Abstract
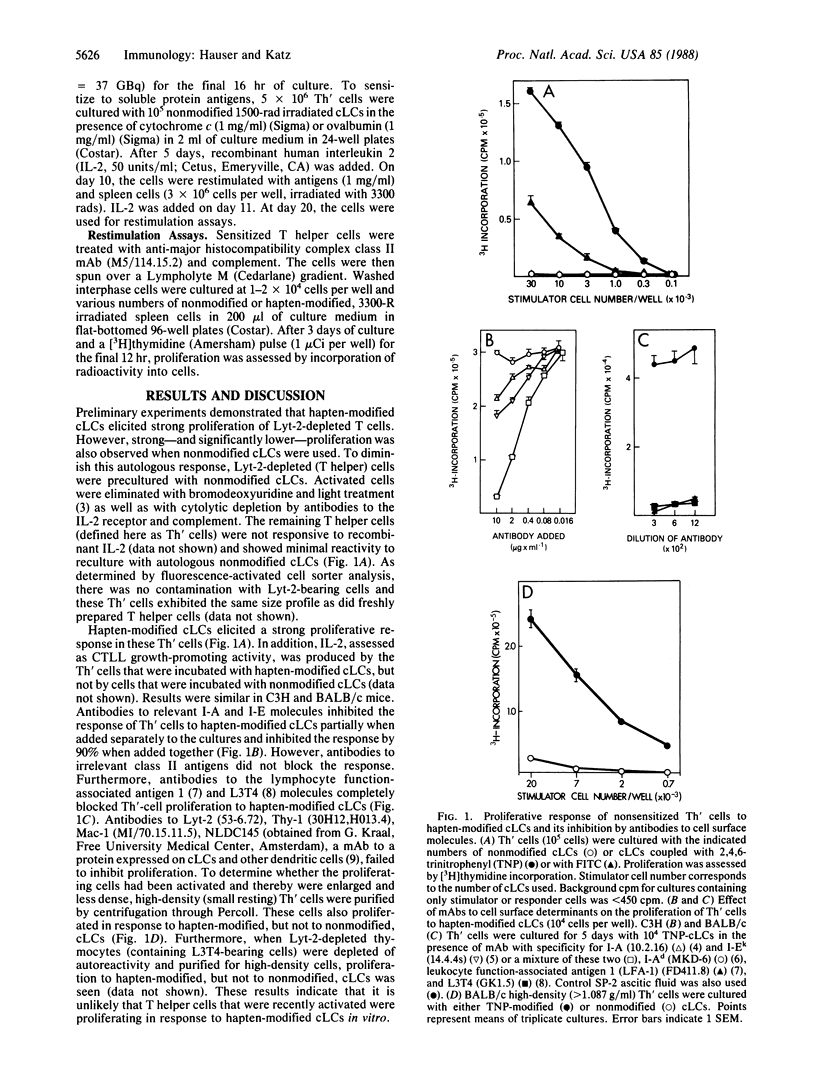
Hapten- and protein-antigen-specific T helper cells are usually expanded in vitro from lymphocytes obtained from sensitized animals. In this paper we report on the primary activation and proliferation in vitro of T helper cells from nonsensitized animals by using syngeneic cultured epidermal Langerhans cells as a source of potent antigen-presenting cells. The primary in vitro proliferation was blocked with monoclonal antibodies to Ia molecules, to lymphocyte function-associated antigen 1 (LFA-1), and to L3T4. T helper cell populations sensitized in vitro to haptens and protein antigens showed hapten- and antigen-specific proliferation when restimulated in vitro with spleen cells. Besides its experimental usefulness, in vitro generation of syngeneic specific T helper cells may afford possibilities for adoptive immunotherapy.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cheever M. A., Thompson D. B., Klarnet J. P., Greenberg P. D. Antigen-driven long term-cultured T cells proliferate in vivo, distribute widely, mediate specific tumor therapy, and persist long-term as functional memory T cells. J Exp Med. 1986 May 1;163(5):1100–1112. doi: 10.1084/jem.163.5.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dialynas D. P., Quan Z. S., Wall K. A., Pierres A., Quintáns J., Loken M. R., Pierres M., Fitch F. W. Characterization of the murine T cell surface molecule, designated L3T4, identified by monoclonal antibody GK1.5: similarity of L3T4 to the human Leu-3/T4 molecule. J Immunol. 1983 Nov;131(5):2445–2451. [PubMed] [Google Scholar]
- Inaba K., Schuler G., Witmer M. D., Valinksy J., Atassi B., Steinman R. M. Immunologic properties of purified epidermal Langerhans cells. Distinct requirements for stimulation of unprimed and sensitized T lymphocytes. J Exp Med. 1986 Aug 1;164(2):605–613. doi: 10.1084/jem.164.2.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii N., Nagy Z. A., Klein J. Absence of Ir gene control of T cells recognizing foreign antigen in the context of allogenic MHC molecules. Nature. 1982 Feb 11;295(5849):531–533. doi: 10.1038/295531a0. [DOI] [PubMed] [Google Scholar]
