Abstract
Background
Quinolone-mediated death of Escherichia coli has been proposed to occur by two pathways. One is blocked by inhibitors of protein synthesis; the other is not. It is currently unknown how these two pathways fit with the recent observation that hydroxyl radical accumulation is associated with quinolone lethality.
Methods
E. coli was treated with thiourea plus 2,2′-bipyridyl to block hydroxyl radical accumulation, and the effect on quinolone lethality was measured for quinolones that distinguished the two lethal pathways: oxolinic acid requires protein synthesis to kill E. coli, while PD161144, a C-8-methoxy fluoroquinolone, does not. The lethal activity of another fluoroquinolone, moxifloxacin, was partially blocked by the presence of chloramphenicol, an inhibitor of protein synthesis. That feature made it possible to determine whether the effects of chloramphenicol and thiourea plus 2,2′-bipyridyl were additive.
Results
Lethal activity of oxolinic acid was completely blocked by thiourea plus 2,2′-bipyridyl and by chloramphenicol. In contrast, PD161144 lethality was unaffected by these treatments. With moxifloxacin, both chloramphenicol and thiourea plus 2,2′-bipyridyl separately exhibited the same partial inhibition of quinolone lethality. No additivity in protection from moxifloxacin lethality was observed when thiourea, 2,2′-bipyridyl and chloramphenicol were combined and compared with the effect of chloramphenicol or thiourea plus 2,2′-bipyridyl used separately.
Conclusions
Inhibitor studies indicated that hydroxyl radical action contributes to quinolone-mediated cell death occurring via the chloramphenicol-sensitive lethal pathway but not via the chloramphenicol-insensitive pathway.
Keywords: oxolinic acid; 8-methoxy fluoroquinolone; hydoxyl radical; thiourea; 2,2′-bipyridyl
Introduction
The quinolones act by trapping DNA gyrase and DNA topoisomerase IV on DNA in complexes that contain broken DNA. Release of the DNA ends from protein constraint fragments bacterial chromosomes under conditions that correlate with cell death.1,2 Two pathways of chromosome fragmentation occur. One is blocked by co-treatment with chloramphenicol (a protein synthesis inhibitor) or by treatment with an anaerobic gas mixture.3 First-generation quinolones (nalidixic and oxolinic acids) act mainly via this pathway.3,4 Third- and fourth-generation compounds, such as ciprofloxacin and the C-8-methoxy derivative PD161144, kill bacteria anaerobically and in the absence of protein synthesis.3 The latter observations define a second, chloramphenicol-insensitive lethal pathway.1 Recent work suggests that reactive oxygen species (ROS) participate in quinolone-mediated cell death. For example, treatment of Escherichia coli with norfloxacin is accompanied by increases in hydroxyl radical concentration.5,6 Moreover, treatment of cells with thiourea or 2,2′-bipyridyl, which interfere with the accumulation of hydroxyl radicals, reduces norfloxacin lethality.5,7 Since norfloxacin behaviour is complex,3 it is not known which pathways of quinolone-mediated cell death involve ROS.
The present work examined the effect of inhibitors of hydroxyl radical accumulation on quinolone-mediated death. Oxolinic acid was chosen to examine the protein synthesis-dependent pathway because the lethality of first-generation quinolones is blocked by chloramphenicol and anaerobiosis.1,3 The C-8-methoxy fluoroquinolone PD161144 was chosen to investigate the other pathway because it exhibits the least sensitivity to chloramphenicol and anaerobiosis.3 Quinolones used clinically, such as norfloxacin and ciprofloxacin, do not discriminate between the two pathways as clearly.3 As a further test for the effects of hydroxyl radicals and chloramphenicol being in the same pathway, we examined moxifloxacin, a quinolone whose lethal activity was only partially blocked by chloramphenicol. The absence of additive effects of chloramphenicol and thiourea plus 2,2′-bipyridyl would support the inhibitors affecting the same pathway.
