Abstract
Introduction
The sensitivity of the in vivo binding of [11C]dihydrotetrabenazine ([11C]DTBZ) and [11C]methylphenidate ([11C]MPH) to their respective targets, the vesicular monoamine transporter (VMAT2) and the neuronal membrane dopamine transporter (DAT), after alterations of endogenous levels of dopamine were examined in the rat brain.
Methods
In vivo binding of [11C]DTBZ and [11C]MPH were determined using a bolus+infusion protocol. In vitro numbers of VMAT2 binding sites were determined by autoradiography.
Results
Repeated dosing with α-methyl-p-tyrosine (AMPT) at doses that significantly (−75%) depleted brain tissue dopamine levels resulted in increased (+36%) in vivo [11C]DTBZ binding to VMAT2 in the striatum. The increase in binding could be completely reversed by treatment with L-DOPA/benserazide to restore dopamine levels. There were no changes in total numbers of VMAT2 binding sites as measured using in vitro autoradiography. No changes were observed for in vivo [11C]MPH binding to the DAT in the striatum following AMPT pretreatment.
Conclusion
These results indicate that large reductions of dopamine concentrations in the rat brain can produce modest but significant changes in binding of radioligands to the VMAT2, which can be reversed by repleneshment of dopamine using exogenous L-DOPA.
Keywords: tetrabenazine; dopamine; tomography,emission computed; vesicular monoamine transporter; alpha-methyl para-tyrosine
1. Introduction
(+)-α-Dihydrotetrabenazine (DTBZ: (+)-2(R)-hydroxy-3(R)-isobutyl-9,10-dimethoxy-1,3,4,6,7-hexahydro-11b(R)H-benzo[a]quinolizine) is a specific and high affinity (Kd=1 nM) ligand for the vesicular monoamine transporter type 2 (VMAT2). In radiolabeled forms it is a useful in vitro and in vivo radioligand for studies of the distribution and concentration of vesicular monoamine transporter binding sites in the mammalian brain. This includes the use of the carbon-11 labeled form for positron emission tomography (PET) imaging of the vesicular monoamine transporter site in the living human brain [1]. Studies of the vesicular monoamine transporter as a marker of monoaminergic nerve terminal densities have been completed in a variety of neurodegenerative and psychiatric diseases [2-9]. [11C]DTBZ has been particularly useful as a marker of dopaminergic terminal densities in the human striatum, due to the high proportion (>95%) of dopaminergic versus other monoaminergic terminals in that brain region.
Although studies have supported the conclusion that the total numbers of VMAT2 in the striatum are not readily (if at all) regulated by a wide variety of acute or chronic pharmacological manipulations of the monoaminergic or cholinergic neurotransmitter systems [10-14], the potential effects of acute drug challenges which significantly alter the cytosolic or vesicular concentrations of dopamine have been less well examined. In studies of DOPA-responsive dystonia, a human neurological disorder resulting from a complete lack of ability to synthesize endogenous dopamine, there was observed a modest (20%) but significant increase in [11C]DTBZ binding in the caudate nucleus and the putamen [5]. The authors concluded that the increased [11C]DTBZ binding reflected, at least in part if not in whole, the greater availability of VMAT2 binding sites due to lack of competition from vesicular dopamine. In recent studies in rats, pharmacological manipulations of dopamine levels using dopamine depleting agents (α-methyl-paratyrosine (AMPT) or amphetamine) or dopamine elevating agents (L-DOPA or γ-hydroxybutyrate) produced modest (12-20%) changes of in vivo [11C]DTBZ binding [15]. Finally, L-DOPA administration to subjects with advanced Parkinson's disease was shown to produce reductions of [11C]DTBZ binding [16].
Depletion of dopamine in the brain of animals and humans is readily accomplished by the administration of α-methyl-para-tyrosine (AMPT), an inhibitor of tyrosine hydroxylase, which is the rate limiting step in the enzymatic synthesis of catecholamines. We report here that severe depletion of brain dopamine levels by AMPT does increase in vivo binding of both the VMAT2 radioligand (+)-[11C]dihydrotetrabenazine, and the increase can be blocked by restoration of dopamine levels. Parts of this work have been previously presented in abstract form [17].
2. Materials and Methods
2.1 Materials
α-Methyl-DL-tyrosine methyl ester hydrochloride (AMPT), DL-serine 2-[2,3,4-trihydroxybenzyl]-hydrazide hydrochloride (benserazide), and L-3,4-dihydroxyphenyl alanine methyl ester (methyl L-DOPA) hydrochloride were obtained from Sigma-Aldrich Corp., St. Louis, MO, USA. Sterile sodium phosphate (45 mM) was obtained from Hospira, Inc., Lake Forest, Il, USA. (+)-α-[11C]Dihydrotetrabenazine and d-threo-[11C]methylphenidate (specific activities 20000-80000 GBq/mmol at end of syntheses) were prepared by [11C]methylation of the appropriate desmethyl precursor using published methods [18]. [3H]Dihydrotetrabenazine (2927 GBq/mmol) was obtained from Amersham Biosciences, Piscataway, NJ, USA.
