Abstract
The matricellular protein thrombospondin-2 (TSP2) has context-dependent effects on osteoblast lineage proliferation and differentiation. Mice lacking TSP2 display increased endocortical bone thickness, which is associated with increased marrow stromal cell (MSC) number and in vitro proliferation. TSP2-null MSC also exhibit delayed osteoblastogenesis and enhanced adipogenesis compared to cells harvested from wild type mice. The goal of the present work was to more precisely characterize the contribution that TSP2 makes to the maturation of osteoblast-derived extracellular matrix (ECM) using a highly characterized pre-osteoblast cell line. Specifically, we asked whether TSP2 influences mineralization indirectly through its known effects on proliferation, or whether TSP2 directly promotes osteoblast differentiation. To pursue these questions, we used RNA-interference (RNAi) to inhibit TSP2 gene expression in MC3T3-E1 pre-osteoblasts. Introduction of siRNA oligonucleotides resulted in reduced TSP2 mRNA expression as early as 24 hours post-transfection, and TSP2 mRNA levels remained low for 10 days. Similarly, TSP2 protein levels in both conditioned medium and the cell-matrix layer were reduced at 24 hours post-transfection and remained reduced for 7 days. At day 21, mineralization was significantly reduced in cells transfected with TSP2 siRNA when compared to cells treated with scrambled siRNA. This decrease in mineralization occurred without a concomitant change in cell number. Twenty-four hours after transfection, runx2 gene expression was transiently enhanced in TSP2 siRNA treated cultures. Between 6 and 14 days post-transfection, runx2, osterix, alkaline phosphatase, type I collagen, osteocalcin and bone sialoprotein all displayed moderate increases in gene expression with TSP2 RNAi. As well, soluble osteocalcin levels were markedly higher in the conditioned medium of cells treated with TSP2 siRNA than in control siRNA-treated cells. Increased soluble osteocalcin occurred without a concomitant change in the levels of osteocalcin in the cell-ECM layer. TSP2 reduction also elicited a transient change in the distribution of collagen between the acid soluble cell-ECM protein fraction and the insoluble matrix. Together, our data suggest that TSP2 may promote mineralization, by facilitating proper organization of the osteoblast-derived ECM.
Keywords: osteoblast, matricellular, thrombospondin-2, osteocalcin, mineralization
Introduction
Thrombospondins (TSPs) are modular extracellular matrix glycoproteins that affect cellular phenotype by modulating cell-ECM interactions in a cell- and environment-dependent manner [1, 2]. TSP1 and TSP2 each form 450 kDa homotrimers, and they display considerable sequence homology, as well as some functional redundancy. For example, TSP1 and 2 are each potent anti-angiogenic factors [3, 4], they inhibit DNA synthesis in multiple cell types [4–6] and they both affect MMP-2 metabolism [7–9]. Despite such functional similarities, several observations suggest distinct roles for TSP1 and 2, especially in the context of skeletal biology. For example, while TSP1-null mice have a mild skeletal phenotype [10], TSP-2 null mice display increased endocortical bone formation rates compared to wild type mice [11]. TSP2 inhibits marrow stromal cell (MSC) proliferation [5], and an increased population of osteoblast precursors within the MSC pool is likely to partially account for increased cortical bone density in TSP2-null mice. TSP2 might also affect osteoblast differentiation more directly, and observations made in vitro support this premise. For example, despite greater MSC numbers and increased rates of proliferation, the onset of terminal osteoblast mineralization is delayed in cultures of TSP2-null MSC [11]. In order to separate the effects of TSP2 on proliferation from its potential direct effects on osteoblast differentiation, we used RNA interference to reduce TSP2 expression in high density cultures of the well-charcterized MC3T3-E1 pre-osteoblast cell line.
TSP2 is expressed by MSC and osteoblast lineage cells [11, 12], but its effects on osteoblasts are incompletely characterized. Osteoblastogenesis is associated with an ordered progression of increasing assembly and deposition of ECM proteins, such as osteocalcin, bone sialoprotein, and type I collagen, but the significance of TSP2 in this process is unknown. In order to determine whether TSP2 directly modulates maturation of the osteoblast-derived ECM, we used siRNA to reduce TSP2 expression in high-density cultures of MC3T3-E1 pre-osteoblasts. Our data indicate that, without affecting cell number, a transient reduction in TSP2 expression leads to a subsequent reduction in mineralization. Gene expression analysis indicates that while TSP1 levels were not affected, decreased TSP2 expression leads to transient increases in runx2, osterix, type I collagen, alkaline phosphatase, bone sialoprotein and osteocalcin gene expression that occur between 6 and 14 days following transfection. TSP2 RNAi resulted in a substantial increase in soluble osteocalcin levels without affecting the amount of osteocalcin present in the cell layer. In contrast, TSP2 reduction lead to modest and transient changes in the distribution of collagen between the medium, the acid soluble cell-ECM fraction and the insoluble matrix fraction. Together, these data suggest that TSP2 affects mineralization, at least partially, through its effects on incorporation of collagen into the extracellular matrix. TSP2 reduction also appears to lead to downstream changes in post-transcriptional regulation of osteocalcin. We hypothesize that changes in cell-ECM interactions secondary to altered collagen organization in the absence of TSP2 lead to compensatory increases in the expression of genes associated with bone matrix assembly and maturation.
Methods
Cell Culture
The highly mineralizing MC3T3-E1 clone 14 cell line (a generous gift from Dr. Renny Franchesci, University of Michigan [13]) was maintained in α-MEM (Invitrogen, Carlsbad, CA) containing 10% fetal bovine serum (Hyclone Laboratories, Logan, UT) and 1% Pennicillin/Streptomycin (Invitrogen, Carlsbad, CA). Cells were maintained under standard cell culture conditions of 5% CO2 and 95% humidity. For experiments, confluent cells were removed using 0.25% trypsin containing 10 mM EDTA, resuspended in antibiotic-free growth medium and plated onto 6 well plates at a density of 150,000 cells per well. 16–20 hours later, cells were transfected with siRNA directed against TSP2, as described below. 24 hours following transfection, the medium was changed to antibiotic-free osteoblastic differentiation medium, which is composed of α- MEM containing 10% FBS plus 10 mM β-glycerolphosphate and 1.25 µM ascorbic acid. Medium was replaced every two to three days. Penicillin/Streptomycin was added back to the differentiation medium with the first post-transfection medium change.
siRNA transfections
Three candidate siRNAs against TSP2 were obtained from Invitrogen (Carlsbad, CA). Preliminary data suggested that one of the three performed optimally with regards to the degree of TSP2 reduction, as well as specificity. This siRNA oligonucleotide was used for all experiments presented here. The sense strand sequence of this siRNA is 5’-UUGGUAUGCUGAUUGCCUUCUACCC-3.’
Transient transfection of TSP2-siRNA or the medium GC-content negative control siRNA (Invitrogen, Carlsbad, CA) was performed using Lipofectamine-2000, according to the manufacturer’s instructions. Briefly, siRNA was diluted to 1 pmol/µl in Optimem reduced serum medium and mixed with pre-formed Lipofectamine-2000 complexes. The siRNA-lipofectamine mixture was added to the antibiotic-free growth medium of cells that had been in culture over-night. Twenty-four hours later, medium was changed to osteoblastic differentiation medium and cell cultures were maintained as described above.