Materials and methods
Strains, drugs and culture conditions
E. coli strain SD1048 was grown at 37°C using Luria–Bertani (LB) broth or agar. Chloramphenicol, thiourea, 2,2′-bipyridyl and oxolinic acid were obtained from Sigma Chemical Co. (St Louis, MO, USA). PD161144 was from Dr John Domagala, Pfizer Pharmaceutical Co. (Ann Arbor, MI, USA) and moxifloxacin was obtained from Bayer AG (Wuppertal, Germany).
Susceptibility measurements
Growth inhibition (MIC) was determined by broth dilution using ∼105 cfu per tube. To measure lethal action, cells were grown aerobically with shaking to mid-log phase, treated for various times and at various concentrations with quinolone, and surviving cells were estimated by colony formation on drug-free agar. The percentage cfu recovered was determined relative to an untreated control sampled at the time of quinolone addition.
The effect of hydroxyl radicals on quinolone-mediated lethality was assessed by treating cells with a subinhibitory combination of 100 mM thiourea plus 0.25 mM 2,2′-bipyridyl (both at 0.5× the MIC) followed by quinolone treatment for various times and at various concentrations. Thiourea plus 2,2′-bipyridyl doubled the MIC for the three quinolones tested.
Results
Effect of thiourea plus 2,2′-bipyridyl on oxolinic acid lethality
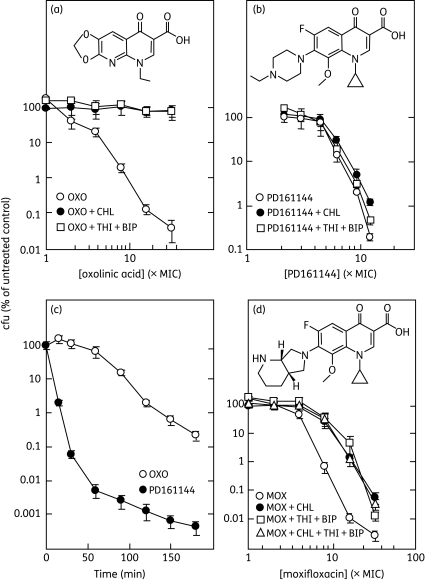
Treatment of wild-type E. coli with thiourea plus 2,2′-bipyridyl at subinhibitory concentrations (0.5× the MIC) completely blocked the lethal activity of oxolinic acid when the quinolone was at various concentrations for 2 h (Figure 1a) or at 10× the MIC for various incubation times (not shown). As expected, pre-treatment with chloramphenicol, an inhibitor of protein synthesis, also blocked the lethal activity of oxolinic acid (Figure 1a). Similar results were obtained when nalidixic acid was used rather than oxolinic acid with strain SD104 and with another E. coli strain (BW25113; not shown). Thus, hydroxyl radicals are important for the lethal action of quinolones that kill E. coli primarily through the protein synthesis-dependent pathway.
Figure 1.
Effect of thiourea plus 2,2′-bipyridyl and chloramphenicol on quinolone lethality. Exponentially growing E. coli cultures were treated with either chloramphenicol or thiourea plus 2,2′-bipyridyl for 10 min, and then quinolone was added. After incubation, the percentage survival was determined as described in the text. (a) Oxolinic acid (OXO). Oxolinic acid alone (MIC = 0.25 mg/L), with 20 mg/L chloramphenicol (CHL; MIC = 2 mg/L) or with 100 mM thiourea (THI; MIC = 200 mM) plus 0.25 mM 2,2′-bipyridyl (BIP; MIC = 0.5 mM) was added at the indicated concentrations to strain SD104 (KD447) followed by incubation for 120 min. (b) PD161144. As in (a) except that PD161144 (MIC = 0.08 mg/L) replaced oxolinic acid and the incubation time was 45 min. (c) Rate of quinolone-mediated killing. Strain SD104 was treated with oxolinic acid or PD161144 at 10× the MIC for the indicated times, after which percentage survival was determined. (d) Moxifloxacin (MOX). Strain SD104 was treated with moxifloxacin (MIC = 0.06 mg/L) for 45 min, with or without pre-treatment as in (a). In addition, cells were pre-treated with chloramphenicol and thiourea plus 2,2′-bipyridyl for 10 min. Error bars represent standard deviations from the mean; similar results were obtained in replicate experiments.