2.2 Animals
Unless otherwise noted, studies were done in mature male CD-1 rats (Charles River Laboratories, Inc., Wilmington, MA) weighing 170-550g. All experiments were approved by the Institutional Animal Care and Use Committees (IACUC) of the University of Michigan and Columbia University, and follow the guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
2.3. AMPT Induced Dopamine Depletion Studies
Male Lewis rats (Charles River Laboratories, Inc., Wilmington, MA) weighing between 250 and 300 g at the start of the experiments were used. Dopamine depletion was effected by systemic administration of AMPT (250 mg/kg/day, in isotonic saline i.p.) for three days. Control rodents received saline i.p. injections alone. On the third day, two hours after the last injection, animals were euthanized by CO2 asphysiation. The brain tissue was harvested by blunt dissection, excess fluids blotted, the tissue weighed and snap frozen in liquid nitrogen. Frozen specimens were then stored at −80 C until further use. Frozen specimens were pulverized in a liquid nitrogen cooled mortar and then homogenized in 10 mls 0.01N HCL with 1 mM EDTA and 4mM sodium metabisulfate. One ml of homogenate was spun at 20,000 × g for 30 min at 4C to clarify. One hundred microliters (1/10) of the supernatant of the extract was then analyzed for dopamine content by ELISA (Rocky Mountain Diagnostics, Colorado Springs, CO ) according to the manufacturers instructions. The total dopamine content of the brain tissue was calculated and normalized to the total wet weight of the brain.
2.4. AMPT Blocking Studies
AMPT (100 mg/kg) was dissolved in 37° C sterile saline and administered i.p. to male CD-1 rats (one injection per day for 3 days prior to radiotracer injection). The third injection of AMPT was administered 2 hours prior to injection of radiotracer. In the studies where both AMPT and L-DOPA were administered, the L-DOPA (100mg/kg)/benserazide (25mg/kg) dose was given i.v. one hour prior to injection of radiotracer. L-DOPA/benserazide were dissolved in 0.1% ascorbic acid in sterile saline and sterile sodium phosphate was added to adjust the pH to approximately 6 for intravenous injection.
2.5. Ex vivo dissection studies
The regional brain distributions of [11C]DTBZ and [11C]MPH were determined following administration of the radioligands using a bolus plus constant infusion technique that has been used for more than ten years [19,20]. Under diethyl ether anesthesia, catheters were inserted in the tail vein for infusion, and the rats were restrained in plastic tubes and allowed to awaken. Four to six animals were done simultaneously. Injected doses of radiopharmaceuticals ranged from 68-133 mBq, with specific activities of 11100-18500 GBq/mmol at the time of administration. All animals were euthanized at 60 minutes by i.v. injection of sodium pentobarbital (50 mg/kg) and brains rapidly dissected into regions of interest (striatum (STR), cortex (CTX) hypothalamus (HYP) and cerebellum (CBL)), which were weighed and counted for carbon-11 radioactivity. Data was calculated as percent injected dose per gram tissue, and converted into distribution volume ratios (DVR) where DVR = (percent injected dose striatum)/(percent injected dose cerebellum), and the specific binding calculated as Binding Potential (BP = DVR –1).
2.6. In vitro autoradiographic binding studies
In vitro binding of [3H]DTBZ was done using an autoradiographic assay previously reported [21]. Rats were euthanized via carbon dioxide asphyxiation and decapitated. Brains were removed and frozen in crushed dry ice, coated with frozen tissue embedding medium (Lipshaw Inc., Detroit, MI) to prevent desiccation, and maintained at −80°C until sectioning. Pairs of adjacent saggital brain sections (20μm thick) were cut on a cryostat (−18°C), thaw-mounted onto polylysine-subbed microscope slides, and allowed to air dry. Sections were preincubated in 137 mM KCl, 3 mM NaCl, 8 mM K2HPO4, 1.5 mM NaH2PO4, 1 mM EDTA, pH 8.0 at 25°C then incubated in 10nM [3H]dihydrotetrabenazine without and with 10μM tetrabenazine (to identify non-specific binding) for 30 minutes at 25°C. Slides were washed twice in cold buffer and dipped in distilled water before air-drying. The slides were then apposed to tritium-sensitive film (Hyperfilm, Amersham) for four weeks. Calibrated plastic radioactive standards were included with each cassette of slides to allow for variation in exposure. Autoradiograms were analyzed by computer-assisted video densitometry (MCID, Imaging Research, St. Catherines, ON, CA). Regions of interest were drawn on the striatum and a section of the occipital-frontal cortex. Imaging plate densities were converted to apparent tissue radioactivity on the bases of the radioactive standards and the specific activity of the [3H]DTBZ used. Radioactive densities were calculated as fmol/μg protein and were averaged across adjacent sections.