Quantitative rtPCR analysis
Total RNA was harvested from cell cultures using the RNeasy RNA isolation kit (Qiagen, Valencia, CA) and DNase treated using the on-column RNAse-free DNase system (Qiagen, Valencia, CA). RNA was quantified by ultraviolet spectrophotometry. One microgram of RNA was then reverse transcribed using oligo (dT) primers and superscript III reverse transcriptase (invitrogen, Carlsbad, CA). The resulting cDNA was used in real time PCR using Power SYBR green (Applied Biosystems, Foster City, CA) and the primer sequences presented in table 1. Thermal cycling was performed using a 7500 Fast Real-Time System (Applied Biosystems, Foster City, CA). Proper amplicon formation was confirmed by melt-curve analysis, and relative mRNA expression levels were calculated using the double delta-Ct method [14].
Table 1.
Primer sequences for rtPCR.
| Gene Product | Forward Primer | Reverse Primer |
|---|---|---|
| TSP2 | tgtctcgctcattgaaaaca | tcttcaatccccgtcaatta |
| Osteocalcin | cgctctgtctctctgacctc | tcacaagcagggttaagctc |
| BSP | ttccatcgaagaatcaaagc | tcgcagtctccattttcttc |
| Alkaline phosphatase | gtcatcatgttcctgggaga | ggcccagcgcaggat |
| Collagen type I | aatggtgctcctggtattgc | ggcaccagtgtctcctttgt |
| osterix | tctctccatctgcctgactc | gtcagcgtatggcttctttg |
| Runx2/cbfa1 | gtcagcaaagcttcttttcg | ttgttgctgttgctgttgtt |
| TSP1 | tatgtgcctaatgccaacca | gccatcaccatcagagtcct |
| β-actin | aagagctatgagctgcctga | tggcatagaggtctttacgg |
Western blot analysis
Medium was collected from cell cultures and equal volumes were diluted in 6X gel sample buffer (3M Tris-HCL (pH 6.8), 30% glycerol, 10% SDS, 5% BME and 0.012% bromphenol blue) containing the protease inhibitors PMSF, sodium fluoride, sodium orthovanadate and the complete EDTA-free protease inhibitor cocktail (Roche Applied Science, Indianapolis, IN). Cell and matrix layers were washed twice in ice-cold PBS and proteins were solubilized in M-PER cell extraction buffer (Fisher Scientific, Pittsburgh, PA) containing protease inhibitors and 1 µl per ml DTT. After boiling, proteins were separated on 7.5% SDS-PAGE gels and transferred to nitrocellulose membranes in transfer buffer (192 mM glycine and 20% methanol in 25 mM Tris, pH 8.3) at 4°C. Non-specific sites were blocked with Tris-buffered saline containing 0.05% Tween 20 and 5% nonfat dry milk. Next, blots were incubated with a monoclonal anti-mouse TSP2 antibody (BD Biosciences, San Jose, CA). The membranes were then washed three times in Tris-buffered saline containing 0.05% Tween 20 and incubated with horseradish peroxidase conjugated goat-anti-mouse immunoglobin-G (Pierce). Membranes were washed again and immunoreactive proteins were detected using enhanced chemiluminescence (ECL; Thermo Scientific, Rockford, IL). Relative band intensities were determined from histograms generated using ImageJ gel analysis software (National Institutes of Health, Bethesda, MD).
Cytochemical detection of mineralized matrix
The alizarin red-S method was used to stain matrix calcium deposits. Cell cultures were rinsed with PBS, air dried and fixed in 50% ethanol. The cells were then stained for five minutes in a 1.0% solution of Alizarin red S, rinsed thoroughly in ddH20 and examined visually for mineralized nodules. Relative amounts of mineral were determined by dissolving the alizarin red dye in 0.5 N HCL containing 5% SDS for 30 minutes at room temperature. Optical densities were read at 415 nm using a SpartSpec 3000 UV spectrophotometer (Biorad Laboratories, Hercules, CA).
Determination of cell number
Cell number was determined by removing the cells with 0.25% trypsin containing 10 mM EDTA and conducting direct cell counts using a hemocytometer.
Determination of DNA content
DNA was extracted from culture wells using a DNA extraction kit (Qiagen, Valencia, CA) and optical densities were read at 260 and 280 nm using a SpectraMax5 spectrophotometer (Molecular Devises, Eugene, Oregon).
Osteocalcin EIA
Conditioned medium was collected and snap frozen at the indicated time points following transfection. Cell and matrix proteins were solubilized for 30 minutes at 37°C in 0.5 N HCL. The acid insoluble protein fraction was removed by centrifugation and the pH of the supernatent was adjusted by adding 50 µl per ml 10N NaOH. Osteocalcin levels in conditioned medium and cell-matrix fractions were determined using an enzyme-linked immunoabsorbent assay kit (Biomedical Technologies, Boston, MA). Concentrations of osteocalcin were determined spectrophotometrically by comparing optical density values of medium and cell-matrix samples to those derived from osteocalcin standards provided with the kit.
Determination of collagen levels
Collagen levels were determined using a sirius red dye binding method (Sircol soluble collagen assay; Biocolor Ltd, Carrickfergus, Northern Ireland). Conditioned medium was collected and snap frozen at the indicated time points following transfection. Acid soluble collagen was extracted from cell layers over night at room temperature in 0.5M acetic acid. The samples were centrifuged for 1 hour at 14,000×g, and levels of acid soluble collagen were determined in the supernatent. Collagen in the insoluble pellet was denatured overnight at 60°C in water containing proteinase K. Concentrations of collagen in conditioned medium and the acid soluble cell layer fraction were determined by comparing optical density values to those derived from collagen standards provided with the kit. Relative amounts of collagen in the insoluble matrix fraction were also determined by spectrophotometry.
Statistics
All data are means ± standard deviation from three to six independent experiments, totaling 6 to 12 observations under each condition. Statistical comparisons between scrambled and TSP2 siRNA treatments were performed using student’s t test. Values were considered to be statistically different at p < 0.05.
