Abstract
Objective
To investigate the association between body mass index (BMI) and histology of renal cell carcinoma (RCC) in a contemporary cohort, as obesity is increasingly prevalent in the USA and might be contributing to the increasing incidence of RCC, but little is known about the relationship of obesity with the different histological subtypes of RCC.
Patients and methods
From January 2000 to December 2007 we identified 1640 patients with renal cortical tumours undergoing surgical extirpation at our institution, and who had their BMI recorded. Multivariable logistic regression models were used to test the association of BMI with RCC histology.
Results
The median (interquartile range) BMI was 28 (25–32) kg/m2 and 38% of patients were classified as obese (BMI > 30 kg/m2). After adjusting for tumour size, age, gender, American Society of Anesthesiologists score, estimated glomerular filtration rate, hypertension, diabetes mellitus and smoking, the BMI was significantly associated with clear-cell histology; the odds ratios were 1.04 for each unit of BMI (95% confidence interval, CI, 1.02–1.06; P < 0.001) and 1.48 when comparing obese vs non-obese patients (95% CI 1.19–1.84; P < 0.001). In the subgroup of patients with RCC (excluding benign renal cortical tumours), BMI was still an independent predictor of clear-cell histology (odds ratio 1.04, 95% CI 1.02–1.06, P = 0.001).
Conclusions
These results suggest that BMI is an independent predictor of clear-cell histology in patients with a renal cortical tumour. While the aetiology of this phenomenon requires further study, these findings might have implications in determining a patient's risk of harbouring a clear-cell RCC and in subsequent treatment recommendations.
Keywords: kidney neoplasms, carcinoma, renal cell, obesity, body mass index, histology
Introduction
The diagnosis of RCC is increasing in the USA; > 54 000 new cases of RCC were expected to be diagnosed in 2008 [1]. The widespread use of abdominal imaging has certainly contributed to the increased detection of RCC but fails to account for it entirely [2]. Part of the increased incidence could result from increasing rates of obesity, which has emerged as a risk factor for RCC in several epidemiological studies [3–9]. Rates of overweight and obesity in the USA have increased in recent decades, reaching > 70% in men and 60% in women [7]. Over a 10-year period, Pettus et al. showed increasing body mass index (BMI) and decreasing baseline renal function in a group of patients with renal cortical tumours undergoing surgical excision [10]. The exact reason for the association between obesity and RCC is not known, but investigators have hypothesized that it might be secondary to hormonal changes (increased levels of IGF or oestrogen), decreased immune function, or associated hypertension/diabetes mellitus in obese patients [5,11].
Clear-cell histology is the most common and one of the more lethal RCC variants. The relatively recent breakthroughs into the molecular pathogenesis of clear-cell RCC have facilitated the development of new targeted therapies. Because of these advances, many of the current clinical trials of RCC are limited to patients with clear-cell histology, underscoring the importance of defining exactly who is at increased risk for this histological variant.
The results of two studies suggested a potential link between obesity and RCC histology. Donat et al. reported a univariate association between clear-cell histology and BMI in a group of surgically treated patients [12]. Also, an Italian study showed a higher risk of RCC in obese patients found to have the clear cell histological subtype [6]. In the present study we used multivariable analyses to examine whether BMI is associated with histology in a contemporary cohort.
Patients and methods
After receiving institutional review board approval, we queried the Memorial Sloan-Kettering Cancer Center prospective renal surgery database for all patients with RCC or a benign renal cortical tumour who underwent surgery between January 2000 and December 2007. We excluded only those patients presenting with a hereditary renal cancer syndrome. Sixty-four patients had missing BMI data, leaving 1640 patients available for analysis.
We defined obese according to the WHO criterion (BMI ≥ 30.0 kg/m2) [13]. The estimated GFR (eGFR) was calculated according to the abbreviated Modified Diet and Renal Disease equation, accounting for patient age, race and last serum creatinine level before renal surgery [14].
