S–247S. doi: 10.1097/00000374-199805001-00005. [DOI] [PubMed] [Google Scholar]
- Oslin DW, Berrettini W, Kranzler HR, Pettinati H, Gelernter J, Volpicelli JR, O'Brien CP. A functional polymorphism of the mu-opioid receptor gene is associated with naltrexone response in alcohol-dependent patients. Neuropsychopharmacology. 2003;28:1546–1552. doi: 10.1038/sj.npp.1300219. [DOI] [PubMed] [Google Scholar]
- Oswald LM, Wand GS. Opioids and alcoholism. Physiol Behav. 2004;81:339–358. doi: 10.1016/j.physbeh.2004.02.008. [DOI] [PubMed] [Google Scholar]
- Parkes H, Sinclair JD. Reduction of alcohol drinking and upregulation of opioid receptors by oral naltrexone in AA rats. Alcohol. 2000;21:215–221. doi: 10.1016/s0741-8329(00)00091-4. [DOI] [PubMed] [Google Scholar]
- Philpot RM, Badanich KA, Kirstein CL. Place conditioning: age-related changes in the rewarding and aversive effects of alcohol. Alcohol Clin Exp Res. 2003;27:593–599. doi: 10.1097/01.ALC.0000060530.71596.D1. [DOI] [PubMed] [Google Scholar]
- Piano MR, Carrigan TM, Schwertz DW. Sex differences in ethanol liquid diet consumption in Sprague-Dawley rats. Alcohol. 2005;35:113–118. doi: 10.1016/j.alcohol.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Prescott CA, Kendler KS. Age at first drink and risk for alcoholism: a noncausal association. Alcohol Clin Exp Res. 1999;23:101–107. [PubMed] [Google Scholar]
- Ray LA, Hutchison KE. A polymorphism of the mu-opioid receptor gene (OPRM1) and sensitivity to the effects of alcohol in humans. Alcohol Clin Exp Res. 2004;28:1789–1795. doi: 10.1097/01.alc.0000148114.34000.b9. [DOI] [PubMed] [Google Scholar]
- Rhodes ME, Rubin RT. Functional sex differences (‘sexual diergism’) of central nervous system cholinergic systems, vasopressin, and hypothalamic-pituitary-adrenal axis activity in mammals: a selective review. Brain Res Brain Res Rev. 1999;30:135–152. doi: 10.1016/s0165-0173(99)00011-9. [DOI] [PubMed] [Google Scholar]
- Roberts AJ, McDonald JS, Heyser CJ, Kieffer BL, Matthes HW, Koob GF, Gold LH. mu-Opioid receptor knockout mice do not self-administer alcohol. J Pharmacol Exp Ther. 2000;293:1002–1008. [PubMed] [Google Scholar]
- Rodd-Henricks ZA, Bell RL, Kuc KA, Murphy JM, McBride WJ, Lumeng L, Li TK. Effects of ethanol exposure on subsequent acquisition and extinction of ethanol self-administration and expression of alcohol-seeking behavior in adult alcohol-preferring (P) rats: I. Periadolescent exposure. Alcohol Clin Exp Res. 2002;26:1632–1641. doi: 10.1097/01.ALC.0000036301.36192.BC. [DOI] [PubMed] [Google Scholar]
- Rutter M. Gene-environment interdependence. Dev Sci. 2007;10:12–18. doi: 10.1111/j.1467-7687.2007.00557.x. [DOI] [PubMed] [Google Scholar]
- Rutter M, Moffitt TE, Caspi A. Gene-environment interplay and psychopathology: multiple varieties but real effects. J Child Psychol Psychiatry. 2006;47:226–261. doi: 10.1111/j.1469-7610.2005.01557.x. [DOI] [PubMed] [Google Scholar]
- Sabol SZ, Hu S, Hamer D. A functional polymorphism in the monoamine oxidase A gene promoter. Hum Genet. 1998;103:273–279. doi: 10.1007/s004390050816. [DOI] [PubMed] [Google Scholar]
- Samochowiec J, Lesch KP, Rottmann M, Smolka M, Syagailo YV, Okladnova O, Rommelspacher H, Winterer G, Schmidt LG, Sander T. Association of a regulatory polymorphism in the promoter region of the monoamine oxidase A gene with antisocial alcoholism. Psychiatry Res. 1999;86:67–72. doi: 10.1016/s0165-1781(99)00020-7. [DOI] [PubMed] [Google Scholar]