Results
TSP2 RNA interference leads to reduced TSP2 mRNA and protein levels
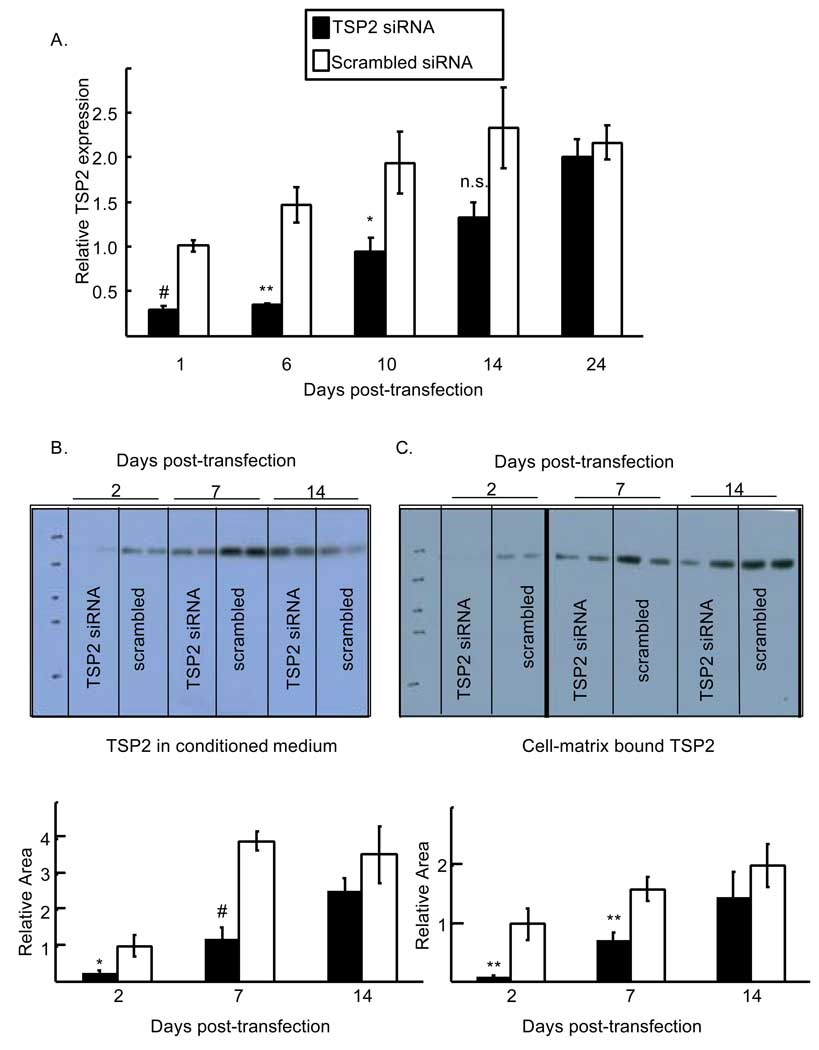
In order to determine whether TSP2 contributes directly to bone cell differentiation, we employed siRNA against TSP2. In the control siRNA treated wells, TSP2 message levels steadily increased between 6 and 24 days in association with osteoblast differentiation (Figure 1). TSP2 RNAi lead to rapid and significant reductions in TSP2 message and protein levels (Figure 1). One day following transfection relative TSP2 mRNA levels were significantly reduced by TSP2 siRNA (0.29 ± 0.05 and 1.01 ± 0.06 for TSP2 siRNA and scrambled siRNA treated wells, respectively; p < 0.001). At day 6 relative levels of TSP2 mRNA remained low in the TSP2 siRNA-treated wells (0.35 ± 0.01 and 1.5 ± 0.19 for TSP2 siRNA and scrambled siRNA treated wells, respectively; p < 0.001.) By day 10 post-transfection, gene expression in the TSP2 siRNA-treated wells was comparable to the day 1 control values (0.94 ± 0.15 and 1.01 ± 0.06 for TSP2 siRNA treated wells at days 10 and day 1, respectively; not significant.) However, TSP2 mRNA levels were still significantly reduced compared to control values at this time (0.94 ± 0.15 and 1.94 ± 0.35 for TSP2 siRNA and scrambled siRNA treated wells, respectively; p < 0.05).
Figure 1.
Time course of TSP2 reduction and recovery following RNA interference. A). TSP2 gene expression levels were determined in TSP2 siRNA- and scrambled siRNA-treated cells by rtPCR. TSP2 mRNA levels, normalized to actin mRNA levels are expressed relative to scrambled siRNA-treated samples at day 1 post-transfection. B). Top: relative soluble TSP2 protein levels were determined in conditioned medium of TSP2 siRNA and scrambled siRNA-treated cells by Western blot. Equal sample volumes were loaded. Bottom: For each lane, histograms were generated using ImageJ and the area under the curve was determined. C). Top: Relative cell-ECM bound TSP2 protein levels were determined in TSP2- and scrambled-siRNA treated wells by Western blot. Equal amounts of protein were loaded. The blot was exposed for 30 seconds to visualize the day 2 samples; the rest of the samples were visualized at 15 seconds. Bottom: For each lane, histograms were generated using ImageJ and the area under the curve was determined. All data are mean ± SEM of three independent experiments totaling 6 to 8 observations under each treatment condition and time point. * p<0.05, ** p<0.01, # p<0.001 as compared to scrambled siRNA treated cells within a time point.
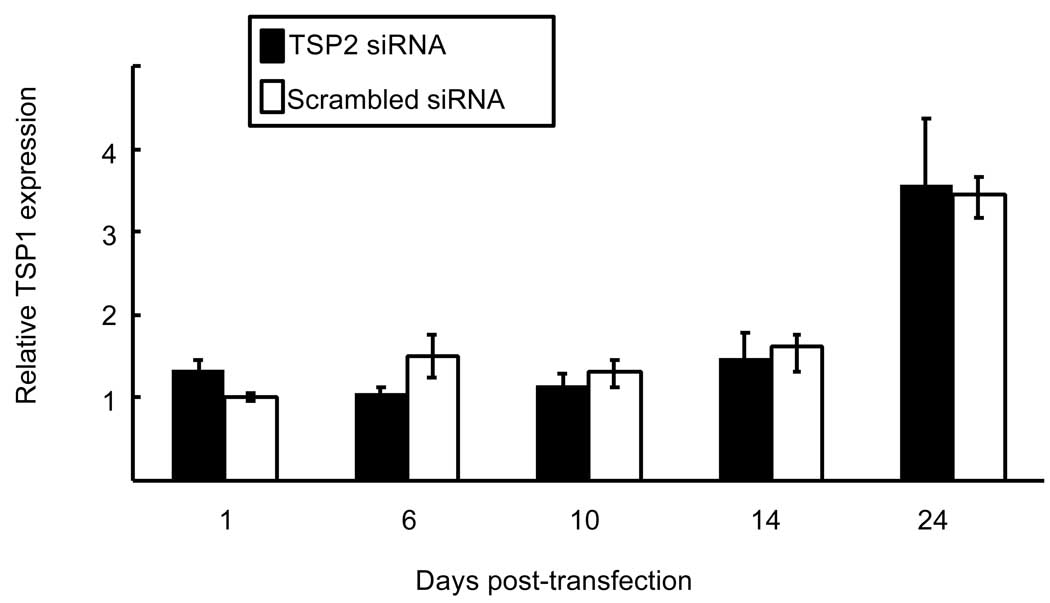
Similar to TSP2 mRNA levels, figure 1B shows that RNAi lead to reduced TSP2 protein levels in culture medium at day 2 (0.24 ± 0.09 and 1.0 ± 0.29 for TSP2 siRNA and scrambled siRNA treated wells, respectively; p < 0.05). At day 7, TSP2 protein levels were still significantly reduced in the conditioned medium of cells treated with TSP2 siRNA (1.18 ± 0.34 and 3.9 ± 0.25 for TSP2 siRNA treated and scrambled siRNA treated wells, respectively; p < 0.001). By day 14, TSP2 protein levels in the siRNA-treated wells were approaching control values and were no longer statistically different (2.55 ± 0.35 and 3.54 ± 0.81 for TSP2 siRNA treated and scrambled siRNA treated wells, respectively). Similarly, figure 1C shows that TSP2 protein levels in cell-matrix protein extracts were reduced at days 2 (0.095 ± 0.02 and 1.0 ± 0.27 for TSP2 siRNA treated and scrambled siRNA treated wells, respectively; p<0.01) and 7 (0.71 ± 0.14 and 1.6 ± 0.21 for TSP2 siRNA and scrambled siRNA treated wells, respectively; p<0.01). By day 14 post-transfection TSP2 levels in the cell layer were returning to control values and were no longer statistically different (1.46 ± 0.44 and 2.0 ± 0.36 for TSP2 siRNA treated and control treated wells, respectively). Together, the data presented in Figure 1 indicate that TSP2 siRNA leads to a rapid reduction in TSP2 mRNA and protein levels, and that it takes approximately two weeks for gene expression to return to control values. Importantly, TSP2 RNAi did not affect TSP1 mRNA levels at any of the times tested (Figure 2).
