Abstract
Objectives
Attachment to the small intestinal mucosa is crucial for initiating and maintaining Giardia infection. We tested the effect of isoflavones on Giardia attachment.
Methods
We evaluated the effect of formononetin on trophozoite attachment to glass, to intestinal epithelial cell layers in vitro and to murine small intestinal explants, and on the intestinal load in mice.
Results
We found that the isoflavone formononetin inhibits both attachment and flagellar motility within minutes and reduces the trophozoite load of Giardia in mice within 1.5 h after treatment.
Conclusions
The antigiardial activity of formononetin is at least partially due to its capacity to rapidly detach trophozoites.
Keywords: attachment, flagella, antigiardial drugs
Introduction
Giardia lamblia is a major cause of diarrhoea in humans. Isoflavones are polyphenols found in soy and red clover plants that reduce growth and viability of Giardia and clear murine infections.1,2 The isoflavone with the most potent antigiardial activity is formononetin, followed by daidzein, biochanin and genistein.1 However, the antigiardial activity of isoflavones has only been evaluated after long-term (1–7 days) treatment and the underlying mechanism is not clear. Sterk et al.2 showed that nucleoside hydrolase is inactivated by isoflavones and that its expression was reduced in formononetin-resistant cells. However, the formononetin concentrations needed to inhibit nucleoside hydrolase activity were substantially higher than growth-inhibitory concentrations, suggesting distinct mechanisms.
To elucidate the mechanism of isoflavone antigiardial activity, we examined the early effects of formononetin on Giardia trophozoites. Attachment of trophozoites to the small intestinal mucosa is a crucial step in establishing and maintaining colonization. Giardia clones deficient in their attachment ability have a reduced capacity to establish infection.3 Trophozoites attach to substrates by their ventral disc and have four distinct pairs of flagella.4 In this study, we examined the effects of the isoflavones formononetin, daidzein and genistein on Giardia trophozoite attachment in vitro, ex vivo and in mice in vivo.
Materials and methods
Cell culture
Giardia trophozoites strain WB (clone C6, ATCC 50803) and strain GS/M-83-H7 (ATCC 50581) were grown to late log phase (∼80% confluence) in modified TYI-S-33 medium with bovine bile.5 The human colon adenocarcinoma cell line Caco-2 (ATCC HTB 37) was grown in Dulbecco's modified Eagle medium supplemented with 10% heat-inactivated fetal calf serum, 2 mM l-glutamine, 1.0 mM sodium pyruvate, 100 U/mL penicillin and 100 µg/mL streptomycin in 95% air/5% CO2 at 37°C.
Detachment assay
WB trophozoites were seeded at 1 × 106 cells/mL on 12-well plastic plates or on confluent Caco-2 cell layers and allowed to attach for at least 30 min under low oxygen conditions. Subsequently, culture supernatants were replaced by medium containing 1–40 µM genistein (Sigma), daidzein (Sigma) or formononetin (Acros Organics), or 1:1000 DMSO as a solvent control. Cultures were incubated at 37°C under low oxygen conditions in a candle jar. At the end of the incubation, phase contrast images of living co-cultures were taken with an inverted microscope or culture supernatants were collected, kept on ice and detached trophozoites were counted. Co-cultures on coverslips were washed in warm PBS, fixed in ice-cold methanol, permeabilized with 0.5% Triton-X 100 for 10 min at room temperature and blocked for 30 min in blocking buffer (5% goat serum, 1% glycerol, 0.1% BSA, 0.1% fish gelatin and 0.04% sodium azide). Coverslips were then incubated for 1 h with a mouse monoclonal antibody against arginine deiminase,6 washed, incubated with goat anti-mouse–Alexa 488 conjugate (Invitrogen), washed, post-fixed with 4% paraformaldehyde, rinsed with PBS and mounted with Prolong Gold (Invitrogen).
Viability assay
Detached trophozoites were chilled on ice, centrifuged for 5 min at 5000 rpm at 4°C and resuspended in PBS with 1 µg/mL propidium iodide. The culture medium on Caco-2 cell layers was replaced by PBS with 1 µg/mL propidium iodide and 0.8 µg/mL fluorescein diacetate. The viability of cell layers or trophozoites was determined under a fluorescence microscope. Viability was also assessed by the BacTiter-Glo cell viability ATP assay (Promega) according to the manufacturer's instructions.
