Abstract
Myocardial stunning is characterized by a metabolic uncoupling from function as mitochondrial tricarboxylic acid (TCA) cycle and oxygen consumption remain normal despite reduced contractility. Overexpression of the sarco-endoplasmic reticulum Ca2+-ATPase (SERCA1) in hearts has recently been reported to reduce dysfunction at reperfusion. In this study we determine whether the metabolic coupling to function improves with SERCA treatment. PBS (control) or adenovirus carrying the cDNA for SERCA1 were delivered via coronary perfusion in vivo to Sprague-Dawley rat hearts. Three days following gene transfer, isolated hearts were perfused with 0.4 mM [2,4,6,8,10,12,14,16-13C8] palmitate and 5 mM glucose, and subjected to 15 min ischemia followed by 40 min reperfusion. Consistent with myocardial stunning, rate-pressure-product (RPP) and left ventricular developed pressure (LVDP) were depressed 30-40% (p<0.05) in the PBS group. With SERCA1 overexpression, dP/dt was 20% greater than controls (p<0.05), and LVDP and RPP recovered to preischemic values. From dynamic 13C NMR, TCA cycle flux at reperfusion was similar to preischemic values for both groups. Therefore, the efficiency of coupling between cardiac work and TCA cycle flux was restored with SERCA1 treatment. Oxidative efficiency was also enhanced with SERCA1 as cytosolic NADH transport into the mitochondria was significantly greater compared to the PBS group. In addition, the phosphocreatine to ATP ratio (PCr/ATP) was not compromised with SERCA1 expression, despite enhanced function, and depressed fatty acid oxidation at 40 min reperfusion in the PBS group was not reversed with SERCA1. These data demonstrate metabolic coupling and NADH transport are significantly improved with SERCA1 treatment.
Keywords: SERCA, fatty acid oxidation, reperfusion, metabolism, PCr/ATP
1. INTRODUCTION
Recent studies show myocardial stunning is significantly attenuated in post-ischemic reperfused hearts overexpressing the endo-sarcoplasmic reticulum Ca2+-ATPase, SERCA (1, 2). SERCA sequesters calcium into the sarcoplasmic reticulum (SR) following contraction, thereby influencing both the cytosolic calcium content and the rate of myocardial relaxation (3). The overexpression of SERCA1 in heart reduces the calcium overload associated with the ischemic insult (1), and leads to accelerated recovery, reduced infarct size, and reduced arrthymogenesis (1,4). What is not known is how this perturbation influences the metabolic response to an ischemic insult. With intracellular calcium as a key regulator of metabolic flux (5,6,7), we hypothesize that the reported perturbations in calcium load with SERCA overexpression may impact several key metabolic processes in reperfused myocardium.
The stunned myocardium is characterized by metabolic inefficiencies as oxygen consumption and tricarboxylic acid (TCA) cycle rates are normal, despite reduced function (8,9,10,11). While the mechanisms for this uncoupling remain largely unknown, we have previously identified a loss in the transport of glycolytic NADH reducing equivalents into the mitochondria to fuel oxidative phosphorylation during reperfusion (9,12,13). This transport of reducing equivalents is balanced to the TCA cycle flux, and is regulated by the coordinated activity between the calcium sensitive mitochondrial matrix enzyme, α-ketoglutarate dehydrogenase (αKGDH), and NADH transport via the α-KG-malate carrier protein (5,6,14). Under the calcium overload condition of ischemia / reperfusion, the calcium activated dehydrogenase out-competes the αKG-malate carrier for mitochondrial αKG, thereby limiting NADH transport (9,13-15). Talukder and colleagues have reported that cytosolic calcium load can be reduced during ischemia in hearts overexpressing SERCA1 (1). Therefore, we investigated whether SERCA overexpression in reperfused myocardium also leads to adjustments in the calcium sensitive balance between TCA cycle flux and transport rates of NADH into the mitochondria.
In addition to the regulatory role calcium plays on TCA cycle flux and NADH transport, intracellular calcium contributes to the balance between fatty acid and glucose oxidation (5,6,7). More specifically, glucose oxidation increases with calcium activation of the mitochondrial bound enzyme, pyruvate dehydrogenase (PDH) (7,16). Early work by our group and others, shows that activation of PDH at reperfusion, shifts substrate oxidation from fatty acids to glucose and significantly aids in recovery (17,18). However, if calcium load decreases with SERCA1 overexpression in the reperfused myocardium as reported (1), the effects on PDH activation could shift the balance of substrate oxidation to fatty acids. This condition could risk metabolic recovery and limit ATP production in meeting the enhanced functional response to SERCA1 treatment. In this study we measured the balance of substrate oxidation as a consequence of SERCA1 overexpression, and determine whether ATP production is compromised in meeting the enhanced function.
The goal of this study was to determine whether the overexpression of SERCA1 in reperfused myocardium; 1) restores the coupling between workload and TCA cycle rates, 2) restores NADH transport into mitochondria, 3) readjusts substrate selection, and 4) compromises the energetic potential (PCr/ATP) in countering post ischemic contractile dysfunction. The findings reveal improvements in metabolic inefficiencies without a permanent shift in substrate selection pathways or energetic states.
2. METHODS
2.1 Animal Models
Three days prior to reperfusion experiments, adenovirus carrying SERCA1 cDNA was delivered in vivo to rat hearts (male, Sprague Dawley, 350-450 gm) by coronary perfusion. This open-chest cross-clamp technique has been described in detail in our previous reports (19,20,21). The adenovirus (generous gift from Dr. G. Inesi) expressed the skeletal muscle isoform of SERCA1a gene (1012 viral particles/ml PBS) under a CMV promoter. As previously reported, total SERCA content increases by 34% for this technique (21). Control groups underwent a similar surgical procedure with delivery of a PBS bolus replacing the adenovirus. The protocol was approved by the Animal Care Policies and Procedures Committee at the University of Illinois in Chicago (Institutional Animal Care and Use Committee accredited), and animals were maintained in accordance with the Guide for the Care and Use of laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996).
2.2 Isolated Perfused Rat Heart
At 48-72 hrs post surgical delivery of the Adv.cmv.SERCA1 (n=6) or the PBS bolus (control, n=6), rats were heparinized (500 U/100 g ip), anesthetized with Nembutal (50 mg/kg ip), and the hearts were excised as previously described (9,13,15,22,23). The aorta was cannulated for retrograde perfusion with media containing 116 mM NaCl, 4 mM KCl, 1.5 mM CaCl2, 1.2 mM MgSO4 and 1.2 mM NaH2PO4, equilibrated with 95% O2/ 5% CO2 with 0.4 mM unlabeled palmitate/albumin complex (3:1 molar ratio) and 5 mM glucose (22,23). The pressure head was set to 80 mmHg. A water-filled latex balloon in the left ventricle set to a diastolic pressure of 5-10 mmHg was connected to a pressure transducer and provided hemodynamic recordings (Powerlab, AD Instruments, Colorado Springs, CO). Left ventricular developed pressure (LVDP), ±dP/dt, and heart rate (HR) were continuously recorded. Perfused hearts were maintained at 37°C and positioned in an NMR spectrometer for metabolic flux measurements.