Figure 2.
Effect of TSP2 RNAi on TSP1 gene expression. TSP1 mRNA levels were determined in TSP2 siRNA and scrambled siRNA-treated cells by rtPCR. TSP1 mRNA levels, normalized to actin mRNA levels are expressed relative to scrambled siRNA treated samples at day 1 post-transfection. Data are mean ± SEM of three independent experiments totaling 6 observations under each treatment condition and time point.
TSP2 RNAi leads to reduced mineral accumulation without affecting cell number
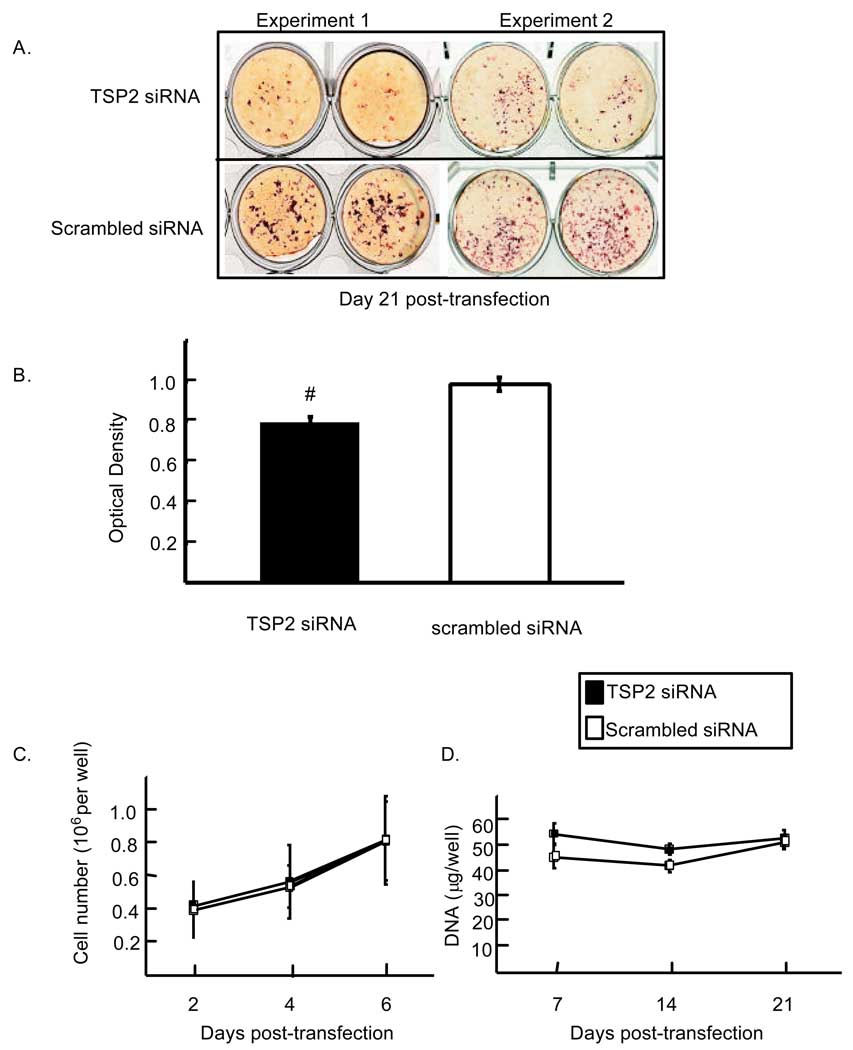
In order to determine the potential contribution that TSP2 makes to bone cell differentiation, we analyzed the effects of TSP2 RNAi on matrix mineralization. Figure 3A shows two representative experiments indicating that a transient reduction of TSP2 expression (figure 1) leads to a subsequent reduction in mineralization at 21 days post-transfection. Relative to cells treated with scrambled siRNA, cells treated with TSP2 siRNA displayed a 20% reduction in mineral (0.80 ± 0.026 and 1.0 ± 0.037 for TSP2 siRNA and scrambled siRNA treated wells, respectively; p < 0.001; Figure 3B). Published data indicate that TSP2 inhibits MSC proliferation, and differences in cell number could potentially account for time-dependent differences in mineralization [5]. However, direct cell counts and DNA quantification by UV spectrophotometry both indicated that TSP2 reduction did not affect MC3T3-E1 cell growth (Figure 3C and D). Subsequent experiments were designed to identify mechanisms by which a reduction in TSP2 expression might lead to reduced mineralization.
Figure 3.
Effect of TSP2 siRNA on mineralization. A). Cells were transfected with TSP2 siRNA or a scrambled siRNA and cultured under osteogenic conditions for 21 days. Mineral nodules were stained with Alizarin Red S dye. B). The dye was solubilized using SDS and acid, and optical densities were recorded at 415 nm. Data are mean ± SEM of five independent experiments totaling 12 observations under each treatment condition. The effect of TSP2 RNAi on proliferation was monitored by direct cell counts (C) and by UV spectrophotometry (D). # p<0.001 compared to scrambled siRNA treated samples.
