Abstract
Objective
We examined the impact of patient adherence and screening test performance on the cost-effectiveness of visual inspection with acetic acid (VIA) and Pap smears when used with colposcopy for diagnosis.
Materials and Methods
Cost-effectiveness analysis was performed using computer modeling. The primary outcome was cancer prevalence in the ten years following screening. Three hypothetical populations of 35-year-old women were compared: never-screened women, women screened with VIA, and women screened with Pap smears. We used community-based data from our screening program in Honduras to estimate screening test sensitivity and specificity, adherence to follow-up, and costs of screening and colposcopy services. Published data were used to model disease outcomes.
Results
VIA was more sensitive than Pap smears (70% vs. 4%), less expensive (U.S. $0.23 vs. $3.17), and the 2-vist VIA system had a higher rate of adherence to follow-up than the 3-visit Pap smear system (84% vs. 38%). VIA had a higher false positive rate than Pap smears resulting in higher colposcopy referral rates, but more dysplasia was detected and treated. Cost-effectiveness analysis revealed that screening with VIA would cost U.S. $3,198 per cancer case avoided and reduce cancer cases by 42%, versus U.S. $36,802 and 2% for Pap screening. Although Pap smear quality was low in Honduras, sensitivity analysis showed that VIA was more cost-effective than Pap smears even when test accuracy was equivalent.
Conclusions
In developing countries, systems barriers can limit the cost-effectiveness of Pap smears. VIA may be a cost-effective alternative for some resource-poor settings, although systems barriers, quality control and feasibility issues must be considered.
INTRODUCTION AND BACKGROUND
Cervical cancer is the most common cancer among women in developing countries, with an estimated lifetime risk of 2–4% (Denny, 2005). Because healthcare resources are scarce, however, the cost-effectiveness of screening programs must be considered. Screening with Papanicolau (Pap) smears, followed by colposcopy with biopsy for diagnosis and LEEP excision for treatment has become the standard of care for developed countries (Wright, 2007) due to its effectiveness in reducing the population-wide incidence of invasive cervical cancer (Laara, Day, & Hakama, 1987) and its overall cost-effectiveness in this setting (Christopherson, 1983). However, there is currently no consensus regarding medical standards for cervical cancer screening in developing countries. Many developing countries in Latin America have existing Pap smear, colposcopy, and pathology services, and physicians follow the standards of care set in developing countries (Agurto, Sandoval, De La Rosa, & Guardado, 2006, Lazcano-Ponce, Moss, Alonso de Ruiz, Salmeron Castro, & Hernandez Avila, 1999; Robles). Unfortunately, cervical cancer mortality has remained high in Latin America despite significant healthcare expenditures due to poor quality services and limited population coverage (Agurto et al, 2006, Lazcano-Ponce et al, 1999, White, & Peruga, 1996).
Visual Inspection with Acetic Acid (VIA) has been proposed as an alternative to Pap smear screening in developing countries. VIA involves washing the cervix with 3–5% acetic acid and then looking for changes indicative of precancerous lesions. The attractive features of VIA include low cost, simple administration, immediate availability of results, and accuracy comparable to that of good quality Pap smears (Denny, 2005; Megevand, Denny, Dehaeck, Soeters, & Bloch, 1996; Sankaranarayanan et al., 2004; University of Zimbabwe/JHPIEGO Cervical Cancer Project, 1999). VIA is typically recommended as part of a “see-and-treat” algorithm. The “see-and-treat” approach involves screening women with VIA and treating those with abnormal exams using cryotherapy ablation in the same visit. This is very inexpensive, maximizes adherence to follow-up therapy, and has been shown to be more cost-effective than screening with Pap smears in low resource settings (Goldie et al., 2005; Goldie, Kuhn, Denny, Pollack, & Wright, 2001; Legood et al., 2005).
“See-and-treat” falls below the current standard of care in developed countries (Wright et al., 2007), however, because no pathologic diagnosis is obtained to ensure the adequacy of treatment. Physicians in developing countries who currently provide Pap smear, colposcopy, and LEEP services may be reluctant to adopt a “see-and-treat” system which falls below their standard of care, even if it is more cost-effective (Suba et al., 2006). To maintain local standards of care, VIA can be used instead of Pap smears for initial screening, with colposcopy and LEEP performed to for diagnosis and treatment. VIA is a less expensive screening test than the Pap smear, but its false positive rate is higher, resulting in more colposcopy referrals which could limit cost savings. To date, no study has examined whether VIA is cost-effective when used with colposcopy and LEEP services.
