Abstract
Serrated polyps of the colorectal mucosa represent a heterogeneous and controversial taxonomic category with variation in histopathologic, molecular, and immunohistochemical characteristics and with incomplete understanding of pathogenesis. A previous study reported that expression of gastric pyloric-type mucin MUC6 characterized sessile serrated adenomas. We therefore evaluated the expression of MUC6 in serrated polyps identified among 2,502 participants in a Phase III chemoprevention trial within the Arizona Cancer Center Colorectal Cancer Prevention Trials Program and characterized the associated histopathologic features and location. We performed immunohistochemistry for MUC6 on 146 serrated lesions and 87 conventional tubular adenomas and assessed the percentage of cells with expression and the grade of staining intensity. Ninety-two hyperplastic polyps, 43 sessile serrated adenomas, and 11 traditional serrated adenomas were included. Polyps ranged in size from 1 to 150 millimeters. The association of MUC6 staining with serrated polyp category was evaluated using classification and regression tree (CART) analysis and two-sided Fisher’s exact test. 53% of sessile serrated adenomas (n=23), 17 % of hyperplastic polyps (n=16), and 18% of traditional serrated adenomas (n=2) but none of 87 tubular adenomas expressed MUC6. Expression was limited to the lower crypts in all serrated polyps. Extent of positive staining ranged from 2–100% of crypt cells and was independent of histopathologic type. MUC6 expression had relatively high specificity for sessile serrated adenoma (82%) but low sensitivity (54%). In CART analysis, proximal location was found to be the best partitioning factor for MUC6, followed by classification as sessile serrated adenoma. We conclude that MUC6 expression is strongly associated with proximal location of serrated polyps but has only modest utility as a tissue biomarker for sessile serrated adenoma.
Keywords: serrated adenoma, hyperplastic polyp, colonic neoplasms, MUC6
Introduction
Serrated adenomas of the colorectum were first described in 1990 by Longacre and Fenoglio-Preiser1. Since the initial description, many attempts have been made to distinguish serrated lesions at the histopathologic, immunologic, and molecular levels.2–15 Although there has been great interobserver variation in classifying serrated lesions, distinguishing between these and other polyps of the colorectum has become increasingly important. Of particular clinical relevance is the proposed origin of sporadic colorectal cancers with high levels of microsatellite instability, (MSI-H), from hyperplastic polyps and sessile serrated adenomas. The recent suggestion that hyperplastic polyps of the proximal colon can serve as the immediate precursor for this molecular subtype of colonic carcinoma raised questions about the inherent malignant potential of hyperplastic polyps previously thought to be benign lesions.16
Recently, the aberrant expression of mucin (MUC) gene products in adenomas and carcinomas of the colorectum has been investigated as a potential source of tissue biomarkers to improve serrated polyp subclassification.5,6 Mucins are high molecular weight epithelial glycoproteins that are classified into two distinct structural and functional classes: secreted gel-forming mucins and transmembrane mucins. Twenty MUC genes have been identified or sequenced to date. The secreted gel-forming mucins include products of the MUC2, MUC5AC, MUC5B, and MUC6 genes that are clustered on chromosome 11p15.5. These mucins play a role in the normal physiologic processes of the gastrointestinal tract, and in neoplastic progression and metastasis of colon cancer cells.17 MUC2 and MUC5AC are expressed in normal colonic epithelium and in colorectal cancers, while MUC5B is only expressed to a minor degree in the colon.17 MUC6 has little expression in the colon, but is expressed in surface foveolar epithelium and deep antral/pyloric glands of the stomach.18
Phenotypically, the cells lining the crypts of hyperplastic polyps and serrated adenomas show differentiation similar to gastric pyloric gland mucous cells. Recent studies have examined the differential expression of MUC gene products, including MUC6, to distinguish sessile serrated adenomas from hyperplastic polyps to improve classification.19–23 Owens et al., for example, recently reported 100% specificity of MUC6 to discriminate between sessile serrated adenoma and hyperplastic polyp.23 In order to provide additional data on the clinical utility of MUC6 as an adjunct tissue biomarker, we examined its expression in relation to histopathologic classification, size, and anatomic location of colorectal serrated lesions in our large studies of subjects with colorectal adenomas who were enrolled in Phase III chemoprevention trials.
Materials and Methods
Patients and Specimens
We examined serrated polyps pooled from 2,502 subjects participating in the Wheat Bran Fiber and the Ursodeoxycholic Acid Phase III polyp prevention trials conducted at the Arizona Cancer Center, the details of which have been reported elsewhere.24,25 Briefly, eligibility criteria included the removal of one or more colorectal adenomas during a colonoscopic examination within the 6-month period before study registration. All colon neoplasms must have been removed completely, except for diminutive (<3 mm) sessile rectal polyps. Subjects with a familial colorectal cancer syndrome or evidence of hyperplastic polyposis were excluded from the trials. Baseline characteristics of the patients enrolled in the two studies are presented in Table 1.