TSP2 reduction leads to modest increases in the expression of osteoblast-specific transcription factors, as well as markers of differentiated osteoblasts
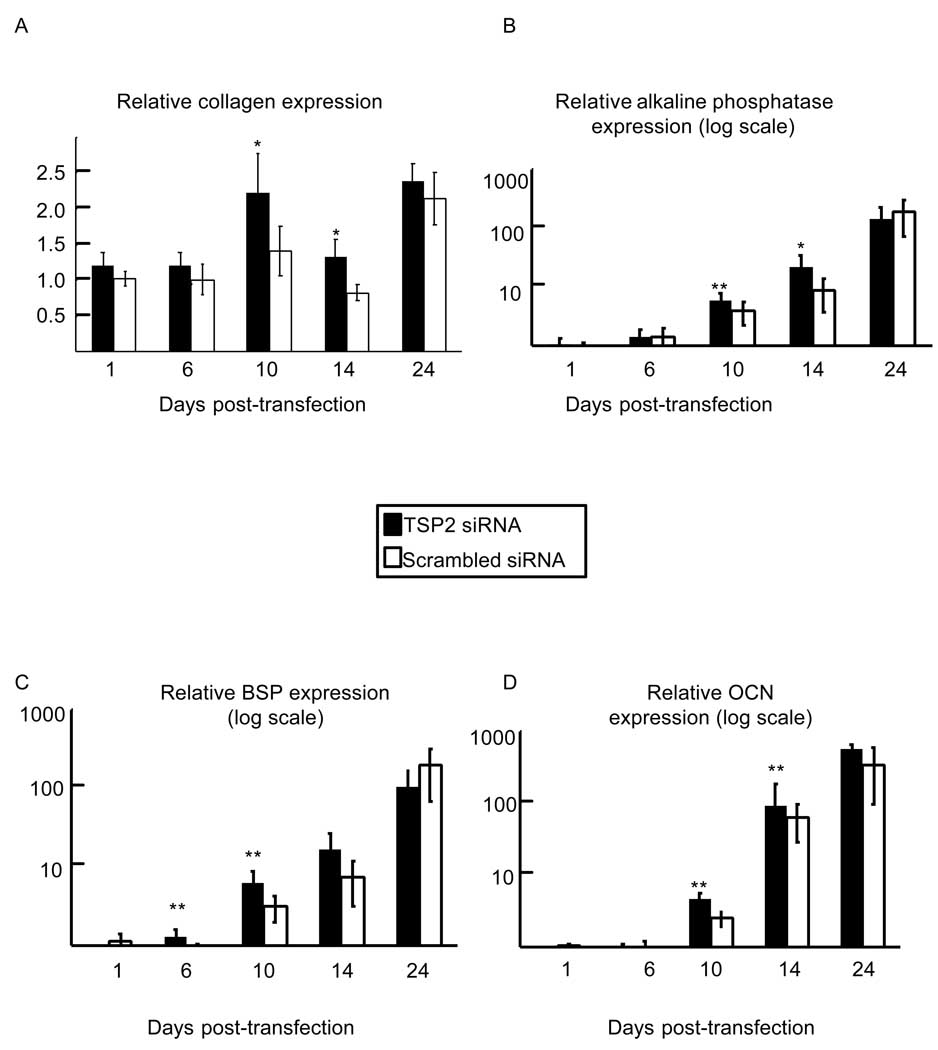
Osteoblast differentiation involves a series of transcriptional activation events that is dependent on runx2 and osterix [15]. Surprisingly, 24 hours following transfection, relative levels of runx2 mRNA were increased in TSP2 siRNA-treated wells (2.13-fold increased; p < 0.05; Figure 4A). This initial increase in mRNA was transient; by day 6 runx2 levels in the TSP2 siRNA treated wells had returned to control values. However, between days 10 and 24, runx2 expression was enhanced with TSP2 RNAi. Specifically, at day 10 there was a trend towards higher runx2 levels with TSP2 reduction (1.57-fold higher at day 10; p=0.05). At days 14 and 24 after transfection, runx2 mRNA levels remained approximately 50% higher in TSP2 siRNA treated wells relative to scrambled siRNA treated wells (p < 0.05).
Figure 4.
Effect of TSP2 RNAi on expression of runx2 and osterix. A. Runx2 mRNA levels (A) and osterix mRNA levels (B), each normalized to actin, are expressed relative to scrambled siRNA treated expression levels at day 1. Data are mean ± SEM of three independent experiments totaling six observations under each treatment condition and time point. * p<0.05, ** p<0.01 as compared to scrambled siRNA treated samples within a time point.
Similar to runx2, osterix mRNA levels were increased in TSP2 siRNA treated wells at day 10 post-transfection (Figure 4B; 2.21-fold higher; p < 0.01). In contrast to runx2; however, the TSP2 RNAi-induced increase in osterix expression was transient, and expression levels had returned to control values by day 14.
We also examined genes associated with osteoblast differentiation and matrix mineralization. Figure 5A shows that type I collagen expression was increased relative to control values at days 10 and 14 post-transfection in TSP2 siRNA treated wells (1.75-fold increased at day 10; p < 0.05. 1.63-fold increased at day 14; p < 0.05). Similarly, at days 10 and 14 following transfection, alkaline phosphatase gene expression was increased relative to control values in TSP2-siRNA treated samples (Figure 5B; 1.78-fold increased at day 10; p < 0.01. 1.74-fold increased at day 14; p < 0.05).
Figure 5.
Effect of TSP2 RNAi on type I collagen (A), alkaline phosphatase (B), bone sialoprotein (C), and osteocalcin gene expression. For each osteoblast marker gene, mRNA levels are normalized to actin mRNA levels and expressed relative to expression levels in scrambled siRNA treated samples at day 1. Data are mean ± SEM of three independent experiments totaling six observations under each treatment condition and time point. * p<0.05, ** p<0.01 as compared to scrambled siRNA treated samples within a time point.
Bone sialoprotein gene expression was also increased in TSP2 siRNA treated wells at days 6 and 10 post-transfection (Figure 5C; 1.67-fold increase at day 6; p < 0.01. 1.95-fold increase at day 10; p < 0.01). Relative osteocalcin gene expression was increased in TSP2 siRNA treated wells at day 14 post-transfection (Figure 4D; 1.82-fold increase; p < 0.01).
Together, gene expression analyses suggest that decreases in TSP2 have modest positive effects on expression of markers of osteoblast differentiation and matrix production, despite the obvious decrease in mineralization observed in Figure 3.
Reduced TSP2 expression affects matrix protein levels and distribution
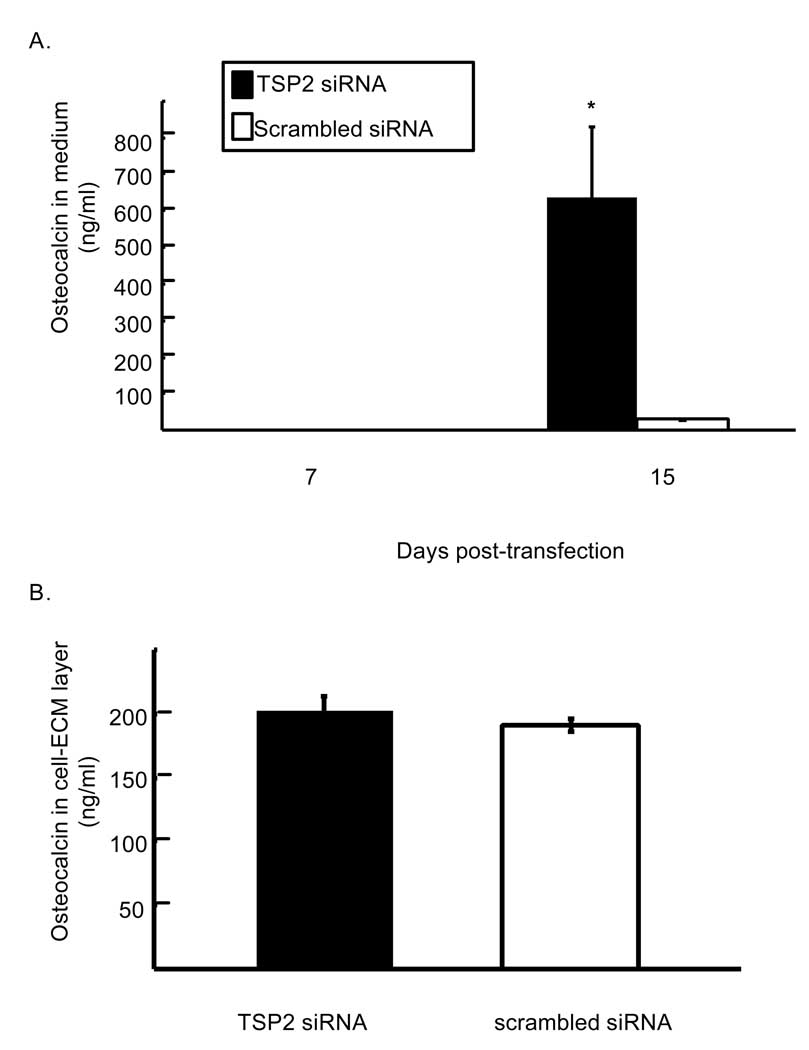
In order to further characterize the relationship between reduced TSP2 gene expression and matrix mineralization, we examined the effects of TSP2 siRNA on accumulation of osteoblast matrix proteins. At day 15 post-transfection, medium removed from cells that were treated with TSP2 siRNA contained markedly higher levels of osteocalcin compared with controls (Figure 6A; 635.2 ± 193.1 and 30.44 ± 2.9 ng osteocalcin per ml conditioned medium for TSP2 siRNA and scrambled siRNA, respectively; p < 0.05). In contrast, in cultures that were beginning to undergo mineralization, levels of osteocalcin in the cell-ECM layer were not affected by TSP2 RNAi (Figure 6B). Together, these data suggest that TSP2 RNAi affects osteocalcin levels through both transcriptional and post-transcriptional mechanisms.