Mouse infections
On day 0, 5-week-old C57 mice (The Jackson Laboratory) were infected by gavage with 1 × 107 GS/M trophozoites and used for experiments on day 4. For treatment of small intestinal explants, small intestine was removed, cut in half, flushed with warm PBS and filled with 1 mL of warm PBS containing 40 µM formononetin or 1:1000 DMSO. After 30 min of incubation at 37°C, the intestine was flushed with 2 mL of warm PBS, and flow-through solutions containing the detached trophozoites were collected. The remaining attached trophozoites were harvested by incubating longitudinally opened explants in cold PBS. The sum of detached trophozoites and those remaining attached was taken as the total number of trophozoites per intestine. A total of 10 small intestines (20 halves) were used in three independent experiments. For in vivo experiments, mice were administered 0.235 mg/g formononetin in 50% DMSO/PBS by gavage on day 4 of infection. We had determined this dose based on drug solubility and DMSO tolerance. After 1.5, 3 and 18 h, trophozoites in the small intestine were counted as described above. A total of 14 mice were used in two independent experiments. All animal studies were reviewed and approved by the UCSD Institutional Animal Care and Use Committee.
Results
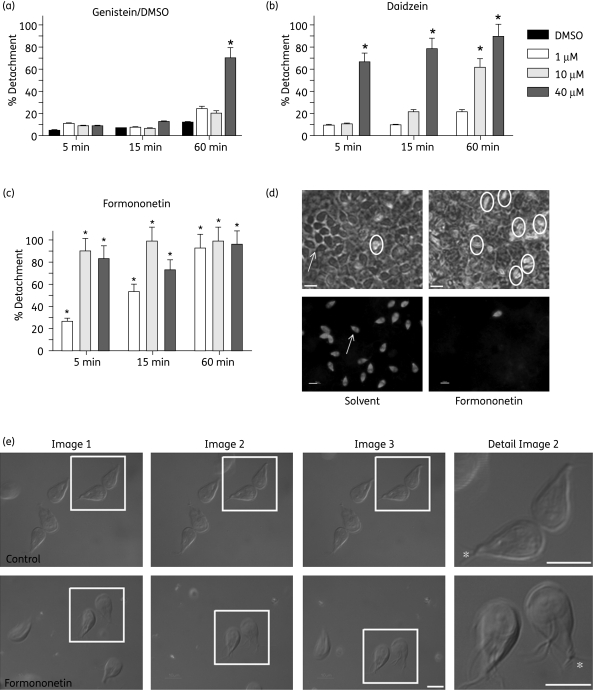
Isoflavones detached trophozoites in a dose- and time-dependent manner (Figure 1a–c). Formononetin was the most potent drug, as it detached >80% of trophozoites within 5 min at 10 µM. Daidzein was less potent, but 40 µM still detached >60% of trophozoites within 5 min. Genistein was the least potent isoflavone and 1 h of treatment with 40 µM was necessary to detach >60% trophozoites. Formononetin- and daidzein-mediated detachment was reversed after removal of the isoflavones [Figure S1, available as Supplementary data at JAC Online (http://jac.oxfordjournals.org/)]. Formononetin also caused rapid and effective (>95%) detachment of trophozoites from Caco-2 cell layers (Figure 1d) and from mouse small intestinal explants (Figure 2a). None of the isoflavones tested caused Giardia or Caco-2 cell death at the doses and times tested. Even after 24 h, Giardia cell death was <2% and there was no decrease in the ATP levels of Caco-2 cell layers treated with 40 µM formononetin, daidzein or genistein. Light microscopic observations showed that in addition to detaching rapidly, isoflavone-treated trophozoites had greatly reduced flagellar motility. The ventral flagella, which under normal conditions beat synchronously in a sigmoidal wave, were twitching or even paralysed in formononetin-treated cells (Figure 1e). Certain isoflavones inhibit phosphodiesterases and increase cAMP activity.7 However, after 30 min of treatment with 40 µM formononetin, cAMP levels in trophozoites remained unchanged (17.4 ± 4.4 nM versus 18.3 ± 4.9 nM in DMSO control) suggesting that cAMP signalling is not involved in Giardia detachment and flagellar paralysis.
Figure 1.
