Abstract
The study of energy expenditure (EE) has deep roots in understanding aging and lifespan in all species. In humans, total EE decreases substantially in advanced age resulting from parallel changes in resting metabolic rate (RMR) and activity EE. For RMR, this reduction appears to be due to a reduction in organ mass and specific metabolic rates of individual tissues. However, these anatomical changes explain very little regarding the decline in activity EE, which is governed by both genetic and environmental sources. The biological control centers for activity EE are closely coupled with body mass fluctuations and seem to originate in the brain. Several candidate neuromodulators may be involved in the age-related reduction of activity EE that include: orexin, agouti-related proteins and dopaminergic pathways. Unfortunately, the existing body of research has primarily focused on how neuromodulators influence weight gain and only a few studies have been performed in aging models. Recent evidence suggests that activity EE has an important role in dictating lifespan and thus places emphasis on future research to uncover the underlying biological mechanisms. The study of EE continues to unlock clues to aging.
1.0 Introduction
Among the enormous literature on theoretical explanations of aging the role of energy expenditure (EE) has received the most attention. For thousands of years, energy expenditure and metabolism have provided an understanding of how we function today, tomorrow, and eventually when we cease to function altogether. The study of EE and aging has been spurred along by the intriguing association between high rates of EE in short-lived mammals and low rates of EE in long-lived mammals. Some propose that EE per lifespan is fixed and abundant usage will accelerate aging (Rubner, 1908). While many works have challenged and severely criticized the rate of living theory (Holloszy and Smith, 1986; Austad and Fischer, 1991), EE and the biological processes that control the machinery are very much under intense study (Speakman, 2005).
Energy expenditure has often been purported as a cause of aging without a complete understanding of changes in each of EE components with age. A thorough understanding of the age-related changes in EE components will help reveal the potential compensatory strategies that preserve lifespan. For example, it remains unclear why EE due to physical activity declines across all mammalian phylogenetic lines. Although many hypotheses on aging have involved in some manner the rates of metabolism, the debate on the role of altering EE for enhancing lifespan continues with new data in humans (Manini et al., 2006). The results add new light to the role of EE in preserving average lifespan among older adults.
Because of rich literature on this topic, a review of “Energy Expenditure and Aging” certainly can’t be performed in the space provided in this article. It is for this reason I have chosen to focus much of my efforts on energy expenditure and aging in humans. There are several reasons for this choice that include: 1.) new data in humans that have begun to address questions regarding theoretical effects of aging and energy metabolism, 2.) methods for assessment of energy expenditure in humans are precise and able to parcel total EE into its individual constituents, and 3.) it has been several years since the previous review of this topic has been performed (Wilson and Morley, 2003).
2. Total energy expenditure and aging
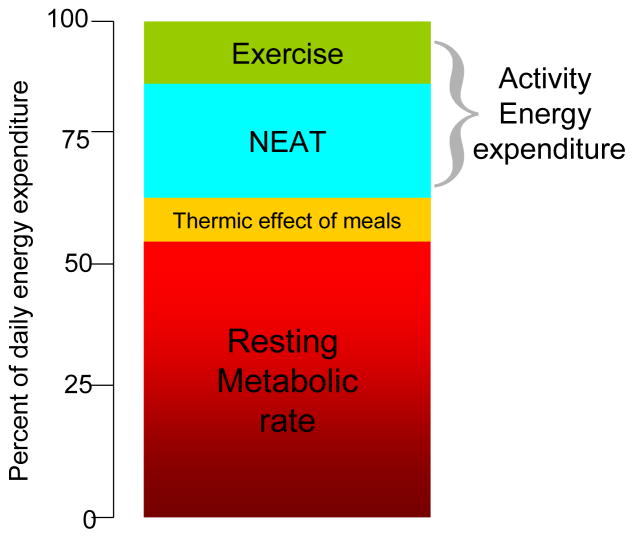
Total energy expenditure is comprised of four major components that include: resting metabolic rate, activity energy expenditure and energy due to the thermic effect of food (Figure 1). Resting metabolic rate (RMR) can be further divided into sleeping and arousal EE, where simple arousal is associated with a 10% increase in EE above that seen during sleeping (Ravussin et al., 1986). Activity EE is composed of EE due to volitional exercise (i.e. jogging, walking for exercise etc) and non-exercise activity thermogenesis (NEAT). NEAT is the energy expended from spontaneous physical activities that include nonvolitional movements (i.e. fidgeting), but is often defined as any EE outside formal volitional exercise programs. More details about each these components and their respective changes with age are outlined in subsequent sections.
Figure 1.
Categories of total energy expenditure. Resting metabolic rate (RMR) accounts for 60–80% of the total, thermic effect of food (TEF) metabolism uses 10% of total, Activity energy expenditure (AEE) is the most variable component and can be divided into volitional exercise and non-exercise activity thermogenesis (NEAT). Exercise and NEAT can comprise 20–50% of total.
Our current understanding of EE has been catapulted with technology that allows precise and accurate assessment of energy metabolism. Therefore, a brief review of the techniques is needed to appreciate the advances that have occurred over the last decades. Initial studies from the Harvard Fatigue Laboratory used whole body calorimetry to assess heat production, a byproduct of energy metabolism. Direct calorimetry, a method used to measure heat loss through convection, evaporation, and radiation uses fresh air to adjust temperature and humidity levels in an air conditioning chamber (Kleiber, 1950). The air is driven through a flow meter into a heat-insulated gradient-free chamber containing the human subject. Temperature and humidity are continuously measured using detectors placed at the fresh air entrance and exit. The difference in values between entrance and exit, the quantity of air flow and the energy input through the heating element are used to determine the heat given off by the subject. However, adequate control for such precise temperature measurements proved to be technically challenging (Kleiber, 1950). Instead, whole body open, indirect calorimetry rose to the forefront because of the rapid development of precise carbon dioxide and oxygen sensors. Tightly sealed rooms were built and fitted with appropriate equipment to assess 24 hour O2 consumption (VO2) and CO2 (VCO2) production continuously. The room is equipped with fresh atmospheric air circulation coupled with heating and cooling coils that keeps the chamber at a constant uniform temperature (e.g. 24 degrees Celsius). The fresh air circulation allows for the replacement of VO2 and VCO2 that is produced and thus the technique is referred to as open calorimetry. Other equipment includes an ultra sensitive turbine for measurement of ventilation, hygrometer, barometer and dust filter that are used to correct for standard temperature pressure conditions and any perturbation to VO2 and VCO2 concentrations. Over a given period of time the VO2 is derived from difference in the decrease in O2 and the net amount of O2 added to the chamber. Similarly, the VCO2 production is determined by the net amount of CO2 accumulation and CO2 extraction by the chamber. Next, a respiratory quotient (RQ) is derived (VCO2/VO2) as an adjustment for fuel utilization. It is then possible to calculate EE using the following equation:
where 4.686 kcal/liter of O2 is the caloric equivalent of a liter of O2 when RQ is 0.707 (RQ of fat oxidation). The ratio of 0.361/0.293 accounts for the differences in oxidation characteristics between carbohydrate and fat where 0.293 is the difference between RQ for carbohydrate (RQ = 1.0) and fat (RQ = 0.707) and 0.361 is the caloric equivalent difference between carbohydrate and fat [0.707 *(5.047 – 4.686 kcal/liter of O2]. This setup allows for precise estimation of energy expenditure over a continuous amount of time. Results from these studies have been instrumental in understanding the components of TEE in many conditions such as obesity, hormone dysregulation and aging.
While the precision and controlled methodology of respiratory chambers has advanced our knowledge of TEE components, they lack ecological value. To assess free-living TEE, water labeled with the stable isotopes of 2H and 18O (doubly labeled water) are used (Schutz et al., 2001). The method, first developed by Lifson, Gordon, and McClintock (Lifson et al., 1955), functions under the dilution principle where oxygen in respired carbon dioxide is in rapid isotopic equilibrium with oxygen in total body water. The difference in turnover rates of isotopic hydrogen (only eliminated as water) and oxygen-labeled water allows for the determination of carbon dioxide. Put more simply, the excess disappearance rate of 18O relative to 2H is a measure of CO2 production rate, a direct measure of total energy expenditure. The equation below depicts the simplistic calculation used to assess the rate of carbon dioxide output (rCO2):
where N is total body water, k18 is oxygen turnover and K2 is hydrogen turnover. Respiratory quotients are calculated using standard indirect calorimetry and used to adjust for fuel metabolism. This method is valid when compared to energy expenditure in respiratory chambers,(Schoeller et al., 1986) has excellent repeatability in younger and older adults (Elia et al., 2000) and is therefore considered the gold-standard measure of free-living activity energy expenditure (Lamonte and Ainsworth, 2001; Schutz et al., 2001). The doubly labeled water method captures any form of physical activity ranging from purposeful (volitional) exercise to simple fidgeting.