Figure 6.
Effect of TSP2 RNAi on osteocalcin protein levels. A). Concentration of osteocalcin in the conditioned medium from TSP2 and scrambled siRNA treated cells as determined by enzyme linked immunoabsorbent assay. B). Concentration of osteocalcin in the cell-ECM layer of TSP2 and scrambled siRNA treated wells undergoing mineralization. Data are mean ± SEM of three independent experiments totaling six observations under each treatment condition and time point. * p< 0.05 compared to scrambled siRNA treated samples within the same time point.
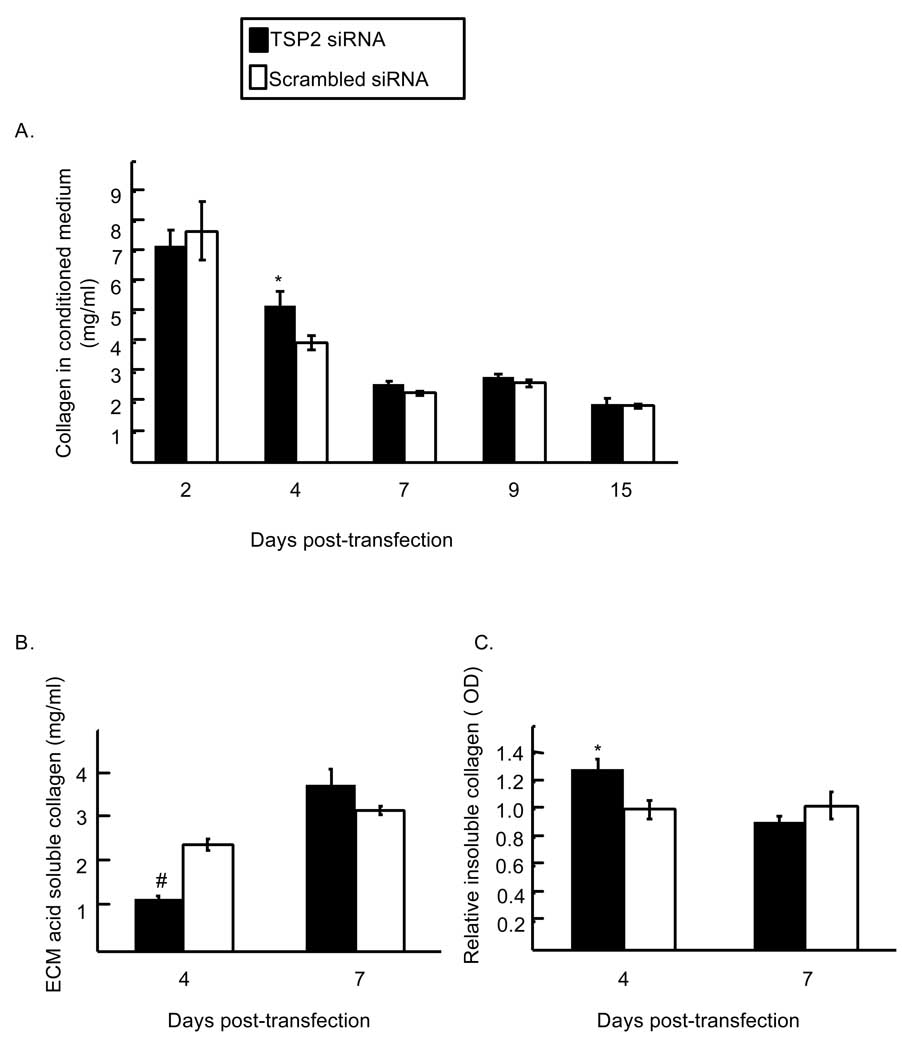
In contrast to the effects on osteocalcin, TSP2 RNAi modestly and transiently increased levels of soluble collagen at day four post-transfection (Figure 7A; 5.3 ± 0.5 and 3.87 ± 0.2 mg collagen per ml conditioned medium for TSP2 siRNA and scrambled siRNA, respectively; p < 0.05). At the same time, levels of acid soluble collagen in the cell-matrix layer of TSP2 siRNA treated wells were reduced compared to control wells (Figure 7B; 1.19 ± 0.07 and 2.43 ± 0.13 mg collagen per ml in TSP2 siRNA and scrambled siRNA treated wells, respectively, p < 0.001). Conversely, relative levels of collagen in the insoluble ECM protein fraction were increased in the TSP2 RNAi treated group at day 4 (Figure 7C; 1.29 ± 0.07 and 1.0 ± 0.07 in TSP2 siRNA and scrambled siRNA treated wells, respectively, p<0.05). Notably, the observed changes in collagen protein levels and distribution occur prior to the increase in type I collagen mRNA levels observed at days 10 and 14 post-transfection. These data implicate a role for TSP2 in promoting matrix mineralization in part by promoting the assembly and/or retention of collagen in the osteoblast-derived ECM.
Figure 7.
Effect of TSP2 RNAi on collagen protein levels A. Concentration of collagen in the conditioned medium of TSP2 and scrambled siRNA treated cells as determined by a sirius red dye binding assay. B. Concentrations of collagen in the acid soluble cell-ECM protein fraction. C. Relative amounts of collagen in the acid insoluble extracellular matrix protein fraction. Data are mean ± SEM of three to four independent experiments totaling six to eight observations under each treatment condition and time point. * p<0.05 compared to scrambled siRNA treated samples within the same time point. # p< 0.001 comapred to scrambled siRNA treated samples within the same time point.
Discussion
In order to identify effects of the matricellular protein TSP2 on osteoblast differentiation, we used siRNA to transiently inhibit TSP2 gene expression in cultures of MC3T3-E1 pre-osteoblasts. TSP2 siRNA leads to reduced TSP2 mRNA and protein expression within 24 hours of transfection, and TSP2 expression levels returned to control values approximately two weeks later. This transient reduction in TSP2 protein expression was associated with changes in mineralization, but did not affect cell number.
Published data suggest that TSP2 contributes to mineralization. For example, TSP2-null MSC display delayed mineralization in culture [5], and exogenous TSP2 restores early mineralization in these cultures (unpublished results). In addition, primary osteoblasts harvested from mice over expressing the transcription factor fra1 display reduced TSP2 mRNA expression, as well as reduced mineralization [16]. Our observations are the first to directly implicate TSP2 in the regulation of mineralization of the osteoblast-derived ECM. This is in marked contrast to Ueno and colleagues [17] who demonstrated that TSP1, which shares significant sequence homology with TSP2, inhibits mineralization in MC3T3-E1 cells.