The primary end point of the study was renal tumour histology, which for descriptive purposes was divided into nine categories: clear-cell, papillary, chromophobe, collecting duct, unclassified RCC, oncocytoma, angiomyolipoma, metanephric adenoma, and other benign. We chose to dichotomize this endpoint into clear cell vs not clear cell, allowing us to use multivariable logistic regression models to explore the association of BMI with RCC histology. Variables within each model were BMI, age (continuous), gender, race (white, non-white), American Society of Anaesthesiologists (ASA) score, eGFR, tumour size (continuous), hypertension, smoking, and diabetes mellitus. The ASA score was used as a surrogate for general comorbidity. Hypertension and smoking were included because both are established risk factors for RCC and could possibly confound the results [5,15]. The presence of diabetes is closely linked to obesity, and might play a role in tumorigenesis and confound the outcome [11]. The 1608 patients with complete data were included in the multivariable analysis.
The initial model was constructed with BMI as a continuous variable, and we included both malignant and benign tumours. We then repeated the analysis, but changed BMI to a binary variable at the threshold for obesity (< 30 vs ≤ 30 kg/m2). We also used the same models to evaluate the subset of patients with RCC (excluding all benign histological variants). The predicted probability of clear-cell histology according to BMI was calculated using a univariate logistic regression model. We assessed any nonlinear relationship between BMI and the probability of clear-cell histology by fitting a model including nonlinear terms for BMI. We found no evidence of a nonlinear relationship, as the risk curves were very similar for the models with and without nonlinear terms; therefore, we only report the risk curve based on the linear model. All statistical tests were two-sided, with P < 0.05 considered to indicate statistical significance.
Results
The study group consisted of 1704 patients, of whom 1640 (96%) had available BMI data. Table 1 outlines the patient demographics and pathological features of the group. The median (interquartile range, IQR) age was 62 (53–70) years. Men comprised 63% (1076) of the cohort; most patients were white (1491; 88%). The median (IQR) eGFR was 65 (56–76) mL/min/1.73 m2. A malignant tumour was present in 1492 patients (88%), and clear-cell RCC was present in 1033 (61%), with non-clear cell histology accounting for the other 671 (39%). The median (IQR) BMI was 28 (25–32) kg/m2; 38% of the patients were classified as obese, and of all patients with clear-cell RCC, 415 (42%) were classified as obese. By contrast, only 62 (31%) of patients with benign pathology were obese (Table 2).
Table 1.
The demographic and pathological characteristics of patients with a renal mass treated surgically between 2000 and 2007
| Characteristic | N (%) or median (IQR) |
|---|---|
| No. of patients | 1704 |
| Age, years | 62 (53–70) |
| Gender | |
| Male | 1076 (63) |
| Female | 628 (37) |
| Race | |
| White | 1491 (88) |
| Black | 79 (5) |
| Other | 134 (8) |
| Hypertension | 894 (52) |
| Diabetes Mellitus | 226 (13) |
| Smoking (ever) | 852 (50) |
| BMI, kg/m2 | 28 (25–32) |
| < 30* | 1018 (62) |
| ≥ 30* | 622 (38) |
| ASA score† | |
| 1 | 69 (4) |
| 2 | 942 (56) |
| 3 | 677 (40) |
| 4 | 7 (0.2) |
| Preoperative: | |
| creatinine, mg/dL | 1.1 (1.0–1.3) |
| eGFR‡ mL/min/1.73 m2 | 65 (56–76) |
| Histology | |
| Malignant | |
| clear Cell | 1033 (61) |
| papillary | 219 (13) |
| chromophobe | 168 (10) |
| collecting Duct | 3 (0.2) |
| unclassified RCC | 69 (4) |
| Benign | |
| oncocytoma | 142 (8) |
| angiomyolipoma | 29 (2) |
| metanephric Adenoma | 8 (0.5) |
| other Benign | 33 (2) |
| Tumour size, cm | 3.8 (2.5–6) |
BMI available for 1640 patients (96%);
ASA available for 1695 patients (99%);
eGFR available for 1681 patients (99%).