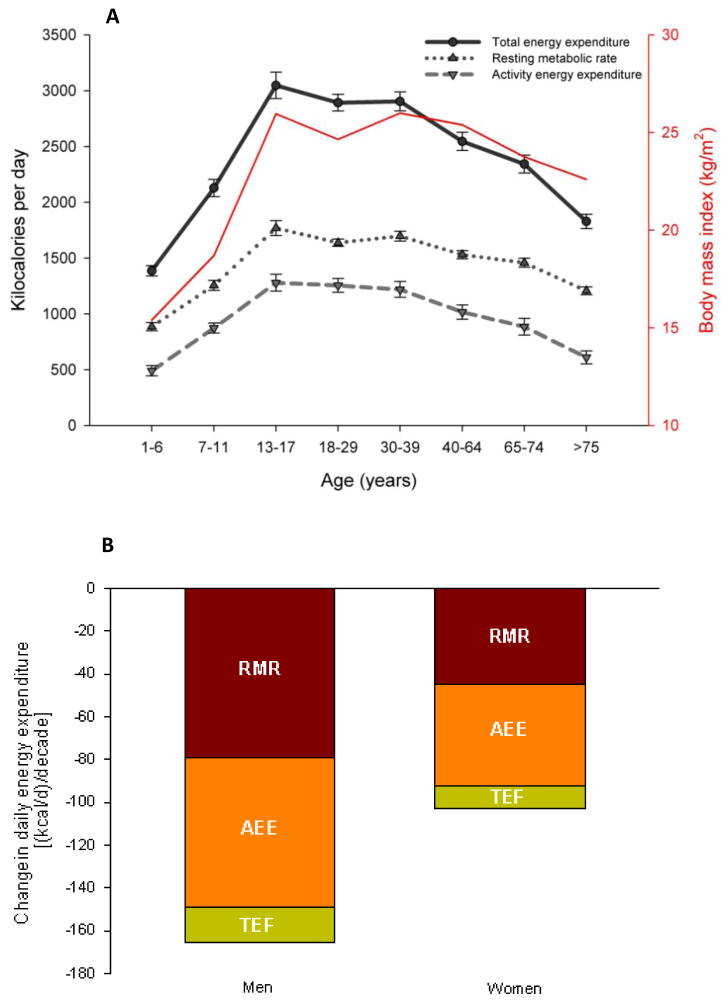
Total EE demonstrates an inverted U pattern across the lifespan. In the first two decades of life, TEE increases 2 fold and mirrors the increase in body mass (see Figure 2a & b). These changes plateau during the next 2 decades of life between 17 and 40 years of age and again this appears to match changes in body mass. Following the age of 40 years, TEE begins to decline quite dramatically where 75 year-olds experience TEE levels similar to a 7–11 year old despite having greater body mass. These changes are also reflected in the current FAO/WHO/UNU nutritional guidelines where adults aged 18–29.9 years and weighing 70 kg are advised to consume 2550 kcal/day whereas adults aged >60 years and weighing the same are asked to ingest 2050 kcal/day. This represents approximately a 500 kcal/day decrease in total EE over 40 years and matches the total EE reduction across the lifespan (see figure 2b for illustration of change in components of total EE (kcal/day) per decade of life).
Figure 2.
Figure 2a & b. a.) Age-related changes in categories of energy expenditure. Body mass index is also plotted as a function of age to evaluate the tracking association between energy expenditure and body mass with age. Data reproduced from Table 3 in (Black et al., 1996) demonstrates a decrease in all components of energy expenditure with increasing age. b.) Summarized changes in daily resting metabolic rate (RMR), activity energy expenditure (AEE) and the thermic effect of food (TEF) in men and women per decade after onset of adulthood (data reproduced from (Elia et al., 2000)).
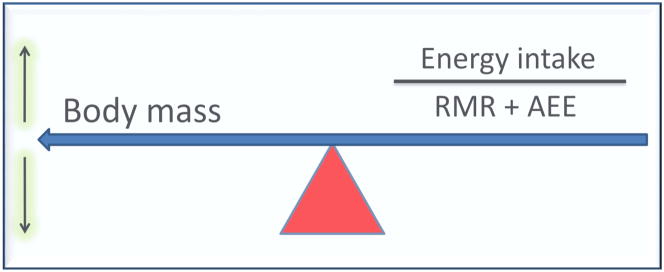
One important aspect that is somewhat revealed in Figure 2a is that changes in total EE are reflective of body mass. It should be noted that there is a constant balance between body mass and components of total EE as demonstrated in Figure 3. The ratio of energy intake to RMR+AEE governs body mass. For example, an increase energy intake without a concomitant change in RMR+AEE results in increased in body mass. Similarly, an increase in activity EE without a change in energy intake decreases the energy intake/RMR+AEE ratio producing a decrease in body mass. Aging is associated with a decrease in all components of the equation: body mass, energy intake, RMR and AEE (Elia et al., 2000). However, it remains unclear what factor initiates the change. Does the decrease in energy intake (Morley, 2001; Wakimoto and Block, 2001) without simultaneous change in RMR+AEE result in a decrease in body mass? Or, does the decline in the ratio of energy intake/RMR+AEE compensate for the decrease in body mass? In summary, the decline in total EE is completely reflective of a combination of decreases in resting metabolic rate and EE due to spontaneous and/or volitional activity. Potential reasons for the age-related changes in each of these components are described in the subsequent sections.
Figure 3.
Energy balance illustrated as a function of body mass and energy intake/[resting metabolic rate (RMR) + activity energy expenditure (AEE)]. The association depicted is unidirectional where the ratio of energy intake/[resting metabolic rate (RMR) + activity energy expenditure (AEE)] balances the variance in body mass.
2.2 Resting metabolic rate and aging
Resting metabolic rate is responsible for 60–80% of total EE and is primarily responsible for the maintenance of organismal homeostasis. Of all components of total EE, RMR has received the most attention because measurement equipment is readily available and fairly inexpensive, and the assessment consumes very little time in humans (about 30–60 minutes). Nevertheless, RMR is most often coupled with measurements of body composition (fat mass and fat-free mass) as an adjustment parameter because the amount of fat-free mass typically explains >60% of daily RMR. Although this adjustment typically corrects for many of the individual differences in RMR, it is not constant across adult humans. It should also be noted that there is a consistent finding that higher body mass in young adults is associated with lower RMR adjusted for fat-free mass (Heymsfield et al., 2002). In animal studies, an increase in body mass is associated with an expansion of lower energetic tissues (fat) without a proportional increase in higher energetic tissues (i.e. brain, liver, kidney etc) (Calder, 1996). Thus, the disproportional increase in skeletal muscle mass that accompanies increased body mass lowers the RMR relative to fat-free mass because the mass of other highly metabolic tissues remain unchanged (Heymsfield et al., 2002). Unfortunately, these data do not apply to aging scenarios where a loss in body mass is typically seen.
Aging is associated with a progressive decline in whole-body RMR at a rate of 1–2% per decade after 20 years of age (see Figure 2a & b) (Elia et al., 2000). This decline is closely linked with the decrease in whole body fat-free mass, which is composed of metabolically active tissues and organs (Poehlman et al., 1993). However, there is some debate whether the decrease in RMR is entirely due to changes in fat-free mass. This debate is triggered by findings that an adjustment for body composition does not fully account for differences in RMR between young and old adults (Krems et al., 2005). In other words, RMR remains significantly lower in the elderly even after correcting for body composition differences. A greater understanding for this finding can be gleaned from the following equation:
where Massi is the mass of that organ and Meti is the specific metabolic rate of an individual organ expressed as kcal · kg−1 · d−1, which represents the metabolic rate of individual cells within that organ. The summation of all organs according this equation yields RMR and is illustrated in Figure 5.
Figure 5.
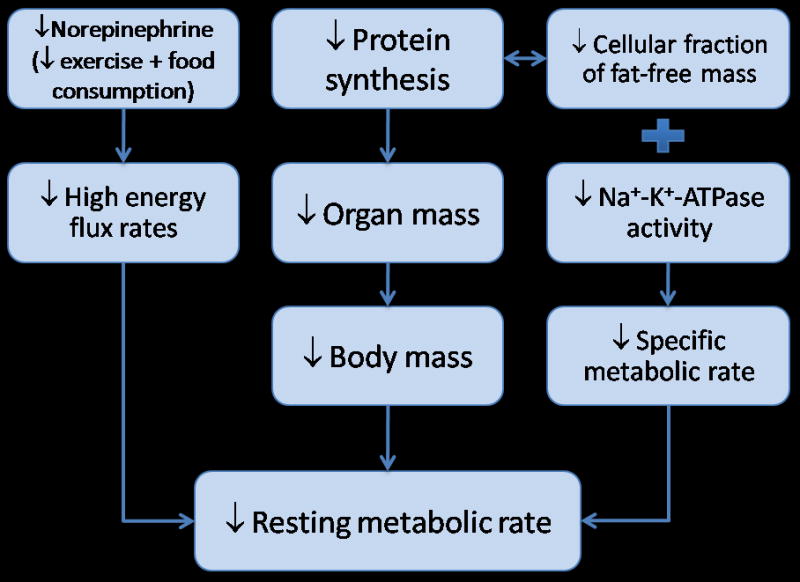
Schematic of biological control processes for age-related decrease in resting metabolic rate.
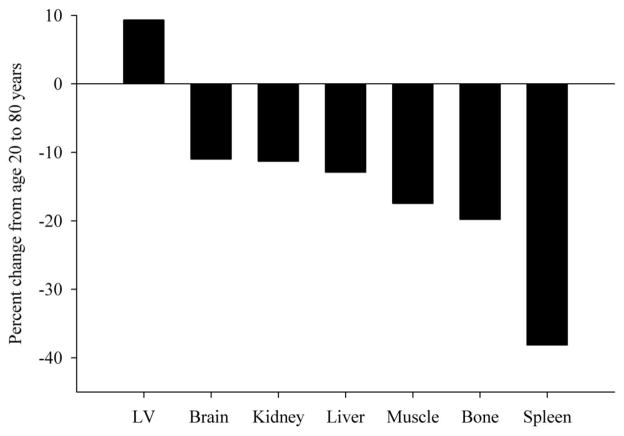
Organ masses (Massi) have been assessed since the first report in 1926 where autopsy studies were performed on approximately 2200 men and women (Bean, 1926). These data suggested a 32% reduction in liver mass, a 23% reduction in kidney mass, a 55% reduction in spleen mass, but a 10% increase in heart mass from age 20 to 80 years. While this and other autopsy studies demonstrated an age-related decline in mass of most organs (Garby et al., 1993), these data are criticized because of the lack of information regarding the cause of death and the delay in which the organs are harvested that may affect fluid retention. Although other evidence exist to support age-related decline in organ mass (Gallagher et al., 1996), there has not been in vivo data until recently. Using the exact, but tedious procedure of analyzing whole body magnetic resonance scans, He and colleagues brought to the forefront that most organs demonstrate a decline with increasing age (He et al., 2009). Figure 4, scaled for percent change from 20 to 80 years of age, was determined using equations by He and coworkers for an African-American female with a body mass of 70 kg and height of 1.6 meters. It should be noted that the results would look similar in men and Caucasians. For comparison purposes, published data on appendicular muscle mass (Gallagher et al., 1997) and bone mass (Rico et al., 1993) were added to Figure 4. Findings suggest that mass of the brain, bone, kidney, liver and muscle decline at relatively similar rates, between 10–20% from the ages of 20 and 80 years. The spleen, however, demonstrates a dramatic 38% loss in tissue mass. These data suggest that the decline in the mass of most organs undoubtedly contributes to the decrease in RMR with age.
Figure 4.