Table 1.
Baseline Characteristics of all patients participating in Wheat Bran Fiber and the Ursodeoxycholic Acid Phase III polyp prevention trials
| WBF (N = 1304) |
UDCA (N = 1193) |
|
|---|---|---|
| Age, mean (SD) (y) | 65.7 ± 8.8 | 65.8 ± 8.5 |
| Sex (%) | ||
| Male | 66.8 | 67.5 |
| Female | 33.2 | 32.5 |
| Mean no. of adenomas | 1.5 ± 0.9 | 1.7 ± 1.1 |
| Location (%) | ||
| Distal colorectum | 46.8 | 37.2 |
| Proximal only | 31.8 | 36.7 |
| Proximal and distal | 15.3 | 19.7 |
| Unknown | 6.1 | 6.4 |
| Size of largest adenoma, mean (SD) (mm) | 8.3 ± 5.9 | 8.9 ± 5.9 |
| Adenoma histology (%) | ||
| Tubular | 78.7 | 79.3 |
| Tubulovillous/villous | 21.3 | 20.7 |
The serrated polyps were identified through review of the biopsy or polypectomy specimens of 3,901 baseline and recurrent lesions. Eighty-seven conventional tubular adenomas with low-grade dysplasia were also selected for comparison from the same study population. None of the tubular adenomas contained high-grade dysplasia. All other histopathologic types of polyps were excluded including inflammatory, hamartomatous, and juvenile polyps.
Only those serrated polyps with at least a portion of well-oriented, full-thickness serrated mucosa in the histopathologic sections were included. Most of the specimens contained adjacent non-lesional colorectal mucosa. Samples with cautery artifact involving the entire serrated epithelial component due to colonoscopic polypectomy or “hot” biopsy procedure were excluded from the study. These inclusion and exclusion criteria resulted in a sample size of 161 serrated polyps. Age and gender of patient as well as size of the lesion and location were recorded. Size was obtained from the colonoscopic measurement or by measurement of the lesion on the histopathologic section if the size was greater than was reported. Polyp site was categorized as distal or proximal relative to the splenic flexure. We were unable to confirm definitively from the endoscopy report the location of 15 serrated polyps, so these were excluded from further analyses. 146 serrated polyps remained for MUC6 staining.
The study was approved by the Institutional Review Boards of the University of Arizona and The University of Texas M. D. Anderson Cancer Center.
Histopathologic Criteria
The classification of sessile serrated adenoma was based on the following criteria of Snover et al: branching of crypts, dilatation of the base of the crypts, crypts extending parallel to the muscularis mucosae, presence of mature cells with gastric foveolar cell phenotype at the base replacing the proliferative zone of non-lesional mucosa, serration at the base of the crypts, and, less commonly, pseudostratification and eosinophilic change of the surface epithelium. 12 Hyperplastic polyps were classified on the basis of serration in only the upper half to one third of the crypts with normal proliferation including a proliferative zone at the base of the crypts that was symmetric and continuous, and the crypts remaining narrow and lined with proliferative cells.8 Traditional serrated adenomas were classified on the basis of villiform configuration that appeared protuberant rather than sessile with a uniform population of abnormal columnar epithelium containing eosinophilic cytoplasm, centrally placed elongated nuclei that were somewhat hyperchromatic, and mild pseudostratification.12 All histopathologic evaluations were performed by two gastrointestinal pathology pathologists (AB and SRH) without knowledge of any clinical characteristics.
Immunohistochemical Analysis of MUC6
Archival paraffin-embedded tissue blocks from each case when available (n=111 serrated polyps, n=60 tubular adenomas) or tissue microarray blocks constructed for cases for which return of blocks was mandated by the enrolling institution (n=35 serrated polyps, n=27 tubular adenomas) were used for immunohistochemistry. The tissue microarrays were constructed using a Beecher instrument from histopathologic areas selected in scout H&E slides from the specimen blocks that were used for classification. The cores were 1.5 millimeters in diameter, and the tissue microarray blocks included gastric and colorectal mucosa as positive and negative controls along with sessile serrated adenoma, hyperplastic polyp, and tubular adenomas with low-grade intra-epithelial neoplasia (dysplasia).
