Abstract
Background
The effect of aging on several pathologic features of allergic-asthma (pulmonary inflammation, eosinophilia, mucus-hypersecretion), and their relationship with airway hyperresponsiveness (AHR) is not well characterized.
Objective
To evaluate lung inflammation, mucus-metaplasia and AHR in relationship to age in murine models of allergic-asthma comparing young and older mice.
Methods
Young (6-week) and older (6-, 12- 18-month) BALB/c mice were sensitized and challenged with ovalbumin (OVA). AHR and bronchoalveolar fluid (BALF) total inflammatory cell count and differential were measured. To evaluate mucus-metaplasia, quantitative PCR for the major airway mucin-associated gene, MUC-5AC, from lung tissue was measured, and lung tissue sections stained with periodic acid-Schiff (PAS) for goblet-cell enumeration. Lung tissue cytokine gene expression was determined by qPCR, and systemic cytokine protein levels by ELISA from spleen-cell cultures. Antigen-specific serum IgE was determined by ELISA.
Results
AHR developed in both aged and young OVA-sensitized/challenged mice (OVA-mice), and was more significantly increased in young OVA-mice than in aged OVA-mice. However, BALF eosinophil numbers were significantly higher, and lung histology showed greater inflammation in aged OVA-mice than in young OVA-mice. MUC-5AC expression and numbers of PAS+ staining bronchial epithelial cells were significantly increased in the aged OVA-mice. All aged OVA-mice had increased IL-5 and IFN-γ mRNA expression in the lung and IL-5 and IFN-γ protein levels from spleen cell cultures compared to young OVA-mice. OVA-IgE was elevated to a greater extent in aged OVA-mice.
Conclusions
Although pulmonary inflammation and mucus-metaplasia after antigen sensitization/challenge occurred to a greater degree in older mice, the increase in AHR was significantly less compared with younger OVA-mice. Antigen treatment produced a unique cytokine profile in older mice (elevated IFN-γ and IL-5) compared with young mice (elevated IL-4 and IL-13). Thus, the airway response to inflammation is lessened in aging animals, and may represent age-associated events leading to different phenotypes in response to antigen provocation.
Keywords: Aging, murine, asthma, airway hyperresponsiveness, eosinophil, inflammation
Introduction
Asthma is frequently thought of as a chronic inflammatory disease that develops in childhood, however, it has been reported that as many as 25% of patients develop symptoms after the age of 401. This number may be an underestimate as there is evidence that in older individuals new onset asthma is underdiagnosed as it may be confused with underlying cardiac disease2. Patients with late onset asthma may have more severe airway disease than patients with earlier onset asthma3, 4, and hospitalization and mortality rates are highest amongst patients with asthma >65 years5, 6. Despite these observations, the effect of aging on allergic airway inflammation in humans or in antigen sensitized aged mice is not well characterized.
For many years, the dogma was that late onset asthma was a non-atopic phenotype. However, several groups have demonstrated that elevated IgE levels and antigen-specific IgE can develop later in life in humans, and in one report, was present in up to 72% of older patients with newly diagnosed asthma2, 5, 7, 8. Furthermore, although it is fairly well established that there is a Th2-cytokine bias early in life9-11, reports on cytokine expression later in life are conflicting. Several studies investigating the cytokine response to nonspecific stimulants in both aged rodents and elderly adults without atopic diseases have demonstrated a bias towards a Th1 effector response 12-14, whereas other studies have suggested that aging is associated with decreased IFN-γ production and higher IL-4, IL-5 and IL-10 responses (a Th2 shift15-17). The effect of aging on cytokine profiles, pulmonary inflammation (including histology and bronchoalveolar lavage fluid (BALF) differential) and airway hyperresponsiveness (AHR) after antigen sensitization and challenge is even less well characterized. Sensitization and challenge of 10- and 12-month old BALB/c mice resulted in a decreased Th2 like profile and significantly decreased BALF total cell count and eosinophil numbers compared to antigen sensitized younger mice (age ranges 1 week to 5 months)18, 19; airway histology was not evaluated.
This study was designed to evaluate the age-dependent inflammatory airway responses in antigen sensitized and challenged mice. Young (6-week) and older (6-, 12- and 18-month old) mice were sensitized and chronically challenged with ovalbumin (OVA), with the goal to evaluate BALF, lung tissue inflammation and mucus cell metaplasia in relationship to AHR.
Methods
Mice and reagents
Young (six-week old) female BALB/c mice were purchased from Jackson Laboratory Bar Harbor, ME. Aged female BALB/c mice (6-, 12- and 18-months old) were obtained from the National Institutes of Aging (N.I.A.), Bethesda, MD. The aged groups of mice represent 20.1, 40.2, and 60.3 human years respectively, based upon the 24 month life span of BALB/c mice and the life expectancy of 80.4 years in a human female (source National Center for Health Statistics, www.cdc.gov/nchs). The ages were chosen to represent early, mid and later adulthood. (Preliminary data on lung histology, lung cytokine expression and airway function revealed no statistical differences between antigen sensitized and challenged mice purchased from Jackson Laboratories who were allowed to age in our facilities, and similarly treated and ages of mice obtained from the N.I.A.) All mice were maintained in the animal facility at the Mount Sinai School of Medicine. Standard guidelines for laboratory animal care were followed (“Guide for the Care and Use of Laboratory Animals,” National Institutes of Health publication no. 86-23)20 and institutional permission for animal handling approved through our animal facilities.
Antigen sensitization and challenge
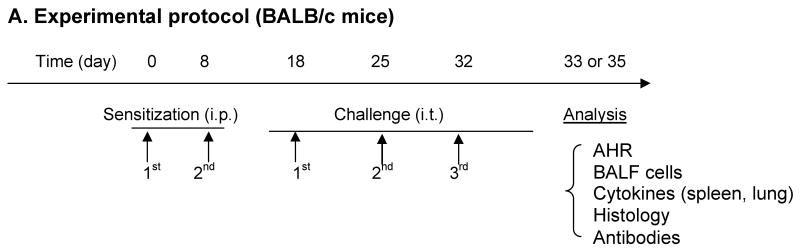
Mice (6-week old, n=5-8/group; 6-, 12- and 18-month, n=10-15/group) were sensitized intraperitoneally (i.p.) with 200μg OVA (Grade V, Sigma) absorbed with 2mg alum (Pierce Biotechnology, Inc.) in 0.4ml PBS on days 0 and 8. Ten days after the last sensitization, mice were anesthetized i.p. with ketamine and xylazine and challenged intratracheally (i.t.) for a total of three times on days 18, 25 and 32 with 100μg OVA (Table I) in 0.05ml PBS, determined to be optimal for distribution to both lobes of the lung21. (Time points for our protocol were selected based on our previous work using young antigen sensitized and challenged AKR22, 23 and BALB/c24 mice (Busse PJ, unpublished data) which produced significant pulmonary inflammation, AHR and antigen specific IgE). Antigen challenge via i.t. administration was performed as previously described25. Control mice were age matched naïve BALB/c mice (n=5/group). (We decided not to administer i.t. PBS to the control mice as we had previously found no significant difference in BALF cell numbers, differential cell count or airway function when comparing i.t. PBS treated naïve mice compared to non-treated naïve mice (unpublished data)). Mice were sacrificed at either 24 or 72 hours after the final OVA challenge.