Organ mass changes from age 20 to 80 years of age. Predicted organ mass change from 20 to 80 years of age. Regression coefficients from He et al.(He et al., 2009) were used to estimate a percent change in the left ventricle (LV) of the heart, brain, kidney, liver and spleen mass. The predicted values were derived by fitting the model for an African-American women with a height of 1.6 meters and body mass of 70 kg. Kidney and spleen masses estimated with the natural logarithm were exponentiated for calculating values presented in the figure. Appendicular muscle mass was estimated using regression coefficients from Gallagher et al.(Gallagher et al., 1997) where body mass and height were used to predict age-related change in African-American women. Bone mass (i.e. bone mineral content) was estimated using data in women from Rico et al.(Rico et al., 1993). Data from Rico et al. were not adjusted for body mass and no information was given as to the race distribution of the subject sample (Reprinted from (Manini, 2009) with permission).
The other component of the RMR equation, Meti, is determined by cellular fractions in tissue, mitochondrial proton leak, protein turnover, and Na+ - K+ - ATPase (Rolfe and Brown, 1997). As such, Meti is not easily measured in vivo and thus much of the literature relies simply on organ mass or whole body fat-free mass assuming that specific metabolic rates are constant across the lifespan. Until recently this assumption has not been challenged. Wang and colleagues performed a comprehensive study of organ mass and estimated specific metabolic rate in adults 23 to 88 years of age. Organ volumes were measured using whole body magnetic resonance imaging and non-energetic components (adipose) were removed. Cellular fraction of organs was also examined with the hypothesis that aging causes an increase in extracellular components that don’t contribute to heat production. The key element of this determination is that reasonable estimates of body cell mass and extracellular water be determined. The investigators used total body potassium for estimation of body cell mass, deuterium for estimation of total body water and a combination of the two to calculate extracellular water. Whole body cellularity was estimated using body cell mass as a function of fat-free mass (BCM/FFM). These factors were added to a statistical model that was constructed as a cellular-level prediction equation of RMR and was then compared to RMR measured by indirect calorimetry. The investigators found that up to the age of 50 years the cellular equation accurately predicted RMR, but severely underestimated RMR after the age of 50 years. This systematic underestimation was thought to be due to lower cell fraction of fat-free mass indicating a low metabolic rate per unit of tissue. Therefore, the homogeneity of specific metabolic rate across the lifespan should not be assumed and this factor may explain the remaining variance associated with age-associated declines in RMR.
Physical activity may play an important role for ameliorating the age-related decrease in RMR because of the strong association between physical activity with preservation of body composition among highly active older adults (Kohrt et al., 1992). Additionally, aerobic capacity and exercise volume are positively correlated with RMR suggesting that physical activity levels contribute to energy flux in the resting state. However, these findings may differ between men and women. In a series of studies performed at the University of Colorado, investigators demonstrated that the effects of chronic physical activity on RMR are different in men and women (Van Pelt et al., 1997; Van Pelt et al., 2001). In old physically active women who had run trained for ~18 years, the decrease in RMR adjusted for fat-free mass was completely absent. Additionally, non-load bearing athletics also demonstrated an effect with competitive older female swimmers having no decrease in RMR adjusted for fat-free mass when compared to younger highly competitive runners (Van Pelt et al., 1997). The researchers concluded that endurance exercise, in general, was responsible for the observed preservation of RMR. Interestingly, the old women also preserved their fat-free mass as compared to young women. In highly physically active men, RMR adjusted for fat-free mass was lower in older adults when compared to a group of young adults. In a sub-analysis, both exercise volume and energy intake were positively correlated with RMR adjusted for fat-free mass. When the young and old groups were matched for exercise volume (in terms of hours per week) or energy intake, the difference in RMR disappeared. Therefore, RMR in men declines with age despite chronic performance of endurance exercise, but this decline is tightly coupled with the decrease in exercise volume and energy intake. It was proposed that men who are able to maintain high levels of exercise and energy intake with age will be able to maintain their RMR levels. Bullough and colleagues investigated the potential mechanisms of increased RMR in trained individuals (Bullough et al., 1995). Using an elegant set of experiments the investigators tested the effects of high energy flux (high energy intake + exercise while in energy balance), low energy flux (low energy intake + no exercise while in energy balance), negative energy flux (low energy intake + exercise), and positive energy flux (High energy intake + no exercise) in trained and untrained individuals. RMR was higher in the trained individuals only during the high energy flux condition where energy consumption matched energy expended during exercise. The mechanism was partially due to the drop in norepinephrine levels that was found in all trained individuals who transitioned from the high to the low energy flux condition. A correlation analysis found that norepinephrine was closely linked with the change in RMR during the high to low energy flux transition. No changes were found in triiodothyronine, glucose, insulin and epinephrine levels and thus these hormones did not modify RMR. These results suggest that total energy flux contributes to RMR even while individuals are kept in energy balance. Regarding age-association effects, RMR may be partially preserved due to the high total energy flux and norepinephrine release that accompanies chronic exercise training.
In many circumstances, aging is tied with onset of diseases that might contribute to a decrease or increase in RMR. Conditions such as Alzheimer s, chronic obstructive pulmonary disease, congestive heart failure, Parkinson s, diabetes, malnourishment and cancer are associated with a change in RMR (Toth and Poehlman, 2000). Disease conditions that elevate RMR have been reported for: chronic obstructive pulmonary disease(Baarends et al., 1997), lung cancer(Gibney et al., 1997), and diabetes (Nair et al., 1984). RMR declines in conditions that are associated with negative energy balance and wasting such as: Alzheimer’s disease (Poehlman and Dvorak, 1998), renal failure(Avesani et al., 2004), and malnourishment (Rigaud et al., 2000). While hypermetabolism is an attractive hypothesis for wasting syndromes, most studies have concluded that reduced energy intake is the mediator for negative energy balance and weight loss that accompanies chronic disease (Toth et al., 2000). It should be noted, however, mechanistic study of RMR is hindered by a lack of studies that have coupled RMR with adjustments of fat-free mass. It also remains unclear whether additional contributions are made by cellular changes or specific metabolic rates.
Specific metabolic rates are also driven by mitochondrial proton leak, protein turnover, and Na+ - K+ - ATPase. Alterations in these processes would lend additional support for a role of specific metabolic rate in contributing to the age-related decline in RMR. When cellular ATP demands are low, mitochondrial proton leak contributes 16–21% of RMR (Rolfe and Brand, 1996). Paradoxically, aging is associated with an increase in proton leak that would theoretically increase RMR (Serviddio et al., 2007; Asami et al., 2008) and thus offers little explanation for observed decreases in specific metabolic rate. Na+ - K+ - ATPase is a key enzyme found in all cells and function to maintain the gradient of Na+ - K+ across the plasma membrane. Activity of Na+ - K+ - ATPase is related to differences in RMR across mammalian and reptilian species (Else and Hulbert, 1987), and body size (Couture and Hulbert, 1995). Several studies in animals demonstrate a reduction in Na+ - K+ - ATPase activity in heart (Poole et al., 1984) and brain (Poole et al., 1984; Tanaka and Ando, 1990) tissue with added support in human erythrocytes (Poehlman et al., 1993) and lymphocytes (Witkowski et al., 1985). Although there is some conflicting evidence (Kennedy and Lever, 1985), the predominance of evidence suggests a reduction Na+ - K+ - ATPase that may contribute to the reduction in RMR seen with aging.
Maintenance of protein balance through the energy-requiring processes that control synthesis and degradation of amino acids are major contributors to RMR (Rolfe et al., 1996). In general, aging in both humans and animals causes a decrease in whole body protein turnover and tissue specific protein turnover (Lewis et al., 1985; Obled and Arnal, 1991; Millward et al., 1997). There is however, a discrepancy on how to normalize for differences in body mass with reports demonstrating no effect of age following an adjustment for fat-free mass (Morais et al., 1997). Consequently, much of the current work is performed on individual tissues, the most popular being skeletal muscle because of its association with sarcopenia and wasting syndromes. In skeletal muscle, it is generally accepted that degradation rates remain constant and muscle protein synthesis rates in both the postabsorbtive and postprandial states are reduced with increasing age (Timmerman and Volpi, 2008). Additionally, when given essential amino acids older adults demonstrate an attenuated increase in muscle protein synthesis (Katsanos et al., 2006). The mechanisms associated with reduced protein synthesis rates are not completely deciphered, but likely candidates are changes in hormones and issues related to nutrient uptake. Testosterone levels are correlated with myosin heavy chain synthesis rates and when levels are replaced in hypogonadal men, mixed muscle protein synthesis is increased (Urban et al., 1995; Brodsky et al., 1996). On the other hand, long-duration growth hormone replacement in older adults do not change muscle protein synthesis rates (Welle et al., 1996). Nutrient uptake into the muscle is also related to the decline in protein synthesis with increased age. In older non-diabetic adults, insulin resistance of muscle protein metabolism and related defects to endothelium-dependent vasodialation are related to reduced protein synthesis (Rasmussen et al., 2006). In further support of this finding, when older adults perform aerobic exercise, a potent vasodialator, insulin resistance of muscle protein metabolism is reduced (Fujita et al., 2007). In summary, age-related changes in specific metabolic rate of tissues seem to be related to decreases in Na+ - K+ - ATPase activity and protein synthesis rates.
2.3 Activity energy expenditure and aging
Activity and locomotion decrease in humans, non-human primates, canines, rodents and insects (for review of non-humans see (Ingram, 2000)). Activity EE comprises two categories: volitional exercise and non-exercise movements. It is highly variable (Levin et al., 1999), the most difficult to measure and thus the least studied component of total EE with regard to aging. Activity EE has a large range in humans, from 262 kcal/day in demented elderly (Prentice et al., 1989), 1434 kcal/day in mountaineers on Mount Everest (Westerterp et al., 1992), and 6333 kcal/day in cyclists in the Tour de France (Westerterp et al., 1986). Similar to other components of EE, activity EE is proportional to change in body mass (Figure 2a). However, the contribution of body mass is much weaker when compared to that of RMR. As discussed above, fat-free mass contributes >60% of RMR, but it only accounts for ~10% of activity EE (Masse et al., 2004). Therefore, the study of activity EE is largely body mass independent and the decrease with age is due to changes in the number and intensity of movements (i.e. locomotion, daily chores, fidgeting, standing etc).