Isoflavones detach Giardia trophozoites. Bar graphs show the percentages of detached trophozoites after 5, 15 and 60 min of treatment with 1, 10 or 40 µM genistein (a), daidzein (b), formononetin (c) or the solvent control (DMSO) (a). Bars show the means of three independent experiments; standard deviations are also shown. *Numbers of detached trophozoites are significantly different compared with the solvent control; P < 0.05, paired t-test. (d) Co-cultures of trophozoites with Caco-2 cell layers 5 min after treatment with 40 µM formononetin or the appropriate solvent dilution. Top panels: phase contrast images of live co-cultures. Detached trophozoites are encircled, and the area with an attached trophozoite is indicated with an arrow. Bottom panels: to facilitate visualization of trophozoites, co-cultures were fixed and immunostained with an antibody against Giardia arginine deiminase. Bars, 10 µm. (e) Attached trophozoites were treated with 20 µM formononetin or not (control), and differential interference contrast (DIC) images were immediately captured with 10 s intervals. Formononetin-treated trophozoites in the box are detached, are turned ventral side up and are slowly floating down the field, whereas untreated trophozoites remain attached. Flagella (except for the caudal, asterisk) in untreated trophozoites were beating too fast to be captured. Flagella in formononetin-treated trophozoites are paralysed and are clearly visible. Bars, 10 µm.
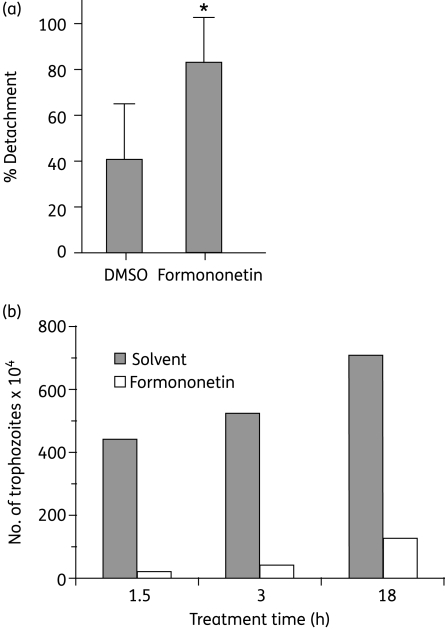
Figure 2.
(a) Percentages of detached trophozoites in small intestinal explants from Giardia-infected mice after 30 min of ex vivo treatment with 40 µM formononetin or solvent control (DMSO). Each bar represents the mean of 10 explants; standard deviations are also shown. *Percentages are significantly different compared with the solvent control; P < 0.05, Mann–Whitney test. (b) Formononetin decreases the number of trophozoites in small intestines of mice compared with the solvent control. At time 0, Giardia-infected mice were treated orally with 0.235 mg/g formononetin or with the solvent control and the numbers of trophozoites in the small intestines were counted 1.5, 3 and 18 h later. The bar graph is representative of two independent experiments with up to four mice per condition.
We next asked whether formononetin treatment would reduce the number of trophozoites in mouse small intestines. After 1.5 and 3 h of formononetin treatment, Giardia-infected mice had 90% fewer trophozoites in their intestines compared with solvent-treated mice (Figure 2b), suggesting that the antigiardial drug effects were rapid and not explained by growth inhibition.
Discussion
Isoflavones act on a variety of pathways and the non-methylated isoflavones (genistein and daidzein) are usually more potent than their methylated structural homologues (formononetin). For example, genistein and daidzein act as selective agonists on β-oestrogen receptors, while formononetin is an inactive precursor.8 Genistein is more potent than daidzein in inhibiting phosphodiesterases7 and T-type calcium channels,9 and inhibits tyrosine kinases, while daidzein and formononetin do not. Surprisingly, we found that formononetin was the most potent isoflavone in inhibiting Giardia attachment, as previously reported for growth and viability.1,2 This, together with our negative cAMP results, suggests that the mechanism underlying isoflavone detachment of Giardia trophozoites involves molecular pathways different from those currently identified.
Cellular attachment and flagellar motility were not affected in the other mucosal protozoan parasites, Entamoeba histolytica and Trichomonas vaginalis (data not shown), suggesting that isoflavones affect Giardia-specific attachment and motility pathways. Since the ventral flagellar movement is proposed to create negative pressure under the disc,4 it is likely that cellular detachment by isoflavones is related to impaired flagellar motility. The motility of eukaryotic flagella is driven by motor proteins that function as ATPases and travel up and down the outer microtubules of the flagellar axoneme. It is regulated by phosphorylation, calcium and adenylyl/guanlylyl cyclase signalling.10 Genistein and daidzein inhibit T-type calcium channels and flagellar motility in sperm.9 However, studies with the calcium channel blocker, verapamil, and the calcium ionophore, ionomycin, suggest that these pathways are not involved in formononetin-mediated detachment of Giardia trophozoites (data not shown).