2.7. Statistics
Statistical significance between groups was performed using an unpaired Student's t-test. A p < 0.5 was considered significant.
3. Results
3.1. AMPT Depletion of brain tissue dopamine
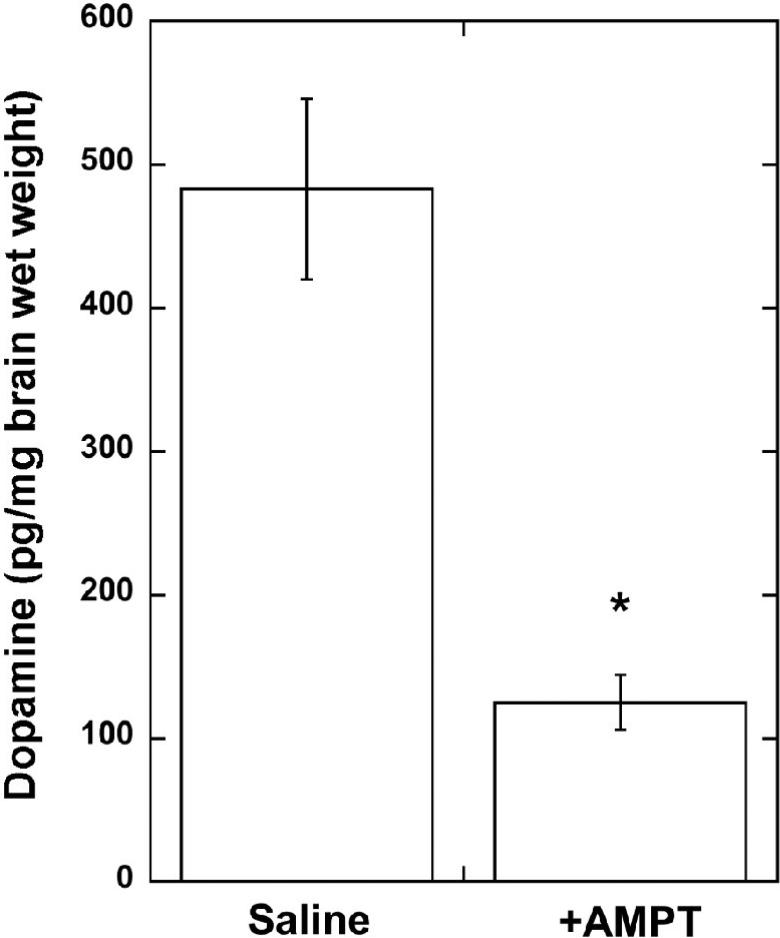
The three-day treatment of rats with 250 mg/kg i.p. doses of AMPT produced a 75% reduction (Fig. 1) in the whole brain dopamine tissue concentrations (p < 0.01 vs. controls).
Fig. 1.
Dopamine tissue concentrations in saline and AMPT-treated rat brains. Data are mean +/1 S.E.M. for N = 4 animals. *P < 0.01 vs. saline-treated.
3.2. AMPT effects on in vivo [11C]DTBZ binding in rat brains
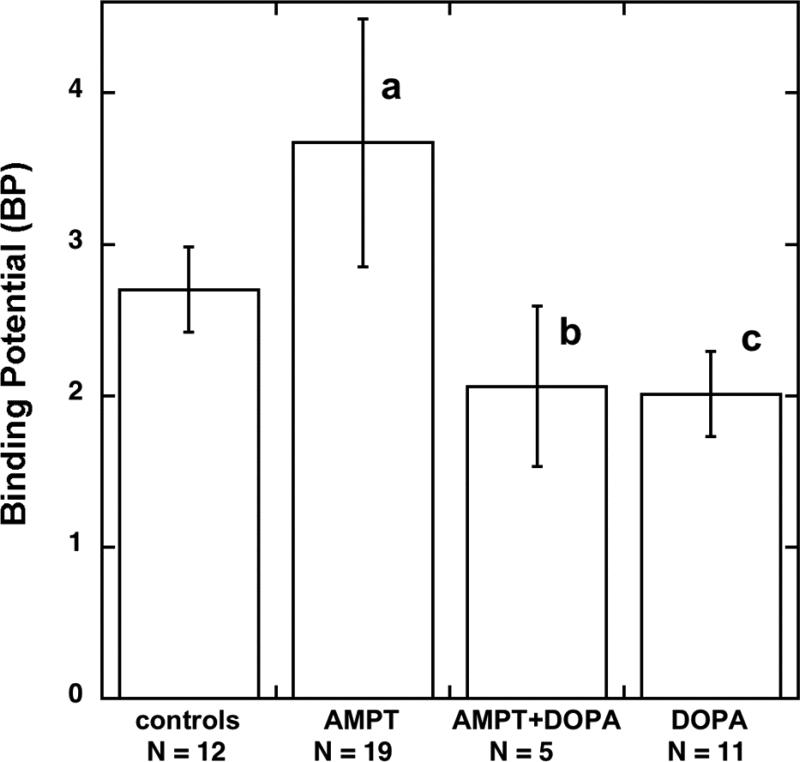
The mean and variance of [11C]DTBZ BP in the control group (2.70 +/− 0.28, N = 12) were essentially identical to a larger group of controls previously done using carbon-11 or tritium labeled dihydrotetrabenazine (2.61 +/− 0.24, N = 35: [20]). Pretreatment of rats with multiple doses of AMPT to deplete brain tissue dopamine levels resulted in a significant 26% increase in the DTBZ BP in the striatum (Fig. 2). The differences in DTBZ BP were entirely due to increases in the striatum (% injected dose/g tissue in controls 0.87 +/− 0.24, drug treated 1.08 +/− 0.33, p < 0.01) and not due to alterations of radiotracer uptake into non-specific regions of the brain such as cerebellum or cortex, as equilibrium concentrations in these tissues were identical in control (% injected dose/g tissue cerebellum 0.23 +/− 0.06, cortex 0.27 +/− 0.07) and AMPT-treated animals (cerebellum 0.24 +/− 0.08, cortex 0.29 +/− 0.09). The treatment with AMPT also did not change radiotracer