Existing studies comparing the cost-effectiveness of VIA and Pap smears primarily used data from large research projects (Goldie et al., 2005; Goldie, Kuhn, Denny, Pollack, & Wright, 2001; Legood et al., 2005) and incorporated good quality Pap smears in their models. During our community-based screening and treatment project in Honduras (Perkins, Langrish, Stern, Figueroa, & Simon, 2007) we found that factors including lack of communication infrastructure (telephone, postal services), routine supplies (spatulas, fixative, laboratory dyes), and up-to-date cytopathologist training and certification negatively impacted both Pap smear quality and patient adherence. The impact of these local factors may be important when comparing the cost-effectiveness of Pap smear and VIA screening programs. The purpose of our study is twofold: 1) to compare the cost-effectiveness of VIA and Pap smears when colposcopy is used for diagnosis, and 2) to examine the impact of systems barriers such as screening test performance and adherence to follow-up on cost-effectiveness.
METHODS
Primary Data Collection: Cervical cancer screening project in Honduras
Honduras is the second poorest country in the Western Hemisphere; two-thirds of the population lives below the poverty line and half lacks basic sanitation and potable water. The average person receives 2–3 years of schooling, and 30% of the population is illiterate ("[Secretary of State of Honduras Presidential Report: Information by Department and Municipality]," 2004). The cervical cancer incidence in Honduras is 39.1/100,000 and the mortality is 16.8/100,000 (Arrossi, Sankaranarayanan, & Parkin, 2003). These rates are over four times higher than in the United States and over ten times higher than in Finland (Aareleid, Pukkala, Thomson, & Hakama, 1993; http://www.healthypeople.gov/document/HTML/Volume1/03Cancer.htm). Honduras has no nationwide cervical cancer screening program, but Pap smears are widely available through local health centers, at private clinics, or at special screening days organized by a variety of private organizations. VIA is available in a handful of clinics nationwide.
The primary data for this cost-effectiveness analysis were collected during our cervical cancer screening and treatment project which took place in Honduras between June 2003 and September 2004 (Perkins et al, 2007). During the project, we provided no-cost screening and treatment services to women in an underserved region of Honduras. We screened 1370 women with Pap smears alone and an additional 339 women with both Pap smears and VIA. Women with abnormal results on either test underwent colposcopy with colposcopic-directed biopsy for diagnosis, and treatment with LEEP excision when appropriate. All pathologic specimens were processed, interpreted, and reported through the main government-associated laboratory in the capital city of Honduras. All Pap smears were taken by Honduran personnel and processed through local laboratories. All 339 Pap smears from the women who underwent Pap smear and VIA as well as 50 Pap smears from the group of 1370 women who underwent Pap screening only were reviewed at an academic medical center in the U.S. to determine the quality of slide preparation and interpretation. Sensitivity and specificity calculations for Pap smear and VIA used data from the 339 women who underwent both tests. Diagnostic and treatment services were provided by a local cancer center that routinely provided outreach services in the area. The data collected during this project were used to estimate the prevalence of different pre-cancerous lesions, to compare the accuracy of VIA and Pap smears for detecting cervical dysplasia, and to determine the costs of screening and treatment.
Overview of cost-effectiveness model
Standard methods of cost-effectiveness analysis were used, and a societal perspective was taken. Using commercially available cost-effectiveness software (TreeAge Pro 2006), we simulated the natural history of HPV-induced cervical dysplasia and the procedures used in the screening, diagnosis and treatment of precancerous lesions of the cervix. The model incorporated primary data from our cervical cancer screening program in Honduras as well as data from the literature (Goldie et al., 2005; Melnikow, Nuovo, Willan, Chan, & Howell, 1998; Ostor, 1993). We assessed alternative strategies by determining the incremental cost-effectiveness ratio, defined as the additional cost of a strategy divided by its additional clinical benefit, as compared with the next-less-expensive strategy. The numerator (cost) represented the average expense of screening for and treating precancerous lesions in a population of 1000 women. The denominator (effectiveness) represented the number of cancer cases avoided over ten years. Base case values and ranges for sensitivity analyses are detailed in Table 1.
Table 1.