Sections were cut at 5 micrometers thickness onto positively charged slides and stained with MUC6 mouse monoclonal antibody VP-M658 (Vector Laboratories, Burlingame, CA) at a dilution of 1:40. The immunogen for the antibody was a synthetic peptide of the MUC6 tandem repeat sequence purified by high performance liquid chromatography. Gastric antral mucosa was used as a positive control with the cytoplasmic staining pattern in the glands. Negative controls consisted of adjoining TMA sections with primary antibodies that were class-matched mouse IgG proteins. Deparaffinization with xylene, antigen retrieval with ethylene diamine tetraacetic acid (EDTA), and immunohistochemistry were performed using the Discovery XT Automated immunohistochemistry system (Ventana Medical Systems, Tucson, AZ). Primary antibody staining, detection and amplification with 3, 3-0-diamnobenzidine (DAB) chromogen, and hematoxylin counterstaining were performed using a multimer based detection system (Ultra-Map) with Ventana Medical Systems validated reagents. Following staining on the instrument, slides were dehydrated through graded alcohols to xylene and a coverslip applied with Pro-Texx mounting medium (Thermo Scientific, Waltham, MA).
Immunoreactivity for MUC6 was evaluated as the percentage of cells with expression and the grade of staining intensity. The extent of staining was assessed as an estimated percentage of total positively stained areas within the lesion, and grade of intensity as weak, moderate, or strong for each positively stained lesion. Predominant expression patterns for MUC6 staining were defined as apical, basolateral, supranuclear or diffuse.
Statistical Analysis
The clinicopathologic features were summarized by calculating mean, median, standard deviation and range for continuous features (e.g. polyp size), and calculating frequencies and relative percentages for categorical features (e.g. histopathologic type). MUC6 staining was first summarized by calculating mean, median, standard deviation and range, i.e. minimum and maximum, of estimated staining percentage. A two-sided Fisher’s exact test was performed to evaluate the statistical significance of differences for categorical outcomes (e.g. large lesion defined as ≥1 centimeter), and a Wilcoxon Rank-Sum test for continuous outcomes (e.g. lesion size). All significance levels were set at 5%. In addition, sensitivity and specificity of MUC6 staining for sessile serrated adenoma were calculated.
A classification and regression tree (CART) analysis, where a deviance method was used to measure the impurity of node, was then performed to explore the factors and the possible interactions between the factors associated with positive MUC6 staining. A multivariate logistic regression model was also fitted to the status of MUC6 staining to compare with the findings from the CART analysis and to study the significance of the factors associated with positive MUC6 staining.
Results
The clinicopathologic features of the colorectal serrated polyps in our study are presented in Table 2. Of the146 serrated polyps, 43 were classified as sessile serrated adenoma (17 proximal, 26 distal), 92 as hyperplastic polyp (20 proximal, 72 distal), and 11 as traditional serrated adenoma (4 proximal, 7 distal). These polyps originated from 24 women and 82 men with a mean age of 64 years (range 45–78 years). No significant associations were seen with gender or age. Polyp size ranged from 1 to 150 millimeters. There were statistically significant differences in lesion size between sessile serrated adenomas (median 5 millimeters) and hyperplastic polyps (median 4 millimeters, p = 0.0002 from Wilcoxon Rank-Sum test). However, differences in the frequency of clinically relevant large lesions of 1 centimeter or more between sessile serrated adenoma (11.6%) and hyperplastic polyp (6.5%) did not reach statistical significance (p = 0.33 by Fisher’s exact test). No intra-epithelial neoplasia (dysplasia) was seen in any sessile serrated adenoma or hyperplastic polyp, and all tubular adenomas selected for comparison had only low-grade dysplasia.
Table 2.
Clinical Characteristics of Patients with Serrated Polyps
| Histology Type | # Polyps |
#Patients, Mean (range) |
Age (Mean +/sd) |
Male (%) |
Proximal (%) |
|---|---|---|---|---|---|
| Hyperplastic Polyp | 92 | 68, 1.3 (1–5) | 63.4 (7.9) | 80.9 | 19.6 |
| Sessile Serrated Adenoma | 43 | 35, 1.3 (1–5) | 66.3 (7.8) | 72.4 | 44.2 |
|
Traditional Serrated Adenoma |
11 | 9, 1.2 (1–3) | 66.7 (6.2) | 66.7 | 36.4 |
| All Serrated | 146 | *106, 1.4 (1–5) | 64.5 (7.8) | 77.4 | 28.1 |
the number of total polyps differs from the number of patients, as some patients contributed more than one polyp to the sample set.
In the sample set of 146 polyps, all lesions that were positive for MUC6 showed only basolateral and diffuse staining patterns (Table 3). 23 of 43 sessile serrated adenomas (53%), 16 of 92 hyperplastic polyps (17%), and 2 of 11 traditional serrated adenomas (18%) had basal staining for MUC6 (Figure 1a–1d). None of the tubular adenomas expressed MUC6. Basal MUC6 staining exhibited high specificity for SSA (82%), but low sensitivity (54%).