TSP2 inhibits MSC proliferation [5, 11], and differences in matrix accumulation and mineralization could potentially be related to differences in cell number. However, in high-density cultures of MC3T3-E1 pre-osteoblasts, TSP2 RNAi did not affect proliferation; whether this discrepancy is related to plating density or to cell-context dependent differences between MSC and osteoblasts remains to be determined.
In order to determine whether TSP2 affects osteoblast mineralization by modulating expression of genes involved in osteoblast maturation, we studied the effects of TSP2 reduction on runx2 and osterix mRNA levels. Runx2 is expressed early in the course of osteoblast differentiation and levels are largely maintained over time. Runx2 activity is controlled post-translationally, both by serine phosphorylation and by the availability of transcription co-factors [18]. While runx2 is essential for normal osteoblast differentiation and bone formation [19], its over expression in osteoblasts leads to a severe osteopenia associated with impaired osteoblast maturation and mineralization, despite increased numbers of neonatal osteoblasts [20]. Here, TSP2 RNAi elicited a transient increase in runx2 gene expression one day after transfection. The mechanism by which the removal of TSP2 from the extracellular environment might elicit such a rapid change in runx2 gene expression remains to be determined.
A second increase in runx2 mRNA levels occurred at approximately day 10 post-transfection and was maintained until day 24. One target of runx2 is osterix, and the two transcription factors are both required for progression of osteoblast lineage cells [18]. At day 10 post-transfection, we did observe a transient increase in osterix gene expression with TSP2 RNAi. However, whether TSP2 regulates runx2 and osterix gene expression directly, and whether the effects of TSP2 on the two genes are independent of each other is unclear. Together, our data indicate that TSP2 has modest effects on expression of runx2 and osterix, two transcription factors required for osteoblast differentiation.
We also evaluated the effects of TSP2 RNAi on expression of markers of differentiating and mineralizing osteoblasts. Osteoblast specific matrix proteins bone sialoprotein (BSP) and osteocalcin are down-stream targets of runx2 and osterix [21]. In vitro, BSP promotes nucleation of hydroxyapatite crystals [22], and osteoblasts derived from BSP transgenic mice display increased mineralization capacity in vitro. However, BSP transgenic mice also display decreased osteoblast numbers and increased osteoclastic activity resulting in uncoupled bone formation and resorption and osteopenia [23]. Temporally, BSP gene expression peaks just prior to the onset of mineralization. While the effect of TSP2 reduction on runx2 and osterix may cause concomitant changes in downstream targets, it is also likely that TSP2 may affect additional genes that were not examined here, and these changes may play important roles in the reduced mineralization that we observe secondary to TSP2 reduction.
Similar to BSP, TSP2 RNAi lead to an increase in relative osteocalcin gene expression in TSP2 RNAi treated wells. This effect was modest at day 14 and it may be the direct result of increased osterix-dependent transcription. In contrast, conditioned medium from cells treated with TSP2 siRNA exhibited on average 20 times more osteocalcin than medium from control-treated cells. Our data indicate that levels of osteocalcin in the cell layer were not affected, and so increased soluble osteocalcin in TSP2 siRNA treated samples may be the result of post-transcriptional effects secondary to TSP2 reduction. Osteocalcin expression correlates with accumulation of mineral in the ECM, but the precise contribution that osteocalcin makes to mineralization in vivo is unclear. Osteocalcin-null mice display age-dependent increases in bone formation at both cortical and cancellous sites. In contrast to TSP2-null mice, increased bone matrix production in osteocalcin-null mice occurs without a concomitant change in osteoblast lineage cell number [24]. The overall increase in bone matrix production in these mice is accompanied by subtle changes in mineral crystal properties [25].
Our data indicate that TSP2 reduction transiently affects organization of the collagenous ECM. Assembly of a type I collagen rich matrix is essential for in vitro maturation of osteoblasts [26], and so changes in the organization of collagen fibrils may have a downstream affect on osteoblast gene expression, matrix maturation and mineralization. Collagen fibrils in the skin and tendons of TSP2-null mice display aberrant fibril sizes and organization [27]. Whether TSP2 also modulates collagen fibril assembly in osteoblast-derived ECM or in bone remains to be determined.
TSP2 binds calcium [28], and so it could potentially interact with mineral crystals and influence mineralization by modulating crystal formation. Like osteocalcin, calcium-binding induces conformational changes in TSP2 [28, 29], but the nature of any direct interactions between TSP2 and other osteoblast matrix proteins has not been well characterized. TSPs interact with a wide-variety of extracellular molecules, including type I collagen [30]. Additional evidence that TSP2 might play an active role in matrix assembly and/or mineralization is provided by the observation that TSP2 is enriched in matrix vesicles isolated from differentiating MC3T3-E1 pre-osteoblasts [31].
Acknowledgements
This work was supported by NIAMS/NIH R01 AR049682 (kdh). We would like to thank Dr. Renny Franceschi for insightful discussions and review of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bornstein P, Agah A, Kyriakides TR. The role of thrombospondins 1 and 2 in the regulation of cell-matrix interactions, collagen fibril formation, and the response to injury. Int J Biochem Cell Biol. 2004;36:1115–1125. doi: 10.1016/j.biocel.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 2.Bornstein P, Sage E. Matricellular proteins: extracellular modulators of cell function. Current Opinion in Cell Biology. 2002;14:608–616. doi: 10.1016/s0955-0674(02)00361-7. [DOI] [PubMed] [Google Scholar]
- 3.Armstrong L, Bornstein P. Thrombospondins 1 and 2 function as inhibitors of angiogenesis. Matrix Biology. 2003;22:63–71. doi: 10.1016/s0945-053x(03)00005-2. [DOI] [PubMed] [Google Scholar]
- 4.Armstrong LC, Bjorkblom B, Hankenson KD, Siadak AW, Stiles CE, Bornstein P. Thrombospondin 2 inhibits microvascular endothelial cell proliferation by a caspase-independent mechanism. Mol Biol Cell. 2002;13:1893–1905. doi: 10.1091/mbc.E01-09-0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hankenson KD, Bornstein P. The secreted protein thrombospondin 2 is an autocrine inhibitor of marrow stromal cell proliferation. J Bone Miner Res. 2002;17:415–425. doi: 10.1359/jbmr.2002.17.3.415. [DOI] [PubMed] [Google Scholar]