Table 1. Experimental protocol (BALB/c mice).
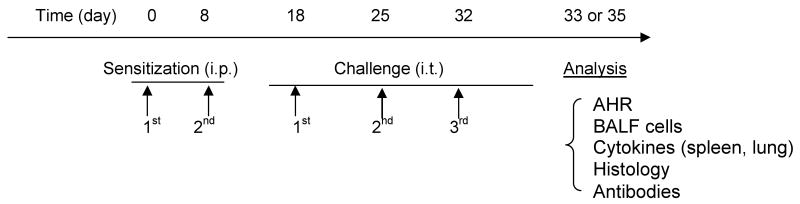 |
Mice (6-week, 6-, 12-, 18-months were sensitized intraperitoneally (i.p.) with OVA (200μg/mouse) twice at weekly intervals and then challenged intratracheally (i.t.) on days 18, 25 and 32 with OVA (100μg/mouse). Analysis was either performed twenty-four hours (BALF) or seventy-two hours after the final challenge (AHR measurement, BALF, lung histology, serum antibodies, spleen cell culture and RNA extraction from lung tissue). Naïve age-matched mice were controls.
Measurement of late-phase airway responsiveness
Seventy-two hours following the final OVA i.t. challenge, airway responsiveness to ACh was measured as previously described26. AHR was defined as the time-integrated change in peak airway pressure (APTI).
BALF preparation and cell differential counts
The chest was opened 24 or 72 hours after the last OVA challenge and BALF collected and total cell number and differential determined as previously described25. The supernatant was transferred and stored at -80°C for IL-5 and murine eotaxin-1 (CCL11) measurement by ELISA according to the manufacturer's directions (R&D Systems).
Lung Histology
Lungs were surgically removed 72 hours post OVA challenge. The left lower lobe was fixed in neutral buffered formaldehyde, and 5-μm paraffin sections were stained with periodic acid-Schiff (PAS) for evaluation of goblet cells, and hematoxylin and eosin for evaluation of inflammation. Slides were examined in a blinded fashion. Mucus expression was calculated adapting a previously published method27, and assessed by determining the percentage of PAS-positive epithelial cells (number of PAS-positive divided by the total cell epithelial cell number) in at least 6 randomly selected bronchioles per animal. Results are expressed as the mean percentage of PAS-positive cells per bronchiole for each group of mice. Perivascular and peribronchial inflammation were graded using a standard scoring method (0=normal, 1=mild, 2=intermediate, 3=severe)28.
RNA extraction and reverse transcription
Seventy-two hours after OVA challenge, lung tissue sections were placed in TRIZOL (Invitrogen, Carlsbad, CA) and frozen at -80°C until RNA extraction. To extract RNA, samples were thawed at 4°C, homogenized and extracted according to manufacturer's direction.
Quantitive real-time PCR
Real time reverse-transcription quantitative PCR (qPCR) was performed using a published SYBR green protocol using a ABI7900 HT machine (Foster City, CA)29, with three housekeeping genes for normalization (beta-actin, RPS11, alpha-tubulin). Primer sequences for MUC-5AC, IL-5, IL-13, RPS11, alpha-tubulin, and beta-actin have been previously published25. Other primer sequences were as follows: IL-4 (sense 5′-ATGGAGCTGCAGAGACTCTT-3′ and anti-sense 5′-AAAGCATGGTGGCTCAGTAC-3′) and IFN-γ (sense 5′-GACTGTATTGCGGGGTTGT-3′ and anti-sense 5′-GGCCCGGAGTGTAGACATCT-3′).
Cell culture and quantification of cytokines
Immediately after APTI measurements, pooled spleen cells from four to five mice were isolated and cultured with either OVA (200μg/ml), Con A (2.5mg/ml, positive control) or medium alone for 72 hours as previously described23. Levels of IL-4, IL-5, IL-13 and IFN-γ in spleen culture supernatants (in triplicate) were determined by ELISA (R&D systems).
Measurement of serum OVA-specific antibodies
Sera were obtained from each group of mice immediately after APTI measurements (72 hours after the third OVA challenge) and stored at -80°C. To determine OVA-specific IgE, plates were coated with OVA and blocked with 1% human serum albumin in PBS. Sera samples (diluted at 1:50) were added and incubated overnight at 4°C. Sheep anti-mouse IgE (0.3μg/ml, BD Pharmigen), followed by biotinylated donkey anti-sheep IgG (0.1μg/ml, BD Pharmigen) were added at room temperature (RT). After washing, avidin-peroxidase (Sigma, 1:1000) was added for 10 minutes at RT and the reactions developed with 2,2′-azine-bis (3-ethylbenzthiazoline-6-sulfonic acid, KPL, Gaithersburg, MD) for 20 minutes in the dark at RT, and read at 405 nm. For measurement of OVA-specific IgG2a and IgG1, plates were coated with OVA and then blocked and washed as above. Serum samples (1:50 for IgG2a, 1:500 for IgG1) were added to the plates in duplicate. Biotinylated rat anti-mouse IgG2a or IgG1 monoclonal antibodies (mAB) (Pharmingen) were added for 40 minutes (IgG2a) or 20 minutes (IgG1) at RT and the reactions developed and read as described above for IgE.
Statistical Analysis
Statistical analyses were performed using one way ANOVA pairwise and multiple comparison testing between groups as well as Mann-Whitney Rank Sum Testing. A p-value <0.05 was considered statistically significant. All statistical analyses were performed using SigmaPlot/Stat software (Systat Software, Inc., Point Richmond, CA).
Results
Induction of airway hyperresponsiveness in aged OVA-mice
Upon sensitization and challenge with OVA, APTI levels were significantly increased in all age groups of mice compared to their naïve age-matched controls (Figure 1). APTI levels were significantly greater (p<0.05) in 6-week old OVA-mice (739.2 ± 162.0 cm H20/sec) compared with each group of aged OVA-mice (394.02 ± 42.1, 321.5 ± 61.8, 324.8 ± 40.5 cm H20/sec, 6-, 12-, 18-months, respectively) (Figure 1). There were no significant differences in APTI levels between naïve control groups.
Figure 1.
Effect of age on airway hyperresponsiveness. APTI after acetylcholine significantly increased in all OVA-mice (greatest in 6-week OVA-mice) compared to naïve age-matched controls, indicating AHR in aged mice. Data are mean ± SEM for 6-9 OVA-mice or 5 naïve mice per groups. *, p<0.05; **, p<0.01; ***, p<0.001 compared to age-matched naïve controls; #, p<0.05 compared to 6-week OVA-mice.