- Sanchez MM, Ladd CO, Plotsky PM. Early adverse experience as a developmental risk factor for later psychopathology. Development and Psychopathology. 2001;13:419–449. doi: 10.1017/s0954579401003029. [DOI] [PubMed] [Google Scholar]
- Sanchez MM, Young LJ, Plotsky PM, Insel TR. Autoradiographic and in situ hybridization localization of corticotropin-releasing factor 1 and 2 receptors in nonhuman primate brain. J Comp Neurol. 1999;408:365–377. [PubMed] [Google Scholar]
- Schaus JF, Sole ML, McCoy TP, Mullett N, Bolden J, Sivasithamparam J, O'Brien MC. Screening for high-risk drinking in a college student health center: characterizing students based on quantity, frequency, and harms. J Stud Alcohol Drugs Suppl. 2009:34–44. doi: 10.15288/jsads.2009.s16.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuckit MA, Gold E, Risch C. Plasma cortisol levels following ethanol in sons of alcoholics and controls. Arch Gen Psychiatry. 1987;44:942–945. doi: 10.1001/archpsyc.1987.01800230022005. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Mazzanti C, Smith TL, Ahmed U, Radel M, Iwata N, Goldman D. Selective genotyping for the role of 5-HT2A, 5-HT2C, and GABA alpha 6 receptors and the serotonin transporter in the level of response to alcohol: a pilot study. Biol Psychiatry. 1999;45:647–651. doi: 10.1016/s0006-3223(98)00248-0. [DOI] [PubMed] [Google Scholar]
- Schwandt ML, Barr CS, Suomi SJ, Higley JD. Age-dependent variation in behavior following acute ethanol administration in male and female adolescent rhesus macaques (Macaca mulatta) Alcohol Clin Exp Res. 2007;31:228–237. doi: 10.1111/j.1530-0277.2006.00300.x. [DOI] [PubMed] [Google Scholar]
- Schwandt ML, Higley JD, Suomi SJ, Heilig M, Barr CS. Rapid tolerance and locomotor sensitization in ethanol-naïve adolescent rhesus macaques. Alcoholism: Clinical and Experimental Research. 2008;32:1217–1228. doi: 10.1111/j.1530-0277.2008.00676.x. [DOI] [PubMed] [Google Scholar]
- Serretti A, Calati R, Mandelli L, De Ronchi D. Serotonin transporter gene variants and behavior: a comprehensive review. Current Drug Targets. 2006;7:1659–1669. doi: 10.2174/138945006779025419. [DOI] [PubMed] [Google Scholar]
- Shannon C, Champoux M, Suomi SJ. Rearing condition and plasma cortisol in rhesus monkey infants. Am J Primatol. 1998;46:311–321. doi: 10.1002/(SICI)1098-2345(1998)46:4<311::AID-AJP3>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Shannon C, Schwandt ML, Champoux M, Shoaf SE, Suomi SJ, Linnoila M, Higley JD. Maternal absence and stability of individual differences in CSF 5-HIAA concentrations in rhesus monkey infants. American Journal of Psychiatry. 2005;162:1658–1664. doi: 10.1176/appi.ajp.162.9.1658. [DOI] [PubMed] [Google Scholar]
- Sillaber I, Rammes G, Zimmermann S, Mahal B, Zieglgansberger W, Wurst W, Holsboer F, Spanagel R. Enhanced and delayed stress-induced alcohol drinking in mice lacking functional CRH1 receptors. Science. 2002;296:931–933. doi: 10.1126/science.1069836. [DOI] [PubMed] [Google Scholar]
- Silveri MM, Spear LP. Decreased sensitivity to the hypnotic effects of ethanol early in ontogeny. Alcohol Clin Exp Res. 1998;22:670–676. doi: 10.1111/j.1530-0277.1998.tb04310.x. [DOI] [PubMed] [Google Scholar]
- Silveri MM, Spear LP. Ontogeny of rapid tolerance to the hypnotic effects of ethanol. Alcohol Clin Exp Res. 1999;23:1180–1184. [PubMed] [Google Scholar]