A previous report found that 1 mg/mouse formononetin for 4 days had no impact on Giardia load, whereas 10 mg for 4 days cleared infection.1 In our studies, 0.235 mg/g formononetin cleared >90% of the Giardia in 1.5 h. The previous study did not examine early times after treatment, so it is unclear whether those findings are in contradiction to ours, or simply explained by different experimental conditions. Nonetheless, since formononetin detached trophozoites rapidly within minutes, and days before the reported trophozoite cell death,1,2 we conclude that antigiardial activity of isoflavones is at least partly due to early detachment of trophozoites. Thus, formononetin could be a valuable drug candidate to treat Giardia infections and a useful tool for future studies on the molecular mechanism underlying Giardia attachment and flagellar motility. Furthermore, our data strongly support the notion that inhibition of attachment is a viable pharmacological strategy in the fight against giardiasis.
Funding
This work was supported by the National Institute of Health (grant numbers UO1 AI75527, DK80506 to L. E. and AI42488, AI51687 to F. D. G.).
Transparency declarations
None to declare.
Supplementary data
Figure S1 is available as Supplementary data at JAC Online (http://jac.oxfordjournals.org/).
Supplementary Material
Acknowledgements
We would like to thank Dr M. Spehlmann for help with experiments.
References
- 1.Khan IA, Avery MA, Burandt CL, et al. Antigiardial activity of isoflavones from Dalbergia frutescens bark. J Nat Prod. 2000;63:1414–6. doi: 10.1021/np000010d. [DOI] [PubMed] [Google Scholar]
- 2.Sterk M, Muller J, Hemphill A, et al. Characterization of a Giardia lamblia WB C6 clone resistant to the isoflavone formononetin. Microbiology. 2007;153:4150–8. doi: 10.1099/mic.0.2007/010041-0. [DOI] [PubMed] [Google Scholar]
- 3.Hernandez-Sanchez J, Linan RF, Salinas-Tobon Mdel R, et al. Giardia duodenalis: adhesion-deficient clones have reduced ability to establish infection in Mongolian gerbils. Exp Parasitol. 2008;119:364–72. doi: 10.1016/j.exppara.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 4.Holberton DV. Attachment of Giardia—a hydrodynamic model based on flagellar activity. J Exp Biol. 1974;60:207–21. doi: 10.1242/jeb.60.1.207. [DOI] [PubMed] [Google Scholar]
- 5.Keister DB. Axenic culture of Giardia lamblia in TYI-S-33 medium supplemented with bile. Trans R Soc Trop Med Hyg. 1983;77:487–8. doi: 10.1016/0035-9203(83)90120-7. [DOI] [PubMed] [Google Scholar]
- 6.Ringqvist E, Palm JE, Skarin H, et al. Release of metabolic enzymes by Giardia in response to interaction with intestinal epithelial cells. Mol Biochem Parasitol. 2008;159:85–91. doi: 10.1016/j.molbiopara.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nichols MR, Morimoto BH. Differential inhibition of multiple cAMP phosphodiesterase isozymes by isoflavones and tyrphostins. Mol Pharmacol. 2000;57:738–45. doi: 10.1124/mol.57.4.738. [DOI] [PubMed] [Google Scholar]
- 8.Beck V, Rohr U, Jungbauer A. Phytoestrogens derived from red clover: an alternative to estrogen replacement therapy? J Steroid Biochem Mol Biol. 2005;94:499–518. doi: 10.1016/j.jsbmb.2004.12.038. [DOI] [PubMed] [Google Scholar]
- 9.Tao J, Zhang Y, Li S, et al. Tyrosine kinase-independent inhibition by genistein on spermatogenic T-type calcium channels attenuates mouse sperm motility and acrosome reaction. Cell Calcium. 2009;45:133–43. doi: 10.1016/j.ceca.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 10.Inaba K. Molecular architecture of the sperm flagella: molecules for motility and signaling. Zoolog Sci. 2003;20:1043–56. doi: 10.2108/zsj.20.1043. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.