Table 3.
Relation between histopathologic polyp type, number of polyps with positive MUC6 expression, and percentage of crypt cells positive for MUC6
| Histology | ||||
|---|---|---|---|---|
| Hyperplastic Polyp |
Sessile Serrated Adenoma |
Traditional Serrated Adenoma |
All (Serrated) |
|
| MUC6 +; n (%) | 16/92 (17.4%) |
23/43 (53.5%) |
2/11 (18.2%) |
41/146 (28.1%) |
|
Crypts staining MUC6
+ Mean (Range)* |
26.4% (2–90%) |
26.8% (2–90%) |
52.5% (5–100%) |
|
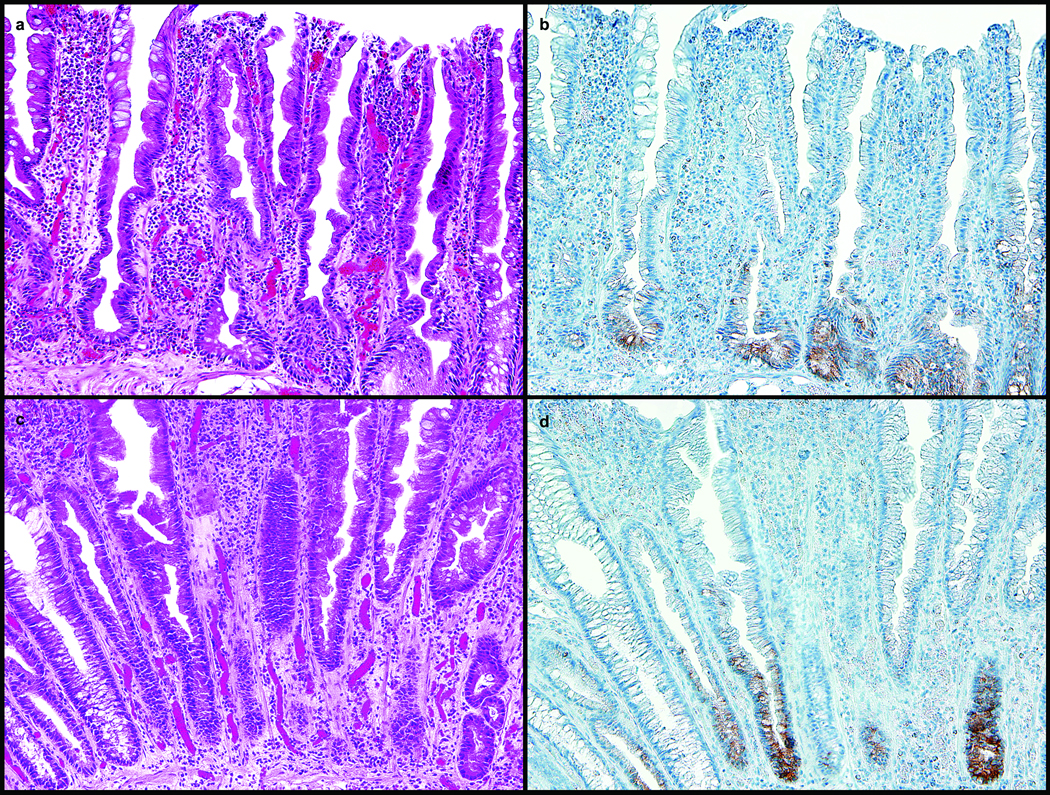
Figure 1.
a. and b. : Sessile Serrated Adenoma, H&E, Proximal Colon, 20X: a. Section of proximal colon stained with hematoxylin and eosin showing some features of sessile serrated adenoma including dilatation of the base of the crypts, crypts extending parallel to the muscularis mucosae, serration at the base of the crypts, and eosinophilic change of the surface epithelium. b. Section of proximal colon sessile serrated adenoma in Figure 1a. with MUC6 immunostaining of the basal crypts.
c. and d. : Hyperplastic Polyp, H&E, Distal Colon, 20X: c. Section of distal colon stained with hematoxylin and eosin showing features of hyperplastic polyp including serration in the upper half to one third of the crypts with normal proliferation including a proliferation zone at the base of the crypts that is symmetric, and crypts that remain narrow and lined with proliferative cells. d. Section of distal colon hyperplastic polyp in Figure 1c. with MUC6 immunostaining of the basal crypts.
Of the lesions staining positive, the percentage of crypt cells expressing MUC6 ranged from 2% to 100%, and all positive lesions showed moderate to strong staining intensity. No significant differences were seen in staining intensity of the MUC6-expressing polyps in relation to their size, anatomic site, or histopathologic subtype. Non-lesional colorectal mucosa, either adjacent to serrated lesions or as a separate specimen, was uniformly negative for MUC6.