Cost-effectiveness model comparing VIA and Pap smears: parameters, base case values, and ranges for sensitivity analysis
| Parameter | Base case (Range) | |
|---|---|---|
| Prevalence of Cervical Dysplasia | ||
| Healthy | 88.5% | (75%–95%) |
| Cervical intraepithelial neoplasia grade 1 | 10.2% | (5%–11%) |
| Cervical intraepithelial neoplasia grade 2 or 3 | 1.15% | (1% – 5%) |
| Cancer | 0.115% | (0.5%–2%) |
| Progression to Cancer without treatment over 10 years | ||
| Healthy | 0% | |
| Cervical intraepithelial neoplasia grade 1 | 2.4% | (1–10%) |
| Cervical intraepithelial neoplasia grade 2 or 3 | 18% | (5–30%) |
| Cancer | 100% | |
| Disease Recurrence following treatment with LEEP excision | ||
| Healthy | N/A | |
| Cervical intraepithelial neoplasia grade 1 | 10% | (2–30%) |
| Cervical intraepithelial neoplasia grade 2 or 3 | 10% | (2–30%) |
| Cancer | N/A | |
| Sensitivity and Specificity of Screening Measures | ||
| No screening | ||
| Sensitivity | N/A | |
| Specificity | N/A | |
| Pap smear | ||
| Sensitivity | 4% | (4–100%) |
| Specificity | 99% | (80–100%) |
| VIA | ||
| Sensitivity | 70% | (60–70%) |
| Specificity | 96% | (66–96%) |
| Adherence to Recommendation for Follow-up Colposcopy | ||
| Pap smear (3 visits required) | 38% | (33–100%) |
| VIA (2 visits required) | 84% | (33–100%) |
| Costs | ||
| Pap smear | 60 L1 / $3.18 U.S. ($0.23 – $3.18) | |
| VIA | 4.33 L / $0.23 U.S. ($0.23 – $4.06) | |
| Colposcopy | 300 L / $15.88 U.S. ($5 – $15.88) | |
| LEEP | 2000 L / $105.88 U.S. ($20 – 105.88) | |
“L” represents Lempiras, the currency of Honduras.
Natural history of cervical disease
Health states among subjects included: healthy (no cervical lesion), cervical intraepithelial neoplasia grades 1, 2, and 3, and invasive cancer. Based on the literature (Goldie et al., 2005, Arrossi et al., 2003) and our primary data, we estimated that 11.5% of the population had cervical disease at baseline. Among women with cervical disease, we estimated that 90% of lesions were cervical intraepithelial neoplasia grade 1, 10% were cervical intraepithelial neoplasia grade 2 or 3, and 1% were cancer.
In our model, subjects progressed from cervical intraepithelial neoplasia grades 1, 2, and 3 to cancer at rates consistent with the published literature: 2.4% of women with cervical intraepithelial neoplasia grade 1 progressed to cancer over ten years, and 18% of women with cervical intraepithelial neoplasia grades 2 and 3 progressed to cancer (Melnikow et al., 1998; Ostor, 1993). Treatment was assumed to be 90% effective in curing cervical intraepithelial neoplasia grades 1, 2, and 3 (Nuovo, Melnikow, Willan, & Chan, 2000). Subjects that were healthy at the time of initial screening were assumed to remain cancer-free throughout the ten-year period (Melnikow et al., 1998). Disease prevalence and rates of progression of pre-cancerous lesions to invasive cancer were varied during sensitivity analysis. Because all costs of screening and treatment occurred within one year, discounting of costs was not required.
Screening strategies and adherence to follow-up
Screening strategies included no screening, screening with Pap smears, and screening with VIA. Each screening strategy was applied to a hypothetical population of 1000 women. Women were screened once at age 35 and followed for ten years for the development of cervical cancer. The Pap smear screening strategy included three visits: 1) for the initial Pap test, 2) to obtain results, and 3) for diagnosis via colposcopy and immediate treatment using Loop Electrosurgical-Excision Procedure (LEEP) when indicated. A separate clinic visit for the communication of results is necessary in Honduras and many other low resource settings because telephone and postal services are rudimentary and face-to-face communication with a healthcare provider is the only reliable and confidential means of obtaining results. The VIA screening strategy included two visits: 1) for the initial VIA screen and communication of results, and 2) for colposcopy with immediate treatment using LEEP excision when indicated. A single-visit strategy with screening and colposcopy services provided in one day was not modeled as this is not currently available in Honduras. Nurses perform Pap smears and VIA at local health outposts, while colposcopy is performed by physicians either in hospital settings or on special outreach days when hospital staff travel to local clinics.
The baseline rates of adherence to follow-up colposcopy were taken directly from our screening and treatment project. Eighty-four percent of women completed follow-up in the two-visit VIA protocol, compared with 38% in the three-visit Pap smear protocol. The decrease in compliance with additional visits is consistent with published literature (Gaffikin, Blumenthal, Emerson, & Limpaphayom, 2003; University of Zimbabwe/JHPIEGO Cervical Cancer Project, 1999). Adherence rates were varied during sensitivity analysis.