Activity EE is tied to underlying genes as evidenced in familial based and monozygotic twin studies (Bouchard et al., 1989; Goran, 1997). More evidence for a genetic link comes from defects in a polygenic mouse model of obesity (New Zealand Obese) where a 72% decrease in physical activity was seen prior to the gain in body fat mass (Thorburn et al., 2000). Additionally, mutations in the larval foraging gene is associated with reduce locomotory behavior in adulthood (Pereira and Sokolowski, 1993). In more recent work, Joosen et al. evaluated dizygotic and monozytotic twins physical activity in a respiratory chamber and free-living conditions (Joosen et al., 2005). No genetic association was found when physical activity from the respiratory chamber was assessed, which is likely due to the confined nature of the testing environment. As the authors expected, environmental variables explained 68% of the variance in activity while in the chamber. However, when the twins were evaluated in free-living conditions, a genetic association was found even after common and unique environmental confounders were considered. This analysis showed that genetics explained 72% of the variance in activity energy expenditure and 78% of the variance in physical activity. This association was reduced, but remained significant, when anthropometric (i.e. body fat) variables were considered. Conversely, another study with similar methods in children found a weaker association between activity EE and genetic factors that disappeared after adjustments for anthropometric and demographic factors (Franks et al., 2005). Overall, while the decision to ambulate originates from higher brain centers, the results presented above suggests that these decisions may be influenced by underlying genetic makeup.
Despite having a genetic component, activity EE continues to decline with age. Using the doubly-labeled water method and indirect calorimetry, Johannsen and colleagues measured total EE and RMR in calculating activity EE using the following equation: Activity EE = Total EE − [RMR + (0.1*Total EE)] (Johannsen et al., 2008). A sample of 206 men and women in three equally sized age groups, 20–34, 60–74 and ≥90 years were compared. Interestingly, only the group of nonagenarian’s had significantly lower activity EE when adjusted for body mass. A longitudinal analysis conducted by Westerp and Meijer added to these findings (Westerterp and Meijer, 2001). Three-hundred and sixteen subjects evaluated over 25 years demonstrated a 9% and 25% lower activity EE at 60–74 and >75 years of age, respectively. This reduction in activity EE accounted for the major determinate of age-associated decline in total EE. Changes in fat-free mass, however, did not explain the reductions in activity EE supporting an innate biological adaptation with age.
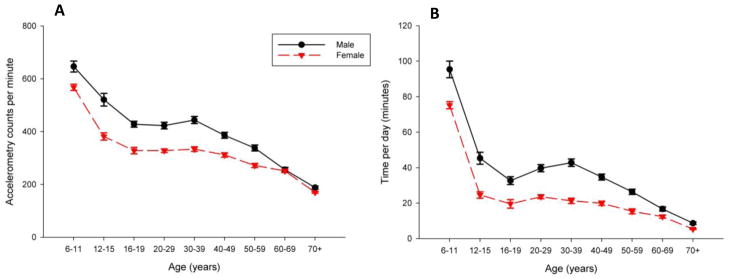
Data from numerous sources, most being epidemiological in origin, suggest a reduction in volitional activity with increasing age, but in most circumstances these sources have used self-reported data that have inherent biases (Verbrugge et al., 1996; Bijnen et al., 1998; Caspersen et al., 2000; Buchman et al., 2007). New objective data on a US national representative sample has confirmed this finding. Troiano and coworkers placed accelerometers on the hip of 4867 individuals across the age spectrum. The equipment functions as a small (2.0 × 1.6 × 0.6 in.) and lightweight (42.5 g) uniaxial accelerometer and can measure accelerations in the range of 0.05–2 G and a band-limited frequency of 0.25–2.5 Hz, which corresponds to movements of most human activities. An analog-to-digital converter collects data at a rate of 10 Hz, and values are summed for the specified time period of 1 min. An important feature of these accelerometers is they measure the number of acceleration counts with more counts in a given period of time corresponding to higher intensity activity. Thresholds for the counts have been derived and were used to determine the intensity of activity into moderate or vigorous activity. Figure 6a illustrates data presented in Troiano et al. where moderate and vigorous activity (combined) of men and women decline in a rapid manner after 11 years-old and again after the age of 39 years. Both men and women had ~55% declines in accelerometer counts originating from moderate or vigorous intensity activity from age 39 to 70+ years (Troiano et al., 2008). The amount of time performing moderate or vigorous intensity activity decreased from 42 min per day (21 min per day in women) to 8.7 min per day (5.4 min per day in women) in men from age 39 to 70+ years. This represents a ~75% decline in time spent performing moderate and vigorous intensity activity in both men and women. Objective data from a nationally representative sample of US persons strongly suggests that volitional activity EE declines drastically with increasing age.
Figure 6.
Figure 6a & b. Volitional physical activity levels across the lifespan in a nationally representative sample of Americans (National Health and Nutrition Examination Survey – NHANES 2003–2004) over 7 days. Physical activity was measured by accelerometry as (a) mean counts per minute and (b) time spent in moderate or vigorous physical activity according to an activity count threshold.
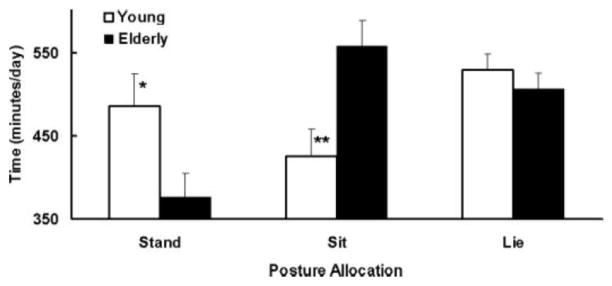
Activity EE contains non-volitional movements known as non-exercise activity thermogenesis (NEAT). In its most stringent definition, NEAT is derived from only unconscious, spontaneous, nonvolitional movements that include fidgeting and restless behaviors (Kotz et al., 2008). While volitional activity EE is largely touted as a major component of total EE in free-living humans, it has less impact on total EE when compared to NEAT (Goran and Poehlman, 1992). This component of activity EE is even more challenging to measure because equipment such as hip accelerometers won’t record many movements that contribute to NEAT (e.g. sitting movements, standing, fidgeting). To overcome these challenges, Harris and colleagues performed a case-control study where a group of healthy older adults (>70 years) were closely matched to a group of young adults (30–40 years old) for fat-free mass and physical activity level. The investigators assessed NEAT using specially designed triaxial accelerometers and inclinometers (captures acceleration against earth’s gravitational field). These monitors were used to examine posture allocation where lying, sitting, standing and locomotion time was calculated over 10 days. Results showed that the elderly walked 3 less miles/day, were in a sitting position 131 more minutes per day and stood 110 minutes less per day when compared to young adults (Figure 7). These data suggest that NEAT, reflected as time sitting, standing and under locomotion is severely reduced even in older adults who have maintained a fat-free mass comparable to the young.
Figure 7.
Posture allocation per day. Time spent standing was significantly greater (*P = 0.04) in young vs. elderly. Time spent sitting was significantly less (**p< 0.01). in the young vs. elderly. Values are means ± standard error. (Reprinted from (Harris et al., 2007) with permission).
2.4 Age-related changes in the biological control of activity energy expenditure
Aging induces a high propensity to be inactive. However, it remains unclear whether aging triggers a biological drive to be less active and if that drive is inherently or evolutionary based. This trigger has not been discovered, but in humans it is likely a complex interaction between biological, psychological, and sociological factors (Sallis and Owen, 1999). Notwithstanding the importance of psychological and sociological factors, the discussion will focus on potential biological origins. Activity EE is closely coupled with regulation of body mass where prolonged caloric restriction reduces activity levels (Keys et al., 1950; de Groot et al., 1989; Martin et al., 2007), although this is not a universal finding in closed room settings (Leibel et al., 1995). Conversely, overfeeding increases activity in lean individuals (Levine et al., 1999). The most recent findings by Martin and colleagues strongly support a balance between activity and body mass (Martin et al., 2007). The investigators found that free-living activity EE assessed using doubly-labeled water was significantly reduced in overweight adults undergoing 3 months of 25% caloric restriction. This finding is consistent with anecdotal reports of apathy and reduced activity in individuals undergoing semistarvation and suggests that reducing physical activity provides a survival advantage to conserve energy (Keys et al., 1950). However, these observations are not in line with the animal literature as caloric restricted non-human primates are known to increase their activity levels (Weed et al., 1997); possibly due to food-seeking behavior. It should be noted, however, that food-seeking behavior and its effect on activity EE is an unlikely factor in human studies because there is ample food supply in our environment. Therefore, restriction of food intake has a suppressive effect on free-living activity EE and might be a method to defend against further losses in body mass (Martin et al., 2007). Considering the close association between body mass and activity EE it seems plausible that age-related decreases in activity EE might be a compensatory strategy for reductions in body mass (Grinker et al., 1995) and fat-free mass (Visser et al., 2003) in late-life. In other words, a reduction in activity EE has an inherent role to conserve energy and defend against the loss in body mass. It is tempting to use this association as a teleological approach to explain age-related declines in activity. However, a recent study showed that old high fat fed rodents were less likely to undergo voluntary wheel running despite having a preserved body mass (Judge et al., 2008).