2.3 Ischemic Reperfusion Protocol
The experimental protocol is illustrated in Figure 1. Hearts perfused with media containing unlabeled palmitate and glucose for 20 minutes to re-establish a metabolic equilibrium and stabilize function. A 31P NMR spectrum was collected at 15 minutes to confirm normal bioenergetic state (phosphocreatine to βATP ratio PCr/ATP).
Figure 1.
Experimental protocol (top) and Western blotting of SERCA isoforms (bottom). Hearts overexpressing SERCA1 and PBS controls were isolated and retrograde perfused. After an initial equilibration period, hearts underwent a 15 min no-flow ischemic insult, followed by a 50 min reperfusion period. 13C NMR spectra were acquired from hearts oxidizing 0.4 mM [2,4,6,8,10,12,14,16-13C8] palmitate and 5 mM glucose throughout reperfusion. Hearts were freeze-clamp for Western blot analysis of SERCA1, SERCA2a, and calsequestrin expression. The adenoviral group (Adv.cmv.SERCA1) demonstrated significant increases in SERCA1 expression relative to the non-viral groups.
Global ischemia was induced by stopping the perfusion supply pump, clamping the aortic line, and maintaining the hearts at 37°C for 15 minutes. 31P NMR spectra were collected every 2 minutes throughout the ischemic insult. Reperfusion was initiated and continued for 10 minutes with perfusate containing unlabeled palmitate and glucose. 31P spectra were acquired the first 5 minutes post-ischemia followed by a 13C NMR spectrum of natural abundance signal 13C (1.1%). The substrate supply was then switched to 0.4 mM [2,4,6,8,10,12,14,16-13C8] palmitate (Isotec Inc, Miamisburg OH) plus 5 mM unlabeled glucose, and sequential 13C spectra were acquired every 2 minutes for the next 40 minutes. At the end of each experiment, an additional 31P spectrum was acquired before rapidly freezing the hearts for in vitro NMR analysis and tissue chemistry. The protocol for the non-ischemic groups (with or without SERCA1 gene transfer) has been previously described (21). In brief, 13C NMR data were acquired for 40 minutes under normoxic conditions in hearts provided 0.4 mM labeled palmitate and 5 mM unlabeled glucose.
2.4 NMR Spectroscopy
Isolated, perfused hearts were positioned in a 20 mm NMR probe within a vertical 89 mm bore, 9.4 T magnet. NMR data from isolated, beating hearts were obtained with a Bruker 400 AVANCE NMR spectrometer (Bruker Daltonics, Billerica, Mass). 31P- and 13C-NMR measurements were acquired by methods extensively described elsewhere (13,15,22,23). Hearts were freeze-clamp at the end of the experiment for in vitro 13C-NMR analysis of heart tissue extract at 14.1 T as previously described (23,24).
2.5 Metabolic Rates Via Kinetic Analysis of 13C-NMR Data
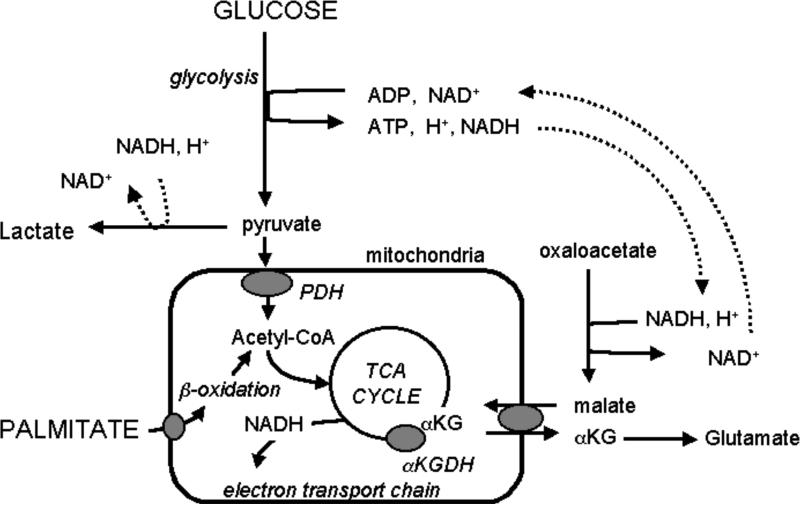
Metabolic flux was measured in the intact, reperfused rat hearts ex vivo using a previously described method for kinetic analysis of the progressive 13C enrichment of glutamate during 13C palmitate and glucose oxidation, as detected via NMR spectroscopy (13,15,22,23,24). Figure 2 illustrates the metabolic pathways involved. The kinetic model enables a simultaneous, 2-parameter, non-linear least-squares fit of 13C-enrichment curves for the 2- and 4-carbon positions of glutamate to provide measures of TCA cycle flux (VTCA) and flux through the αKG-malate carrier, F1 (25).
Figure 2.
Metabolic pathway schematic. Two key regulatory enzymes of mitochondrial metabolism include the αketoglutarate dehydrogenase (αKGDH) and pyruvate dehydrogenase (PDH). αKGDH balances TCA cycle flux to exchange of metabolite across the αKG-malate carrier. PDH is a key enzyme regulating the balance between glucose and fatty acid (palmitate) oxidation. Both αKGDH and PDH are sensitive to Ca2+ load in normal heart. In this study, we determined if both responded to the reported reduction in calcium overload observed in reperfused hearts overexpressing SERCA1 (1). We found metabolite exchange across the αKG-malate carrier increased with SERCA1 expression, while the balance between palmitate and glucose oxidation via PDH was unaffected.
2.6 Tissue Biochemistry
Tissue concentrations of glutamate, aspartate, citrate, malate, and α-ketoglutarate were determined spectrophotometrically and fluorometrically from perchloric acid extracts of frozen left ventricle using previously described assays (25). The metabolite data were used for kinetic analysis of the 13C NMR data. The percent of labeled acetyl-CoA entering the TCA cycle (Fc) from [2,4,6,8,10,12,14,16-13C8] palmitate was determined from high resolution in vitro 13C NMR spectra (23,24). Western blot analysis of SERCA1, SERCA2a, and calsequestrin (protein reference) were performed as recently reported (21), and content was assessed by standard semiquantitative densitometric analysis.
2.7 Statistical Analysis
Data is presented as mean ± standard error unless otherwise stated. Mean values were compared using paired or unpaired, two-tailed students t-test. Differences were considered statistically significant at a probability level of less then 5% (P<0.05).