Test Characteristics
The sensitivity and specificity of VIA and Pap smears in Honduras were estimated based on data from our screening and treatment project and the literature (Perkins et al., 2007, Goldie et al, 2005). All Pap smears were processed through the central laboratories in the capital city of Honduras which receive the majority of Pap smears taken throughout the country. Pap smear sensitivity was estimated at 4% and specificity at 100%, compared with a sensitivity of 70% and specificity of 96% for VIA.
We found the Pap smears in Honduras to be of very low quality, and therefore significantly less accurate than VIA in this setting (Perkins et al., 2007). Pathologic review in the U.S. highlighted deficiencies in both slide preparation and interpretation. Many clinics lacked spatulas, cytobrushes, and fixative, and instead used tongue depressors, Q-tips, and hair spray. Specimens were often stored in warm, humid conditions for one or more weeks prior to transport to the central processing facility, resulting in fungal contamination. The central laboratory facilities lacked the resources to purchase new supplies on a regular basis, so dyes and stains were re-used and recycled to extend their use; this resulted in poor quality staining. At the time that this study was performed, Honduras had no cytopathologist training facilities. The fourteen cytopathologists who were employed in Honduras at that time were trained in neighboring countries and did not have regular access to continuing education. We reviewed all 339 slides from the study group that underwent both Pap smear and VIA as well as 50 additional slides from the 1370 women who underwent Pap smear only at a U.S. teaching hospital. The pathologists commented on the poor quality of slide preparation and staining, but were also able to identify a number of abnormal Pap smears which had been read as negative for intraepithelial lesion or malignancy in Honduras.
VIA was performed by a nurse practitioner working within the Honduran Ministry of Health clinic system who was trained in VIA prior to initiating the study protocol. The accuracy of VIA in our study (Perkins et al., 2007) was consistent with other published studies from Latin America (Claeys et al., 2003; Jeronimo et al., 2005).
Cost Data
Costs are presented in both Honduran Lempiras and in US dollars1. Pap smear costs used in our model included the cost of the nurse visit to collect specimen, disposable supplies (spatula, slide fixative), specimen transport, and specimen processing and analysis. These costs were incorporated into a single global Pap smear fee (US$3.17). VIA costs included the costs of the nurse visit and disposable supplies (acetic acid, cotton swabs), which were also included as a single global VIA fee (US$0.23). The cost of colposcopy (US$15.88) included physician’s time, supplies, and the cost for processing one biopsy specimen2. The cost of LEEP (US$105.88) includes the physician’s time, supplies, and pathology costs. The costs associated with clinic visits, including nurses’ time, Pap smear and VIA supplies were obtained from the Ministry of Health clinic staff with whom we worked. Costs of colposcopy, biopsy and LEEP services were obtained from the private referral hospital that performed these procedures and the pathology laboratory that processed the specimens during our screening and treatment project. Because cost-effectiveness analysis focuses on cost differences, expenses that were common to both screening tests were excluded from the final analysis3. The costs of both screening and treatment services were varied during sensitivity analysis.
RESULTS
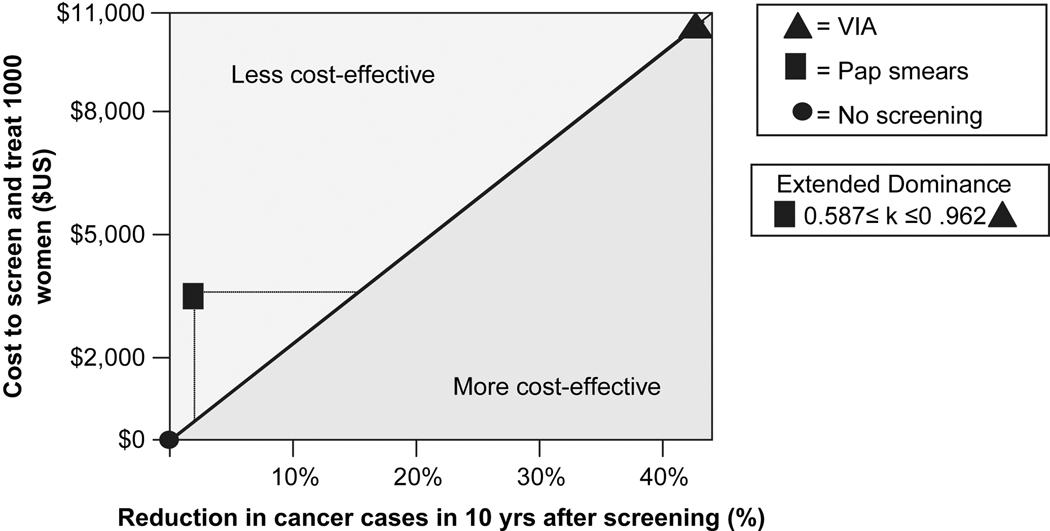
Screening with VIA reduced the ten-year cancer rate by 42%, from 5.7 to 3.3 per 1000. The cost to avoid one cancer case was U.S. $3,198. Screening with Pap smears reduced the cancer rate by only 2%, from 5.7 to 5.6 per 1000. The cost to avoid one cancer case was U.S. $36,802. Thus, VIA screening was more cost-effective than Pap smear screening by an order of magnitude. Population-wide screening with VIA was more costly than screening with Pap smears because more women received treatment. However, the cost per cancer case avoided was far lower because VIA was much more successful at detecting pre-cancerous lesions (extended dominance). See Figure 1.