Effect of thiourea plus 2,2′-bipyridyl on lethality of PD161144
PD161144 is an investigational, ciprofloxacin-like quinolone (for its structure see Figure 1b). With strain SD104, no effect of thiourea plus 2,2′-bipyridyl was observed on the lethal activity of PD161144 (Figure 1b). As expected, chloramphenicol also had no effect on PD161144 lethality (Figure 1b). With another strain of E. coli, the lethal activity of PD161144 is also unaffected by the presence of chloramphenicol or anaerobic conditions, two treatments that block killing by first-generation compounds such as nalidixic and oxolinic acids.3 PD161144 also killed E. coli more rapidly than oxolinic acid (Figure 1c). Collectively, these data support the conclusion that quinolones kill E. coli via two distinct pathways, with the relative use of a particular pathway depending on quinolone structure.4 Moreover, they suggest that the chloramphenicol-insensitive lethal pathway does not require hydroxyl radical accumulation.
Effect of chloramphenicol, thiourea and 2,2′-bipyridyl on moxifloxacin lethality
To further test the idea that hydroxyl radicals contribute to only one of the lethal pathways, we examined the behaviour of moxifloxacin. This potent fourth-generation quinolone (for its structure see Figure 1d) killed E. coli with about the same drug concentration dependence seen for PD161144 after data were normalized to the MIC to correct for differences in intracellular drug concentration (Figure 1). However, separate treatment with either chloramphenicol or thiourea plus 2,2′-bipyridyl partially inhibited the ability of moxifloxacin to kill E. coli (Figure 1d). Partial inhibition of moxifloxacin lethality then provided a way to determine whether inhibitor effects were additive. When E. coli was treated with the three inhibitors and then moxifloxacin, survival was the same as when only chloramphenicol or only thiourea plus 2,2′-bypyridyl was used to block moxifloxacin lethality (Figure 1d). Thus, no additivity was evident, which supports the assertion that the chloramphenicol-sensitive and hydroxyl radical-mediated steps are in the same pathway.
Effect of quinolone treatment on hydroxyl radical accumulation
Since inhibitor results indicated that hydroxyl radical accumulation affects only the chloramphenicol-sensitive pathway, we next assayed9 hydroxyl radicals to determine whether oxolinic acid and PD161144 both cause their accumulation. After 45 min of oxolinic acid treatment, hydroxyl radical-mediated fluorescence was 18 times background; co-treatment with thiourea plus 2,2′-bipyridyl reduced the fluorescence by 98%. A similar response was seen with PD161144 and moxifloxacin: the hydroxyl radical signal increased 20- and 30-fold, respectively, after 30 min of quinolone treatment; co-treatment with thiourea plus 2,2′-bipyridyl reduced it by 90%–96%. Thus, quinolones cause accumulation of hydroxyl radicals, and thiourea plus 2,2′-bipyridyl blocks accumulation. Chloramphenicol reduced the hydroxyl radical signal by 98% for oxolinic acid, while the reduction was only 30% with PD161144. Thus, oxolinic acid and PD161144 differ with respect to the chloramphenicol sensitivity of hydroxyl radical accumulation and quinolone-mediated lethality. As expected, a quinolone-resistant gyrA mutant (strain KD3258, S83L D87Y) showed no hydroxyl radical accumulation when treated for 1 h with oxolinic acid at 4 mg/L, conditions that caused a 50-fold increase in the wild-type strain BW25113.