Table 2.
Histological variants of renal masses according to BMI (data available for 1640 patients, 96%)
| Histology | Not obese* | Obese† |
|---|---|---|
| No. of patients | 1018 | 622 |
| Malignant | ||
| Clear cell | 580 (57) | 415 (67) |
| Papillary | 145 (14) | 66 (11) |
| Chromophobe | 109 (11) | 55 (9) |
| Collecting duct | 3 (0.3) | 0 (0) |
| Unclassified RCC | 44 (4) | 24 (4) |
| Benign | ||
| Oncocytoma | 90 (9) | 44 (7) |
| Angiomyolipoma | 21 (2) | 6 (1) |
| Metanephric adenoma | 7 (1) | 1 (0.2) |
| Other benign | 19 (2) | 11 (2) |
defined as BMI < 30 kg/m2;
defined as BMI ≥ 30 kg/m2.
The results of the multivariable logistic regression analyses (including both benign and malignant tumours) are shown in Table 3; initially BMI was treated as a continuous variable. While controlling for age, gender, ASA score, tumour size, eGFR, hypertension, diabetes mellitus and smoking, for every 1 kg/m2 increase in BMI there was a 4% increase in the odds of having clear cell RCC (odds ratio 1.04, 95% CI 1.02–1.06, P < 0.001). The only other independent predictors of clear-cell histology in this model were tumour size (odds ratio 1.04, 95% CI 1.01–1.07, P = 0.02) and male gender (1.26, 1.02–1.56, P = 0.03). In addition, obese patients were almost 50% more likely to have clear-cell RCC than those with a BMI of < 30 kg/m2 (odds ratio 1.48, 95% CI 1.19–1.84, P < 0.001; Table 3).
Table 3.
Multivariable logistic regression analysis of factors possibly affecting clear-cell histology among all renal masses, with BMI as a continuous or categorical variable (complete data available for 1608 patients, 94%)
| Variable | Odds ratio (95% CI) | P |
|---|---|---|
| BMI (continuous) | 1.04 (1.02–1.06) | < 0.001 |
| Age | 0.99 (0.98–1.00) | 0.10 |
| Male gender | 1.26 (1.02–1.56) | 0.03 |
| ASA score | 1.13 (0.89–1.42) | 0.30 |
| Tumour size | 1.04 (1.01–1.07) | 0.02 |
| eGFR | 1.0 (0.99–1.00) | 0.50 |
| Hypertension | 0.87 (0.69–1.09) | 0.20 |
| Diabetes mellitus | 1.35 (0.97–1.86) | 0.07 |
| Smoker | 1.0 (0.81–1.23) | 0.90 |
| BMI categorical | ||
| < 30 vs ≥ 30 kg/m2 | 1.48 (1.19–1.84) | < 0.001 |
| Age | 0.99 (0.98–1.00) | 0.07 |
| Male gender | 1.27 (1.02–1.57) | 0.03 |
| ASA Score | 1.15 (0.91–1.45) | 0.20 |
| Tumour size | 1.04 (1.00–1.07) | 0.02 |
| eGFR | 1.0 (0.99–1.00) | 0.50 |
| Hypertension | 0.88 (0.70–1.10) | 0.30 |
| Diabetes mellitus | 1.39 (1.00–1.92) | 0.05 |
| Smoker | 1.0 (0.81–1.23) | 0.90 |
Tumour size, eGFR, and age were entered as continuous variables.
Figure 1 shows the predicted probability of clear-cell histology according to BMI, showing only BMI values of 20–40 kg/m2, as the predicted probabilities for extreme BMI values become quite unstable.
Fig. 1.