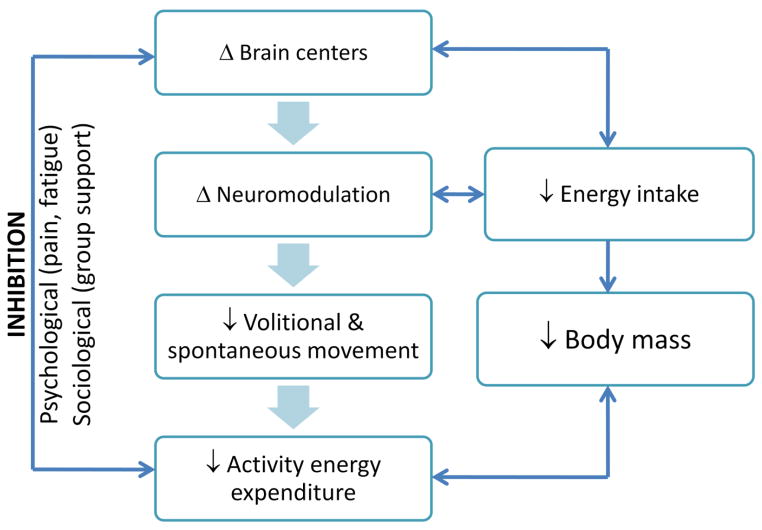
The biological origins of activity EE are thought to arise in higher brain centers and are coupled with energy intake (Kotz et al., 2008). Work being done to understand the regulation of activity has experienced a surge with the increasing obesity epidemic, of which some information can be applied to the occurrences seen with aging. Brain neurotransmitters have received the most attention with cholecystokinin, corticotropin-releasing hormone, neuromedin U, neuropeptide Y, Leptin, Agouti-related protein (AGRP), orexin, ghrelin and arousal systems controlled through the dopaminergic pathways leading the obesity-driven research (Teske et al., 2008). While these neuromodulators (and even more not mentioned here) have provided a basis for understanding development of obesity, very little has carried over into aging literature. The few that have received attention are orexin, AGRP and dopamine. Orexin-A and –B are neuropeptides located in the hypothalamus and spinal cord that have important roles in energy balance through cortical arousal (Lubkin and Stricker-Krongrad, 1998; Brown et al., 2002). Transgenic mice with deficiencies in the orexin producing system display reduced spontaneous activity and have a higher body mass despite a lower food intake (Chemelli et al., 1999). With regard to aging, the density of orexin axons is reduced across the lifespan of rhesus macaque’s (Downs et al., 2007). Additionally, mRNA expression of orexin levels in the thalamus, hippocampus, pons and medulla were reduced in aged rat brains (Terao et al., 2002). AGRP’s are endogenous antagonists for the melanocyte-stimulating hormones and are primarily located in the arcuate nucleaus of the hypothalamus (Fong et al., 1997). These proteins are thought to regulate energy balance through locomotor activity that develops late in life as found in AGRP deficient mice (Wortley et al., 2005). This unique development in late-life suggests that compensatory strategies for overcoming the AGRP deficiency may be removed with aging. Motivational factors controlled through the dopaminergic system provide the willfulness to expend energy through activity and may have an important role in age-related decline in activity. For instance, the concentration of the D2 dopamine receptor declines with age in a wide-variety of species (Roth and Joseph, 1994). Activation with quinpirole infusion (a D2 receptor agonist) increases exploratory activity, but to a lower extent in old versus young rats (Crawford and Levine, 1995). Additional evidence showed that when dopamine was directly injected into the brain, younger rats increased their exploratory activity while this response was blunted in older rats (Cousin et al., 1985). It should be noted that the specific mechanistic study of each of these neuromodulators is complicated by their overlapping roles. For example, dopamine terminals innervate ARGP neurons (Korotkova et al., 2006) and orexin axons are covered with dopamine receptors (Nakamura et al., 2000). In summary, the biological origins of reduced activity and its role in phenotypical changes with aging are still in their infancy. However, it is clear that fluctuations in activity EE are responsive to reductions in body mass, and these responses are under brain control as illustrated in Figure 8.
Figure 8.
Schematic of the control processes for decreases in activity energy expenditure with age.
3.0 Longevity and energy expenditure
Much attention has been paid on EE as a predominate predictor of lifespan. Volumes of information have been written on this topic arguing for and against the importance of energy expenditure as a factor to explain underpinnings of aging (Ramsey et al., 2000). This section will likely add to the confusion, at least in humans, as it supports a growing body of literature that activity EE might be an important determinant of lifespan (Byberg et al., 2009). Before this argument can be made, a brief review of the rate of living (ROL) theory, no matter how controversial, is required to set the stage for further discussion.
Rubner was the first to document an association between rate of metabolism of animals and body size. He also observed that EE expressed as function of body size and lifespan was relatively fixed (Rubner, 1908) and thus energy used up faster will shorten lifespan. Additional work by Loeb and Northrop demonstrated that poikilotherms exposed to increased ambient temperature had a decreased lifespan (Loeb and Northrop, 1917). Pearl expanded on this initial work in two books that built the foundation for the rate of living theory (Pearl, 1922; Pearl, 1928). Pearl wrote after performing experiments in flies and cantaloupe seedlings that “Duration of life is determined by two variables, the constitution of the individuals, genetically determined, and the average rate of metabolism or rate of energy expenditure.” The ROL theory is supported by mammalian correlative studies (Cutler, 1982), on manipulation of temperature environments (Atlan et al., 1976; Miquel et al., 1976) and mutant D. melanogaster (shaker mutants that are highly active and sleepless) (Cirelli et al., 2005). The shaker mutants have a reduced lifespan that is inversely proportional to their metabolic rate (Trout and Kaplan, 1970). However, it is refuted by inconsistencies among birds and bats which live several fold longer than do mammals of comparable body size and RMR (Austad et al., 1991). Additionally, rats exposed to long duration cold exposure increased their EE, but did not have a shortened lifespan (Holloszy et al., 1986). Nevertheless, the ROL theory, while largely scrutinized under its original pretenses (Lints, 1989), has provided a foundation on which to develop new ideas on which energy may contribute to aging through the cumulative effects of toxic by-products of oxygen utilization (Harman, 1956).
As discussed in several review articles, the ROL theory is hindered by problems associated with correction of body size, accurate definitions of lifespan, appropriate methods for comparing species, and choice of the most appropriate component of EE: Resting, total or activity (Speakman et al., 2002; Speakman, 2005). Regarding the latter, Speakman and coworkers made the argument that daily EE, as opposed to RMR, is the most appropriate measure for testing the ROL theory. When adjusted for fat-free mass, the variability is RMR is low within species and does not reflect the total EE of a particular organism. Additionally, total EE is highly variable within and across species even after correcting for body mass (or heat producing mass) (Ravussin et al., 1986), refuting the fixed EE hypotheses posed by Rubner. Speakman compiled total EE data, calculated with doubly-labeled water method, on mammals and birds to determine the association between lifespan and total EE. In small mammals (weighing less than 4kg) total EE was inversely associated with lifespan even after removing the shared variance of body mass and effects of ambient temperature on total EE (Speakman, 2005). Barring the inherent limitations of not accounting of age-related changes in total EE and inconsistent results in birds, the data support the ROL theory in that the rate of daily EE in mammals is associated with shortened lifespan.
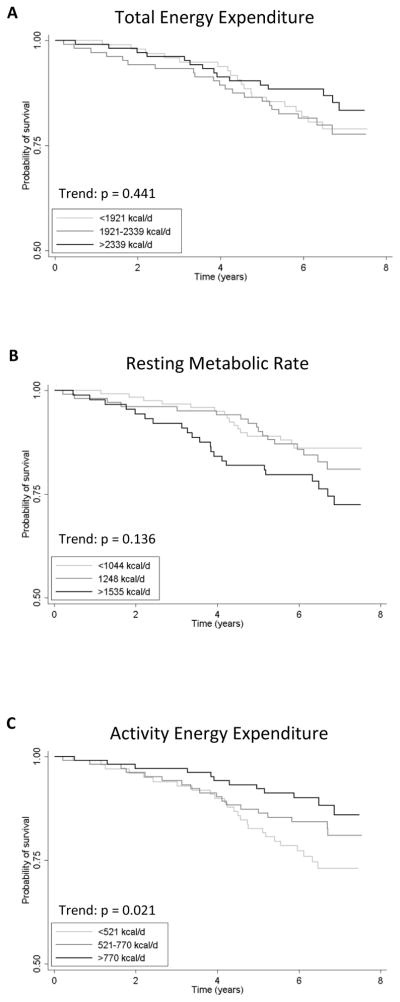
Energy expenditure data in humans support a completely opposite view of the ROL theory. In the study by Manini and colleagues (Manini et al., 2006), total EE and RMR were assessed in 302 older adults (+70 years) using doubly-labeled water and indirect calorimetry, respectively. These two measurements were used to calculate energy due to activity alone with the equation: (total EE × 0.90) - RMR. Mortality was ascertained over an 8 year period and time to death was statistically modeled with discreet-time hazard rates, which uses the probability that death will occur at a particular time to an individual, given that the individual is at risk at that time. Each EE component unadjusted and adjusted for body mass was assessed for its association with mortality as demonstrated in Figure 9. When expressed into equal thirds, neither resting nor total EE expended per unit tissue was associated with the occurrence of death. Only after partitioning activity EE from total EE was an association found. Individuals in the lowest third of activity EE had approximately three times greater risk of death when compared to the upper third. When data were expressed as standard deviation units, the results showed that a 287 kcal/day increase in activity EE was associated with a 32% reduction in mortality risk. Statistical adjustments for gender, health status and body composition did not affect the results. Additionally, the effects were unlikely due to a predominance of physically fit individuals who regularly exercised as the prevalence of individuals performing this type of activity was low (17%) and similar across all activity EE groups. On the contrary, light intensity activities (i.e. household chores) separated the groups suggesting that these have a major role in determining activity EE among older adults. Individuals in the lowest third of activity EE had a similar number of health conditions as compared to the upper third and thus the results were unlikely affected by underlying impairments. In additional work, the investigators explored whether activity EE was associated with mobility performance (Manini et al., 2009). The findings suggest that activity EE is also associated with reduced mobility impairment and thus preserved physical function, although the effects were stronger in men. The limitations of such work are numerous and include the inability to assess activity EE across the lifespan and the investigation of a single species with a short age-range. With the exception of these limitations, the data support a general finding that activity EE levels are associated with a reduced rate of death and preserved health (Paffenbarger et al., 1993). However, the results add an important detail that the accumulated amount of activity EE may be a strong predictor of lifespan. The data also refute a direct quote from Pearl who wrote, There is a direct and positive relation between the magnitude of the age specific death rates and the average expenditure of physical energy in occupation… This relation is of the sort that associates high mortality with high physical labour. Similar to findings 40 yrs ago where Paffenbarger et al. found a inverse association between physical work performed in longshoreman and mortality rates (Paffenbarger et al., 1971), these new data suggest that any energy expended through physical activity may have a role in determining lifespan.
Figure 9.
Figure 9a, b, & c. Kaplan-Meier survival plots adjusted for body mass along with mortality rates. Each component of energy expenditure was divided into tertiles of (a) total energy expenditure, (b) resting metabolic rate and (c) activity energy expenditure. Trend tests, which adjusted for the effect of body mass on both energy expenditure and mortality, were calculated with Cox regression analysis and used to determine the equality of survivor functions between the tertiles. Data for Figure 9c are recreated from (Manini et al., 2006). Data from 9a and b are unpublished.