3. RESULTS
3.1 Cardiac Function of Isolated Hearts and SERCA expression
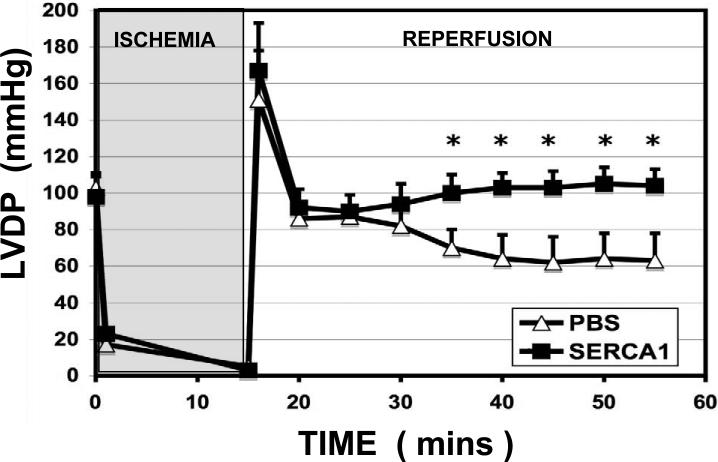
Hemodynamics for preischemic and reperfusion conditions are listed in Table 1. Figure 3 displays LVDP. In support of earlier work (21), the +dP/dt and –dP/dt were 20% greater (p<0.05) with the overexpression of SERCA1 compared to the PBS group prior to ischemia. The faster relaxation supports an augmented rate of calcium uptake into the sarcoplasmic reticulum with SERCA overexpression (26,27,28,29). Also in support of our earlier work and that of others (21,30), RPP, LVDP, and heart rate were similar between PBS and SERCA1. During reperfusion, the PBS group showed a 40% depression in developed pressure (p<0.05) and 30% reduction in RPP (ns), consistent with myocardial stunning. Diastolic pressure was also significantly elevated in the reperfusion group relative to pre-ischemic values, consistent with elevated cytosolic calcium levels. Importantly, LVDP, RPP, and diastolic pressure all recovered to preischemic values in the reperfusion group overexpressing SERCA1. Enhanced contractility, ±dP/dt, in the SERCA1 group was also sustained during reperfusion.
Table 1.
Contractile parameters from pre-ischemic isolated hearts and at 40 min reperfusion following a 15 minute ischemic insult. Experiments were performed 3 days following gene transfer of SERCA1 cDNA or PBS (control) to rat heart in vivo. (mean ± S.E.).
| Parameters | PBS (n=6) | SERCA1 (n=6) |
|---|---|---|
| Pre-ischemic | ||
| RPP (mmHg*beats/min) | 31500 ± 3800 | 27900 ± 4100 |
| LVDP (mmHg) | 103 ± 8 | 98 ± 11 |
| LVEDP (mmHg) | 5 ± 2 | 5 ± 2 |
| Heart Rate (beats/min) | 270 ± 20 | 270 ± 20 |
| −dP/dt (mmHg/s) | 2660 ± 250 | 3650 ± 380† |
| +dP/dt (mmHg/s) |
2960 ± 119 |
3950 ± 150† |
| Reperfusion (40 min) | ||
| RPP (mmHg*beats/min) | 22300 ± 7600 | 35900 ± 2200 |
| LVDP (mmHg) | 63 ± 15* | 104 ± 9† |
| LVEDP (mmHg) | 20 ± 11* | 5 ± 4† |
| Heart Rate (beats/min) | 290 ± 40 | 330 ± 20 |
| −dP/dt (mmHg/s) | 2280 ± 410 | 3340 ± 160† |
| +dP/dt (mmHg/s) | 2430 ± 206 | 3940 ± 100† |
RPP, rate pressure product; LVDP, left ventricle developed pressure; LVEDP, left ventricle end diastolic pressure; ±dP/dt, change in pressure with change in time.
(p<0.05) Significant difference between pre-ischemic PBS vs reperfused PBS group.
(p<0.05) Significant difference between SERCA1 and PBS group.
Figure 3.
LVDP. Pre-ischemic left ventricle developed pressure (LVDP) in isolated hearts overexpressing SERCA1 were similar to PBS controls, as expected (33,35). However, LVDP was significantly enhanced in the SERCA1 group throughout reperfusion, thereby revealing the cardioprotective effects of the Ad.cmv.SERCA1 treatment. (Mean± S.E. *Significant difference, p<0.05).
Hearts were freeze-clamped following the protocol and Western blot analysis of SERCA isoform expression were perform from tissue extracts. Westerns are illustrated in Figure 1, and demonstrate significant increases in SERCA1 expression (3 days post gene transfer). Consistent with our recent report, semiquantitative densitometric analysis indicated total SERCA content (SERCA2a + SERCA1) increased 25% with no reduction in SERCA2a content.
3.2 13C NMR Spectroscopy, Isotope Kinetics, and Metabolic Rates
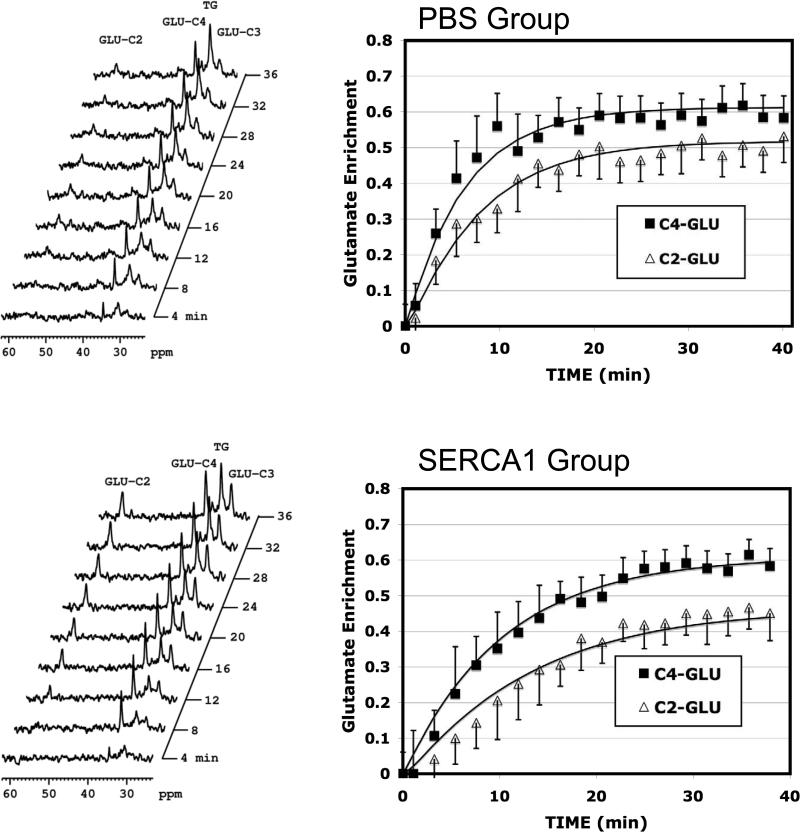
Figure 4 graphically displays the time course of 13C enrichment of the glutamate (mean ± SD) at 2- and 4-carbons from all acquired spectra of hearts in each group shown along with the least-squares fitting of the kinetic model. Note the enrichment profile for the reperfused PBS group display a faster enrichment to steady state compared to the reperfused SERCA1. However, the glutamate pool was significantly reduced in the reperfused PBS group relative to the SERCA1 group. Metabolic calculations by the kinetic model take into account both these enrichment profiles and the pool size of glutamate and other key metabolites. Output from the model provided measures of TCA cycle flux, VTCA, and the rate of glycolytic NADH reducing equivalent transport into the mitochondrial via the αKG-malate transporter. The results are illustrated in Figure 5.