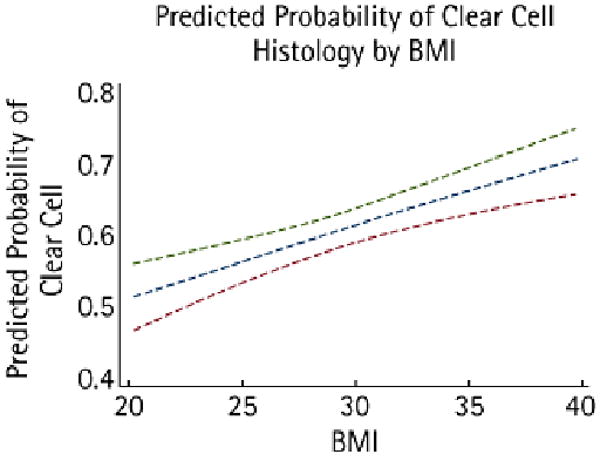
The predicted probability of clear-cell histology according to BMI, showing an estimate based on a univariate logistic regression model. The dashed lines indicate the 95% CI.
We repeated the analysis using only the subgroup of patients with RCC. BMI as a continuous variable remained an independent predictor of clear cell RCC (odds ratio 1.04, 95% CI 1.02–1.06, P = 0.001). Tumour size and male gender were no longer significant predictors. In this subgroup, obese patients remained almost 50% more likely to harbour clear cell RCC than those with a BMI of < 30 kg/m2 in a multivariable analysis (odds ratio 1.48, 95% CI 1.16–1.89, P = 0.002).
Discussion
Several case-control and prospective observational studies have established obesity as a risk factor for RCC, but little is known about its specific association with the different histological subtypes of RCC [3–6,8,9]. In a group of patients with a renal mass treated with surgery, we found increasing BMI to be independently associated with the clear-cell variant. While controlling for all measured covariates, we found that for every 1 kg/m2 increase in BMI there was a 4% increase in the odds of having conventional clear-cell RCC histology. This statistically and clinically significant association remained even when BMI was treated as a dichotomous variable within our models. Obese patients (with a BMI of ≥30 kg/m2) had a 48% greater odds of having clear cell histology than non-obese patients.
Our results confirm and extend the findings of two other studies. Donat et al. [12] reported that a BMI of ≥ 30 kg/m2 was associated with an increased proportion of clear-cell renal cancers. Survival was the primary endpoint of their study, and therefore their multivariate analyses did not specifically explore predictors of clear-cell histology in patients with RCC. Although they found no association between BMI and survival in the patients studied, the univariate association of obesity with clear-cell tumours remained intriguing, as this histology is generally considered more virulent than are the papillary and chromophobe variants. In an Italian case-control study, RCC was associated with obesity, with the association strongest among patients with the clear-cell subtype (odds ratio 1.84, 95% CI 1.09–3.11) [6]. Combining our findings from a contemporary cohort of patients with these two cited studies provides more evidence of a link between obesity and clear cell histology.
Based on the work many investigators, obesity is widely considered to increase the risk of RCC [3–6,8,9]. In a meta-analysis examining 17 different datasets, Renehan et al. [8] confirmed a strong association between obesity and RCC for both men and women. Chow et al. [5] studied a large Swedish cohort and found that higher BMI independently increased the risk of RCC. Interestingly, there was a direct relationship; even with small increases in BMI there were increases in cancer risk. Similarly, we showed an increasing risk of clear cell histology with incremental increases in BMI.
The exact biological mechanisms linking obesity to increased risk of RCC and of clear cell histology are unknown, but there are several theories. An increased incidence of hypertension and diabetes, and increased levels of IGF and oestrogen in obese patients might contribute to tumorigenesis [5,11]. Interestingly, obesity is also associated with impaired immune function, and recent studies of T cell co-stimulation suggest that negative regulators of T cell activation are present on clear-cell RCC cells and are associated with adverse pathological features and poor survival [16,17]. Our refined understanding of the association of BMI with clear-cell RCC will hopefully help to elucidate the mechanism behind the effect of obesity on RCC risk.