3.0 Conclusion
Energy expenditure continues to be of major interest in aging research. Novel techniques that employ respiratory chambers and non-radioactive isotopes for precise estimation of EE have allowed a substantial degree of study into the causes and consequences of aging. The current discoveries in EE research have the potential for building a basic understanding for the control processes that govern changes with age. The age-associated reduction in total EE is caused by a combination of decreases in RMR and activity EE, which are both dictated in part to reductions in body mass and fat-free mass. The decline in RMR is manifested through reductions in organ mass and specific metabolic rate of tissues without a clear understanding about the contribution of each. Activity EE is carried as a familial trait and typically declines in a rapid manner after the age of 40 years and contributes substantially to the overall reduction in total EE. More importantly, activity EE may be a biomarker of aging as it is strongly associated with death in humans and declines in most species with senescence. While new research is beginning to uncover the biological control of non-exercise activity, very little is known about whether these same biochemical markers have a role in age-related reductions in activity EE. Understanding of such mechanisms may hold promise for identifying key regulators of lifespan.
Acknowledgments
I would like to thank Thomas Buford, Ph.D., Steve Anton Ph.D. and for the insightful comments on drafts of this article and intellectual discussion regarding energy expenditure and aging. This work was supported by the National Institute on Aging contracts NO1-AG-6-2101, NO1-AG-6-2103, and NO1-AG-6_2106. This research was supported in part by the Intramural Research Program of the NIH, National Institute on Aging. Todd Manini was supported by the University of Florida’s Claude D. Pepper Older Americans Independence Center, P30AG028740.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Asami DK, McDonald RB, et al. Effect of aging, caloric restriction, and uncoupling protein 3 (UCP3) on mitochondrial proton leak in mice. Exp Gerontol. 2008;43:1069–76. doi: 10.1016/j.exger.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atlan H, Miquel J, et al. Thermodynamics of aging in Drosophila melanogaster. Mech Ageing Dev. 1976;5:371–87. doi: 10.1016/0047-6374(76)90035-x. [DOI] [PubMed] [Google Scholar]
- Austad SN, Fischer KE. Mammalian aging, metabolism, and ecology: evidence from the bats and marsupials. J Gerontol. 1991;46:B47–53. doi: 10.1093/geronj/46.2.b47. [DOI] [PubMed] [Google Scholar]
- Avesani CM, Draibe SA, et al. Decreased resting energy expenditure in non-dialysed chronic kidney disease patients. Nephrol Dial Transplant. 2004;19:3091–7. doi: 10.1093/ndt/gfh547. [DOI] [PubMed] [Google Scholar]
- Baarends EM, Schols AM, et al. Total daily energy expenditure relative to resting energy expenditure in clinically stable patients with COPD. Thorax. 1997;52:780–5. doi: 10.1136/thx.52.9.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean B. Composite study of weight of vital organs in man. Am J Phys Anthrop. 1926;9:293–317. [Google Scholar]
- Bijnen FC, Feskens EJ, et al. Age, period, and cohort effects on physical activity among elderly men during 10 years of follow-up: the Zutphen Elderly Study. J Gerontol A Biol Sci Med Sci. 1998;53:M235–41. doi: 10.1093/gerona/53a.3.m235. [DOI] [PubMed] [Google Scholar]
- Black AE, Coward WA, et al. Human energy expenditure in affluent societies: an analysis of 574 doubly-labelled water measurements. Eur J Clin Nutr. 1996;50:72–92. [PubMed] [Google Scholar]
- Bouchard C, Tremblay A, et al. Genetic effect in resting and exercise metabolic rates. Metabolism. 1989;38:364–70. doi: 10.1016/0026-0495(89)90126-1. [DOI] [PubMed] [Google Scholar]
- Brodsky IG, Balagopal P, et al. Effects of testosterone replacement on muscle mass and muscle protein synthesis in hypogonadal men--a clinical research center study. J Clin Endocrinol Metab. 1996;81:3469–75. doi: 10.1210/jcem.81.10.8855787. [DOI] [PubMed] [Google Scholar]
- Brown RE, Sergeeva OA, et al. Convergent excitation of dorsal raphe serotonin neurons by multiple arousal systems (orexin/hypocretin, histamine and noradrenaline) J Neurosci. 2002;22:8850–9. doi: 10.1523/JNEUROSCI.22-20-08850.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchman AS, Boyle PA, et al. Physical activity and motor decline in older persons. Muscle Nerve. 2007;35:354–62. doi: 10.1002/mus.20702. [DOI] [PubMed] [Google Scholar]
- Bullough RC, Gillette CA, et al. Interaction of acute changes in exercise energy expenditure and energy intake on resting metabolic rate. Am J Clin Nutr. 1995;61:473–81. doi: 10.1093/ajcn/61.3.473. [DOI] [PubMed] [Google Scholar]
- Byberg L, Melhus H, et al. Total mortality after changes in leisure time physical activity in 50 year old men: 35 year follow-up of population based cohort. Bmj. 2009;338:b688. doi: 10.1136/bmj.b688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calder W. Size, Function, and Life History. Dover Publications; New York: 1996. [Google Scholar]
- Caspersen CJ, Pereira MA, et al. Changes in physical activity patterns in the United States, by sex and cross-sectional age. Med Sci Sports Exerc. 2000;32:1601–9. doi: 10.1097/00005768-200009000-00013. [DOI] [PubMed] [Google Scholar]
- Chemelli RM, Willie JT, et al. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999;98:437–51. doi: 10.1016/s0092-8674(00)81973-x. [DOI] [PubMed] [Google Scholar]
- Cirelli C, Bushey D, et al. Reduced sleep in Drosophila Shaker mutants. Nature. 2005;434:1087–92. doi: 10.1038/nature03486. [DOI] [PubMed] [Google Scholar]
- Cousin KM, Uretsky NJ, et al. Locomotor response of nialamide pretreated old rats to intraaccumbens dopamine. Pharmacol Biochem Behav. 1985;22:461–8. doi: 10.1016/0091-3057(85)90048-6. [DOI] [PubMed] [Google Scholar]
- Couture P, Hulbert AJ. Relationship between body mass, tissue metabolic rate, and sodium pump activity in mammalian liver and kidney. Am J Physiol. 1995;268:R641–50. doi: 10.1152/ajpregu.1995.268.3.R641. [DOI] [PubMed] [Google Scholar]
- Crawford C, Levine M. Dopaminergic function in the neostriatum and nucleus accumbens of young and aged Fischer-344 rates. Neurobiol Aging. 1995;18:57–66. doi: 10.1016/s0197-4580(96)00210-2. [DOI] [PubMed] [Google Scholar]
- Cutler R. Anti-oxidants, aging and longevity. In: Pryor W, editor. Free Radicals in Biology. Academic Press; Orlando: 1982. [Google Scholar]
- de Groot LC, van Es AJ, et al. Adaptation of energy metabolism of overweight women to alternating and continuous low energy intake. Am J Clin Nutr. 1989;50:1314–23. doi: 10.1093/ajcn/50.6.1314. [DOI] [PubMed] [Google Scholar]
- Downs JL, Dunn MR, et al. Orexin neuronal changes in the locus coeruleus of the aging rhesus macaque. Neurobiol Aging. 2007;28:1286–95. doi: 10.1016/j.neurobiolaging.2006.05.025. [DOI] [PubMed] [Google Scholar]
- Elia M, Ritz P, et al. Total energy expenditure in the elderly. Eur J Clin Nutr 54 Suppl. 2000;3:S92–103. doi: 10.1038/sj.ejcn.1601030. [DOI] [PubMed] [Google Scholar]
- Else PL, Hulbert AJ. Evolution of mammalian endothermic metabolism: “leaky” membranes as a source of heat. Am J Physiol. 1987;253:R1–7. doi: 10.1152/ajpregu.1987.253.1.R1. [DOI] [PubMed] [Google Scholar]
- Fong TM, Mao C, et al. ART (protein product of agouti-related transcript) as an antagonist of MC-3 and MC-4 receptors. Biochem Biophys Res Commun. 1997;237:629–31. doi: 10.1006/bbrc.1997.7200. [DOI] [PubMed] [Google Scholar]
- Franks PW, Ravussin E, et al. Habitual physical activity in children: the role of genes and the environment. Am J Clin Nutr. 2005;82:901–8. doi: 10.1093/ajcn/82.4.901. [DOI] [PubMed] [Google Scholar]
- Fujita S, Rasmussen BB, et al. Aerobic exercise overcomes the age-related insulin resistance of muscle protein metabolism by improving endothelial function and Akt/mammalian target of rapamycin signaling. Diabetes. 2007;56:1615–22. doi: 10.2337/db06-1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher D, Visser M, et al. Appendicular skeletal muscle mass: effects of age, gender, and ethnicity. J Appl Physiol. 1997;83:229–39. doi: 10.1152/jappl.1997.83.1.229. [DOI] [PubMed] [Google Scholar]
- Gallagher D, Visser M, et al. Metabolically active component of fat-free body mass: influences of age, adiposity, and gender. Metabolism. 1996;45:992–7. doi: 10.1016/s0026-0495(96)90269-3. [DOI] [PubMed] [Google Scholar]
- Garby L, Lammert O, et al. Weights of brain, heart, liver, kidneys, and spleen in health and apparently healthy adult danish subjects. Am J Hum Biol. 1993;5:291–296. doi: 10.1002/ajhb.1310050307. [DOI] [PubMed] [Google Scholar]
- Gibney E, Elia M, et al. Total energy expenditure in patients with small-cell lung cancer: results of a validated study using the bicarbonate-urea method. Metabolism. 1997;46:1412–7. doi: 10.1016/s0026-0495(97)90140-2. [DOI] [PubMed] [Google Scholar]