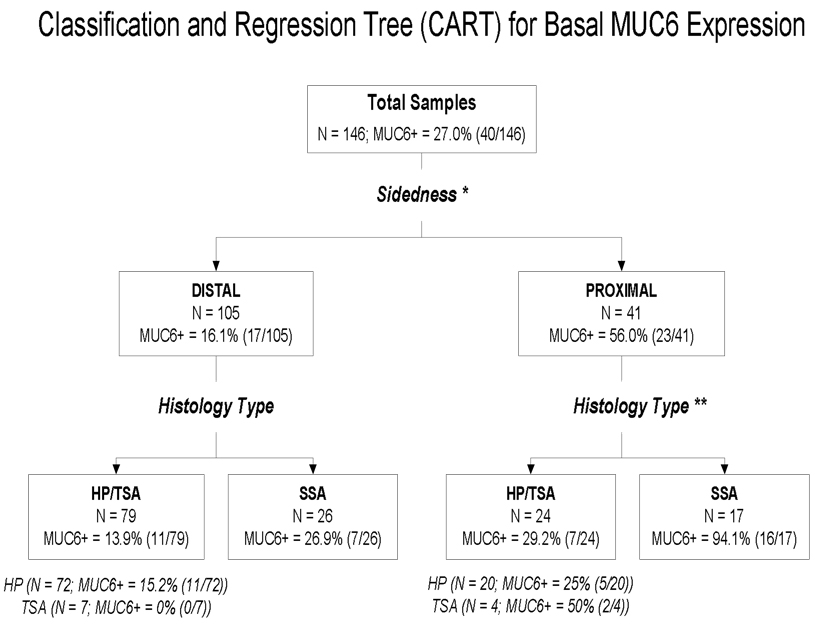
A classification and regression tree (CART) analysis was performed to explore the factors associated with MUC6 expression status without relying on a specific distribution or model. Lesion characteristics (e.g. anatomic location as proximal or distal, and lesion morphology as hyperplastic polyp, sessile serrated adenoma or traditional serrated adenoma) and the corresponding demographic information for each patient (e.g. age and gender) were the independent variables (predictors) to build the tree for MUC6 staining status. The results are shown in Figure 2. The analysis indicates that anatomic location (proximal vs. distal) was the optimal first split, followed by lesion morphology, where traditional serrated adenoma and hyperplastic polyp were grouped together by the splitting algorithm (i.e. sessile serrated adenoma vs. hyperplastic polyp/traditional serrated adenoma).
Figure 2. Classification and Regression Tree Analysis.
CART model of basal MUC6 staining and patient clinical characteristics. The model indicates that a polyp staining positive for MUC6 was explained by an interaction between proximal location and sessile serrated adenoma category. When sidedness was proximal, the proportion of MUC6-positive specimens was 56.0% compared to 16.1% in specimens from the distal colon. When histology was included, 94.1% of specimens that were MUC6-positive in the proximal colon were sessile serrated adenomas compared to only 29.2% of proximal specimens that were hyperplastic polyps or traditional serrated adenomas. In the distal specimens, the corresponding frequencies were 26.9% and 13.9% No further splits were found to significantly improve the homogeneity of the subgroup outcomes for MUC6 staining. Abbreviation Key: HP- hyperplastic polyp, SSA- sessile serrated adenoma, TSA- traditional serrated adenoma
Based on a multivariate logistic regression model (Table 4), proximal lesions had a significantly higher rate of MUC6 expression compared to distal lesions with an odds ratio of 5.79 (p = 0.0001; 95% CI 2.35–14.25), and sessile serrated adenoma had a significantly higher rate of MUC6 expression compared to hyperplastic polyp with an odds ratio of 5.18 (p = 0.001; 95% CI 2.04–13.15). MUC6 positivity did occur in distal sessile serrated adenomas (7 of 26, 27%) and hyperplastic polyps (11 of 72, 15%), but to a lesser extent than proximal lesions and with no significant association for sessile serrated adenoma compared to hyperplastic polyp or traditional serrated adenoma. To account for potential dependence between polyps within the same subject, analyses were conducted using a generalized linear mixed effects model with random intercept. These results were nearly identical to those shown for the analysis assuming independence between polyps within the same subject in Table 4.
Table 4.
Association of clinicopathologic features and MUC6 expression
| Variable | Odds Ratio (95% CI) | p value |
|---|---|---|
| Age ( ≥64 years) | 0.34 (0.14–0.86) | 0.02 |
| Male v. Female | 0.52 (0.19–1.46) | 0.21 |
| Proximal v. Distal | 5.79 (2.35–14.25) | 0.0001 |
| Histology Type | ||
| TSA v. HP | 1.06 (0.17–6.53) | 0.95 |
| SSA v. HP | 5.18 (2.04–13.15) | 0.001 |
| TSA v. SSA | 0.21 (0.03–1.26) | 0.09 |
Age (≥64 years), gender, location, and histology type were included in the multivariate logistic regression model.