localization in the hypothalamus (DTBZ BP values: AMPT-treated 1.09 +/− 0.09 (N = 4), controls 1.20 +/− 0.34, (N = 23)). The administration of L-DOPA and benserazide after the third dose of AMPT, but one hour before the start of the [11C]DTBZ infusion, resulted in striatal DTBZ BP values that were significantly reduced (−44%) from the AMPT-treated group. In a group of animals treated only with L-DOPA plus beserazide one hour prior to [11C]DTBZ infusion, DTBZ BP values were also lower (−26%) than in control untreated animals. However, this was the result of a non-significant decrease (−7%) of radioligand concentration in the striatum (controls 0.87 +/− 0.24, DOPA-treated 0.81 +/− 0.13, p = 0.49) coupled with a larger but again non-significant increase (+17%) in radioligand concentration in the cerebellum controls 0.23 +/− 0.06, DOPA-treated 0.27 +/− 0.06, P = 0.14), and the combination of these two non-significant tissue concentrations resulted in the observed change in their ratio.
Fig. 2.
Effects of α -methyl-p-tyrosine (AMPT) and L-DOPA treatments on in vivo specific binding of [11C]DTBZ to the vesicular monoamine transporter in striatum of the rat brain. BP = Binding Potential: error bars represent S.D. for controls, N = 12; AMPT, N = 19; AMPT+DOPA, N = 5; DOPA, N = 11. a P < 0.001 vs controls, b P < 0.05 vs AMPT treated, c P < 0.05 vs controls
3.3 AMPT effects on in vitro [3H]DTBZ binding rat brains
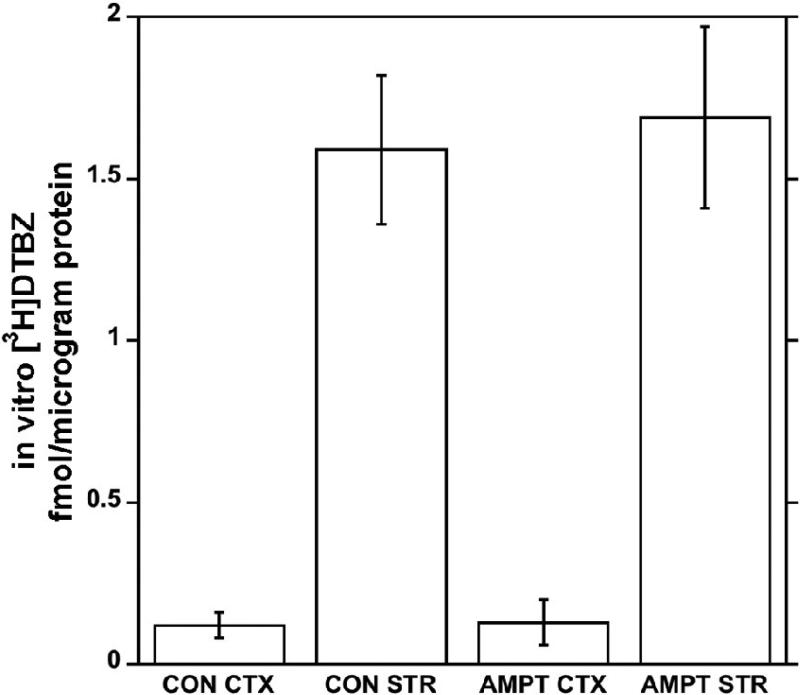
Using the in vitro autoradiographic assay on rat brain slices, the binding of [3H]DTBZ in control and AMPT-treated rats was identical in both the cortex and striatum (Fig. 3).