Figure 4.
13C NMR. Spectra and enrichment curves for the 4- and 2-carbon positions of glutamate throughout the reperfusion period. Top, PBS control group. Bottom, SERCA1 overexpression group. Identifiable resonance peaks in the 13C spectra include: GLU-C2, 2-carbon of glutamate; GLU-C4, 4-carbon of glutamate; GLU-C3, 3-carbon of glutamate; TG, triacylglyceride. The enrichment curves shown include a solid line through the data which represents least–squares fitting of the kinetic model to the enrichment data. ■, 4-Carbon of glutamate;  , 2-carbon of glutamate. Note the relative rate of enrichment is slower for the SERCA1 groups compared to the PBS group. However, glutamate content was nearly 3 fold greater in the SERCA1 group (see Table 1), thereby accounting for the appearance of a slower enrichment rate despite similar TCA cycle rates between groups. (means ± S.D.).
, 2-carbon of glutamate. Note the relative rate of enrichment is slower for the SERCA1 groups compared to the PBS group. However, glutamate content was nearly 3 fold greater in the SERCA1 group (see Table 1), thereby accounting for the appearance of a slower enrichment rate despite similar TCA cycle rates between groups. (means ± S.D.).
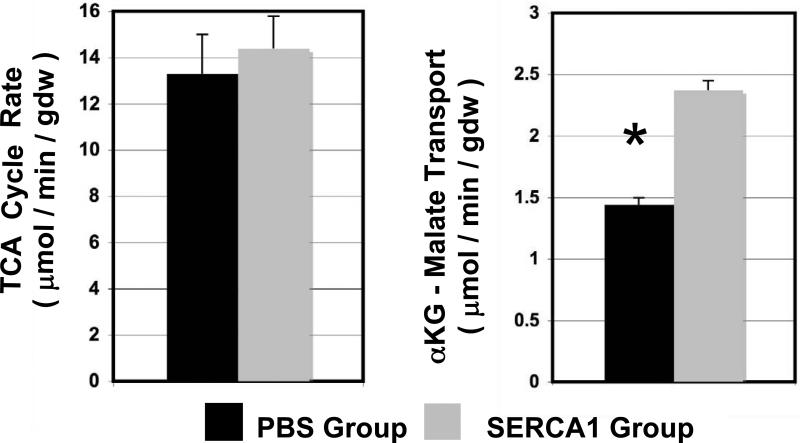
Figure 5.
Metabolic rates. TCA cycle rates and cytosolic NADH transport rate into the mitochondria via the αKG-malate transporter. TCA cycle rates were similar between SERCA1 and PBS groups, though cardiac function was significantly improved for the SERCA1 group. This result indicates TCA coupling to cardiac function is improved with SERCA1 overexpression. In addition, NADH shuttling rate into the mitochondria (via the αKG-malate carrier) to fuel oxidative phosphorylation was significantly increased in the SERCA1 group. These finding indicates metabolic efficiency is significantly improved with SERCA1 treatment of reperfused heart. (means ± S.E., * significant difference p<0.05)
Values for VTCA were not significantly different between the SERCA1 and PBS postischemic hearts. As expected, VTCA in the postischemic PBS hearts was not proportional to the large reduction in RPP. This is consistent with previous observations of stunned myocardium that TCA cycle rates are normal despite reduced function (9,10). In addition, the transport of glycolytic NADH reducing equivalent into the mitochondria via the αKG-malate transporter, F1, was significantly lower (67% reduced, p<0.05) relative to preischemic values. This finding supports our earlier reports for post ischemic hearts (9,13). Importantly, this rate for F1 was significantly greater (p<0.05) in the reperfused SERCA1 group. While F1 did not completely recovery to non-ischemic values, this improvement indicates enhanced efficiency of the mitochondria to transport cytosolic reducing equivalent into the mitochondria with the overexpression of SERCA1.
Consistent with the greater αKG-malate carrier rates in the SERCA1 group, glutamate content was also significantly increased in the reperfusion group overexpressing SERCA1 (see Table 2). Glutamate is formed from the transamination of αKG. Thus, a greater transport of αKG from the mitochondria in exchange for cytosolic malate (see Figure 2), increases the αKG pool available for the formation of glutamate (9,15). αKG, citrate, and aspartate content were also assayed and are listed in Table 2. There was no change in the concentration between groups for these metabolites.
Table 2.
Steady-state metabolite content (μmol/gdw) in intact heart at 60 min reperfusion.
| Group | Glutamate | αKG | Citrate | Aspartate |
|---|---|---|---|---|
| PBS | 8. 4 ± 2.7 | 0.21 ± 0.05 | 0.97 ± 0.16 | 3.13 ± 0.21 |
| SERCA1 | 20.8 ± 1.5* | 0.25 ± 0.02 | 1.12 ± 0.06 | 3.36 ± 0.14 |
All values are means ± SE.
p<0.005, significant difference between SERCA1 and PBS group.
3.3 Mitochondrial Oxidation of Palmitate
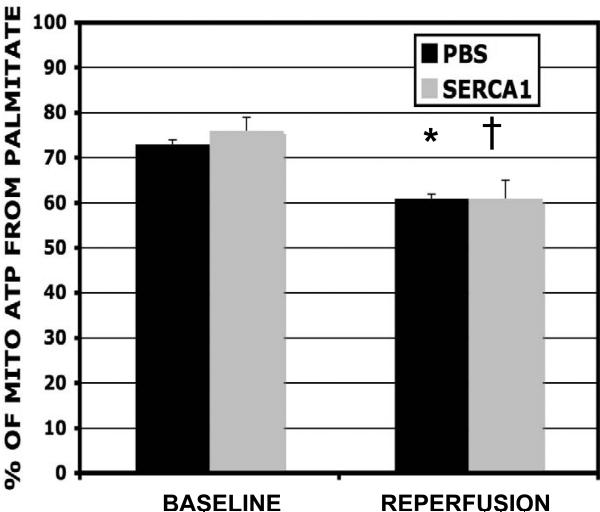
The fractional enrichments of acetyl-CoA produced from 13C palmitate are illustrated in Figure 6. Palmitate contributed 73% to mitochondrial ATP production prior to the ischemic insult in both PBS and SERCA1 groups. The contribution reduced to 61% during reperfusion (measured at 40 min post-ischemia) in both groups. Thus, SERCA1 overexpression in the reperfused hearts did not affect the balance between fat and carbohydrate oxidation despite improved cardiac function.