- Goran MI. Genetic influences on human energy expenditure and substrate utilization. Behav Genet. 1997;27:389–99. doi: 10.1023/a:1025644215744. [DOI] [PubMed] [Google Scholar]
- Goran MI, Poehlman ET. Endurance training does not enhance total energy expenditure in healthy elderly persons. Am J Physiol. 1992;263:E950–7. doi: 10.1152/ajpendo.1992.263.5.E950. [DOI] [PubMed] [Google Scholar]
- Grinker JA, Tucker K, et al. Body habitus changes among adult males from the normative aging study: relations to aging, smoking history and alcohol intake. Obes Res. 1995;3:435–46. doi: 10.1002/j.1550-8528.1995.tb00173.x. [DOI] [PubMed] [Google Scholar]
- Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- Harris AM, Lanningham-Foster LM, et al. Nonexercise movement in elderly compared with young people. Am J Physiol Endocrinol Metab. 2007;292:E1207–12. doi: 10.1152/ajpendo.00509.2006. [DOI] [PubMed] [Google Scholar]
- He Q, Heshka S, et al. Smaller Organ Mass with Greater Age, Except for Heart. J Appl Physiol. 2009 doi: 10.1152/japplphysiol.90454.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heymsfield SB, Gallagher D, et al. Body-size dependence of resting energy expenditure can be attributed to nonenergetic homogeneity of fat-free mass. Am J Physiol Endocrinol Metab. 2002;282:E132–8. doi: 10.1152/ajpendo.2002.282.1.E132. [DOI] [PubMed] [Google Scholar]
- Holloszy JO, Smith EK. Longevity of cold-exposed rats: a reevaluation of the “rate-of-living theory”. J Appl Physiol. 1986;61:1656–60. doi: 10.1152/jappl.1986.61.5.1656. [DOI] [PubMed] [Google Scholar]
- Ingram DK. Age-related decline in physical activity: generalization to nonhumans. Med Sci Sports Exerc. 2000;32:1623–9. doi: 10.1097/00005768-200009000-00016. [DOI] [PubMed] [Google Scholar]
- Johannsen DL, DeLany JP, et al. Physical activity in aging: comparison among young, aged, and nonagenarian individuals. J Appl Physiol. 2008;105:495–501. doi: 10.1152/japplphysiol.90450.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joosen AM, Gielen M, et al. Genetic analysis of physical activity in twins. Am J Clin Nutr. 2005;82:1253–9. doi: 10.1093/ajcn/82.6.1253. [DOI] [PubMed] [Google Scholar]
- Judge MK, Zhang J, et al. Prolonged hyperphagia with high-fat feeding contributes to exacerbated weight gain in rats with adult-onset obesity. Am J Physiol Regul Integr Comp Physiol. 2008;295:R773–80. doi: 10.1152/ajpregu.00727.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsanos CS, Kobayashi H, et al. A high proportion of leucine is required for optimal stimulation of the rate of muscle protein synthesis by essential amino acids in the elderly. Am J Physiol Endocrinol Metab. 2006;291:E381–7. doi: 10.1152/ajpendo.00488.2005. [DOI] [PubMed] [Google Scholar]
- Kennedy BG, Lever JE. Transport by the (Na+,K+) ATPase: modulation by differentiation inducers and inhibition of protein synthesis in the MDCK kidney epithelial cell line. J Cell Physiol. 1985;123:410–6. doi: 10.1002/jcp.1041230317. [DOI] [PubMed] [Google Scholar]
- Keys A, Brozek J, et al. The Biology of Human Starvation. University of Minnesota Press; Minneapolis: 1950. [Google Scholar]
- Kleiber M. Calorimetric measurements. In: Uber F, editor. Biophysical Research Methods. Interscience; New York: 1950. [Google Scholar]
- Kohrt WM, Obert KA, et al. Exercise training improves fat distribution patterns in 60- to 70-year-old men and women. J Gerontol. 1992;47:M99–105. doi: 10.1093/geronj/47.4.m99. [DOI] [PubMed] [Google Scholar]
- Korotkova TM, Brown RE, et al. Effects of arousal- and feeding-related neuropeptides on dopaminergic and GABAergic neurons in the ventral tegmental area of the rat. Eur J Neurosci. 2006;23:2677–85. doi: 10.1111/j.1460-9568.2006.04792.x. [DOI] [PubMed] [Google Scholar]
- Kotz CM, Teske JA, et al. Neuroregulation of nonexercise activity thermogenesis and obesity resistance. Am J Physiol Regul Integr Comp Physiol. 2008;294:R699–710. doi: 10.1152/ajpregu.00095.2007. [DOI] [PubMed] [Google Scholar]
- Krems C, Luhrmann PM, et al. Lower resting metabolic rate in the elderly may not be entirely due to changes in body composition. Eur J Clin Nutr. 2005;59:255–62. doi: 10.1038/sj.ejcn.1602066. [DOI] [PubMed] [Google Scholar]
- Lamonte MJ, Ainsworth BE. Quantifying energy expenditure and physical activity in the context of dose response. Med Sci Sports Exerc. 2001;33:S370–8. doi: 10.1097/00005768-200106001-00006. discussion S419–20. [DOI] [PubMed] [Google Scholar]
- Leibel RL, Rosenbaum M, et al. Changes in energy expenditure resulting from altered body weight. N Engl J Med. 1995;332:621–8. doi: 10.1056/NEJM199503093321001. [DOI] [PubMed] [Google Scholar]
- Levin S, Jacobs DR, Jr, et al. Intra-individual variation and estimates of usual physical activity. Ann Epidemiol. 1999;9:481–8. doi: 10.1016/s1047-2797(99)00022-8. [DOI] [PubMed] [Google Scholar]
- Levine JA, Eberhardt NL, et al. Role of nonexercise activity thermogenesis in resistance to fat gain in humans. Science. 1999;283:212–4. doi: 10.1126/science.283.5399.212. [DOI] [PubMed] [Google Scholar]
- Lewis SE, Goldspink DF, et al. The effects of aging and chronic dietary restriction on whole body growth and protein turnover in the rat. Exp Gerontol. 1985;20:253–63. doi: 10.1016/0531-5565(85)90050-6. [DOI] [PubMed] [Google Scholar]
- Lifson N, Gordon GB, et al. Measurement of total carbon dioxide production by means of D2O18. J Appl Physiol. 1955;7:704–10. doi: 10.1152/jappl.1955.7.6.704. [DOI] [PubMed] [Google Scholar]
- Lints FA. The rate of living theory revisited. Gerontology. 1989;35:36–57. doi: 10.1159/000212998. [DOI] [PubMed] [Google Scholar]
- Loeb J, Northrop J. On the influence of food and temperature on the duration of life. J Biol Chem. 1917;32:103–121. [Google Scholar]
- Lubkin M, Stricker-Krongrad A. Independent feeding and metabolic actions of orexins in mice. Biochem Biophys Res Commun. 1998;253:241–5. doi: 10.1006/bbrc.1998.9750. [DOI] [PubMed] [Google Scholar]
- Manini TM. Organ-o-penia. J Appl Physiol. 2009;106:1759–60. doi: 10.1152/japplphysiol.00315.2009. [DOI] [PubMed] [Google Scholar]
- Manini TM, Everhart JE, et al. Daily activity energy expenditure and mortality among older adults. Jama. 2006;296:171–9. doi: 10.1001/jama.296.2.171. [DOI] [PubMed] [Google Scholar]
- Manini TM, Everhart JE, et al. Activity energy expenditure and mobility limitation in older adults: differential associations by sex. Am J Epidemiol. 2009;169:1507–16. doi: 10.1093/aje/kwp069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin CK, Heilbronn LK, et al. Effect of calorie restriction on resting metabolic rate and spontaneous physical activity. Obesity (Silver Spring) 2007;15:2964–73. doi: 10.1038/oby.2007.354. [DOI] [PubMed] [Google Scholar]
- Masse LC, Fulton JE, et al. Influence of body composition on physical activity validation studies using doubly labeled water. J Appl Physiol. 2004;96:1357–64. doi: 10.1152/japplphysiol.00901.2003. [DOI] [PubMed] [Google Scholar]
- Millward DJ, Fereday A, et al. Aging, protein requirements, and protein turnover. Am J Clin Nutr. 1997;66:774–86. doi: 10.1093/ajcn/66.4.774. [DOI] [PubMed] [Google Scholar]
- Miquel J, Lundgren PR, et al. Effects of temperature on the life span, vitality and fine structure of Drosophila melanogaster. Mech Ageing Dev. 1976;5:347–70. doi: 10.1016/0047-6374(76)90034-8. [DOI] [PubMed] [Google Scholar]
- Morais JA, Gougeon R, et al. Whole-body protein turnover in the healthy elderly. Am J Clin Nutr. 1997;66:880–9. doi: 10.1093/ajcn/66.4.880. [DOI] [PubMed] [Google Scholar]
- Morley JE. Decreased food intake with aging. J Gerontol A Biol Sci Med Sci. 2001;56(Spec No 2):81–8. doi: 10.1093/gerona/56.suppl_2.81. [DOI] [PubMed] [Google Scholar]
- Nair KS, Halliday D, et al. Increased energy expenditure in poorly controlled Type 1 (insulin-dependent) diabetic patients. Diabetologia. 1984;27:13–6. doi: 10.1007/BF00253494. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Uramura K, et al. Orexin-induced hyperlocomotion and stereotypy are mediated by the dopaminergic system. Brain Res. 2000;873:181–7. doi: 10.1016/s0006-8993(00)02555-5. [DOI] [PubMed] [Google Scholar]
- Obled C, Arnal M. Age-related changes in whole-body amino acid kinetics and protein turnover in rats. J Nutr. 1991;121:1990–8. doi: 10.1093/jn/121.12.1990. [DOI] [PubMed] [Google Scholar]
- Paffenbarger RS, Gima AS, et al. Characteristics of longshoremen related fatal coronary heart disease and stroke. Am J Public Health. 1971;61:1362–70. doi: 10.2105/ajph.61.7.1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paffenbarger RS, Jr, Hyde RT, et al. The association of changes in physical-activity level and other lifestyle characteristics with mortality among men. N Engl J Med. 1993;328:538–45. doi: 10.1056/NEJM199302253280804. [DOI] [PubMed] [Google Scholar]
- Pearl R. The Biology of Death. J.B. Lippincott; Philadelphia: 1922. [Google Scholar]
- Pearl R. The Rate of Living. University London Press; London: 1928. [Google Scholar]