Clear-cell RCC is the most common and probably the best understood variant of RCC. It accounts for 70–80% of RCC cases; the next most common histological variants are papillary, chromophobe and collecting-duct carcinoma. Clear-cell RCC typically results from a somatic mutation within the von Hippel-Lindau tumour-suppressor gene found on the short arm of chromosome 3 (3p25) [18,19]. Activation of the von Hippel-Lindau gene results in up-regulation of hypoxia-inducible factor, leading to the increased transcription of genes like vascular endothelial growth factor, that play a key role in renal cell tumorigenesis [20]. This new understanding of the molecular pathogenesis of clear-cell RCC has led to rapid advances in targeted therapy. Drugs like sunitinib, sorafenib, temsirolimus and bevacizumab block specific molecular targets and impede tumour angiogenesis. These drugs are effective in the clear-cell variants of RCC, but the same may not be true for the other histological subtypes. Early identification of patients with or at risk of clear-cell RCC is needed as the role of targeted agents expands.
Our findings could have an influence on the management of small renal masses. Although the prognostic significance of RCC histological subtypes has been disputed [21], clear-cell tumours are generally considered to be more aggressive than are papillary or chromophobe cancers [22,23]. Although the diagnostic capabilities of percutaneous renal biopsy are improving, they are not perfect. If obese patients are definitively shown to be at an increased risk for clear-cell RCC, providers might be more aggressive in treating these patients and less inclined to actively survey them.
Our study has some limitations; it is a retrospective case series, and as such lacks a control group. Our results could be explained by a lower prevalence of non-clear-cell histological subtypes in obese patients rather than higher prevalence of the clear-cell variant. However, we consider this explanation to be unlikely, given the well documented association between obesity and increased risk of RCC in general. As the Memorial Sloan-Kettering Cancer Center is a tertiary-care centre, this study is subject to referral/selection bias. BMI was calculated by height and weight at one point in time (just before surgery); weights can certainly fluctuate over time. Our findings need to be corroborated by others before strong conclusions are made about the importance of this association with clear-cell RCC and obesity.
In conclusion, BMI appears to be an independent predictor of conventional clear-cell histology in patients undergoing removal of a renal cortical tumour. As BMI increased, the odds of having a clear-cell RCC also increased. Although the mechanism of pathogenesis requires further study, this finding might have implications in determining a patient's risk of harbouring a clear-cell RCC and in subsequent treatment recommendations.
Acknowledgments
The authors thank Janet Novak, PhD, of Helix Editing, for substantive editing of the manuscript, and Melanie Bernstein for her assistance with data collection. We also thank Angel Cronin for her statistical assistance.
Sources of support: The Stephen Hanson Family Fellowship, NIH T32 CA82088, and the Sidney Kimmel Center for Prostate and Urologic Cancers
Abbreviations
- IQR
interquartile range
- BMI
body mass index
- ASA
American Society of Anesthesiologists
- eGFR
estimated GFR
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Chow WH, Devesa SS, Warren JL, Fraumeni JF., Jr Rising incidence of renal cell cancer in the United States. JAMA. 1999;281:1628–31. doi: 10.1001/jama.281.17.1628. [DOI] [PubMed] [Google Scholar]
- 3.Bergstrom A, Hsieh CC, Lindblad P, Lu CM, Cook NR, Wolk A. Obesity and renal cell cancer – a quantitative review. Br J Cancer. 2001;85:984–90. doi: 10.1054/bjoc.2001.2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bjorge T, Tretli S, Engeland A. Relation of height and body mass index to renal cell carcinoma in two million Norwegian men and women. Am J Epidemiol. 2004;160:1168–76. doi: 10.1093/aje/. [DOI] [PubMed] [Google Scholar]