Figure 6.
Palmitate oxidation. Contributions from palmitate to mitochondrial ATP production (%) for both non-ischemic and reperfused hearts. The balanced is attributed to carbohydrates and endogenous fats. Experimental groups include hearts overexpressing SERCA1 (grey bars) and a PBS control group (black bars). The contribution from palmitate to mitochondrial ATP production was reduced for both groups during the reperfusion group. Thus, the overexpression of SERCA1 had no affect on substrate selection during reperfusion despite enhanced function. (mean ± S.E., * significant difference p<0.05).
3.4 Energetic State
Figure 7 shows PCr / βATP ratios through out the protocol. Preischemic values were similar for PBS and SERCA1 groups (1.6±0.2 and 1.6±0.1 respectively). While more typical PCr/ATP ratios are reported to range from 1.8 to 2, our measurements here are consistent with the reported post-surgical ratio observed following the open-chest procedures for adenoviral and PBS delivery (21,26). The PCr/ATP ratio at reperfusion was greater than preischemic values, as expected for non-treated reperfused hearts (12). There was no difference between the ratios of PCr/ATP during reperfusion though the SERCA group showed a lower trend (non-significant difference).
Figure 7.
Energy potential. The energetic potential (PCr/ATP) was determined from 31P NMR spectra acquired from SERCA1 and PBS hearts throughout the protocol. The energetic ratio during reperfusion was greater than preischemic values as expected for the PBS group. The energetic potential also recovered for the SERCA group. The observed difference between the two groups, non-signficant, was accounted for by a modest improvement in ATP (ns) for the SERCA1 group, while PCr was similar between groups. (mean ± S.E.).
4. DISCUSSION
In this study the skeletal muscle isoform of SERCA1 was expressed in rat heart. Compared to the cardiac isoform (SERCA2a) SERCA1 is not regulated by phospholamban (27), and has a higher activity with a two-fold greater calcium uptake relative to SERCA2a (28,29). SERCA1 is also more resistant to oxidative stress (31) and acidosis (32) (ie., two key characteristics of ischemic reperfusion) than SERCA2a. Several groups, including our own, have shown that increased expression of SERCA improves myocardial contractility and Ca2+ handling in several models of heart disease including ischemic reperfused injury (1,2,4), diabetes (33), and heart failure (21,26).
4.1 SERCA1 Protects Against Myocardial Stunning
Consistent with our earlier report (21), overexpression of SERCA1 resulted in a 20% increase in myocardial contractility (±dP/dt). The accelerated relaxation of the heart following contraction is consistent with a faster sequestration of calcium into the sarcoplasmic reticulum as a consequence of SERCA1 overexpression. This improvement in contractility at baseline was not accompanied by a significant change in RPP or developed pressure, consistent with findings of Logeart and colleagues (30). Using a similar method of SERCA1 gene delivery to rabbit heart, they found left ventricular contractility (±dP/dt) increased 15% while left ventricular systolic pressure was unchanged. In contrast, several groups report a greater functional response in transgenic mice overexpressing SERCA1. Huke et al has reported a 20% increase LVDP with ±dP/dt nearly doubling (34), and Talukder reported a 100% increase in LVDP with ±dP/dt increasing three fold (1).
An important distinction between the transgenic mice and the rat/rabbit model relates to the extent of SERCA1 overexpression. Whereas, Logeart and our group report a 21-35% increase in SERCA content using adenoviral delivery (21,30), SERCA content increased 100-150% in the transgenic mouse models (1,34). Thus, improvements in function are linked to the extent of SERCA overexpression. In support of this suggestion, He et al developed a transgenic mouse model with SERCA protein content increasing 20%. They found dP/dt increased 15 - 30% without a change in LVDP (35). Nevertheless, the level of SERCA expression induced by our method was sufficient to provide significant protection against myocardial stunning.
The PBS group showed a rate-pressure-product (RPP) during reperfusion 30% lower than pre-ischemic values. Consistent with myocardial stunning, this loss was attributed to a significant drop (40% reduction, p<0.05) in LVDP. The overexpression of SERCA1 protected the hearts from cardiac dysfunction. RPP, LVDP, and ±dP/dt all recovered to preischemic values in the SERCA1 group.
The recovery with SERCA1 supports the trend observed by Talukder for SERCA1 transgenic mice after a 30 min ischemic insult (1). While recovery was not complete for the length of ischemia in their model, they did observe an accelerated and markedly improved postichemic myocardial contractile function, diminished incidence of abnormal ventricular rhythms, and smaller infarct size. They attributed the recovery to a measured reduction in the accumulation of intracellular Ca2+ compared with non-transgenics. This reduction in calcium load was the basis for our suggestion that metabolic activity would be influenced at reperfusion in hearts overexpressing SERCA.
4.2 Metabolic Flux Rate During Reperfusion
The reducing equivalents of cytosolic NADH are transported into the mitochondria via the αKG-malate transporter as part of the malate-asparate shuttle. In earlier work, we examined how perturbation in myocardial calcium load would influence the transport of cytosolic NADH into the mitochondria (15). Cytosolic and mitochondrial calcium loads were increased in isolated retrograde perfused hearts by increasing the perfusate calcium content, while workloads were held constant with pH reduced. We found the transport of reducing equivalents into the mitochondria was reduced as αKG-malate transporter activity decreased. Both reperfused hearts (9,13) and in hearts at high workloads (adrenergic challenge) (36), both conditions characterized by high calcium load, display comparable reductions in the transport of reducing equivalents into mitochondria. Thus, we speculated whether the reduced calcium load, as reported for reperfused hearts overexpressing SERCA1, would preserve the transport of cytosolic NADH reducing equivalents into the mitochondria.
The transport of reducing equivalent into the mitochondria is balanced to TCA cycle flux by the coordinated activity of the mitochondrial matrix enzyme, α-KG dehydrogenase, and the α-KG-malate transporter of the mitochondrial membrane. The calcium activated dehydrogenase competes with the reversible α-KG-malate carrier for efflux of αKG through this mitochondrial membrane transporter. The process is regulated by intramitochondrial calcium, pH+ (5,6), αKG availability, and redox state (NADH/NAD+) (15,37). Conversely, the α-KG-malate transporter is not sensitive to pH+ or calcium, yet is limited by α-KG and cytosolic redox state (38). Thus an increase in α-KG dehydrogenase activity due to Ca2+ load shifts the efflux of α-KG from the mitochondria to oxidation by the dehydrogenase in postischemic myocardium.