Fig. 3.
Effects of pretreatments with α-methyl-p-tyrosine on in vitro binding of [3H]DTBZ to the vesicular monoamine transporter in the striatum and cortex of the rat brain. Error bars represent S.D. for N = 8 (controls) or N = 16 (AMPT-treated).
3.4. AMPT effects on in vivo [11C]MPH binding in rat brains
In rats treated with the three injections of AMPT using the same protocol as for the [11C]DTBZ studies, regional brain uptake and BP values for [11C]methylphenidate were identical in AMPT-treated (1.05 +/− 0.23, N = 6) and control animals (1.07 +/− 0.12, N = 9) for in vivo binding to the neuronal membrane dopamine transporter.
4. Discussion
This study was undertaken to determine if pharmacological treatments known to alter cytosolic and vesicular concentrations of dopamine would affect the in vivo binding of [11C]dihydrotetrabenazine (DTBZ), a radioligand for the vesicular monoamine transporter type 2 in the rat brain, or [11C]methylphenidate (MPH), a radioligand for the neuronal membrane dopamine transporter. Studies were done using a well-established quantitative in vivo radioligand methodology that provides reproducible estimates of in vivo specific binding (DVR values) which are insensitive to wide ranges of injected mass amounts [19,20].
The repeated treatment of rats with AMPT, an inhibitor of the first enzymatic step in the biosynthesis of dopamine, produced the expected significant reductions (−75%) in brain tissue dopamine levels (Fig. 1). The dose of AMPT used in this experiment was the highest tolerated by the animals, and has been reported to produce maximal inhibition (87%) of biosynthesis of dopamine in the rat brain [22]. Application of a multi-day repeated administration of AMPT has been previously reported to produce a lasting and significant (78%) reduction in brain tissue dopamine levels [23].
The AMPT dose protocol employed here also resulted in an increase of in vivo specific binding of the [11C]DTBZ in the striatum, a region of the brain with a high concentration of VMAT2 in dopaminergic terminals (Fig. 2). The increased specific binding, measured as the Binding Potential (BP), was entirely attributable to increases in [11C]DTBZ binding in the striatum, as there were no changes seen for radioligand uptake and retention in the regions of non-specific binding (cortex and cerebellum). Further support that the changes in striatal radioligand binding reflect alterations of dopamine is that no changes were observed for the hypothalamus, a region of the brain containing intermediate concentrations of the VMAT2 but where serotonin and norepinephrine are the predominant endogenous monoamine neurotransmitters, and treatments with AMPT do not affect the biosynthesis of serotonin.
The changes of in vivo binding of [11C]DTBZ are not the result of rapid up-regulation of the concentrations of vesicular monoamine transporters, as the studies of in vitro binding of [3H]DTBZ in AMPT-treated animals demonstrated no differences from controls (Fig. 3). This result is consistent with the experience of Naudon et al [24], who demonstrated that the de novo synthesis of vesicular monoamine transporter molecules is quite slow following irreversible inhibition by reserpine, supporting a slow turnover of these proteins in neurons.
The AMPT-induced increase in [11C]DTBZ binding in vivo in the striatum can be completely reversed by administration of L-DOPA, the availability of which by-passes the enzymatic step blocked by the AMPT. This precursor to dopamine was administered at a sufficient time after the last dose of AMPT to avoid potential complications from competition of AMPT and L-DOPA for the large amino acid transporter, which both compounds share as the mechanism for transport across the blood brain barrier. The reversal of the striatal BP value for the AMPT+DOPA group was due to reductions of striatal concentrations of radioligand (−38%, p < 0.01 vs. AMPT group) with only small and non-significant increases (7-8%) in radioligand uptake and retention in cortex or cerebellum (p > 0.5 for both).
Treatment of control animals with a high dose of L-DOPA, which should increase the tissue concentrations of dopamine in the rat brain, produced a significant (−26%) decrease of the in vivo BP for [11C]DTBZ. Although this result is consistent with the experience of De La Fuentes-Fernandez [16] in L-DOPA treatment in Parkinson's disease, the significance here was the result of a very small and non-significant decrease of radioligand concentration in the striatum coupled with a larger but again non-significant increase in radioligand concentration in the cerebellum, and the combination of these two non-significant tissue concentrations resulted in the observed change in their ratio. As the cerebellum was used in this study as an estimate of non-specific radiotracer distribution in the rat brain, ratios calculated from data where cerebellar concentrations are altered by the drug treatment may no longer be valid estimates of specific binding. Further studies are needed to confirm that increasing dopamine concentrations can affect in vivo binding of VMAT2 radioligands in the rat brain.