Induction of airway inflammation in aged OVA-mice
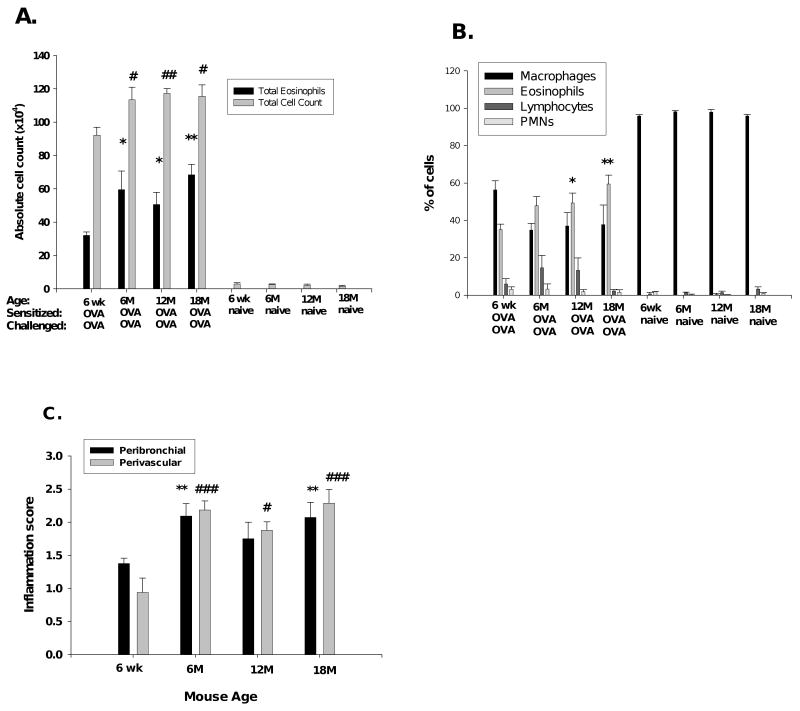
To evaluate the basis for the difference in APTI as well as the effect of aging on pulmonary inflammation after antigen sensitization and challenge, we first determined the total numbers of cells in BALF immediately following APTI measurement. The mean total number of leukocytes and eosinophils were increased significantly in the aged OVA-mice compared with the 6-week OVA-mice (Figure 2A). There was no significant difference in the total cell count between all naïve groups, and no eosinophils were detected. The mean eosinophil percentage was elevated (significantly in the 12- and 18-month mice) in aged OVA-mice compared with the 6-week old OVA-mice (Figure 2B). There were no significant differences in BALF differential counts between all age groups of naïve mice. There was no correlation between total eosinophil numbers and AHR in aged OVA-mice. The Pearson product correlation coefficient between APTI and absolute eosinophil number was 0.884, -0.543, 0.168 and 0.299 for the 6-week, 6-, 12- and 18-month old OVA mice, respectively.
Figure 2.
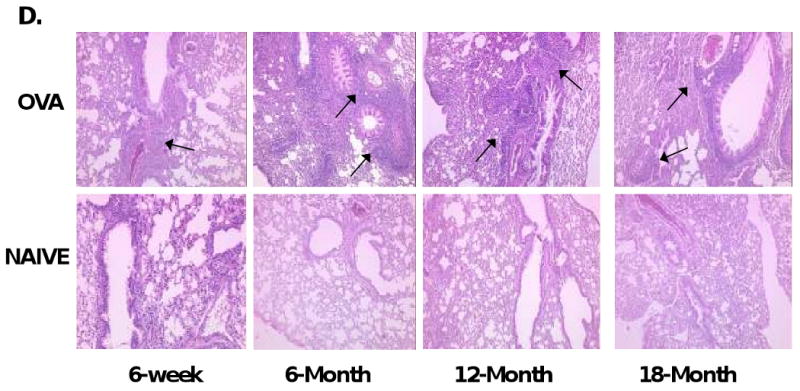
Pulmonary inflammation with aging. (A) Increased total cell and eosinophil counts and (B) eosinophil percentage in aged OVA-mice compared with 6-week OVA-mice. (C) Inflammation grades for peribronchial and perivascular inflammation in OVA-mice. (D) H&E staining (arrows marking increased inflammation) in OVA-mice (10×). One representative histologic sample per group. Data in mean ± SEM for 6-9 OVA-mice or 5 naïve mice per groups. *, #, p<0.05, **, ##, p<0.01, ***, ###, p<0.001 compared to 6-week OVA-mice. wk=week, M=month.
To correlate BALF total cell counts with lung tissue inflammation, H&E staining of lung sections collected 72 hours after OVA-challenge was performed. All OVA-mice demonstrated peribronchial (PB) and perivascular (PV) inflammation, which was not seen in the naïve control mice (Figure 2C). Inflammation grades were greatest in aged OVA-mice (2.091 ± 0.19 (PB, p<0.01), 2.182 ± 0.14 (PV, p<0.001), 6-month; 1.750 ± 0.25 (PB, n.s.), 1.875 ± 0.13 (PV, p<0.05), 12-month; 2.071 ± 0.23 (PB, p<0.001), 2.286 ± 0.21 (PV, p<0.001), 18-month mice) compared to the 6-week OVA-mice (1.375 ± 0.082 (PB), 0.938 ± 0.22 (PV)). Representative examples of histologic findings are shown in Figure 2D. These results demonstrate that i.t. antigen challenge of sensitized aged mice produces increased BALF leukocyte and eosinophil counts and pulmonary inflammation in the aged OVA-mice as compared to young OVA-mice. However, despite increased inflammation, aged OVA-mice exhibited less AHR.
Lung and systemic cytokine production
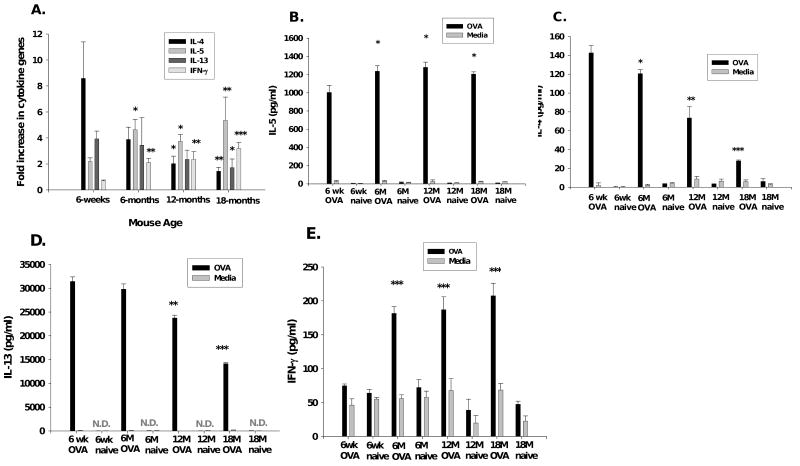
In an attempt to understand the mechanisms of increased lung inflammation and its dichotomy with AHR we studied the induction of cytokines in this model. Cytokine expression following OVA sensitization/challenge was measured in lung tissue using qPCR. (Results are expressed in fold increase in mRNA copy number from OVA-mice compared to mRNA copy number from age-matched naïve mice.) In the aged OVA-mice, the fold increase in IL-5 mRNA expression in lung tissue was significantly increased compared to young 6-week old OVA-mice, suggesting a potential mechanism for the increased airway eosinophils in the aged mice (Figure 3A). The fold expression of mRNA for IL-4 and IL-13 was greatest in the young OVA-mice (Figure 3A). However, the fold increase in lung mRNA for IFN-γ increased with increasing age in the OVA-mice (Figure 3A). To examine cytokine protein levels, we stimulated pooled spleen cells with OVA. Expression of IL-5 significantly increased (p<0.05) with age in the OVA-mice groups (Figure 3B). IL-4, IL-13 and IFN-γ protein also correlated with lung tissue mRNA (Figure 3C, 3D, 3E). Con A stimulation (positive control) of splenocyte cultures produced increased expression of all cytokines (data not shown).