In the current reperfusion study, the overexpression of SERCA1 resulted in a significant increase in αKG-malate transporter activity. While VTCA cycle activity was unchanged, αKG-malate activity increased 65% (p<0.05) relative to the reperfused PBS group. The increase in αKG-malate activity, as part of the malate-aspartate shuttle, provides a mechanism for increased shuttling of cytosolic NADH reducing equivalent into the mitochondria to support oxidative phosphorylation. Consistent with this faster exchange of metabolite across the αKG-malate transporter, glutamate content (21±2 μmol/gdw) completely recovered to pre-ischemic values. These findings support the mechanism suggested, namely, that a lower calcium load reported for reperfused heart overexpressing SERCA will alter metabolic activity, particularly at the level of metabolite exchange between the mitochondria and cytosol. With an increase in the transport of glycolytic NADH into the mitochondria to fuel oxidative metabolism, metabolic efficiency is enhance with SERCA overexpression in reperfused myocardium.
4.3 Fatty Acid Oxidation During Reperfusion
In addition to the regulatory role calcium plays on mitochondrial dehydrogenase activity and NADH transport, calcium activation of PDH contributes to regulation of the balance between fatty acid and glucose oxidation. Indeed, Belke and Dillmann have shown that a high level of SERCA2 overexpression leads to activation of PDH as a consequence of increased cytosolic calcium load (39). This resulted in an increase in glucose oxidation for their transgenic model. Under reperfusion conditions, Talukder has reported that cytosolic calcium loads are reduced in transgenic mice overexpressing SERCA1 (1). It was not known whether glucose oxidation would subsequently be reduced. A reduction in glucose oxidation could potentially be detrimental to recovery, and evoke the opposite affect of the many therapeutic approaches (DCA, ranolazine, trimetazidine) to increase glucose oxidation at reperfusion (17,40).
To the contrary, we found SERCA overexpression did not alter the balance between palmitate and glucose oxidation. Under pre-ischemic conditions, palmitate contributed 73% to mitochondrial ATP production in both SERCA1 and PBS groups. After a 40 min reperfusion period, the contributions from palmitate was reduced to 63% in both groups. The drop in palmitate oxidation during reperfusion supports earlier work by Tamm et al for isolated hearts studied under experimental conditions similar to our own (41). The ineffective influence of SERCA over palmitate oxidation, contrary to the Belke study cited above, is consistent with our earlier report and likely reflects the lower levels of SERCA overexpression with our animal model (21,39). Thus, with improvements in metabolic coupling and efficiency, the balance of fat and carbohydrate oxidation by the mitochondria is not influenced at 40 min reperfusion for this level of SERCA1 expression. It remains to be seen if this is a dose response, and whether higher levels of SERCA expression leads to reduced glucose oxidation and limits the extent of recovery in reperfused heart.
4.4 Energetic Costs
31P NMR is the only noninvasive technique capable of measuring PCr, ATP, and inorganic phosphate in the intact beating heart, and it is one indicator of the energetic state of the myocardium. A reduction in PCr/ATP is interpreted as a loss of energy reserve. Measuring PCr/ATP values has the disadvantage that it underestimates the change in PCr when ATP levels are also reduced. Though direct quantification of high-energy metabolites concentrations were beyond the scope of this study, we did assess the percent change in PCr and ATP level referenced to pre-ischemic values (Figure 7). We found no significant difference in recovery of the PCr pool between PBS and SERCA1 group. ATP recovery was not significantly different between groups, though the SERCA1 group showed a trend toward greater recovery. This would account for the lower PCr/ATP ratio trend observed for the SERCA1 group in Figure 7. While the rate of recovery or level of high energy metabolites are not significantly different between groups, it is important to recognize that the SERCA1 group had much greater demands, with higher developed pressures, and these energetic demands were being met without a loss in energy potential.
4.5 Animal Model Considerations
An important consideration involves the level of SERCA overexpression at reperfusion (ie., dose response). While our group and Logeart reported a 20-34% increase in SERCA content following the gene transfer approach taken in this study (21,30), others find SERCA content increasing 100-400% in SERCA overexpressing transgenic mice and infected isolated cardiomyocytes (1,28,29,34,39). Thus, it remains to be seen whether higher levels of SERCA overexpression further affect the metabolic outcome. In addition, while the transgenic model provides a robust level of SERCA overexpression, the adenoviral approach provides a much more “targeted” model for examination. The physiological changes are acute and in direct response to SERCA overexpression. The transgenic approach represents chronic overexpression for which whole body physiological and biochemical changes occur. It is not always clear whether these changes observed with transgenics are direct or a secondary adaptation to protein overexpression.
Changes in calcium handling measured directly in intact functioning rat heart overexpressing SERCA1 during ischemia / reperfusion has not been reported. However, in mice Talukder reported intra-ischemic Ca2+ levels were significantly lower in isolated transgenic hearts overexpressing SERCA (1). This finding is consistent with reports from our group and others that calcium uptake by the SR is accelerated with SERCA1 overexpression in isolated cardiomyocytes (28,29). Unfortunately the cardiomyocyte model cannot be extended for an ischemic / reperfusion condition. Whereas a model of hypoxia offers an alternative, hypoxic responses cannot be used for comparison of calcium and metabolic function of an ischemic model. Glycolytic activity remains intact during hypoxia and toxic byproducts (lactate, H+) are readily release contrary to the ischemic condition which results in stunning. Additional studies are required to establish direct changes in calcium handling for the intact functioning rat heart overexpressing SERCA1 for the ischemia / reperfusion model which would support the results reported for mice.
4.6 Conclusions
The major finding in this study was that the uncoupling between TCA cycle flux and cardiac function, in the stunned myocardium, is reversed in hearts overexpressing SERCA1. We have identified the transport of glycolytic NADH reducing equivalents into the mitochondria, which is depressed in stunned myocardium, as one mechanism for improved recovery. This represents an improvement in cardiac metabolic efficiency as reducing equivalents from glycolysis are better utilized to support oxidative phosphorylation. Interestingly, the balance between fatty acid and carbohydrate oxidation was not altered with SERCA overexpression in reperfused heart. This lack of change in substrate selection is important, because it was unknown whether glucose oxidation decreased as a result of the reported reduction in calcium load observed for reperfused hearts overexpressing SERCA, thereby potentially limiting recovery. While much is still to be learned, this study finds the cardioprotective and metabolic effects of SERCA overexpresssion support the increasing potential of its therapeutic utility.
The improvement in metabolic efficiency we report with SERCA overexpression is clearly an outcome of a multifactorial process, involving calcium removal from the cytosol. The noted improvements in NADH transport are a new factor that contributes to this efficiency, but clearly not the sole event to account for enhanced function. Future studies are necessary to examine whether mitochondrial membrane damage and myofilament dysfunction are also directly prevented by limiting calcium overload at reperfusion with SERCA overexpression.