Figure 1. Cost-effectiveness analysis of VIA and Pap smears.
Cost-effectiveness of no screening, screening with Pap smears (3-visits), and screening with VIA (2-visits). Effectiveness is measured as the percentage of cancer cases avoided in the 10 years following screening. Costs are represented in U.S. dollars. VIA demonstrated extended dominance over the Pap smear.
Sensitivity Analysis
One-way sensitivity analysis did not change results. The following parameters were varied (see ranges in Table 1): baseline cervical dysplasia rate, relative proportions of cervical intraepithelial neoplasia grades 1, 2, 3 and cancer among the abnormal results, proportions of women with cervical intraepithelial neoplasia 1, 2 and 3 progressing to cancer, recurrence of disease following LEEP excision, sensitivity and specificity of VIA and Pap smears, adherence to follow-up colposcopy after VIA and Pap smears, and the costs of VIA, Pap smears, colposcopy, and LEEP.
Two-way sensitivity analysis was undertaken to determine the effects of simultaneously changing the cost, adherence to follow-up, and sensitivity of the Pap smear. The Pap smear cost was varied from U.S. $0.23–$3.17, Pap smear sensitivity and specificity were varied from 4%–100%, and adherence to follow-up was varied from 33%–100%. VIA remained more cost-effective than Pap smears even when the cost of the Pap smear was reduced to that of VIA and the adherence to follow-up was increased to 100%. This is due to the low sensitivity of the test. Even when the Pap smear was made into a “perfect” test (both sensitivity and specificity of 100%) and the cost was decreased to that of VIA, VIA still remained superior to the Pap smear due to low adherence to follow-up.
DISCUSSION
This study demonstrates the impact of systems barriers on the cost-effectiveness of cervical cancer screening methods. Although Pap smears are considered the standard of care for cervical cancer screening in countries with adequate resources (http://www.who.int/reproductive-health/publications/cervical_cancer_gep/text.pdf), the use of Pap smears in Latin America over the past several decades has failed to lower cervical cancer rates (Agurto et al, 2006, Lazcano-Ponce et al, 1999, White, & Peruga, 1996). In our model, VIA was more cost-effective than Pap smears, even when combined with expensive diagnostic and treatment options such as colposcopy and LEEP. The key advantages of VIA were its high sensitivity (70% vs. 4%), low cost per woman screened (U.S. $0.23 vs. $3.17) and high rate of adherence to follow-up (84% vs. 38%). We found the Pap smears in Honduras to be of extremely low quality (Perkins et al, 2007), so VIA performed well in comparison. However, VIA was more cost-effective even when good quality Pap smears were modeled due to other systems barriers including relatively high cost and low adherence to follow-up.
Systems factors are largely responsible for the failure of Pap smears in the developing world, and systems factors relevant to VIA must be considered when determining whether screening programs using VIA could be viable alternatives. The accuracy of VIA has primarily been tested in large, well-designed research studies (University of Zimbabwe/JHPIEGO Cervical Cancer Project, 1999, Megevand et al, 1996), the results of which may be difficult to replicate in community settings. In fact, some experts fear that the quality of visual screening methods will be more difficult to control than the quality of Pap smears (Wright, 2003). Because VIA is based entirely on the provider’s visual impression of the cervix, VIA programs must have systems in place to ensure screening quality (Jeronimo et al, 2005; http://www.who.int/reproductive-health/publications/cervical_cancer_gep/text.pdf). VIA training should be limited to practitioners who see an adequate volume of screen-eligible patients, practitioners should be formally certified through programs with both didactic and clinical proficiency requirements, and ongoing training should occur on a regular basis. Didactic materials and training programs are available through international agencies (http://www.iarc.fr/en/about/index.php; http://www.jhpiego.org/media/featarticles/ft20081218.htm). Pap smears will still be required in post-menopausal women, for whom VIA is inappropriate(http://www.who.int/reproductive-health/ publications/cervical_cancer_gep/text.pdf). Other innovations not currently being used in Honduras, such as two visit Pap smear protocols rather than the current three visit protocols, could also be developed and tested.