Figure 3.
Pulmonary tissue and spleen-cell culture cytokines. (A) qPCR for IL-4, -5, -13, IFN-γ, expressed as fold increase in copy number. Each OVA-mice group=6-9 mice. Spleen-cells cultured with OVA or medium alone, supernant ELISA for (B) IL-5, (C) IL-4, (D) IL-13, (E) IFN-γ. Culture results from pooled data from three experiments are presented as mean±standard error. *, p<0.05; **, p<0.01; ***, p<0.001 compared to 6-week OVA-mice. N.D.=non-detectable.
Modulation of lung eosinophilia by eotaxin and IL-5
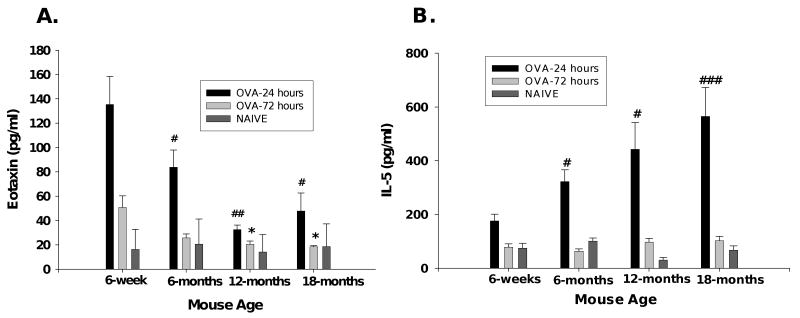
To evaluate the mechanism underlying the differences in lung eosinophilic inflammation between young and aged OVA-mice, we measured the eosinophil chemotaxic factors eotaxin and IL-5 in BALF. Both eotaxin and IL-5 expression was greater at the 24 hours than at 72-hours (Figure 4A, 4B), as found previously in young OVA-mice30. BALF eotaxin was significantly elevated in 6-week old OVA-mice at 24-hours (135.5 ± 22.86 pg/ml) compared to the aged OVA-mice (83.93 ± 13.94, 32.67 ± 3.66, 47.85 ± 14.75 pg/ml, 6-, 12-, 18-months respectively). By 72 hours, BALF eotaxin returned to baseline (similar to that of naïve mice) in all of the aged mice, but was still elevated in 6-week old mice. In contrast, mean IL-5 protein levels in BALF collected 24 hours after the final OVA challenge significantly increased with increasing age (176.2 ± 24.92, 323.2 ± 42.82, 443.1 ± 98.89, 565.2 ± 107.59, 6-week, 6-, 12- and 18-month, respectively). BALF IL-5 returned to baseline at 72 hours. Thus, the increase in BALF eosinophils in aged mice correlates with increased IL-5, but not eotaxin.
Figure 4.
Eosinophil chemoattractant factors. BALF fluid supernatant collected 24- or 72-hours after OVA-challenge; eotaxin and IL-5 protein expression determined by ELISA. (A) Eotaxin protein decreases in aged OVA-mice compared to young OVA-mice. (B) IL-5 protein increases in aged OVA-mice. Data are mean ± SEM using 5-9 mice/group. *, #, p<0.05; **, ##, p<0.01; ###, p<0.001.
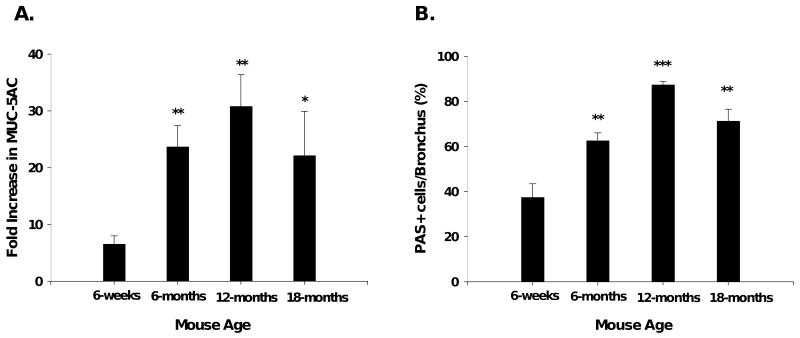
Mucus cell metaplasia increased with age in OVA-mice
As mucus cell metaplasia has been associated with increased inflammation and eosinophilia31, 32, we performed real time PCR for the major airway mucus producing gene, MUC-5AC33, performed on lung tissue. Results are expressed as the fold change in mRNA copy number of MUC-5AC in the OVA-mice compared to their age-matched naïve controls. MUC-5AC mRNA expression was significantly increased in aged OVA-mice compared to the young OVA-mice (Figure 5A). To correlate the increase in mRNA for MUC-5AC with histologic changes, lung tissue sections were stained for PAS. The number of positively staining PAS cells in bronchioles were significantly increased in aged OVA-mice, consistent with the pattern of the mRNA MUC-5AC expression (Figure 5B). There were no PAS positive airway epithelial cells in naïve mice. Figure 5C shows representative histology of OVA-sensitized/challenged and naïve mice, demonstrating increased PAS staining in lungss of the aged OVA-groups.
Figure 5.
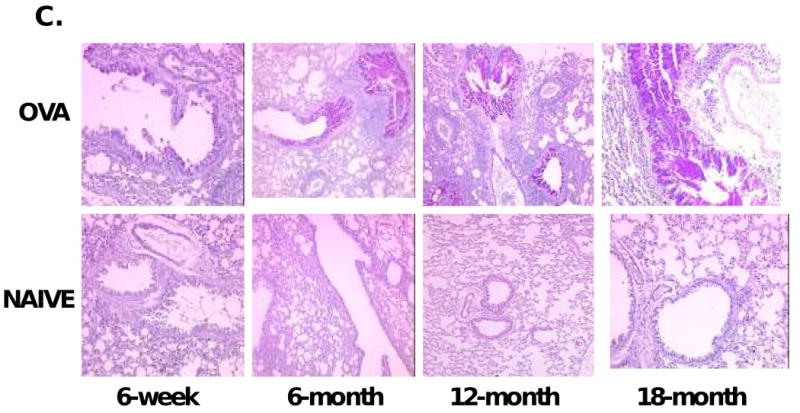
Airway mucus metaplasia. (A) qPCR for MUC-5AC expressed as fold increase in copy number; n=5-8 mice/group. (B) PAS grading. (C) PAS positive (deep purple) staining demonstrating mucus metaplasia in 6-week (10×), 6-month (10×), 12-month (10X), 18-month (20×) OVA-mice; none in age-matched naïve mice. One representative histologic sample per group shown. *, p<0.05; **, p<0.01; ***, p<0.001 compared to 6-week OVA-mice.