Discussion
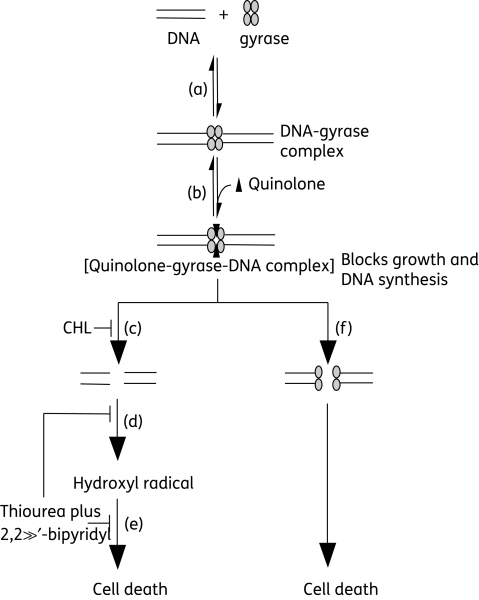
The data described above fit into a scheme containing two lethal pathways (Figure 2). Prior to divergence of the two pathways, all quinolones are postulated to form reversible quinolone–gyrase–DNA complexes. These complexes block growth, as measured by the MIC. Since thiourea plus 2,2′-bipyridyl has no more than a 2-fold effect on the quinolone MIC, hydroxyl radicals probably act largely after cleaved complex formation. Lethal effects of quinolones, which are seen when the drug concentration is raised above bacteriostatic levels, correlate with chromosome fragmentation.1,2 As discussed previously,6,7,10 that fragmentation appears to lead to production of superoxide, which dismutates to peroxide. Peroxide then generates highly toxic hydroxyl radicals through the Fenton reaction. The present work indicated that hydroxyl radicals account for most of the oxolinic acid-mediated lethality, since a combination of thiourea plus 2,2′-bipyridyl, at subinhibitory concentrations, blocked death of wild-type E. coli. Such a result is consistent with anaerobic growth blocking the lethal activity of first-generation quinolones.3 Further support for the chloramphenicol-sensitive event and hydroxyl radical accumulation being in the same pathway was obtained by examination of moxifloxacin: its lethality, which was partially blocked by chloramphenicol, was the same in the presence of chloramphenicol or thiourea plus 2,2′-bipyridyl as in the presence of all three inhibitors (thiourea, 2,2′-bipyridyl and chloramphenicol; Figure 1d). The lack of additivity was also observed with oxolinic acid treatment of a gyrB-225 mutant11 in which the protective effect of chloramphenicol is partially relieved (not shown).
Figure 2.
Schematic representation of quinolone-mediated lethality. (a) Complex formed by gyrase interacting with DNA. (b) Quinolones trapping gyrase on DNA; DNA is broken with its ends constrained, which causes inhibition of DNA replication and bacterial growth. (c) First-generation quinolones (nalidixic and oxolinic acids) cause chromosome fragmentation that is blocked by chloramphenicol (CHL), a protein synthesis inhibitor. (d) Chromosome fragmentation stimulates a surge of ROS with hydroxyl radicals as the terminal product. (e) Formation of highly toxic hydroxyl radicals, the end product of an ROS cascade, is blocked by 2,2′-bipyridyl, an iron chelator, and thiourea, a hydroxyl radical scavenger, which prevent cell death. (f) Fluoroquinolones also kill bacterial cells by a protein synthesis-insensitive, ROS-independent chromosome fragmentation pathway that is postulated to occur by gyrase subunit dissociation.
In Figure 2 we show chloramphenicol-sensitive chromosome fragmentation being upstream from hydroxyl radical effects, because preliminary experiments show that oxolinic acid-mediated DNA fragmentation, measured by a cell lysate viscosity assay, is inhibited by chloramphenicol but not by thiourea plus 2,2′-bipyridyl (X. Wang, unpublished results). Chloramphenicol also inhibited the accumulation of hydroxyl radicals, an observation that is consistent with oxolinic acid-mediated, chloramphenicol-sensitive chromosome fragmentation being upstream from hydroxyl radical formation.