If colposcopy is to be used for the triage of positive VIA results, one must also consider the potential for overwhelming existing colposcopy resources due to the higher false positive rate of VIA when compared with Pap smears (University of Zimbabwe/JHPIEGO Cervical Cancer Project, 1999; Perkins et al., 2007). Although options exist for overcoming this systems barrier, each has limitations. The least expensive option is to combine VIA with immediate cryotherapy, the traditional “see-and-treat” model. Although this system compares favorably with Pap smears in terms of cost and efficacy (Goldie et al., 2005; Legood et al., 2005; Mandelblatt et al., 2002), no pathologic diagnosis is obtained to assess the adequacy of treatment, and providers may not accept what they view as a reduced level of care (Suba et al., 2006). Another option would be to limit the number of women screened each year to avoid overburdening existing colposcopy systems. For example, ten percent of the highest-risk female population (ages 35–45) could be screened annually with the goal of screening the entire population each decade. Although each woman would receive fewer exams, overall mortality should still be reduced as one lifetime screening with VIA is estimated to reduce the population-wide cervical cancer incidence by one third and two screenings by up to 70% (Goldie et al, NEJM, 2005). An important challenge would be ensuring the equitable distribution of limited screening resources. A third option would be a see-biopsy-treat model, in which the mid-level provider performing VIA biopsies suspicious lesions at the time of the VIA exam (visit 1), and then performs cryotherapy if necessary based on biopsy results (visit 2). This algorithm would reduce costs by eliminating the need for physicians and colposcopic equipment, comply with healthcare standards by providing pathologic confirmation of the diagnosis, and improve practitioners’ VIA skills through ongoing comparison of their visual impressions with pathologic diagnoses. The disadvantages of this system include the need for additional training of mid-level providers in taking cervical biopsies, the increased cost of the additional equipment (re-usable biopsy forceps, monsel’s solution, and jars of formalin), and the potential to overwhelm existing pathology services with large numbers of biopsies.
These obstacles are substantial, but the alternative is the status quo: a screening system which is expensive in itself yet fails to lower the cervical cancer rate (Agurto et al, 2006, Lazcano-Ponce et al, 1999, White, & Peruga, 1996). VIA requires a substantial investment in provider training and ongoing education, but it has the advantages of requiring fewer disposable resources and providing immediate results. Although systems barriers must be considered prior to implementing a VIA system, VIA may be a potentially cost- and life-saving alternative to Pap smears in some resource-poor settings.
Cost-effectiveness analysis is subject to a number of limitations. Data must be combined from various sources with different study designs, and values that cannot be calculated directly must be estimated. During our project, colposcopy was not offered to women with negative screening tests. Therefore, we extrapolated the results of relevant studies to estimate the baseline rate of cervical dysplasia and the sensitivity of VIA (Arrossi et al., 2003; Goldie et al., 2005) and used primary data to calculate the sensitivity of Pap smears, the specificities of both methods, adherence to follow-up, and costs. The combination of primary and secondary data is a well-established technique in cost-effectiveness analysis (Goldie et al., 2005; Goldie et al., 2001).
Conclusions
In developing countries, systems barriers can limit the cost-effectiveness of Pap smears. VIA may be a cost-effective alternative for some resource-poor settings, although systems barriers, quality control and feasibility issues must be considered.
Acknowledgments
Sources of financial support:
Funding for the cervical cancer prevention project from which primary data was gathered was provided by the Alliance for Cervical Cancer Prevention Small Grants Program. Funding for research was provided by the Building Interdisciplinary Careers in Women’s Health (BIRCWH) Scholar Program K12-HD43444.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
April 2008 exchange rate
We included the cost of processing one specimen as this was the median number of biopsies per colposcopy in our project.
The following costs were excluded: private examination areas, speculums (including costs of sterilization), and light sources.
