Abstract
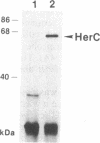
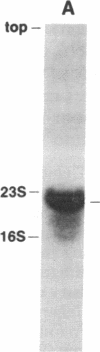
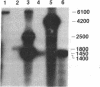
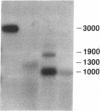
The prfB gene encodes peptide-chain-release factor 2 of Escherichia coli, which catalyzes translation termination at UGA and UAA codons. The gene, identified by sequencing, is located at the 62-min region of the E. coli chromosome. The prfB gene is followed by an open reading frame encoding a 57,603-Da protein. This downstream open reading frame was identified as herC, a gene defined by a suppressor mutation that restores replication of a ColE1 plasmid mutant. RNA blot hybridization and S1 nuclease protection analyses of in vivo transcripts showed that prfB and herC are cotranscribed into a 2800-base transcript in the counterclockwise direction with respect to the E. coli genetic map. Thus, we refer to the two genes as the prfB-herC operon. Data are presented that suggest that supK, a mutation in Salmonella typhimurium that suppresses UGA termination, is the structural gene for Salmonella release factor 2. Translation control within the prfB-herC operon and the relationship of these genes to a tRNA methyltransferase are discussed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aiba H., Adhya S., de Crombrugghe B. Evidence for two functional gal promoters in intact Escherichia coli cells. J Biol Chem. 1981 Nov 25;256(22):11905–11910. [PubMed] [Google Scholar]
- Amann E., Brosius J., Ptashne M. Vectors bearing a hybrid trp-lac promoter useful for regulated expression of cloned genes in Escherichia coli. Gene. 1983 Nov;25(2-3):167–178. doi: 10.1016/0378-1119(83)90222-6. [DOI] [PubMed] [Google Scholar]
- Appleyard R K. Segregation of Lambda Lysogenicity during Bacterial Recombination in Escherichia Coli K12. Genetics. 1954 Jul;39(4):429–439. doi: 10.1093/genetics/39.4.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björk G. R. A novel link between the biosynthesis of aromatic amino acids and transfer RNA modification in Escherichia coli. J Mol Biol. 1980 Jul 5;140(3):391–410. doi: 10.1016/0022-2836(80)90391-5. [DOI] [PubMed] [Google Scholar]
- Björk G. R., Ericson J. U., Gustafsson C. E., Hagervall T. G., Jönsson Y. H., Wikström P. M. Transfer RNA modification. Annu Rev Biochem. 1987;56:263–287. doi: 10.1146/annurev.bi.56.070187.001403. [DOI] [PubMed] [Google Scholar]
- Burton Z. F., Gross C. A., Watanabe K. K., Burgess R. R. The operon that encodes the sigma subunit of RNA polymerase also encodes ribosomal protein S21 and DNA primase in E. coli K12. Cell. 1983 Feb;32(2):335–349. doi: 10.1016/0092-8674(83)90453-1. [DOI] [PubMed] [Google Scholar]
- Caskey C. T., Forrester W. C., Tate W., Ward C. D. Cloning of the Escherichia coli release factor 2 gene. J Bacteriol. 1984 Apr;158(1):365–368. doi: 10.1128/jb.158.1.365-368.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang A. C., Cohen S. N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978 Jun;134(3):1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craigen W. J., Caskey C. T. Expression of peptide chain release factor 2 requires high-efficiency frameshift. Nature. 1986 Jul 17;322(6076):273–275. doi: 10.1038/322273a0. [DOI] [PubMed] [Google Scholar]
- Craigen W. J., Cook R. G., Tate W. P., Caskey C. T. Bacterial peptide chain release factors: conserved primary structure and possible frameshift regulation of release factor 2. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3616–3620. doi: 10.1073/pnas.82.11.3616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorini L. Ribosomal discrimination of tRNAs. Nat New Biol. 1971 Dec 29;234(52):261–264. doi: 10.1038/newbio234261a0. [DOI] [PubMed] [Google Scholar]
- Hirsh D. Tryptophan tRNA of Escherichia coli. Nature. 1970 Oct 3;228(5266):57–57. doi: 10.1038/228057a0. [DOI] [PubMed] [Google Scholar]
- Johnston H. M., Roth J. R. UGA suppressor that maps within a cluster of ribosomal protein genes. J Bacteriol. 1980 Oct;144(1):300–305. doi: 10.1128/jb.144.1.300-305.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein H., Capecchi M. R. Polypetide chain termination. Purification of the release factors, R1 and R2, from Escherichia coli. J Biol Chem. 1971 Feb 25;246(4):1055–1061. [PubMed] [Google Scholar]
- Masukata H., Tomizawa J. Control of primer formation for ColE1 plasmid replication: conformational change of the primer transcript. Cell. 1986 Jan 17;44(1):125–136. doi: 10.1016/0092-8674(86)90491-5. [DOI] [PubMed] [Google Scholar]