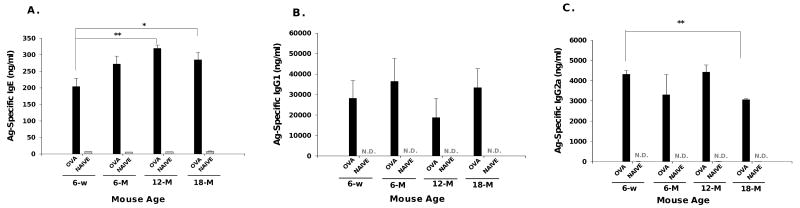
Development of antigen-specific IgE in aged mice
To assess the degree of sensitization of aged mice, we measured OVA-specific serum IgE. All mice developed significantly elevated serum specific IgE to OVA in response to OVA sensitization and challenge (Figure 6A). Aged OVA-mice exhibited higher levels of OVA specific serum IgE than 6-week old OVA-mice, reaching statistical significance in the 12- and 18-month old groups. OVA-specific IgE was virtually undetectable in all aged naïve mice. Serum OVA-specific IgG1 levels were not significantly different in young and aged groups of OVA-mice, but were significantly increased compared to naïve age-matched control mice, where OVA-specific IgG1 was nearly undetectable (Figure 6B). Similar to IgG1, all OVA-mice had significantly increased OVA-specific IgG2a compared to their age-matched controls (Figure 6C). OVA-specific serum IgG2a was significantly (p<0.01) elevated in 6-week OVA-mice compared to 18-month old OVA-mice, but not significantly different from the 6- or 12-month OVA-mice. OVA-specific IgG2a was nearly undetectable in all naïve mice.
Figure 6.
Serum OVA-specific immunoglobulin. OVA-specific (A) IgE, (B) IgG1 and (C) IgG2a determined by ELISA from sera collected 72 hours after final OVA-challenge. OVA-specific IgG1, IgG2a were undetectable for naïve mice, labeled as N.D (non-detectable). Data expressed in mean ± SEM for using 5-9 mice per group. *, p<0.05, **, p<0.01 compared to 6-week OVA-mice. w=week, M=month
Discussion
Asthma is a heterogeneous disease with several phenotypes including childhood onset, late onset, allergen-induced, nocturnal, and exercise-induced. For many years, the dogma was that late onset asthma is rare and not atopic. However, several reports in humans have suggested otherwise2, 5, 7, 8. As part of the Normative Aging Study, the presence of IgE antibody to dust mite antedated the development new-onset wheezing in late adulthood8, and in a Japanese cohort of asthmatics, the proportion of skin-test positive patients among late onset asthmatics was significantly larger than that seen in a younger-onset group34. Despite the occurrence of late onset allergic asthma, the effect of aging on allergic airway inflammation in humans or in aged murine asthma models is not well characterized.
In the present study we sensitized and intratracheally challenged young (6-week) and older (6, 12, 18 month) mice with OVA and demonstrated that aged mice develop a different pulmonary pathology with greater inflammation, notably increased BALF eosinophils, and increased mucus cell metaplasia compared to the younger mice. Furthermore, the aged antigen treated mice also developed a distinct cytokine pattern (elevated IL-5 and IFN-γ) in their lung tissue and spleen-cell cultures, whereas similarly treated younger mice responded with a Th2 pattern alone. Despite this, antigen sensitization and challenge produced significantly lower AHR in the aged OVA-mice compared with the young-OVA mice. The difference in AHR between older and young mice was antigen-specific because there was no difference in airway responses (APTI) to ACh provocation between aged and young naïve mice. These age-related inflammatory changes in response to antigen sensitization and challenge are illustrated in Table II.
Table 2. Features of airway inflammation in different ages of antigen treated mice.
| Tissue | BALF | Mucus | Airway cytokine | Spleen cell culture | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | Inflam‡ | Eosinophils | Metaplasia* | IL-5 | IL-4 | IL-13 | IL-5γ | IL-5 | IL-4 | IL-13 | IL-5γ | AHR | OVA- IgE¶ |
| 6 week | + | + | + | + | +++ | ++ | - | +++ | +++ | +++ | + | +++ | + |
| 6 month | ++ | ++ | ++ | ++ | ++ | ++ | + | +++ | ++ | +++ | ++ | ++ | ++ |
| 12 month | ++ | ++ | +++ | ++ | + | + | + | +++ | + | ++ | ++ | ++ | +++ |
| 18 month | ++ | +++ | ++ | ++ | + | + | + | +++ | + | + | +++ | ++ | ++ |
Determined by histology and BALF total cell count
Determined by PAS staining and MUC-5AC mRNA expression
Serum levels
There are only a few reports in the literature investigating airway inflammation in aged antigen sensitized and challenged rodents; one in aged Brown Norway rats (100-120 weeks)35 and two in BALB/c mice (10 or 12-months)18, 19. These studies measured airway inflammation by BALF collection and histology (in one study), and found increased total leukocyte cell count and eosinophil percentage in the young, but not in the aged antigen sensitized rodents. Our protocol using BALB/c mice differed slightly in that we employed a more rigorous and chronic antigen challenge protocol (over 3 weeks) compared to two or three consecutive days and we used several groups of aging mice which included older groups (12- and 18-months). Additionally, the mice were antigen challenged intratracheally, which directly introduces the antigen into the airways and allows for less gastric deposition of antigen21, whereas the previous two studies in BALB/c mice used aerosol challenges.
Although our findings of greater lung eosinophilia and inflammation in antigen sensitized and challenged aged mice differs from previous aged rodent studies, it is similar to a human study which compared characteristics of adult-onset to childhood-onset asthma. Miranda et al found that there was significantly greater airway tissue and lavage fluid eosinophilia in patients who presented with asthma at a mean age of 27 ± 1.3 years, than in asthmatics who developed airway disease at a mean age of 2.6 ± 1.0 years4. (However, it should be noted that our aged mice represent a relatively older age group).
To further define eosinophil recruitment to the lungs of our aged antigen sensitized mice, we measured two eosinophil chemoattractants, IL-5 and eotaxin in the BALF supernatant. We found that IL-5 levels were higher and eotaxin levels were lower in the lungs of aged OVA-mice than young OVA-mice. Our data suggest that IL-5 may be more important than eotaxin in airway eosinophil recruitment in aged OVA-mice. One potential explanation for a decrease in eotaxin production in the aged OVA-mice is that fibroblasts, which are one source of airway eotaxin36, decrease with aging37.
Despite increased pulmonary inflammation and eosinophilia in the aged OVA-mice, AHR was more pronounced in the young OVA-mice. However, unlike previous murine studies19, 38, we found that antigen sensitization and challenge of aged mice, produced AHR. The relationship between airway inflammation, specifically eosinophilia, and AHR in asthma is not entirely clear, and although a causal relationship has been proposed, this hypothesis is controversial39-41. Furthermore, the role of the eosinophil in AHR of aged patients or in animal models of asthma may differ. In the Miranda et al study, patients with later-onset disease showed a weaker association between asthma symptoms and eosinophilia compared to those with early-onset disease. In addition, the presence or absence of eosinophils in the late-onset group was not associated with significant differences in pulmonary function4. Our results potentially argue against a direct relationship between pulmonary inflammation and eosinophils in AHR in aged antigen-challenged sensitized animals, although further studies are necessary.
The mechanisms of AHR are complex, and other factors besides airway inflammation and eosinophilia are involved in its induction, including direct cytokine modulation. We demonstrated that antigen sensitization and challenge of older mice produced a unique cytokine profile as determined by gene expression in lung tissue and protein levels in spleen cell cultures.