- Pereira HS, Sokolowski MB. Mutations in the larval foraging gene affect adult locomotory behavior after feeding in Drosophila melanogaster. Proc Natl Acad Sci U S A. 1993;90:5044–6. doi: 10.1073/pnas.90.11.5044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poehlman ET, Dvorak RV. Energy expenditure in Alzheimer’s disease. J Nutr Health Aging. 1998;2:115–8. [PubMed] [Google Scholar]
- Poehlman ET, Goran MI, et al. Determinants of decline in resting metabolic rate in aging females. Am J Physiol. 1993;264:E450–5. doi: 10.1152/ajpendo.1993.264.3.E450. [DOI] [PubMed] [Google Scholar]
- Poehlman ET, Toth MJ, et al. Sodium-potassium pump activity contributes to the age-related decline in resting metabolic rate. J Clin Endocrinol Metab. 1993;76:1054–7. doi: 10.1210/jcem.76.4.8386182. [DOI] [PubMed] [Google Scholar]
- Poole LB, Liu MS, et al. Kinetic studies of the Na+-K+-ATPase enzyme system in brain and heart of aging rats. Am J Physiol. 1984;247:R850–5. doi: 10.1152/ajpregu.1984.247.5.R850. [DOI] [PubMed] [Google Scholar]
- Prentice AM, Leavesley K, et al. Is severe wasting in elderly mental patients caused by an excessive energy requirement? Age Ageing. 1989;18:158–67. doi: 10.1093/ageing/18.3.158. [DOI] [PubMed] [Google Scholar]
- Ramsey JJ, Harper ME, et al. Restriction of energy intake, energy expenditure, and aging. Free Radic Biol Med. 2000;29:946–68. doi: 10.1016/s0891-5849(00)00417-2. [DOI] [PubMed] [Google Scholar]
- Rasmussen BB, Fujita S, et al. Insulin resistance of muscle protein metabolism in aging. Faseb J. 2006;20:768–9. doi: 10.1096/fj.05-4607fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravussin E, Lillioja S, et al. Determinants of 24-hour energy expenditure in man. Methods and results using a respiratory chamber. J Clin Invest. 1986;78:1568–78. doi: 10.1172/JCI112749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rico H, Revilla M, et al. Age-related differences in total and regional bone mass: a cross-sectional study with DXA in 429 normal women. Osteoporos Int. 1993;3:154–9. doi: 10.1007/BF01623277. [DOI] [PubMed] [Google Scholar]
- Rigaud D, Hassid J, et al. A paradoxical increase in resting energy expenditure in malnourished patients near death: the king penguin syndrome. Am J Clin Nutr. 2000;72:355–60. doi: 10.1093/ajcn/72.2.355. [DOI] [PubMed] [Google Scholar]
- Rolfe DF, Brand MD. Contribution of mitochondrial proton leak to skeletal muscle respiration and to standard metabolic rate. Am J Physiol. 1996;271:C1380–9. doi: 10.1152/ajpcell.1996.271.4.C1380. [DOI] [PubMed] [Google Scholar]
- Rolfe DF, Brown GC. Cellular energy utilization and molecular origin of standard metabolic rate in mammals. Physiol Rev. 1997;77:731–58. doi: 10.1152/physrev.1997.77.3.731. [DOI] [PubMed] [Google Scholar]
- Roth GS, Joseph JA. Cellular and molecular mechanisms of impaired dopaminergic function during aging. Ann N Y Acad Sci. 1994;719:129–35. doi: 10.1111/j.1749-6632.1994.tb56824.x. [DOI] [PubMed] [Google Scholar]
- Rubner M. Das problem der Lebensdauer und seine Beziehungen sum Wachstum under Ehrnarung. Oldenburg: Munich; 1908. [Google Scholar]
- Sallis J, Owen N. Physical Activity and Behavioral Medicine. Sage; Thousand Oaks, CA: 1999. [Google Scholar]
- Schoeller DA, Ravussin E, et al. Energy expenditure by doubly labeled water: validation in humans and proposed calculation. Am J Physiol. 1986;250:R823–30. doi: 10.1152/ajpregu.1986.250.5.R823. [DOI] [PubMed] [Google Scholar]
- Schutz Y, Weinsier RL, et al. Assessment of free-living physical activity in humans: an overview of currently available and proposed new measures. Obes Res. 2001;9:368–79. doi: 10.1038/oby.2001.48. [DOI] [PubMed] [Google Scholar]
- Serviddio G, Bellanti F, et al. Bioenergetics in aging: mitochondrial proton leak in aging rat liver, kidney and heart. Redox Rep. 2007;12:91–5. doi: 10.1179/135100007X162112. [DOI] [PubMed] [Google Scholar]
- Speakman JR. Body size, energy metabolism and lifespan. J Exp Biol. 2005;208:1717–30. doi: 10.1242/jeb.01556. [DOI] [PubMed] [Google Scholar]
- Speakman JR, Selman C, et al. Living fast, dying when? The link between aging and energetics. J Nutr. 2002;132:1583S–97S. doi: 10.1093/jn/132.6.1583S. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Ando S. Synaptic aging as revealed by changes in membrane potential and decreased activity of Na+,K(+)-ATPase. Brain Res. 1990;506:46–52. doi: 10.1016/0006-8993(90)91197-o. [DOI] [PubMed] [Google Scholar]
- Terao A, Apte-Deshpande A, et al. Age-related decline in hypocretin (orexin) receptor 2 messenger RNA levels in the mouse brain. Neurosci Lett. 2002;332:190–4. doi: 10.1016/s0304-3940(02)00953-9. [DOI] [PubMed] [Google Scholar]
- Teske JA, Billington CJ, et al. Neuropeptidergic mediators of spontaneous physical activity and non-exercise activity thermogenesis. Neuroendocrinology. 2008;87:71–90. doi: 10.1159/000110802. [DOI] [PubMed] [Google Scholar]
- Thorburn AW, Holdsworth A, et al. Differential and genetically separable associations of leptin with obesity-related traits. Int J Obes Relat Metab Disord. 2000;24:742–50. doi: 10.1038/sj.ijo.0801213. [DOI] [PubMed] [Google Scholar]
- Timmerman KL, Volpi E. Amino acid metabolism and regulatory effects in aging. Curr Opin Clin Nutr Metab Care. 2008;11:45–9. doi: 10.1097/MCO.0b013e3282f2a592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth MJ, Poehlman ET. Energetic adaptation to chronic disease in the elderly. Nutr Rev. 2000;58:61–6. doi: 10.1111/j.1753-4887.2000.tb01840.x. [DOI] [PubMed] [Google Scholar]
- Troiano RP, Berrigan D, et al. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc. 2008;40:181–8. doi: 10.1249/mss.0b013e31815a51b3. [DOI] [PubMed] [Google Scholar]
- Trout WE, Kaplan WD. A relation between longevity, metabolic rate, and activity in shaker mutants of Drosophila melanogaster. Exp Gerontol. 1970;5:83–92. doi: 10.1016/0531-5565(70)90033-1. [DOI] [PubMed] [Google Scholar]
- Urban RJ, Bodenburg YH, et al. Testosterone administration to elderly men increases skeletal muscle strength and protein synthesis. Am J Physiol. 1995;269:E820–6. doi: 10.1152/ajpendo.1995.269.5.E820. [DOI] [PubMed] [Google Scholar]
- Van Pelt RE, Dinneno FA, et al. Age-related decline in RMR in physically active men: relation to exercise volume and energy intake. Am J Physiol Endocrinol Metab. 2001;281:E633–9. doi: 10.1152/ajpendo.2001.281.3.E633. [DOI] [PubMed] [Google Scholar]
- Van Pelt RE, Jones PP, et al. Regular exercise and the age-related decline in resting metabolic rate in women. J Clin Endocrinol Metab. 1997;82:3208–12. doi: 10.1210/jcem.82.10.4268. [DOI] [PubMed] [Google Scholar]
- Verbrugge LM, Gruber-Baldini AL, et al. Age differences and age changes in activities: Baltimore Longitudinal Study of Aging. J Gerontol B Psychol Sci Soc Sci. 1996;51:S30–41. doi: 10.1093/geronb/51b.1.s30. [DOI] [PubMed] [Google Scholar]
- Visser M, Pahor M, et al. One- and two-year change in body composition as measured by DXA in a population-based cohort of older men and women. J Appl Physiol. 2003;94:2368–74. doi: 10.1152/japplphysiol.00124.2002. [DOI] [PubMed] [Google Scholar]
- Wakimoto P, Block G. Dietary intake, dietary patterns, and changes with age: an epidemiological perspective. J Gerontol A Biol Sci Med Sci. 2001;56(Spec No 2):65–80. doi: 10.1093/gerona/56.suppl_2.65. [DOI] [PubMed] [Google Scholar]
- Weed JL, Lane MA, et al. Activity measures in rhesus monkeys on long-term calorie restriction. Physiol Behav. 1997;62:97–103. doi: 10.1016/s0031-9384(97)00147-9. [DOI] [PubMed] [Google Scholar]
- Welle S, Thornton C, et al. Growth hormone increases muscle mass and strength but does not rejuvenate myofibrillar protein synthesis in healthy subjects over 60 years old. J Clin Endocrinol Metab. 1996;81:3239–43. doi: 10.1210/jcem.81.9.8784075. [DOI] [PubMed] [Google Scholar]
- Westerterp KR, Kayser B, et al. Energy expenditure climbing Mt. Everest. J Appl Physiol. 1992;73:1815–9. doi: 10.1152/jappl.1992.73.5.1815. [DOI] [PubMed] [Google Scholar]
- Westerterp KR, Meijer EP. Physical activity and parameters of aging: a physiological perspective. J Gerontol A Biol Sci Med Sci. 2001;56A:7–12. doi: 10.1093/gerona/56.suppl_2.7. [DOI] [PubMed] [Google Scholar]
- Westerterp KR, Saris WH, et al. Use of the doubly labeled water technique in humans during heavy sustained exercise. J Appl Physiol. 1986;61:2162–7. doi: 10.1152/jappl.1986.61.6.2162. [DOI] [PubMed] [Google Scholar]
- Wilson MM, Morley JE. Invited review: Aging and energy balance. J Appl Physiol. 2003;95:1728–36. doi: 10.1152/japplphysiol.00313.2003. [DOI] [PubMed] [Google Scholar]
- Witkowski JM, Mysliwski A, et al. Decrease of lymphocyte (Na+,K+)ATP-ase activity in aged people. Mech Ageing Dev. 1985;33:11–7. doi: 10.1016/0047-6374(85)90105-8. [DOI] [PubMed] [Google Scholar]
- Wortley KE, Anderson KD, et al. Agouti-related protein-deficient mice display an age-related lean phenotype. Cell Metab. 2005;2:421–7. doi: 10.1016/j.cmet.2005.11.004. [DOI] [PubMed] [Google Scholar]