Finally, the effects of AMPT are not observed with the neuronal membrane dopamine transporter radioligand [11C]methylphenidate. This is consistent with the study of DOPA-responsive dystonia patients, where no changes of [11C]MPH binding were observed, as well as with previous animal studies which have reported insensitivity of [11C]MPH to synaptic dopamine levels [25,26], although the literature is inconsistent on the sensitivity of other radioligands for this transporter [27,28].
Taken in composite, the in vivo and in vitro studies reported here support that pharmacological (α-methyl-p-tyrosine) treatments that dramatically decrease rat brain dopamine levels can produce modest changes in the in vivo binding of (+)-[11C]DTBZ to VMAT2 sites of dopaminergic terminals in the striatum. The converse effect of increasing dopamine levels through administration of L-DOPA needs further evaluation to verify possible changes in radioligand binding. The changes in dopamine levels produced by the pharmacological treatments utilized here are quite large (75% or more decreases). Although there is no consistency in reported changes of brain dopamine levels after exogenous L-DOPA administration, due to widely varying doses, routes of administration, timing of measurements, and methodologies for measuring dopamine in different compartments (e.g., whole tissue, extracellular, cytosolic, releasable, or vesicular) [29-31], the magnitude of such changes are in general of the same magnitude (increases of about 100%) as the decreases produced by the AMPT depletion protocols. Thus, for both conditions of large decreases or increases of dopamine levels, the percent change of the in vivo vesicular monoamine transporter binding of [11C]dihydrotetrabenazine is only a fraction of the alteration of the endogenous neurotransmitter level. Further studies will be needed to determine any dose-response relationship, and in particular whether changes in [11C]DTBZ binding are observed with smaller and more physiologically relevant changes in dopamine concentrations.
The experimental results obtained here confirm and significantly extend the observations originally reported by Tong et al [15] regarding the sensitivity of [11C]DTBZ to endogenous dopamine levels. In this study, different drug (AMPT, L-DOPA) doses were employed and a different in vivo radioligand binding assay performed, but the results are very similar. Importantly, and not shown in the Tong et al (2008) paper, the changes in endogenous dopamine levels were shown to be very high (75% decreased), tissue concentrations of VMAT2 are not altered by AMPT treatment, and the increased in vivo [11C]DTBZ binding could be reversed in AMPT-treated animals by an intervening treatment with L-DOPA to restore tissue dopamine levels.
The results of our experiments do have relevance to the use of [11C]DTBZ and [11C]MPH as potential quantitative imaging agents for dopaminergic nerve terminals in human PET imaging studies. In the rat, large changes of brain dopamine levels produce small but measurable changes in [11C]DTBZ binding to the vesicular monoamine transporter. The study of De la Fuente-Fernandez et al [5] in DOPA-reponsive dystonia demonstrated changes of similar magnitude (20%) for in vivo [11C]DTBZ binding in that severe example of dopamine deficiency. There is however no change in the total numbers of transporters in the rat striatum, consistent with prior studies showing that the vesicular monoamine transporter of the rat brain is not readily regulated by acute or chronic dopaminergic drug treatments [10-14]. The lack of any change of [11C]MPH binding after AMPT treatment is consistent with prior experiments using this radioligand, but generalization of these findings to all radioligands for the neuronal membrane dopamine transporter [32] may be unwarranted and such dopamine sensitivity should be evaluated for each and every one.