The aged OVA-mice all developed increased IL-4, -5, -13 when compared to their age-matched naïve controls, suggesting that antigen sensitization stimulates a Th2 like response in aged mice. However, the young OVA-mice had a greater fold change in mRNA gene expression of IL-4 and IL-13, but not for IL-5. IL-5 protein levels from lung tissue and spleen cell cultures in the young OVA-mice paralleled lung tissue mRNA expression. The aged OVA-mice also produced more IFN-γ than the younger OVA-mice. One previous study of antigen sensitized/challenged aged BALB/c mice examined cytokine profiles in BALF by ELISA and found both IL-4 and IL-5 to be significantly decreased compared to the young antigen sensitized mice19. Another study of aged mice demonstrated decreased IL-4, -5, -13 cytokine expression in purified naïve CD4+ T cell by splenocytes of aging OVA-specific TCR αβ transgenic mice stimulated with OVA peptide and irradiated APCs from young BALB/c mice18, a protocol which may not reflect cytokine changes in lungs following antigen sensitization and challenge. These studies also found increased IFN-γ in the aged OVA-mice compared to the young OVA-mice, similar to our data.
The distinct cytokine pattern in the antigen sensitized and challenged aged mice could explain why the AHR in these groups was not as significantly elevated when compared with that of the young OVA-mice. IL-13 can induce AHR independently of eosinophilia42, and like IL-4, directly stimulates airway smooth muscle cells41 43. Therefore, the lower IL-4 and IL-13 might explain a decreased AHR independent of inflammation in the aged OVA-mice. Furthermore, the effects of IL-4 and IL-13 on airway smooth muscle may be important as its function and response may change with aging. Additionally, IFN-γ, elevated in both the lung tissue and spleen cell culture in the aged OVA-mice, may offer protection from AHR44, 45. A recent study demonstrated that over-expression of IFN-γ in the airways of antigen sensitized and challenged mice increased airway inflammation, but decreased AHR compared to wild-type mice46.
Decreased gastric mucus production with aging has been reported47, but mucus production has not been evaluated in the airway of aged rodents. None of the previous studies using aged antigen sensitized rodents have evaluated mucus cell metaplasia. The mRNA expression of the airway mucin-producing gene, MUC-5AC, was increased in the aged OVA-mice compared with the younger OVA-mice. Quantification of positive PAS airway staining paralleled the MUC-5AC mRNA expression. IL-13 and IL-5 have been suggested to be important cytokines inducing mucus metaplasia and MUC-5AC expression in the airways31, 48; the role of IL-4 in mucus hypersecretion is less well established49. However, in aged mice, IL-13 and IL-4 may not be as critical for mucus induction, and IL-5 may be more important; this requires further investigation. Several other cytokines including IL-6, IL-9, TNF-α, interleukin 1β, and factors including oxidant stress, proteases, and histamine have also been shown to induce airway mucus cell metaplasia/hyperplasia and mucin-gene expression25, 48, and may be more relevant factors for mediating these effects during aging. Furthermore, the eosinophil may induce mucus cell metaplasia31, 32, 50, offering an explanation in our model. Finally in the aged OVA-mice, mucus hypersecretion did not appear to directly correlate with AHR. The effect of mucus hypersecretion on AHR is not clearly established51, 52 (unpublished data), and may differ in an aged population.
Finally, we demonstrated that antigen sensitized and chronically challenged aged mice develop Ag-specific IgE. A previous study showed that OVA-specific IgE was detected in sensitized aged rats, but was decreased compared to younger rats38. One of the studies using BALB/c mice found a lower total serum IgE in the aged OVA-treated mice compared to the younger mice19. They did not, however, measure OVA-specific IgE. In our study, the aged OVA-mice produced more OVA-specific IgE than younger mice, suggesting that although sensitization can occur later in life, other factors besides, or in conjunction with, IgE regulate AHR in aged rodents. The mechanism of increased OVA-specific IgE production in the aged OVA-mice despite lower lung tissue mRNA IL-4 expression and IL-4 in splenocyte culture is more difficult to explain. However, it may be that the level of IL-4 protein expression was sufficient to induce class switching toward IgE in the plasma cells of the aged OVA-mice.
In conclusion, this is the first study to demonstrate increased pulmonary eosinophilia, inflammation and increased mucus cell metaplasia in antigen sensitized and challenged older mice. In addition, antigen sensitization/challenge of aged mice produced an altered cytokine pattern. Contrary to previous reports, we demonstrated that aged mice are able to develop AHR. Although increased AHR was found in the aged antigen sensitized/challenged mice, it was not as great as younger antigen sensitized/challenged mice, suggesting discordance between airway inflammation and AHR with aging. This study is important because it suggests that antigen sensitization and challenge of aged mice produces a distinct pattern of pulmonary inflammation and cytokine expression and offers a murine model of late onset allergic asthma.
Acknowledgments
We thank Dr. Stuart Sealfon and Bernard Lin from the Mount Sinai School of Medicine Real Time core PCR facility, and Sami Termani for their excellent technical assistance.
This work was funded by a grant from the A.A.A.A.I and the Hartford Foundation for Aging (T. Franklin Williams Scholars Program) (P.B.), and P01 AT002647 (Li).
Abbreviations
- APTI
Airway pressure time index
- AHR
Airway hyperresponsiveness
- BALF
Bronchoalveolar fluid
- i.t.
Intratracheal
- i.p.