- 5.Chow WH, Gridley G, Fraumeni JF, Jr, Jarvholm B. Obesity, hypertension, and the risk of kidney cancer in men. N Engl J Med. 2000;343:1305–11. doi: 10.1056/NEJM200011023431804. [DOI] [PubMed] [Google Scholar]
- 6.Dal Maso L, Zucchetto A, Tavani A, et al. Renal cell cancer and body size at different ages: an Italian multicenter case-control study. Am J Epidemiol. 2007;166:582–91. doi: 10.1093/aje/kwm108. [DOI] [PubMed] [Google Scholar]
- 7.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295:1549–55. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 8.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569–78. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 9.Setiawan VW, Stram DO, Nomura AM, Kolonel LN, Henderson BE. Risk factors for renal cell cancer: the multiethnic cohort. Am J Epidemiol. 2007;166:932–40. doi: 10.1093/aje/kwm170. [DOI] [PubMed] [Google Scholar]
- 10.Pettus JA, Jang TL, Thompson RH, Yossepowitch O, Kagiwada M, Russo P. Effect of baseline glomerular filtration rate on survival in patients undergoing partial or radical nephrectomy for renal cortical tumors. Mayo Clin Proc. 2008;83:1101–6. doi: 10.4065/83.10.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moyad MA. Obesity, interrelated mechanisms, and exposures and kidney cancer. Semin Urol Oncol. 2001;19:270–9. [PubMed] [Google Scholar]
- 12.Donat SM, Salzhauer EW, Mitra N, Yanke BV, Snyder ME, Russo P. Impact of body mass index on survival of patients with surgically treated renal cell carcinoma. J Urol. 2006;175:46–52. doi: 10.1016/S0022-5347(05)00054-6. [DOI] [PubMed] [Google Scholar]
- 13.World Health Organization. BMI Classification. Updated 12/16/08. Available at http://www.who.int/bmi/index.jsp?introPage=intro_3.html.
- 14.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet and Renal Disease Study Group Ann Intern Med. 1999;130:461–70. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 15.Lipworth L, Tarone RE, McLaughlin JK. The epidemiology of renal cell carcinoma. J Urol. 2006;176:2353–8. doi: 10.1016/j.juro.2006.07.130. [DOI] [PubMed] [Google Scholar]
- 16.Thompson RH, Gillett MD, Cheville JC, et al. Costimulatory B7–H1 in renal cell carcinoma patients. Indicator of tumor aggressiveness and potential therapeutic target. Proc Natl Acad Sci USA. 2004;101:17174–9. doi: 10.1073/pnas.0406351101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thompson RH, Kuntz SM, Leibovich BC, et al. Tumor B7–H1 is associated with poor prognosis in renal cell carcinoma patients with long-term follow-up. Cancer Res. 2006;66:3381–5. doi: 10.1158/0008-5472.CAN-05-4303. [DOI] [PubMed] [Google Scholar]
- 18.Anglard P, Tory K, Brauch H, et al. Molecular analysis of genetic changes in the origin and development of renal cell carcinoma. Cancer Res. 1991;51:1071–7. [PubMed] [Google Scholar]
- 19.Gnarra JR, Tory K, Weng Y, et al. Mutations of the VHL tumour suppressor gene in renal carcinoma. Nat Genet. 1994;7:85–90. doi: 10.1038/ng0594-85. [DOI] [PubMed] [Google Scholar]
- 20.Hutson TE. Targeted therapy for renal cell carcinoma: a new treatment paradigm. Proc (Bayl University Med Cent) 2007;20:244–8. doi: 10.1080/08998280.2007.11928297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patard JJ, Leray E, Rioux-Leclercq N, et al. Prognostic value of histologic subtypes in renal cell carcinoma: a multicenter experience. J Clin Oncol. 2005;23:2763–71. doi: 10.1200/JCO.2005.07.055. [DOI] [PubMed] [Google Scholar]
- 22.Beck SD, Patel MI, Snyder ME, et al. Effect of papillary and chromophobe cell type on disease-free survival after nephrectomy for renal cell carcinoma. Ann Surg Oncol. 2004;11:71–7. doi: 10.1007/BF02524349. [DOI] [PubMed] [Google Scholar]
- 23.Cheville JC, Lohse CM, Zincke H, Weaver AL, Blute ML. Comparisons of outcome and prognostic features among histologic subtypes of renal cell carcinoma. Am J Surg Pathol. 2003;27:612–24. doi: 10.1097/00000478-200305000-00005. [DOI] [PubMed] [Google Scholar]