Contributor Information
R.B. Perkins, Department of Obstetrics and Gynecology, Boston University Medical Center, 85 E. Concord St. 6th Floor, Boston, MA 02118, Tel: 617-710-7446, Fax: 617-467-5805, rebecca.perkins@bmc.org
S.M. Langrish, Emma Romero de Callejas Centro de Cancer, TGU000187, 6834 NW 77 Ct, Miami, Florida 33166, wanderinginhonduras@yahoo.com
L.J. Stern, Brigham and Women’s Hospital- PROMESA project, 2908 Hemingford Lane, Oklahoma City, OK 73120, lindajostern@yahoo.com
J.F. Burgess, Department of Health Policy and Management, Boston University School of Public Health, 715 Albany Street, Boston, MA 02118, jfburges@bu.edu
C.J. Simon, Department of Health Policy and Management, Boston University School of Public Health, 715 Albany Street, Boston, MA 02118, cjsimon@bu.edu
References
- Aareleid T, Pukkala E, Thomson H, Hakama M. Cervical cancer incidence and mortality trends in Finland and Estonia: a screened vs. an unscreened population. Eur J Cancer. 1993;29A(5):745–749. doi: 10.1016/s0959-8049(05)80359-4. [DOI] [PubMed] [Google Scholar]
- Agurto I, Sandoval J, De La Rosa M, Guardado ME. Improving cervical cancer prevention in a developing country. Int J Qual Health Care. 2006;18(2):81–86. doi: 10.1093/intqhc/mzi100. [DOI] [PubMed] [Google Scholar]
- Arrossi S, Sankaranarayanan R, Parkin DM. Incidence and mortality of cervical cancer in Latin America. Salud Publica Mex. 2003;45 Suppl 3:S306–S314. doi: 10.1590/s0036-36342003000900004. [DOI] [PubMed] [Google Scholar]
- Christopherson WM. Lucy Wortham James Award. Cytologic detection and diagnosis of cancer. Its contributions and limitations. Cancer. 1983;51(7):1201–1208. doi: 10.1002/1097-0142(19830401)51:7<1201::aid-cncr2820510706>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Claeys P, De Vuyst H, Gonzalez C, Garcia A, Bello RE, Temmerman M. Performance of the acetic acid test when used in field conditions as a screening test for cervical cancer. Trop Med Int Health. 2003;8(8):704–709. doi: 10.1046/j.1365-3156.2003.01082.x. [DOI] [PubMed] [Google Scholar]
- Denny L. The prevention of cervical cancer in developing countries. BJOG. 2005;112(9):1204–1212. doi: 10.1111/j.1471-0528.2005.00713.x. [DOI] [PubMed] [Google Scholar]
- Denny L, Kuhn L, De Souza M, Pollack AE, Dupree W, Wright TC., Jr Screen-and-treat approaches for cervical cancer prevention in low-resource settings: a randomized controlled trial. JAMA. 2005;294(17):2173–2181. doi: 10.1001/jama.294.17.2173. [DOI] [PubMed] [Google Scholar]
- Gaffikin L, Blumenthal PD, Emerson M, Limpaphayom K. Safety, acceptability, and feasibility of a single-visit approach to cervical-cancer prevention in rural Thailand: a demonstration project. Lancet. 2003;361(9360):814–820. doi: 10.1016/s0140-6736(03)12707-9. [DOI] [PubMed] [Google Scholar]
- Goldie SJ, Gaffikin L, Goldhaber-Fiebert JD, Gordillo-Tobar A, Levin C, Mahe C, et al. Cost-effectiveness of cervical-cancer screening in five developing countries. N Engl J Med. 2005;353(20):2158–2168. doi: 10.1056/NEJMsa044278. [DOI] [PubMed] [Google Scholar]
- Goldie SJ, Kuhn L, Denny L, Pollack A, Wright TC. Policy analysis of cervical cancer screening strategies in low-resource settings: clinical benefits and cost-effectiveness. JAMA. 2001;285(24):3107–3115. doi: 10.1001/jama.285.24.3107. [DOI] [PubMed] [Google Scholar]
- [Accessed July 18, 2009];Healthy People 2010 Vol 3 - Cancer. http://www.healthypeople.gov/document/HTML/Volume1/03Cancer.htm.
- International Agency for Research on Cancer. [Accessed July 28, 2009]; http://www.iarc.fr/en/about/index.php.