- Silveri MM, Spear LP. Ontogeny of ethanol elimination and ethanol-induced hypothermia. Alcohol. 2000;20:45–53. doi: 10.1016/s0741-8329(99)00055-5. [DOI] [PubMed] [Google Scholar]
- Sommer WH, Rimondini R, Hansson AC, Hipskind PA, Gehlert DR, Barr CS, Heilig MA. Upregulation of voluntary alcohol intake, behavioral sensitivity to stress, and amygdala crhr1 expression following a history of dependence. Biol Psychiatry. 2008;63:139–145. doi: 10.1016/j.biopsych.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Sorocco KH, Lovallo WR, Vincent AS, Collins FL. Blunted hypothalamic-pituitary-adrenocortical axis responsivity to stress in persons with a family history of alcoholism. Int J Psychophysiol. 2006;59:210–217. doi: 10.1016/j.ijpsycho.2005.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear L. Modeling adolescent development and alcohol use in animals. Alcohol Res Health. 2000a;24:115–123. [PMC free article] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000b;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Spear LP. Alcohol's effects on adolescents. Alcohol Res Health. 2002;26:287–291. [PMC free article] [PubMed] [Google Scholar]
- Stoltenberg SF, Twitchell GR, Hanna GL, Cook EH, Fitzgerald HE, Zucker RA, Little KY. Serotonin transporter promoter polymorphism, peripheral indexes of serotonin function, and personality measures in families with alcoholism. Am J Med Genet. 2002;114:230–234. doi: 10.1002/ajmg.10187. [DOI] [PubMed] [Google Scholar]
- Stromberg MF, Rukstalis MR, Mackler SA, Volpicelli JR, O'Brien CP. A comparison of the effects of 6-beta naltrexol and naltrexone on the consumption of ethanol or sucrose using a limited-access procedure in rats. Pharmacol Biochem Behav. 2002;72:483–490. doi: 10.1016/s0091-3057(02)00721-9. [DOI] [PubMed] [Google Scholar]
- Stromberg MF, Volpicelli JR, O'Brien CP. Effects of naltrexone administered repeatedly across 30 or 60 days on ethanol consumption using a limited access procedure in the rat. Alcohol Clin Exp Res. 1998;22:2186–2191. [PubMed] [Google Scholar]
- Suomi SJ. Early determinants of behaviour: evidence from primate studies. British Medical Bulletin. 1997;53:170–184. doi: 10.1093/oxfordjournals.bmb.a011598. [DOI] [PubMed] [Google Scholar]
- Tabakoff B, Hoffman PL. Animal models in alcohol research. Alcohol Res Health. 2000;24:77–84. [PMC free article] [PubMed] [Google Scholar]
- Thomasson HR. Gender differences in alcohol metabolism. Physiological responses to ethanol. Recent Dev Alcohol. 1995;12:163–179. doi: 10.1007/0-306-47138-8_9. [DOI] [PubMed] [Google Scholar]
- Tolliver GA, Samson HH. The influence of early postweaning ethanol exposure on oral self-administration behavior in the rat. Pharmacol Biochem Behav. 1991;38:575–580. doi: 10.1016/0091-3057(91)90016-u. [DOI] [PubMed] [Google Scholar]
- Turker T, Sodmann R, Goebel U, Jatzke S, Knapp M, Lesch KP, Schuster R, Schutz H, Weiler G, Stober G. High ethanol tolerance in young adults is associated with the low-activity variant of the promoter of the human serotonin transporter gene. Neurosci Lett. 1998;248:147–150. doi: 10.1016/s0304-3940(98)00347-4. [DOI] [PubMed] [Google Scholar]
- van den Wildenberg E, Wiers RW, Dessers J, Janssen RG, Lambrichs EH, Smeets HJ, van Breukelen GJ. A functional polymorphism of the mu-opioid receptor gene (OPRM1) influences cue-induced craving for alcohol in male heavy drinkers. Alcohol Clin Exp Res. 2007;31:1–10. doi: 10.1111/j.1530-0277.2006.00258.x. [DOI] [PubMed] [Google Scholar]