Discussion
We demonstrate that gastric pyloric mucin MUC6 is expressed in all categories of serrated lesions of the colorectum including sessile serrated adenoma, hyperplastic polyp, and traditional serrated adenoma, but not in conventional tubular adenomas in our study. The basal expression of MUC6 was shown by immunohistochemistry to be more frequent in proximal serrated lesions regardless of category. Expression was more often positive in sessile serrated adenomas of the proximal colon, with high specificity (82%). However, basal MUC6 expression was seen in proximal hyperplastic polyps and in distal serrated polyps including both sessile serrated adenomas and hyperplastic polyps. MUC6 staining was not seen in distal traditional serrated adenomas. Patient gender, age, or size of the polyp was not significantly associated with MUC6 expression.
Our results contrast with those of Owens et al. who reported 100% specificity when using MUC6 to distinguish sessile serrated adenomas from hyperplastic polyps.23 In their study, none of 48 hyperplastic polyps or 13 traditional serrated adenomas were positive for MUC6. Neither anatomic location nor polyp size appeared to account for the apparent differences in MUC6 expression. Also in their study, the authors found sessile serrated adenomas to be significantly larger than hyperplastic polyps and theorized that MUC6 staining could be related to polyp size rather than morphological polyp subtype. While they found that sessile serrated adenomas tended to be slightly larger than hyperplastic polyps23, we did not find the difference to be clinically meaningful.
Percinel et al. similarly found no MUC6 expression in hyperplastic polyps or normal mucosa. Twenty percent of the sessile serrated adenomas in their study were positive for MUC6.22 These authors concluded that hyperplastic epithelium may give rise to adenomatous change, and that MUC6 may take part later in the neoplastic continuum. Bartman et al. found no MUC6 expression in hyperplastic polyps or normal mucosa by immunohistochemistry and found minimal amounts of MUC6 mRNA in samples of normal mucosa by slot blot analysis.19 They found significantly higher levels of MUC6 mRNA in adenomas but did not specifically analyze sessile serrated adenomas or hyperplastic polyps.
In contrast to these reports, we found MUC6 positivity in 17% of hyperplastic polyps regardless of colorectal location. This finding suggests that MUC6 lacks sufficient discrimination for use as a diagnostic tool to definitively differentiate sessile serrated adenoma from hyperplastic polyp. In agreement with our findings, Hirono et al. found MUC6 expression in 68% of hyperplastic polyps, 71% of sessile serrated adenomas, and 9.5% of tubular adenomas, 20 and Mochizuka et al. found MUC6 expression in 27% of hyperplastic polyps and 76% of sessile serrated adenomas. In their study, sessile serrated adenomas had significantly higher staining scores for MUC6. In addition, right-sided sessile serrated adenomas had a higher staining score than left-sided sessile serrated adenomas, but there was no significant difference between right-sided and left-sided hyperplastic polyps.21 Neither study found MUC6 expression in non-lesional colonic mucosa.
Also in agreement with several previous studies, 2,8–10, 21,22 we found that sessile serrated adenomas occurred at higher frequency in the distal colon than in the proximal colon, but sessile serrated adenomas showed a greater predilection for the proximal colon when compared to hyperplastic polyps. Other studies show sessile serrated adenomas predominantly located in the proximal colon. 23, 26 These differences could be attributed to sample selection as well as the use of differing diagnostic criteria for classifying serrated polyps. Nonetheless, the detection of MUC6 in distal and proximal sessile serrated adenomas as well as hyperplastic polyps limits the utility of MUC6 immunostaining to differentiate between these morphologic subtypes.
Potential limitations of our study that may explain differences from other studies include the selection of a patient population that includes only those patients with known previous adenomas and the age of the histologic blocks used in the study (ranging from 1991–2007). Methodologic differences in the immunohistochemical preparation of the slides, including the antibodies used and staining methods that are not uniform in all of the studies, could contribute as well. These limitations, however, cannot explain the strong association with anatomic location in the proximal colon nor the clearly positive staining of serrated polyps other than sessile serrated adenomas. The low sensitivity in our sample, however, most likely reflects the older age of our specimens and failure to retrieve antigen, effects that are random and not likely to contribute to significant bias in the overall study results, with the exception of the sensitivity of the antibody method of MUC6 staining.