- 6.Lopes N, Gregg D, Vasudevan S, Hassanain H, Goldschmidt-Clermont P, Kovacic H. Thrombospondin 2 regulates cell proliferation induced by Rac1 redox-dependent signaling. Mol Cell Biol. 2003;23:5401–5408. doi: 10.1128/MCB.23.15.5401-5408.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bein K, Simons M. Thrombospondin type 1 repeats interact with matrix metalloproteinase 2. Regulation of metalloproteinase activity. J Biol Chem. 2000;275:32167–32173. doi: 10.1074/jbc.M003834200. [DOI] [PubMed] [Google Scholar]
- 8.Yang Z, Kyriakides TR, Bornstein P. Matricellular proteins as modulators of cellmatrix interactions: adhesive defect in thrombospondin 2-null fibroblasts is a consequence of increased levels of matrix metalloproteinase-2. Mol Biol Cell. 2000;11:3353–3364. doi: 10.1091/mbc.11.10.3353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee T, Esemuede N, Sumpio BE, Gahtan V. Thrombospondin-1 induces matrix metalloproteinase-2 activation in vascular smooth muscle cells. J Vasc Surg. 2003;38:147–154. doi: 10.1016/s0741-5214(02)75468-2. [DOI] [PubMed] [Google Scholar]
- 10.Lawler J, Sunday M, Thibert V, Duquette M, George EL, Rayburn H, Hynes RO. Thrombospondin-1 is required for normal murine pulmonary homeostasis and its absence causes pneumonia. J Clin Invest. 1998;101:982–992. doi: 10.1172/JCI1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hankenson KD, Bain SD, Kyriakides TR, Smith EA, Goldstein SA, Bornstein P. Increased marrow-derived osteoprogenitor cells and endosteal bone formation in mice lacking thrombospondin 2. J Bone Miner Res. 2000;15:851–862. doi: 10.1359/jbmr.2000.15.5.851. [DOI] [PubMed] [Google Scholar]
- 12.Carron JA, Bowler WB, Wagstaff SC, Gallagher JA. Expression of members of the thrombospondin family by human skeletal tissues and cultured cells. Biochem Biophys Res Commun. 1999;263:389–391. doi: 10.1006/bbrc.1999.1380. [DOI] [PubMed] [Google Scholar]
- 13.Wang D, Christensen K, Chawla K, Xiao G, Krebsbach PH, Franceschi RT. Isolation and characterization of MC3T3-E1 preosteoblast subclones with distinct in vitro and in vivo differentiation/mineralization potential. J Bone Miner Res. 1999;14:893–903. doi: 10.1359/jbmr.1999.14.6.893. [DOI] [PubMed] [Google Scholar]
- 14.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using realtime quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 15.Lian JB, Stein GS, Javed A, van Wijnen AJ, Stein JL, Montecino M, Hassan MQ, Gaur T, Lengner CJ, Young DW. Networks and hubs for the transcriptional control of osteoblastogenesis. Rev Endocr Metab Disord. 2006;7:1–16. doi: 10.1007/s11154-006-9001-5. [DOI] [PubMed] [Google Scholar]
- 16.Nishiwaki T, Yamaguchi T, Zhao C, Amano H, Hankenson KD, Bornstein P, Toyama Y, Matsuo K. Reduced expression of thrombospondins and craniofacial dysmorphism in mice overexpressing Fra1. J Bone Miner Res. 2006;21:596–604. doi: 10.1359/jbmr.051216. [DOI] [PubMed] [Google Scholar]
- 17.Ueno A, Miwa Y, Miyoshi K, Horiguchi T, Inoue H, Ruspita I, Abe K, Yamashita K, Hayashi E, Noma T. Constitutive expression of thrombospondin 1 in MC3T3-E1 osteoblastic cells inhibits mineralization. J Cell Physiol. 2006;209:322–332. doi: 10.1002/jcp.20735. [DOI] [PubMed] [Google Scholar]
- 18.Franceschi RT, Ge C, Xiao G, Roca H, Jiang D. Transcriptional Regulation of Osteoblasts. Cells Tissues Organs. 2008 doi: 10.1159/000151747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ducy P, Zhang R, Geoffroy V, Ridall AL, Karsenty G. Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell. 1997;89:747–754. doi: 10.1016/s0092-8674(00)80257-3. [DOI] [PubMed] [Google Scholar]
- 20.Liu W, Toyosawa S, Furuichi T, Kanatani N, Yoshida C, Liu Y, Himeno M, Narai S, Yamaguchi A, Komori T. Overexpression of Cbfa1 in osteoblasts inhibits osteoblast maturation and causes osteopenia with multiple fractures. J Cell Biol. 2001;155:157–166. doi: 10.1083/jcb.200105052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roca H, Phimphilai M, Gopalakrishnan R, Xiao G, Franceschi RT. Cooperative interactions between RUNX2 and homeodomain protein-binding sites are critical for the osteoblast-specific expression of the bone sialoprotein gene. J Biol Chem. 2005;280:30845–30855. doi: 10.1074/jbc.M503942200. [DOI] [PubMed] [Google Scholar]
- 22.Hunter GK, Goldberg HA. Nucleation of hydroxyapatite by bone sialoprotein. Proc Natl Acad Sci U S A. 1993;90:8562–8565. doi: 10.1073/pnas.90.18.8562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Valverde P, Zhang J, Fix A, Zhu J, Ma W, Tu Q, Chen J. Overexpression of bone sialoprotein leads to an uncoupling of bone formation and bone resorption in mice. J Bone Miner Res. 2008;23:1775–1788. doi: 10.1359/JBMR.080605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ducy P, Desbois C, Boyce B, Pinero G, Story B, Dunstan C, Smith E, Bonadio J, Goldstein S, Gundberg C, Bradley A, Karsenty G. Increased bone formation in osteocalcin-deficient mice. Nature. 1996;382:448–452. doi: 10.1038/382448a0. [DOI] [PubMed] [Google Scholar]
- 25.Boskey AL, Gadaleta S, Gundberg C, Doty SB, Ducy P, Karsenty G. Fourier transform infrared microspectroscopic analysis of bones of osteocalcin-deficient mice provides insight into the function of osteocalcin. Bone. 1998;23:187–196. doi: 10.1016/s8756-3282(98)00092-1. [DOI] [PubMed] [Google Scholar]
- 26.Xiao G, Gopalakrishnan R, Jiang D, Reith E, Benson MD, Franceschi RT. Bone morphogenetic proteins, extracellular matrix, and mitogen-activated protein kinase signaling pathways are required for osteoblast-specific gene expression and differentiation in MC3T3-E1 cells. J Bone Miner Res. 2002;17:101–110. doi: 10.1359/jbmr.2002.17.1.101. [DOI] [PubMed] [Google Scholar]
- 27.Kyriakides TR, Zhu YH, Smith LT, Bain SD, Yang Z, Lin MT, Danielson KG, Iozzo RV, LaMarca M, McKinney CE, Ginns EI, Bornstein P. Mice that lack thrombospondin 2 display connective tissue abnormalities that are associated with disordered collagen fibrillogenesis, an increased vascular density, and a bleeding diathesis. J Cell Biol. 1998;140:419–430. doi: 10.1083/jcb.140.2.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Misenheimer TM, Mosher DF. Biophysical characterization of the signature domains of thrombospondin-4 and thrombospondin-2. J Biol Chem. 2005;280:41229–41235. doi: 10.1074/jbc.M504696200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hauschka PV, Wians FH., Jr Osteocalcin-hydroxyapatite interaction in the extracellular organic matrix of bone. Anat Rec. 1989;224:180–188. doi: 10.1002/ar.1092240208. [DOI] [PubMed] [Google Scholar]
- 30.Kyriakides TR, Tam JW, Bornstein P. Accelerated wound healing in mice with a disruption of the thrombospondin 2 gene. J Invest Dermatol. 1999;113:782–787. doi: 10.1046/j.1523-1747.1999.00755.x. [DOI] [PubMed] [Google Scholar]
- 31.Xiao Z, Camalier CE, Nagashima K, Chan KC, Lucas DA, de la Cruz MJ, Gignac M, Lockett S, Issaq HJ, Veenstra TD, Conrads TP, Beck GR., Jr Analysis of the extracellular matrix vesicle proteome in mineralizing osteoblasts. J Cell Physiol. 2007;210:325–335. doi: 10.1002/jcp.20826. [DOI] [PubMed] [Google Scholar]