Intraperitoneal
- OVA
Ovalbumin
- PMNs
Polymononuclear cells
- RT
Room temperature
References
- 1.Broder I, Higgins MW, Mathews KP, Keller JB. Epidemiology of asthma and allergic rhinitis in a total community, Tecumseh, Michigan. 3. Second survey of the community. J Allergy Clin Immunol. 1974;53(3):127–38. doi: 10.1016/0091-6749(74)90001-3. [DOI] [PubMed] [Google Scholar]
- 2.Enright PL, McClelland RL, Newman AB, Gottlieb DJ, Lebowitz MD. Underdiagnosis and undertreatment of asthma in the elderly. Cardiovascular Health Study Research Group. Chest. 1999;116(3):603–13. doi: 10.1378/chest.116.3.603. [DOI] [PubMed] [Google Scholar]
- 3.Hizawa N, Yamaguchi E, Konno S, Tanino Y, Jinushi E, Nishimura M. A functional polymorphism in the RANTES gene promoter is associated with the development of late-onset asthma. Am J Respir Crit Care Med. 2002;166(5):686–90. doi: 10.1164/rccm.200202-090OC. [DOI] [PubMed] [Google Scholar]
- 4.Miranda C, Busacker A, Balzar S, Trudeau J, Wenzel SE. Distinguishing severe asthma phenotypes: role of age at onset and eosinophilic inflammation. J Allergy Clin Immunol. 2004;113(1):101–8. doi: 10.1016/j.jaci.2003.10.041. [DOI] [PubMed] [Google Scholar]
- 5.Ariano R, Panzani RC, Augeri G. Late onset asthma clinical and immunological data: importance of allergy. J Investig Allergol Clin Immunol. 1998;8(1):35–41. [PubMed] [Google Scholar]
- 6.Quadrelli SA, Roncoroni AJ. Is asthma in the elderly really different? Respiration. 1998;65(5):347–53. doi: 10.1159/000029294. [DOI] [PubMed] [Google Scholar]
- 7.Burrows B, Barbee RA, Cline MG, Knudson RJ, Lebowitz MD. Characteristics of asthma among elderly adults in a sample of the general population. Chest. 1991;100(4):935–42. doi: 10.1378/chest.100.4.935. [DOI] [PubMed] [Google Scholar]
- 8.Litonjua AA, Sparrow D, Weiss ST, O'Connor GT, Long AA, Ohman JL., Jr Sensitization to cat allergen is associated with asthma in older men and predicts new-onset airway hyperresponsiveness. The Normative Aging Study. Am J Respir Crit Care Med. 1997;156(1):23–7. doi: 10.1164/ajrccm.156.1.9608072. [DOI] [PubMed] [Google Scholar]
- 9.Prescott SL, Macaubas C, Holt BJ, et al. Transplacental priming of the human immune system to environmental allergens: universal skewing of initial T cell responses toward the Th2 cytokine profile. J Immunol. 1998;160(10):4730–7. [PubMed] [Google Scholar]
- 10.Holt PG, Macaubas C, Prescott SL, Sly PD. Primary sensitization to inhalant allergens. Am J Respir Crit Care Med. 2000;162(3 Pt 2):S91–4. doi: 10.1164/ajrccm.162.supplement_2.ras-7. [DOI] [PubMed] [Google Scholar]
- 11.Forsthuber T, Yip HC, Lehmann PV. Induction of TH1 and TH2 immunity in neonatal mice. Science. 1996;271(5256):1728–30. doi: 10.1126/science.271.5256.1728. [DOI] [PubMed] [Google Scholar]
- 12.Adkins B, Du RQ. Newborn mice develop balanced Th1/Th2 primary effector responses in vivo but are biased to Th2 secondary responses. J Immunol. 1998;160(9):4217–24. [PubMed] [Google Scholar]
- 13.Poynter ME, Daynes RA. Age-associated alterations in splenic iNOS regulation: influence of constitutively expressed IFN-gamma and correction following supplementation with PPARalpha activators or vitamin E. Cell Immunol. 1999;195(2):127–36. doi: 10.1006/cimm.1999.1525. [DOI] [PubMed] [Google Scholar]
- 14.Bandres E, Merino J, Vazquez B, et al. The increase of IFN-gamma production through aging correlates with the expanded CD8(+high)CD28(-)CD57(+) subpopulation. Clin Immunol. 2000;96(3):230–5. doi: 10.1006/clim.2000.4894. [DOI] [PubMed] [Google Scholar]
- 15.Rink L, Cakman I, Kirchner H. Altered cytokine production in the elderly. Mech Ageing Dev. 1998;102(23):199–209. doi: 10.1016/s0047-6374(97)00153-x. [DOI] [PubMed] [Google Scholar]
- 16.Kubo M, Cinader B. Polymorphism of age-related changes in interleukin (IL) production: differential changes of T helper subpopulations, synthesizing IL 2, IL 3 and IL 4. Eur J Immunol. 1990;20(6):1289–96. doi: 10.1002/eji.1830200614. [DOI] [PubMed] [Google Scholar]
- 17.Lio D, D'Anna C, Scola L, et al. Interleukin-5 production by mononuclear cells from aged individuals: implication for autoimmunity. Mech Ageing Dev. 1999;106(3):297–304. doi: 10.1016/s0047-6374(98)00122-5. [DOI] [PubMed] [Google Scholar]
- 18.Hasegawa A, Miki T, Hosokawa H, et al. Impaired GATA3-dependent chromatin remodeling and Th2 cell differentiation leading to attenuated allergic airway inflammation in aging mice. J Immunol. 2006;176(4):2546–54. doi: 10.4049/jimmunol.176.4.2546. [DOI] [PubMed] [Google Scholar]
- 19.Gelfand EW, Joetham A, Cui ZH, et al. Induction and maintenance of airway responsiveness to allergen challenge are determined at the age of initial sensitization. J Immunol. 2004;173(2):1298–306. doi: 10.4049/jimmunol.173.2.1298. [DOI] [PubMed] [Google Scholar]
- 20.Institute of Laboratory Animal Resources Commissions and Life Sciences, NCR. Guide for the care and use of laboratory animals. Washington (DC): National Academy Press; 1996. National Institutes of Health publication 86-23. [Google Scholar]
- 21.Eyles JE, Spiers ID, Williamson ED, Alpar HO. Tissue distribution of radioactivity following intranasal administration of radioactive microspheres. J Pharm Pharmacol. 2001;53(5):601–7. doi: 10.1211/0022357011775929. [DOI] [PubMed] [Google Scholar]
- 22.Serebrisky D, Teper AA, Huang CK, et al. CpG oligodeoxynucleotides can reverse Th2-associated allergic airway responses and alter the B7.1/B7.2 expression in a murine model of asthma. J Immunol. 2000;165(10):5906–12. doi: 10.4049/jimmunol.165.10.5906. [DOI] [PubMed] [Google Scholar]
- 23.Li XM, Huang CK, Zhang TF, et al. The chinese herbal medicine formula MSSM-002 suppresses allergic airway hyperreactivity and modulates TH1/TH2 responses in a murine model of allergic asthma. J Allergy Clin Immunol. 2000;106(4):660–8. doi: 10.1067/mai.2000.110102. [DOI] [PubMed] [Google Scholar]
- 24.Busse PJ, Wen MC, Huang CK, Srivastava KD, Zhang TF, Schofield B, Sampson HA, Li XM. Therapuetic effects of the Chinese herbal formula, MSSM-003d, on persistent airway hyperresponsiveness and airway remodeling. J Allergy Clin Immunol. 2004;113(2):S220. [Google Scholar]
- 25.Busse PJ, Zhang TF, Srivastava K, et al. Chronic exposure to TNF-alpha increases airway mucus gene expression in vivo. J Allergy Clin Immunol. 2005;116(6):1256–63. doi: 10.1016/j.jaci.2005.08.059. [DOI] [PubMed] [Google Scholar]
- 26.Li XM, Chopra RK, Chou TY, Schofield BH, Wills-Karp M, Huang SK. Mucosal IFN-gamma gene transfer inhibits pulmonary allergic responses in mice. J Immunol. 1996;157(8):3216–9. [PubMed] [Google Scholar]