SOURCES OF FUNDING
This work was supported in part by National Heart, Lung, and Blood Institute Grants RO1 HL-079415 (J.M. O'Donnell) and HL-56178 (E.D. Lewandowski).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Talukder MAH, Kalyanasundaram A, Zhao X, Zuo L, Bhupathy P, Babu GJ, Cardounel AJ, Periasamy M, Zweier JL. Expression of SERCA isoform with faster Ca2+ transport properties improves postischemic cardiac function and Ca2+ handling and decreases myocardial infarction. Am J Physiol Heart Circ Physiol. 2007;293:H2418–H2428. doi: 10.1152/ajpheart.00663.2007. [DOI] [PubMed] [Google Scholar]
- 2.Chen Y, Escoubet B, Prunier F, Amour J, Simonides WS, Vivien B, Lenoir C, Heimburger M, Choqueux C, Gellen B, Riou B, Michel JB, Franz WM, Mercadier JJ. Constitutive cardiac overexpression of sarcoplasmic/endoplasmic reticulum Ca2+-ATPase delays myocardial failure after myocardial infarction in rats at a cost of increased acute arrhythmias. Circulation. 2004;109:1898–1903. doi: 10.1161/01.CIR.0000124230.60028.42. [DOI] [PubMed] [Google Scholar]
- 3.Periasamy M, Huke S. SERCA pump level is a critical determinant of Ca2+ homeostasis and cardiac contractility. J Mol Cell Cardiol. 2001;33:1053–1063. doi: 10.1006/jmcc.2001.1366. [DOI] [PubMed] [Google Scholar]
- 4.Del Monte F, Lebeche D, Guerrero JL, Tsuji T, Doye AA, Gwathmey JK, Hajjar RJ. Abrogation of ventricular arrhythmias in a model of ischemia and reperfusion by targeting myocardial calcium cycling. Proc Natl Acad Sci. 2004;101:5622–5627. doi: 10.1073/pnas.0305778101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McCormack JG, Halestrap AP, Denton RM. Role of calcium ions in regulation of mammalian intramitochondrial metabolism. Physiol Rev. 1990;70:391–417. doi: 10.1152/physrev.1990.70.2.391. [DOI] [PubMed] [Google Scholar]
- 6.Wan B, LaNoue KF, Cheung JY, Scaduto JR RC. Regulation of citric acid cycle by calcium. J Biol Chem. 1989;264:13430–13439. [PubMed] [Google Scholar]
- 7.Balaban RS. Cardiac energy metabolism homeostasis: role of cytosolic calcium. J Mol Cell Cardiol. 2002;34:1259–1271. doi: 10.1006/jmcc.2002.2082. [DOI] [PubMed] [Google Scholar]
- 8.Laster SB, Becker LC, Ambrosio G, Jacobus WE. Reduced aerobic metabolic efficiency in globally “stunned” myocardium. J Mol Cell Cardiol. 1989;21:419–426. doi: 10.1016/0022-2828(89)90652-4. [DOI] [PubMed] [Google Scholar]
- 9.Lewandowski ED, Yu X, LaNoue KF, White LT, Doumen C, O'Donnell JM. Altered metabolite exchange between subcellular compartments in intact postischemic rabbit hearts. Circ Res. 1997;81:165–175. doi: 10.1161/01.res.81.2.165. [DOI] [PubMed] [Google Scholar]
- 10.Liu B, elAlaoui-Talibi Z, Clanachan AS, Schulz R, Lopaschuk GD. Uncoupling of contractile function from mitochondiral TCA cycle activity and MVO2 during reperfusion of ischemic hearts. Am J Physiol (Heart Circ Physiol) 1996;270(39):H72–H80. doi: 10.1152/ajpheart.1996.270.1.H72. [DOI] [PubMed] [Google Scholar]
- 11.Weiss RG, Kalil-Filho R, Herskowitz A, Chacko VP, Litt M. Tricarboxylic acid cycle activitiy in postischemic rat hearts. Circulation. 1993;87:270–282. doi: 10.1161/01.cir.87.1.270. [DOI] [PubMed] [Google Scholar]
- 12.Lewandowski ED, Johnston DL. Reduced substrate oxidation in postischemic myocardium 13C and 31P NMR analyses. Am J Physiol (Heart Circ. Physiol) 1990;2598(27):H1357–H1365. doi: 10.1152/ajpheart.1990.258.5.H1357. [DOI] [PubMed] [Google Scholar]
- 13.O'Donnell JM, White LT, Lewandowski ED. Mitochondrial Transporter Responsiveness and Metabolic Flux Homeostasis in Postischemic Hearts. Amer J Physiol. 1999;277:H866–H873. doi: 10.1152/ajpheart.1999.277.3.H866. [DOI] [PubMed] [Google Scholar]
- 14.LaNoue DF, Williamson JR. Interrelationships between malate-aspartate shuttle and citric acid cycle in rat heart mitochondrial. Metabolism. 1992;20:119–140. doi: 10.1016/0026-0495(71)90087-4. [DOI] [PubMed] [Google Scholar]
- 15.O'Donnell JM, Doumen C, LaNoue KF, White LT, Yu X, Alpert NM, Lewandowski ED. Dehydrogenase Regulation of Metabolite Oxidation and Efflux From Mitochondria in Intact Hearts. Amer J Physiol. 1998;43:H467–H476. doi: 10.1152/ajpheart.1998.274.2.H467. [DOI] [PubMed] [Google Scholar]
- 16.Terrand J, Papageorgiou I, Rosenblatt-Velin N, Lerch R. Calcium-mediated activation of pyruvate dehydrognase in severly injured postischemic myocardium. Am J Physiol Heart Circ Physiol. 2001;281:H722–H730. doi: 10.1152/ajpheart.2001.281.2.H722. [DOI] [PubMed] [Google Scholar]
- 17.Lewandowski ED, White LT. Pyruvate dehydrogenase influences postichemic heart function. Circulation. 1995;91:2071–2079. doi: 10.1161/01.cir.91.7.2071. [DOI] [PubMed] [Google Scholar]
- 18.Wambolt RB, Lopaschuk GD, Brownsey RW, Allard MF. Dichloroacetate improves postischemic function of hypertrophied rat hearts. J Am Coll Cardiol. 2000;36(4):1378–85. doi: 10.1016/s0735-1097(00)00856-1. [DOI] [PubMed] [Google Scholar]
- 19.O'Donnell JM, Lewandowski ED. Efficient, Cardiac-Specific Adenoviral Gene Transfer by Isolated Retrograde Perfusion In Vivo. Gene Therapy. 2005;12:958–965. doi: 10.1038/sj.gt.3302477. [DOI] [PubMed] [Google Scholar]
- 20.O'Donnell JM, Lewandowski ED. Controlling Specificity and Efficiency of Adenoviral Gene Transfer in Heart by Catheter Based Coronary Perfusion. In: Niewohner & Tannert, editor. Gene Therapy - Prospective assessment in its societal context. Elsevier (pub); 2006. pp. 33–46. [Google Scholar]