The chloramphenicol-insensitive lethal pathway associated with PD161144 appeared to be independent of hydroxyl radical effects even though this fluoroquinolone stimulated the accumulation of hydroxyl radicals. Apparently the lethal damage caused by PD161144, which occurs more rapidly than that caused by oxolinic acid (Figure 1c), is fast enough to take precedence over the lethal effects of hydroxyl radicals. PD161144-mediated damage is characterized by chromosome fragmentation that is not blocked by chloramphenicol and by sensitivity to the lexA3 mutation,2 which prevents induction of the SOS response. We have speculated that this chromosome fragmentation arises from quinolone-mediated dissociation of gyrase subunits, since fluoroquinolones can fragment purified chromosomes when gyrase is present.2
Fluoroquinolones used clinically, such as norfloxacin, ciprofloxacin and moxifloxacin, appear to act by both pathways, since thiourea plus 2,2′-bipyridyl partially blocks the lethal action. Therefore, small molecules that enhance the induction of ROS may prove useful for improving quinolone lethality.
Funding
This work was supported by the National Institutes of Health (RO1 AI073491 and R21 AI 068014 to K. D. and X. Z., respectively).
Transparency declarations
None to declare.
Acknowledgements
We thank Marila Gennaro, Shajo Kunnath and Richard Pine for critical comments on the manuscript prior to submission.
References
- 1.Chen C-R, Malik M, Snyder M, et al. DNA gyrase and topoisomerase IV on the bacterial chromosome: quinolone-induced DNA cleavage. J Mol Biol. 1996;258:627–37. doi: 10.1006/jmbi.1996.0274. [DOI] [PubMed] [Google Scholar]
- 2.Malik M, Zhao X, Drlica K. Lethal fragmentation of bacterial chromosomes mediated by DNA gyrase and quinolones. Mol Microbiol. 2006;61:810–25. doi: 10.1111/j.1365-2958.2006.05275.x. [DOI] [PubMed] [Google Scholar]
- 3.Malik M, Hussain S, Drlica K. Effect of anaerobic growth on quinolone lethality with Escherichia coli. Antimicrob Agents Chemother. 2007;51:28–34. doi: 10.1128/AAC.00739-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Drlica K, Malik M, Kerns R J, et al. Quinolone-mediated bacterial death. Antimicrob Agents Chemother. 2008;52:385–92. doi: 10.1128/AAC.01617-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dwyer D, Kohanski M, Hayete B, et al. Gyrase inhibitors induce an oxidative damage cellular death pathway in Escherichia coli. Mol Syst Biol. 2007;3:91. doi: 10.1038/msb4100135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kohanski M, Dwyer D, Hayete B, et al. A common mechanism of cellular death induced by bactericidal antibiotics. Cell. 2007;130:797–810. doi: 10.1016/j.cell.2007.06.049. [DOI] [PubMed] [Google Scholar]
- 7.Wang X, Zhao X. Contribution of oxidative damage to antimicrobial lethality. Antimicrob Agents Chemother. 2009;53:1395–402. doi: 10.1128/AAC.01087-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DiNardo S, Voelkel K, Sternglanz R, et al. Escherichia coli DNA topoisomerase I mutants have compensatory mutations in DNA gyrase genes. Cell. 1982;31:43–51. doi: 10.1016/0092-8674(82)90403-2. [DOI] [PubMed] [Google Scholar]
- 9.Setsukinai K-I, Urano Y, Kakinuma K, et al. Development of novel fluorescence probes that can reliably detect reactive oxygen species and distinguish specific species. J Biol Chem. 2003;278:3170–5. doi: 10.1074/jbc.M209264200. [DOI] [PubMed] [Google Scholar]
- 10.Kohanski M, Dwyer D, Wierzbowski J, et al. Mistranslation of membrane proteins and two-component system activation trigger antibiotic-mediated cell death. Cell. 2008;135:679–90. doi: 10.1016/j.cell.2008.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heddle J, Lu T, Zhao X, et al. gyrB-225, a mutation of DNA gyrase that compensates for topoisomerase I deficiency: investigation of its low activity and quinolone hypersensitivity. J Mol Biol. 2001;309:1219–31. doi: 10.1006/jmbi.2001.4733. [DOI] [PubMed] [Google Scholar]