In summary, this is one of the largest study groups to date on MUC6 immunostaining in serrated polyps of the colorectum. Although we confirm some of the findings of smaller studies in the recent literature, including the predominant association of MUC6 with proximal or right-sided lesions, we did not observe exclusivity of MUC6 staining for polyps of the sessile serrated adenoma type. Further studies, including incorporation of molecular characteristics, are needed to determine if aberrant expression of gastric mucins would prove helpful as an adjunct to morphology in the clinical discrimination of these polyps. Of particular interest, the question of the relationship between MUC6 expression and origin of adenocarcinoma in serrated polyps of the colorectum remains to be answered.
Acknowledgements
The authors thank Dr. Ray Nagle and staff for performing the immunostaining for this project, and Kim-Anh Vu for color figures.
This work was supported by National Cancer Institute grant CA-41108.
Immunohistochemical data were generated by the TACMASS Core (Tissue Acquisition and Cellular/Molecular Analysis Shared Service) that is supported by the Arizona Cancer Center Support Grant, NIH CA023074.
Dr. Hamilton is supported by the Frederick F. Becker Distinguished University Chair for Cancer Research.
References
- 1.Longacre TA, Fenoglio-Preiser CM. Mixed hyperplastic adenomatous polyps/serrated adenomas. A distinct form of colorectal neoplasia. Am J Surg Pathol. 1990;14:524–537. doi: 10.1097/00000478-199006000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Yao T, Kouzuki T, Kajiwara M, Matsui N, Oya M, Tsuneyoshi M. 'Serrated' adenoma of the colorectum, with reference to its gastric differentiation and its malignant potential. J Pathol. 1999;187:511–517. doi: 10.1002/(SICI)1096-9896(199904)187:5<511::AID-PATH308>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 3.Matsumoto T, Mizuno M, Shimizu M, Manabe T, Iida M. Clinicopathological features of serrated adenoma of the colorectum: comparison with traditional adenoma. J Clin Pathol. 1999;52:513–516. doi: 10.1136/jcp.52.7.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hawkins NJ, Gorman P, Tomlinson IP, Bullpitt P, Ward RL. Colorectal carcinomas arising in the hyperplastic polyposis syndrome progress through the chromosomal instability pathway. Am J Pathol. 2000;157:385–392. doi: 10.1016/S0002-9440(10)64551-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sylvester PA, Myerscough N, Warren BF, Carlstedt I, Corfield AP, Durdey P, Thomas MG. Differential expression of the chromosome 11 mucin genes in colorectal cancer. J Pathol. 2001;195:327–335. doi: 10.1002/path.951. [DOI] [PubMed] [Google Scholar]
- 6.Gurbuz Y, Kloppel G. Differentiation pathways in duodenal and ampullary carcinomas: a comparative study on mucin and trefoil peptide expression, including gastric and colon carcinomas. Virchows Arch. 2004;444:536–541. doi: 10.1007/s00428-004-1008-2. [DOI] [PubMed] [Google Scholar]
- 7.Tanaka M, Kusumi T, Sasaki Y, Yamagata K, Ichinohe H, Nishida J, Kudo H. Colonic intra-epithelial carcinoma occurring in a hyperplastic polyp via a serrated adenoma. Pathol Int. 2001;51:215–220. doi: 10.1046/j.1440-1827.2001.01177.x. [DOI] [PubMed] [Google Scholar]
- 8.Torlakovic E, Skovlund E, Snover DC, Torlakovic G, Nesland JM. Morphologic reappraisal of serrated colorectal polyps. Am J Surg Pathol. 2003;27:65–81. doi: 10.1097/00000478-200301000-00008. [DOI] [PubMed] [Google Scholar]
- 9.Bariol C, Hawkins NJ, Turner JJ, Meagher AP, Williams DB, Ward RL. Histopathological and clinical evaluation of serrated adenomas of the colon and rectum. Mod Pathol. 2003;16:417–423. doi: 10.1097/01.MP.0000068236.47471.DB. [DOI] [PubMed] [Google Scholar]