- Masukata H., Tomizawa J. Effects of point mutations on formation and structure of the RNA primer for ColE1 DNA replication. Cell. 1984 Feb;36(2):513–522. doi: 10.1016/0092-8674(84)90244-7. [DOI] [PubMed] [Google Scholar]
- Murray N. E., Murray K. Manipulation of restriction targets in phage lambda to form receptor chromosomes for DNA fragments. Nature. 1974 Oct 11;251(5475):476–481. doi: 10.1038/251476a0. [DOI] [PubMed] [Google Scholar]
- Naito S., Kitani T., Ogawa T., Okazaki T., Uchida H. Escherichia coli mutants suppressing replication-defective mutations of the ColE1 plasmid. Proc Natl Acad Sci U S A. 1984 Jan;81(2):550–554. doi: 10.1073/pnas.81.2.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y., Uchida H. Isolation of conditionally lethal amber mutations affecting synthesis of the nusA protein of Escherichia coli. Mol Gen Genet. 1983;190(2):196–203. doi: 10.1007/BF00330640. [DOI] [PubMed] [Google Scholar]
- Oeschger M. P., Oeschger N. S., Wiprud G. T., Woods S. L. High efficiency temperature-sensitive amber suppressor strains of Escherichia coli K12: isolation of strains with suppressor-enhancing mutations. Mol Gen Genet. 1980;177(4):545–552. doi: 10.1007/BF00272662. [DOI] [PubMed] [Google Scholar]
- Oeschger M. P., Wiprud G. T. High efficiency temperature-sensitive amber suppressor strains of Escherichia coli K12: construction and characterization of recombinant strains with suppressor-enhancing mutations. Mol Gen Genet. 1980;178(2):293–299. doi: 10.1007/BF00270475. [DOI] [PubMed] [Google Scholar]
- Piepersberg W., Böck A., Wittmann H. G. Effect of different mutations in ribosomal protein S5 of Escherichia coli on translational fidelity. Mol Gen Genet. 1975 Sep 29;140(2):91–100. doi: 10.1007/BF00329777. [DOI] [PubMed] [Google Scholar]
- Pope W. T., Brown A., Reeves R. H. The identification of the tRNA substrates for the supK tRNA methylase. Nucleic Acids Res. 1978 Mar;5(3):1041–1057. doi: 10.1093/nar/5.3.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope W. T., Reeves R. H. Purification and characterization of a tRNA methylase from Salmonella typhimurium. J Bacteriol. 1978 Oct;136(1):191–200. doi: 10.1128/jb.136.1.191-200.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratliff J. C., Caskey C. T. Immunologic evidence for structural homology between the release factors of Escherichia coli. Arch Biochem Biophys. 1977 Jun;181(2):671–677. doi: 10.1016/0003-9861(77)90273-9. [DOI] [PubMed] [Google Scholar]
- Reeves R. H., Roth J. R. A recessive UGA suppressor. J Mol Biol. 1971 Mar 28;56(3):523–533. doi: 10.1016/0022-2836(71)90399-8. [DOI] [PubMed] [Google Scholar]
- Reeves R. H., Roth J. R. Transfer ribonucleic acid methylase deficiency found in UGA supressor strains. J Bacteriol. 1975 Oct;124(1):332–340. doi: 10.1128/jb.124.1.332-340.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddle D. L., Roth J. R. Frameshift suppressors. II. Genetic mapping and dominance studies. J Mol Biol. 1972 May 28;66(3):483–493. doi: 10.1016/0022-2836(72)90428-7. [DOI] [PubMed] [Google Scholar]
- Ryden M., Murphy J., Martin R., Isaksson L., Gallant J. Mapping and complementation studies of the gene for release factor 1. J Bacteriol. 1986 Dec;168(3):1066–1069. doi: 10.1128/jb.168.3.1066-1069.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rydén S. M., Isaksson L. A. A temperature-sensitive mutant of Escherichia coli that shows enhanced misreading of UAG/A and increased efficiency for some tRNA nonsense suppressors. Mol Gen Genet. 1984;193(1):38–45. doi: 10.1007/BF00327411. [DOI] [PubMed] [Google Scholar]
- Sancar A., Hack A. M., Rupp W. D. Simple method for identification of plasmid-coded proteins. J Bacteriol. 1979 Jan;137(1):692–693. doi: 10.1128/jb.137.1.692-693.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scolnick E., Tompkins R., Caskey T., Nirenberg M. Release factors differing in specificity for terminator codons. Proc Natl Acad Sci U S A. 1968 Oct;61(2):768–774. doi: 10.1073/pnas.61.2.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. The 3'-terminal sequence of Escherichia coli 16S ribosomal RNA: complementarity to nonsense triplets and ribosome binding sites. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1342–1346. doi: 10.1073/pnas.71.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss R. B., Murphy J. P., Gallant J. A. Genetic screen for cloned release factor genes. J Bacteriol. 1984 Apr;158(1):362–364. doi: 10.1128/jb.158.1.362-364.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T. T. A model for three-point analysis of random general transduction. Genetics. 1966 Aug;54(2):405–410. doi: 10.1093/genetics/54.2.405. [DOI] [PMC free article] [PubMed] [Google Scholar]