Acknowledgements
This work was supported by a grant from the Office of Science (BER), U.S. Dept. of Energy, DE-FG02-87ER60561, and the National Institutes of Health NS-15655. The authors thank the radiochemistry staff of the University of Michigan PET Program for synthesis of the carbon-11 radiotracers.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Frey KA, Koeppe RA, Kilbourn MR. Advances in Neurology. Lippincott Williams and Wilkins; Philadelphia: 2001. Imaging the Vesicular Monoamine Transporter. pp. 237–247. [PubMed] [Google Scholar]
- 2.Albin RL, Koeppe RA, Bohnen NI, Nichols TE, Meyer P, Wernette K, Minoshima S, Kilbourn MR, Frey KA. Increased ventral striatal monoaminergic innervation in Tourette syndrome. Neurology. 2003;61:310–315. doi: 10.1212/01.wnl.0000076181.39162.fc. [DOI] [PubMed] [Google Scholar]
- 3.Bohnen NI, Koeppe RA, Meyer P, Ficaro E, Wernette K, Kilbourn MR, Kuhl DE, Frey KA, Albin RL. Decreased striatal monoaminergic terminals in Huntington disease. Neurology. 2006;54:1753–1759. doi: 10.1212/wnl.54.9.1753. [DOI] [PubMed] [Google Scholar]
- 4.Bohnen NI, Albin RL, Koeppe RA, Wernette KA, Kilbourn MR, Minoshima S, Frey KA. Positron emission tomography of monoaminergic vesicular binding in aging and Parkinson disease. J Cerebral Blood Flow Metab. 2006;26:1198–1212. doi: 10.1038/sj.jcbfm.9600276. [DOI] [PubMed] [Google Scholar]
- 5.De La Fuente-Fernandez R, Furtado S, Guttman M, Furukawa Y, Lee CS, Calne DB, Ruth TJ, Stoessl AJ. VMAT2 binding is elevated in dopa-responsive dystonia: visualizing empty vesicles by PET. Synapse. 2003;49:20–28. doi: 10.1002/syn.10199. [DOI] [PubMed] [Google Scholar]
- 6.Gilman S, Koeppe RA, Chervin RD, Consens FB, Little R, An H, Junck L, Heumann M. REM sleep behavior disorder is related to striatal monoaminergic deficit in MSA. Neurology. 2003;61:29–33. doi: 10.1212/01.wnl.0000073745.68744.94. [DOI] [PubMed] [Google Scholar]
- 7.Koeppe RA, Gilman S, Joshi A, Liu S, Little R, Junck L, Heumann M, Frey KA, Albin RL. 11C-DTBZ and 18F-FDG PET measures in differentiating dementias. J Nucl Med. 2005;46:936–944. [PubMed] [Google Scholar]
- 8.Martin WRW, Wieler M, Stoessl J, Schulzer M. Dihydrotetrabenazine positron emission tomography imaging in early, untreated Parkinson's disease. Ann Neurol. 2008;63:388–394. doi: 10.1002/ana.21320. [DOI] [PubMed] [Google Scholar]
- 9.Zubieta JK, Taylor SF, Huguelet P, Koeppe RA, Kilbourn MR, Frey KA. Vesicular monoamine transporter concentrations in bipolar disorder type I, schizophrenia, and healthy subjects. Biol Psych. 2001;49:110–116. doi: 10.1016/s0006-3223(00)00981-1. [DOI] [PubMed] [Google Scholar]
- 10.Kilbourn MR, Frey KA, Vander Borght T, Sherman PS. Effects of dopaminergic drug treatments on in vivo radioligand binding to brain vesicular monoamine transporters. Nucl Med Biol. 1996;23:467–471. doi: 10.1016/0969-8051(96)00023-6. [DOI] [PubMed] [Google Scholar]
- 11.Naudon L, Leroux-Nicollet I, Costentin J. Short-term treatments with haloperidol or bromocriptine do not alter the density of the monoamine vesicular transporter. Neurosci Lett. 1994;173:1–4. doi: 10.1016/0304-3940(94)90136-8. [DOI] [PubMed] [Google Scholar]
- 12.Vander Borght TM, Kilbourn MR, Desmond TJ, Kuhl DE, Frey KA. The vesicular monoamine transporter is not regulated by dopaminergic drug treatments. Eur J Pharmacol. 1995;294:577–583. doi: 10.1016/0014-2999(95)00594-3. [DOI] [PubMed] [Google Scholar]
- 13.Vilpoux C, Leroux-Nicollet I, Naudon L, Raisman-Vozari R, Costentin J. Reserpine or chronic fluoxetine treatments do not modify the vesicular monoamine transporter 2 expression in serotonin-containing regions of the rat brain. Neuropharm. 2000;39:1075–1082. doi: 10.1016/s0028-3908(99)00210-5. [DOI] [PubMed] [Google Scholar]
- 14.Wilson JM, Kish SJ. The vesicular monoamine transporter, in contrast to the dopamine transporter, is not altered by chronic cocaine self-administration in the rat. J Neurosci. 1996;16:3507–3510. doi: 10.1523/JNEUROSCI.16-10-03507.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tong J, Wilson AA, Boileau I, Houle S, Kish SJ. Dopamine modulating drugs influence striatal (+)-[11C]DTBZ binding in rats: VMAT2 binding is sensitive to changes in vesicular dopamine concentration. Synapse. 2008;62:873–876. doi: 10.1002/syn.20573. [DOI] [PubMed] [Google Scholar]