- Kappler J. W., Skidmore B., White J., Marrack P. Antigen-inducible, H-2-restricted, interleukin-2-producing T cell hybridomas. Lack of independent antigen and H-2 recognition. J Exp Med. 1981 May 1;153(5):1198–1214. doi: 10.1084/jem.153.5.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraal G., Breel M., Janse M., Bruin G. Langerhans' cells, veiled cells, and interdigitating cells in the mouse recognized by a monoclonal antibody. J Exp Med. 1986 Apr 1;163(4):981–997. doi: 10.1084/jem.163.4.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougal J. S., Gordon D. S. Generation of T-helper cells in vitro. I. Cellular and antigen requirements. J Exp Med. 1977 Mar 1;145(3):676–692. doi: 10.1084/jem.145.3.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishell R. I., Dutton R. W. Immunization of normal mouse spleen cell suspensions in vitro. Science. 1966 Aug 26;153(3739):1004–1006. doi: 10.1126/science.153.3739.1004. [DOI] [PubMed] [Google Scholar]
- Oi V. T., Jones P. P., Goding J. W., Herzenberg L. A., Herzenberg L. A. Properties of monoclonal antibodies to mouse Ig allotypes, H-2, and Ia antigens. Curr Top Microbiol Immunol. 1978;81:115–120. doi: 10.1007/978-3-642-67448-8_18. [DOI] [PubMed] [Google Scholar]
- Ozato K., Mayer N., Sachs D. H. Hybridoma cell lines secreting monoclonal antibodies to mouse H-2 and Ia antigens. J Immunol. 1980 Feb;124(2):533–540. [PubMed] [Google Scholar]
- Rosenberg S. A., Spiess P., Lafreniere R. A new approach to the adoptive immunotherapy of cancer with tumor-infiltrating lymphocytes. Science. 1986 Sep 19;233(4770):1318–1321. doi: 10.1126/science.3489291. [DOI] [PubMed] [Google Scholar]
- Sanchez-Madrid F., Simon P., Thompson S., Springer T. A. Mapping of antigenic and functional epitopes on the alpha- and beta-subunits of two related mouse glycoproteins involved in cell interactions, LFA-1 and Mac-1. J Exp Med. 1983 Aug 1;158(2):586–602. doi: 10.1084/jem.158.2.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shevach E. M. Elimination of specific immunoreactive T lymphocytes with 5-bromodeoxyuridine and light. Methods Enzymol. 1984;108:262–270. doi: 10.1016/s0076-6879(84)08090-3. [DOI] [PubMed] [Google Scholar]
- Shimada S., Caughman S. W., Sharrow S. O., Stephany D., Katz S. I. Enhanced antigen-presenting capacity of cultured Langerhans' cells is associated with markedly increased expression of Ia antigen. J Immunol. 1987 Oct 15;139(8):2551–2555. [PubMed] [Google Scholar]
- Thomas D. W., Clement L., Shevach E. M. T lymphocyte stimulation by hapten-conjugated macrophages: a model system for the study of immunocompetent cell interactions. Immunol Rev. 1978;40:181–204. doi: 10.1111/j.1600-065x.1978.tb00406.x. [DOI] [PubMed] [Google Scholar]