- JHPIEGO: Regional Single Visit Approach using VIA & Cryotherapy Clinical Training Course. [Accessed July 28, 2009]; http://www.jhpiego.org/media/featarticles/ft20081218.htm. [Google Scholar]
- Jeronimo J, Morales O, Horna J, Pariona J, Manrique J, Rubinos J, et al. Visual inspection with acetic acid for cervical cancer screening outside of low-resource settings. Rev Panam Salud Publica. 2005;17(1):1–5. doi: 10.1590/s1020-49892005000100001. [DOI] [PubMed] [Google Scholar]
- Laara E, Day NE, Hakama M. Trends in mortality from cervical cancer in the Nordic countries: association with organised screening programmes. Lancet. 1987;1(8544):1247–1249. doi: 10.1016/s0140-6736(87)92695-x. [DOI] [PubMed] [Google Scholar]
- Lazcano-Ponce EC, Moss S, Alonso de Ruiz P, Salmeron Castro J, Hernandez Avila M. Cervical cancer screening in developing countries: why is it ineffective? The case of Mexico. Arch Med Res. 1999;30(3):240–250. doi: 10.1016/s0188-0128(99)00006-8. [DOI] [PubMed] [Google Scholar]
- Legood R, Gray AM, Mahe C, Wolstenholme J, Jayant K, Nene BM, et al. Screening for cervical cancer in India: How much will it cost? A trial based analysis of the cost per case detected. Int J Cancer. 2005;117(6):981–987. doi: 10.1002/ijc.21220. [DOI] [PubMed] [Google Scholar]
- Mandelblatt JS, Lawrence WF, Gaffikin L, Limpahayom KK, Lumbiganon P, Warakamin S, et al. Costs and benefits of different strategies to screen for cervical cancer in less-developed countries. J Natl Cancer Inst. 2002;94(19):1469–1483. doi: 10.1093/jnci/94.19.1469. [DOI] [PubMed] [Google Scholar]
- Megevand E, Denny L, Dehaeck K, Soeters R, Bloch B. Acetic acid visualization of the cervix: an alternative to cytologic screening. Obstet Gynecol. 1996;88(3):383–386. doi: 10.1016/0029-7844(96)00189-5. [DOI] [PubMed] [Google Scholar]
- Melnikow J, Nuovo J, Willan AR, Chan BK, Howell LP. Natural history of cervical squamous intraepithelial lesions: a meta-analysis. Obstet Gynecol. 1998;92(4 Pt 2):727–735. doi: 10.1016/s0029-7844(98)00245-2. [DOI] [PubMed] [Google Scholar]
- Nuovo J, Melnikow J, Willan AR, Chan BK. Treatment outcomes for squamous intraepithelial lesions. Int J Gynaecol Obstet. 2000;68(1):25–33. doi: 10.1016/s0020-7292(99)00162-9. [DOI] [PubMed] [Google Scholar]
- Ostor AG. Natural history of cervical intraepithelial neoplasia: a critical review. Int J Gynecol Pathol. 1993;12(2):186–192. [PubMed] [Google Scholar]
- Perkins RB, Langrish SM, Stern LJ, Figueroa J, Simon CJ. Comparison of visual inspection and Papanicolau (PAP) smears for cervical cancer screening in Honduras: should PAP smears be abandoned? Trop Med Int Health. 2007;12(9):1018–1025. doi: 10.1111/j.1365-3156.2007.01888.x. [DOI] [PubMed] [Google Scholar]
- Robles SC, White F, Peruga A. Trends in cervical cancer mortality in the Americas. Bull Pan Am Health Organ. 1996;30(4):290–301. [PubMed] [Google Scholar]
- Sankaranarayanan R, Basu P, Wesley RS, Mahe C, Keita N, Mbalawa CC, et al. Accuracy of visual screening for cervical neoplasia: Results from an IARC multicentre study in India and Africa. Int J Cancer. 2004;110(6):907–913. doi: 10.1002/ijc.20190. [DOI] [PubMed] [Google Scholar]
- Secretary of State of Honduras Presidential Report: Information by Department and Municipality. 2004:1–37. Appendix 4.
- Suba EJ, Murphy SK, Donnelly AD, Furia LM, Huynh ML, Raab SS. Systems analysis of real-world obstacles to successful cervical cancer prevention in developing countries. Am J Public Health. 2006;96(3):480–487. doi: 10.2105/AJPH.2004.061606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- University of Zimbabwe/JHPIEGO Cervical Cancer Project. Visual inspection with acetic acid for cervical-cancer screening: test qualities in a primary-care setting. Lancet. 1999;353(9156):869–873. [PubMed] [Google Scholar]
- World Health Organization; Comprehensive Cervical Cancer Control, a guide to essential practice. [Accessed July 28, 2006]; http://www.who.int/reproductive-health/publications/cervical_cancer_gep/text.pdf. [PubMed]
- Wright TC, Jr, Massad LS, Dunton CJ, Spitzer M, Wilkinson EJ, Solomon D. 2006 consensus guidelines for the management of women with abnormal cervical cancer screening tests. Am J Obstet Gynecol. 2007;197(4):346–355. doi: 10.1016/j.ajog.2007.07.047. [DOI] [PubMed] [Google Scholar]
- Wright TC., Jr Cervical cancer screening using visualization techniques. Review. J Natl Cancer Inst Monogr. 2003;(31):66–71. doi: 10.1093/oxfordjournals.jncimonographs.a003485. [DOI] [PubMed] [Google Scholar]