- 27.Cho JY, Miller M, Baek KJ, et al. Immunostimulatory DNA sequences inhibit respiratory syncytial viral load, airway inflammation, and mucus secretion. J Allergy Clin Immunol. 2001;108(5):697–702. doi: 10.1067/mai.2001.119918. [DOI] [PubMed] [Google Scholar]
- 28.Bachelet I, Munitz A, Levi-Schaffer F. Abrogation of allergic reactions by a bispecific antibody fragment linking IgE to CD300a. J Allergy Clin Immunol. 2006;117(6):1314–20. doi: 10.1016/j.jaci.2006.04.031. [DOI] [PubMed] [Google Scholar]
- 29.Yuen T, Wurmbach E, Pfeffer RL, Ebersole BJ, Sealfon SC. Accuracy and calibration of commercial oligonucleotide and custom cDNA microarrays. Nucleic Acids Res. 2002;30(10):e48. doi: 10.1093/nar/30.10.e48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eum SY, Maghni K, Hamid Q, et al. Inhibition of allergic airways inflammation and airway hyperresponsiveness in mice by dexamethasone: role of eosinophils, IL-5, eotaxin, and IL-13. J Allergy Clin Immunol. 2003;111(5):1049–61. doi: 10.1067/mai.2003.1416. [DOI] [PubMed] [Google Scholar]
- 31.Shen HH, Ochkur SI, McGarry MP, et al. A causative relationship exists between eosinophils and the development of allergic pulmonary pathologies in the mouse. J Immunol. 2003;170(6):3296–305. doi: 10.4049/jimmunol.170.6.3296. [DOI] [PubMed] [Google Scholar]
- 32.Burgel PR, Lazarus SC, Tam DC, et al. Human eosinophils induce mucin production in airway epithelial cells via epidermal growth factor receptor activation. J Immunol. 2001;167(10):5948–54. doi: 10.4049/jimmunol.167.10.5948. [DOI] [PubMed] [Google Scholar]
- 33.Zuhdi Alimam M, Piazza FM, Selby DM, Letwin N, Huang L, Rose MC. Muc-5/5ac mucin messenger RNA and protein expression is a marker of goblet cell metaplasia in murine airways. Am J Respir Cell Mol Biol. 2000;22(3):253–60. doi: 10.1165/ajrcmb.22.3.3768. [DOI] [PubMed] [Google Scholar]
- 34.Atsuta R, Akiyama K, Shirasawa T, Okumura K, Fukuchi Y, Ra C. Atopic asthma is dominant in elderly onset asthmatics: possibility for an alteration of mast cell function by aging through Fc receptor expression. Int Arch Allergy Immunol. 1999;120 1:76–81. doi: 10.1159/000053600. [DOI] [PubMed] [Google Scholar]
- 35.Ide K, Hayakawa H, Yagi T, et al. Decreased expression of Th2 type cytokine mRNA contributes to the lack of allergic bronchial inflammation in aged rats. J Immunol. 1999;163(1):396–402. [PubMed] [Google Scholar]
- 36.Sato E, Nelson DK, Koyama S, Hoyt JC, Robbins RA. Inflammatory cytokines modulate eotaxin release by human lung fibroblast cell line. Exp Lung Res. 2001;27(2):173–83. doi: 10.1080/019021401750069401. [DOI] [PubMed] [Google Scholar]
- 37.Hasenmaile S, Pawelec G. The concept of telomeric non-reciprocal recombination (TENOR) applied to human fibroblasts grown in serial cultures: concordance with genealogical data. Rejuvenation Res. 2005;8(3):154–71. doi: 10.1089/rej.2005.8.154. [DOI] [PubMed] [Google Scholar]
- 38.Yagi TSA, Hayakawa H, Ide K. Failure of aged rats to accomulate eosinophils in allergic inflammation of the airway. J Allergy Clin Immunol. 1997;99:38. doi: 10.1016/s0091-6749(97)70298-7. [DOI] [PubMed] [Google Scholar]
- 39.Brusasco V, Crimi E, Pellegrino R. Airway hyperresponsiveness in asthma: not just a matter of airway inflammation. Thorax. 1998;53(11):992–8. doi: 10.1136/thx.53.11.992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Flood-Page P, Menzies-Gow A, Phipps S, et al. Anti-IL-5 treatment reduces deposition of ECM proteins in the bronchial subepithelial basement membrane of mild atopic asthmatics. J Clin Invest. 2003;112(7):1029–36. doi: 10.1172/JCI17974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Venkayya R, Lam M, Willkom M, Grunig G, Corry DB, Erle DJ. The Th2 lymphocyte products IL-4 and IL-13 rapidly induce airway hyperresponsiveness through direct effects on resident airway cells. Am J Respir Cell Mol Biol. 2002;26(2):202–8. doi: 10.1165/ajrcmb.26.2.4600. [DOI] [PubMed] [Google Scholar]
- 42.Walter DM, McIntire JJ, Berry G, et al. Critical role for IL-13 in the development of allergen-induced airway hyperreactivity. J Immunol. 2001;167(8):4668–75. doi: 10.4049/jimmunol.167.8.4668. [DOI] [PubMed] [Google Scholar]
- 43.Eum SY, Maghni K, Tolloczko B, Eidelman DH, Martin JG. IL-13 may mediate allergen-induced hyperresponsiveness independently of IL-5 or eotaxin by effects on airway smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2005;288(3):L576–84. doi: 10.1152/ajplung.00380.2003. [DOI] [PubMed] [Google Scholar]
- 44.Yoshida M, Leigh R, Matsumoto K, et al. Effect of interferon-gamma on allergic airway responses in interferon-gamma-deficient mice. Am J Respir Crit Care Med. 2002;166(4):451–6. doi: 10.1164/rccm.200202-095OC. [DOI] [PubMed] [Google Scholar]
- 45.Patel HJ, Belvisi MG, Donnelly LE, Yacoub MH, Chung KF, Mitchell JA. Constitutive expressions of type I NOS in human airway smooth muscle cells: evidence for an antiproliferative role. Faseb J. 1999;13(13):1810–6. doi: 10.1096/fasebj.13.13.1810. [DOI] [PubMed] [Google Scholar]
- 46.Koch M, Witzenrath M, Reuter C, et al. Role of local pulmonary IFN-gamma expression in murine allergic airway inflammation. Am J Respir Cell Mol Biol. 2006;35(2):211–9. doi: 10.1165/rcmb.2005-0293OC. [DOI] [PubMed] [Google Scholar]
- 47.Newton JL. Changes in upper gastrointestinal physiology with age. Mech Ageing Dev. 2004;125(12):867–70. doi: 10.1016/j.mad.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 48.Rogers DF. Airway mucus hypersecretion in asthma: an undervalued pathology? Curr Opin Pharmacol. 2004;4(3):241–50. doi: 10.1016/j.coph.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 49.Chen Y, Thai P, Zhao YH, Ho YS, DeSouza MM, Wu R. Stimulation of airway mucin gene expression by interleukin (IL)-17 through IL-6 paracrine/autocrine loop. J Biol Chem. 2003;278(19):17036–43. doi: 10.1074/jbc.M210429200. [DOI] [PubMed] [Google Scholar]
- 50.Lee JJ, Dimina D, Macias MP, et al. Defining a link with asthma in mice congenitally deficient in eosinophils. Science. 2004;305(5691):1773–6. doi: 10.1126/science.1099472. [DOI] [PubMed] [Google Scholar]
- 51.Bai TR, Knight DA. Structural changes in the airways in asthma: observations and consequences. Clin Sci (Lond) 2005;108(6):463–77. doi: 10.1042/CS20040342. [DOI] [PubMed] [Google Scholar]
- 52.Wu CA, Puddington L, Whiteley HE, et al. Murine cytomegalovirus infection alters Th1/Th2 cytokine expression, decreases airway eosinophilia, and enhances mucus production in allergic airway disease. J Immunol. 2001;167(5):2798–807. doi: 10.4049/jimmunol.167.5.2798. [DOI] [PubMed] [Google Scholar]