- 16.De La Fuentes-Fernandez R, Sossi V, McCormick S, Shulzer M, Ruth TJ, Stoessl AJ. Visualizing vesicular dopamine dynamics in Parkinson's disease. Synapse. 2009;63:713–716. doi: 10.1002/syn.20653. [DOI] [PubMed] [Google Scholar]
- 17.Kilbourn MR, Butch ER, Desmond T, Sherman P, Frey KA. Dopamine depletion increases in vivo [11C]DTBZ binding in awake rat brain. NeuroImage. 2008;41(Suppl 2):T54. [Google Scholar]
- 18.Jewett DM, Kilbourn MR, Lee LC. A simple synthesis of [11C]dihydrotetrabenazine. Appl Rad Isot. 1997;24:197–199. doi: 10.1016/s0969-8051(96)00213-2. [DOI] [PubMed] [Google Scholar]
- 19.Kilbourn M, Sherman P. In vivo binding of (+)-α-[3H]dihydrotetrabenazine to the vesicular monoamine transporter of rat brain: bolus vs. equilibrium studies. Eur J Pharm. 1997;331:161–168. doi: 10.1016/s0014-2999(97)01054-6. [DOI] [PubMed] [Google Scholar]
- 20.Kilbourn MR. Long-term reproducibility of in vivo measures of specific binding of radioligands in rat brain. Nucl Med Biol. 2004;31:591–595. doi: 10.1016/j.nucmedbio.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 21.Frey K, Kilbourn M, Robinson T. Reduced striatal vesicular monoamine transporters after neurotoxic but not after behaviorally-sensitizing doses of methamphetamine. Eur J Pharm. 1997;334:273–279. doi: 10.1016/s0014-2999(97)01152-7. [DOI] [PubMed] [Google Scholar]
- 22.Widerlov E, Lewander T. Inhibition of the in vivo biosynthesis and changes of catecholamine levels in rat brain after α-methyl-p-tyrosine: time and dose-response relationships. Naunym-Schmeidbergs Arch Pharmacol. 1978;304:111–123. doi: 10.1007/BF00495547. [DOI] [PubMed] [Google Scholar]
- 24.Naudon L, Leroux-Nicollet I, Raisman-Vozari R, Botton D, Costentin J. Time-course of modifications elicited by reserpine on the density and mRNA synthesis of the vesicular monoamine transporter, and on the density of the membrane dopamine uptake complex. Synapse. 1995;21:29–36. doi: 10.1002/syn.890210105. [DOI] [PubMed] [Google Scholar]
- 25.Gatley SJ, Volkow ND, Fowler JS, Dewey SL, Logan J. Sensitivity of striatal [11C]cocaine binding to decreases in synaptic dopamine. Synapse. 1995;20:137–144. doi: 10.1002/syn.890200207. [DOI] [PubMed] [Google Scholar]
- 26.Gatley SJ, Ding YS, Volkow ND, Chen R, Sugano Y, Fowler JS. Binding of d-threo-[11C]methylphenidate to the dopamine transporter in vivo: insensitivity to synaptic dopamine. Eur J Pharmacol. 1995;281:141–149. doi: 10.1016/0014-2999(95)00233-b. [DOI] [PubMed] [Google Scholar]
- 27.Ikawa K, Watanabe A, Kaneno S, Toru M. Modulation of [3H]mazindol binding sites in rat striatum by dopaminergic agents. Eur J Pharmacol. 1993;250:261–266. doi: 10.1016/0014-2999(93)90390-4. [DOI] [PubMed] [Google Scholar]
- 28.Scheffel U, Steinart C, Kim SE, Ehlers MD, Boja JW, Kuhar MJ. Effect of dopaminergic drugs on the in vivo binding of [3H]WIN 35,428 to the central dopamine transporters. Synapse. 1996;23:61–69. doi: 10.1002/(SICI)1098-2396(199606)23:2<61::AID-SYN1>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 29.Buu NT. Vesicular accumulation of dopamine following L-DOPA administration. Biochem Pharmacol. 1989;38:1787–1792. doi: 10.1016/0006-2952(89)90413-9. [DOI] [PubMed] [Google Scholar]
- 30.Doshi PS, Edwards DJ. Effects of L-DOPA on dopamine and norepinephrine concentrations in rat brain assessed by gas chromatography. J Chrom. 1981;210:505–551. doi: 10.1016/s0021-9673(00)80342-8. [DOI] [PubMed] [Google Scholar]
- 31.Wachtel SR, Abercrombie ED. L-3,4-Dihydroxyphenylalanine-induced dopamine release in the striatum of intact and 6-hydroxydopamine-treated rats: differential effects of monoamine oxidase A and B inhibitors. J Neurochem. 1994;63:108–117. doi: 10.1046/j.1471-4159.1994.63010108.x. [DOI] [PubMed] [Google Scholar]
- 32.Zahniser NR, Doolen S. Chronic and acute regulation of Na /Cl- -dependent neurotransmitter transporters: drugs, substrates, presynaptic receptors and signaling systems. Pharmacol Ther. 2001;92:21–55. doi: 10.1016/s0163-7258(01)00158-9. [DOI] [PubMed] [Google Scholar]