- 21.O'Donnell JM, Fields A, Xu X, Chowdhury SAK, Geenen DL, Bi J. Limited functional and metabolic improvements in hypertrophic and healthy rat heart overexpressing the skeletal muscle isoform of SERCA1 by adenoviral gene trasfer in vivo. Am J Physiol (Heart Circ Physiol) 2008;295:H2483–H2494. doi: 10.1152/ajpheart.01023.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O'Donnell JM, Fields A, Sorokina N, Lewandowski ED. The absence of endogenous lipid oxidation in early stage heart failure exposes limits in lipid storage and turnover. J Mol Cell Physiol. 2008;44(2):315–322. doi: 10.1016/j.yjmcc.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O'Donnell JM, White LT, Alpert NM, Griffin JL, Lewandowski ED. Coupling of Mitochondrial Fatty Acid Uptake to Oxidative Flux in the Intact Heart. Biophys J. 2002 Jan;82(1):11–18. doi: 10.1016/S0006-3495(02)75369-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Malloy CR, Sherry AD, Jeffrey FMH. Evaluation of carbon flux and substrate selection through alternate pathways involving the citric acid cycle of the heart by 13C NMR spectroscopy. J Biol Chem. 1989;263:6965–6971. [PubMed] [Google Scholar]
- 25.Yu X, Alpert NM, Lewandowski ED. Modeling enrichment kinetics from dynamic 13C NMR spectra: theoretical analysis and practical considerations. Am J Physiol Cell Physiol. 1997;272:C2037–C2048. doi: 10.1152/ajpcell.1997.272.6.C2037. [DOI] [PubMed] [Google Scholar]
- 26.Del Monte F, Williams E, Lebeche D, Schmidt U, Rosenzweig A, Gwathmey JK, Lewandowski ED, Hajjar RJ. Improvement in survival and cardiac metabolism after gene transfer of sarcoplasmic reticulum Ca(2+)-ATPase in a rat model of heart failure. Circulation. 2001 Sep 18;104(12):1424–1429. doi: 10.1161/hc3601.095574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Periasamy M, Kalyanasundaram A. SERCA pump isoforms: their role in calcium transport and disease. Muscle Nerve. 2007;35:430–442. doi: 10.1002/mus.20745. [DOI] [PubMed] [Google Scholar]
- 28.Cavagna M, O'Donnell JM, Sumbilla C, Inesi G, Klein MG. Exogenous Ca2+-ATPase Isoform Effects on Ca2+ Transients of Embryonic Chicken and Neonatal Rat Cardiac Myocytes. J of Physiol. 2000;528(1):53–63. doi: 10.1111/j.1469-7793.2000.00053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Inesi G, Cavagna M, O'Donnell JM, Sumbilla C, Zhong L, Ma H, Klein MG. Adenovirus Mediated Gene Transfer of SERCA Isoforms. In: Hasenfuss G, Marban E, editors. Molecular Approaches to the Therapy of Heart Failure. Springer-Steinkopff; Germany: 2000. pp. 76–88. [Google Scholar]
- 30.Logeart D, Vinet L, Ragot T, Heimburger M, Louedec L, Michel JB, Escoubet B, Mercadier JJ. Percutaneous intracoronary delivery of SERCA gene increases myocardial function: a tissue Doppler imaging echocardiographic study. Am J Physiol Heart Circ Physiol. 2006;291:H1773–1779. doi: 10.1152/ajpheart.00411.2006. [DOI] [PubMed] [Google Scholar]
- 31.Viner RI, Ferrington DA, Willioms TD, Bigelow DJ, Schoneich C. Protein modification during biological aging: selective tyrosine nitration of the SERCA2a isoform of the sarcoplasmic reticulum Ca2+-ATPase in skeletal muscle. Biochem J. 1999;340:667–669. [PMC free article] [PubMed] [Google Scholar]
- 32.Wolosker H, Rocha JBT, Engelender S, Panizzutti R, Miranda JD, de Meis L. Sarco/endoplasmic reticulum Ca2+-ATPase isoforms: diverse responses to acidosis. Biochem J. 1997;321:545–550. doi: 10.1042/bj3210545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vetter R, Rehfeld U, Reissfelder C, Weiss W, Wagner KD, Gunther J, Hammes A, Tschope C, Dillmann W, Paul M. Transgenic overexpression of the sarcoplasmic reticulum Ca2+ATPase improves reticular Ca2+ handling in normal and diabetic reat hearts. FASEB J. 2002 Oct;16(12):1657–1659. doi: 10.1096/fj.01-1019fje. [DOI] [PubMed] [Google Scholar]
- 34.Huke S, Prasad V, Nieman ML, Nattamai KJ, Grupp IL, Lorenz JN, Periasamy M. Altered dose response to β-agonists in SERCA1-expressing hearts ex vivo and in vivo. Am J Physiol Heart Circ Physiol. 2002;283:H958–H966. doi: 10.1152/ajpheart.00078.2002. [DOI] [PubMed] [Google Scholar]
- 35.He H, Giordano FJ, Hilal-Dandan R, Choi DJ, Rockman HA, McDonough PM, Fluhm WF, Meyer M, Sayen MR, Swanson E, Dillmann WH. Overexpression of the rat sarcoplasmic reticulum Ca2+ ATPase gene in the heart of transgenic mice accelerates calcium transients and cardiac relaxation. J Clin Invest. 1997;100:380–389. doi: 10.1172/JCI119544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O'Donnell JM, Kudej RK, White LT, LaNoue KF, Vatner SF, Lewandowski ED. Limited Transfer of Cytosolic NADH into Mitochondria at High Cardiac Workload. Amer J Physiol (Heart Circ Physiol) 2004;286(6):H2237–H2242. doi: 10.1152/ajpheart.01113.2003. [DOI] [PubMed] [Google Scholar]
- 37.Dawson AG. Oxidation of cytosolic NADH formed during aerobic metabolism in mammalian cells. Trends Biochem Sci. 1979;4:171–176. [Google Scholar]
- 38.Sluse FE, Goffart G, Liebecq C. Mechanism of the exchanges catalysed by the oxoglutarate translocator of rat heart mitochondria. Kinetics of the external-product inhibition. Eur J Biochem. 1973;32:283–291. doi: 10.1111/j.1432-1033.1973.tb02609.x. [DOI] [PubMed] [Google Scholar]
- 39.Belke DD, Swanson E, Suarez J, Scott BT, Stenbit AE, Dillmann WH. Increased expression of SERCA in the hearts of transgenic mice results in increased oxidation of glucose. Am J Physiol Heart Circ Physiol. 2007;292:H1755–H1763. doi: 10.1152/ajpheart.00884.2006. [DOI] [PubMed] [Google Scholar]
- 40.Van der Vusse GJ, van Bilsen M. Free fatty acids and postischemic myocardial function. Sem Cardiothor Basc Anesthesia. 2006;10(3):231–235. doi: 10.1177/1089253206291319. [DOI] [PubMed] [Google Scholar]
- 41.Tamm C, Benzi R, Papageorgiou I, Tardy I, Lerch R. Substrate competition in postischemic myocardium. Effect of substrate availability during reperfusion on metabolic and contractile recovery in isolated rat hearts. Circ Res. 1994;75:1103–1112. doi: 10.1161/01.res.75.6.1103. [DOI] [PubMed] [Google Scholar]