- Vetter CS, Doremus-Fitzwater TL, Spear LP. Time course of elevated ethanol intake in adolescent relative to adult rats under continuous, voluntary-access conditions. Alcohol Clin Exp Res. 2007;31:1159–1168. doi: 10.1111/j.1530-0277.2007.00417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virkkunen M, Linnoila M. Serotonin in early-onset alcoholism. Recent Dev Alcohol. 1997;13:173–189. doi: 10.1007/0-306-47141-8_10. [DOI] [PubMed] [Google Scholar]
- Vivian JA, Green HL, Young JE, Majersky LS, Thomas BW, Shively CA, Tobin JR, Nader MA, Grant KA. Induction and maintenenace of ethanol self-administration in cynomolgus monkeys (Macaca fascicularis): long-term characterization of sex and individual differences. Alcoholism: Clinical and Experimental Research. 2001;25:1087–1097. [PubMed] [Google Scholar]
- Watts ES, Gavan JA. Postnatal growth of nonhuman primates: the problem of the adolescent spurt. Hum Biol. 1982;54:53–70. [PubMed] [Google Scholar]
- Wendland JR, Lesch KP, Newman TK, Timme A, Gachot-Neveu H, Thierry B, Suomi SJ. Differential functional variability of serotonin transporter and monoamine oxidase A genes in macaque species displaying contrasting levels of aggression-related behavior. Behavior Genetics. 2006;36:163–172. doi: 10.1007/s10519-005-9017-8. [DOI] [PubMed] [Google Scholar]
- White AM, Truesdale MC, Bae JG, Ahmad S, Wilson WA, Best PJ, Swartzwelder HS. Differential effects of ethanol on motor coordination in adolescent and adult rats. Pharmacol Biochem Behav. 2002;73:673–677. doi: 10.1016/s0091-3057(02)00860-2. [DOI] [PubMed] [Google Scholar]
- White HR, Brick J, Hansell S. A longitudinal investigation of alcohol use and aggression in adolescence. J Stud Alcohol Suppl. 1993;11:62–77. doi: 10.15288/jsas.1993.s11.62. [DOI] [PubMed] [Google Scholar]
- Wills TA, Walker C, Mendoza D, Ainette MG. Behavioral and emotional self-control: relations to substance use in samples of middle and high school students. Psychol Addict Behav. 2006;20:265–278. doi: 10.1037/0893-164X.20.3.265. [DOI] [PubMed] [Google Scholar]
- Wilsnack RW, Vogeltanz ND, Wilsnack SC, Harris TR, Ahlstrom S, Bondy S, Csemy L, Ferrence R, Ferris J, Fleming J, et al. Gender differences in alcohol consumption and adverse drinking consequences: cross-cultural patterns. Addiction. 2000;95:251–265. doi: 10.1046/j.1360-0443.2000.95225112.x. [DOI] [PubMed] [Google Scholar]
- Witt ED. Mechanisms of alcohol abuse and alcoholism in adolescents: a case for developing animal models. Behav Neural Biol. 1994;62:168–177. doi: 10.1016/s0163-1047(05)80015-9. [DOI] [PubMed] [Google Scholar]
- Young SE, Friedman NP, Miyake A, Willcutt EG, Corley RP, Haberstick BC, Hewitt JK. Behavioral disinhibition: liability for externalizing spectrum disorders and its genetic and environmental relation to response inhibition across adolescence. J Abnorm Psychol. 2009;118:117–130. doi: 10.1037/a0014657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann U, Spring K, Kunz-Ebrecht SR, Uhr M, Wittchen HU, Holsboer F. Effect of ethanol on hypothalamic-pituitary-adrenal system response to psychosocial stress in sons of alcohol-dependent fathers. Neuropsychopharmacology. 2004;29:1156–1165. doi: 10.1038/sj.npp.1300395. [DOI] [PubMed] [Google Scholar]