- 10.Higuchi T, Jass JR. My approach to serrated polyps of the colorectum. J Clin Pathol. 2004;57:682–686. doi: 10.1136/jcp.2003.015230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baker K, Zhang Y, Jin C, Jass JR. Proximal versus distal hyperplastic polyps of the colorectum: different lesions or a biological spectrum? J Clin Pathol. 2004;57:1089–1093. doi: 10.1136/jcp.2004.016600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Snover DC, Jass JR, Fenoglio-Preiser C, Batts KP. Serrated polyps of the large intestine: a morphologic and molecular review of an evolving concept. Am J Clin Pathol. 2005;124:380–391. doi: 10.1309/V2EP-TPLJ-RB3F-GHJL. [DOI] [PubMed] [Google Scholar]
- 13.Sandmeier D, Seelentag W, Bouzourene H. Serrated polyps of the colorectum: is sessile serrated adenoma distinguishable from hyperplastic polyp in a daily practice? Virchows Arch. 2007;450:613–618. doi: 10.1007/s00428-007-0413-8. [DOI] [PubMed] [Google Scholar]
- 14.Torlakovic EE, Gomez JD, Driman DK, Parfitt JR, Wang C, Benerjee T, Snover DC. Sessile serrated adenoma (SSA) vs. traditional serrated adenoma (TSA) Am J Surg Pathol. 2008;32:21–29. doi: 10.1097/PAS.0b013e318157f002. [DOI] [PubMed] [Google Scholar]
- 15.Wu JM, Montgomery EA, Iacobuzio-Donahue CA. Frequent beta-catenin nuclear labeling in sessile serrated polyps of the colorectum with neoplastic potential. Am J Clin Pathol. 2008;129:416–423. doi: 10.1309/603UQKM7C2KELGJU. [DOI] [PubMed] [Google Scholar]
- 16.Hawkins NJ, Ward RL. Sporadic colorectal cancers with microsatellite instability and their possible origin in hyperplastic polyps and serrated adenomas. J Natl Cancer Inst. 2001;93:1307–1313. doi: 10.1093/jnci/93.17.1307. [DOI] [PubMed] [Google Scholar]
- 17.Byrd JC, Bresalier RS. Mucins and mucin binding proteins in colorectal cancer. Cancer Metastasis Rev. 2004;23:77–99. doi: 10.1023/a:1025815113599. [DOI] [PubMed] [Google Scholar]
- 18.Reid CJ, Harris A. Developmental expression of mucin genes in the human gastrointestinal system. Gut. 1998;42:220–226. doi: 10.1136/gut.42.2.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bartman AE, Sanderson SJ, Ewing SL, Niehans GA, Wiehr CL, Evans MK, Ho SB. Aberrant expression of MUC5AC and MUC6 gastric mucin genes in colorectal polyps. Int J Cancer. 1999;80:210–218. doi: 10.1002/(sici)1097-0215(19990118)80:2<210::aid-ijc9>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 20.Hirono H, Ajioka Y, Watanabe H, Baba Y, Tozawa E, Nishikura K, Mukai G, Honma T, Aoyagi Y. Bidirectional gastric differentiation in cellular mucin phenotype (foveolar and pyloric) in serrated adenoma and hyperplastic polyp of the colorectum. Pathol Int. 2004;54:401–407. doi: 10.1111/j.1440-1827.2004.01639.x. [DOI] [PubMed] [Google Scholar]
- 21.Mochizuka A, Uehara T, Nakamura T, Kobayashi Y, Ota H. Hyperplastic polyps and sessile serrated 'adenomas' of the colon and rectum display gastric pyloric differentiation. Histochem Cell Biol. 2007;128:445–455. doi: 10.1007/s00418-007-0326-2. [DOI] [PubMed] [Google Scholar]
- 22.Percinel S, Savas B, Ensari A, Kuzu I, Kuzu MA, Bektas M, Cetinkaya H, Kursun N. Mucins in the colorectal neoplastic spectrum with reference to conventional and serrated adenomas. Turk J Gastroenterol. 2007;18:230–238. [PubMed] [Google Scholar]
- 23.Owens SR, Chiosea SI, Kuan SF. Selective expression of gastric mucin MUC6 in colonic sessile serrated adenoma but not in hyperplastic polyp aids in morphological diagnosis of serrated polyps. Mod Pathol. 2008;21:660–669. doi: 10.1038/modpathol.2008.55. [DOI] [PubMed] [Google Scholar]
- 24.Alberts DS, Martínez ME, Hess LM, Einspahr JG, Green SB, Bhattacharyya AK, Guillen J, Krutzsch M, Batta AK, Salen G, Fales L, Koonce K, Parish D, Clouser M, Roe D, Lance P. Phase III trial of ursodeoxycholic acid to prevent colorectal adenoma recurrence. J. Natl. Cancer Inst. 2005;97:846–853. doi: 10.1093/jnci/dji144. [DOI] [PubMed] [Google Scholar]
- 25.Alberts DS, Martínez ME, Roe DJ, Guillén-Rodríguez JM, Marshall JR, van Leeuwen JB, Reid ME, Ritenbaugh C, Vargas PA, Bhattacharyya AB, Earnest DL, Sampliner RE. Lack of effect of a high-fiber cereal supplement on the recurrence of colorectal adenomas. N Engl J Med. 2000;342:1156–1162. doi: 10.1056/NEJM200004203421602. [DOI] [PubMed] [Google Scholar]
- 26.Parfitt JR, Driman DK. Survivin and hedgehog protein expression in serrated colorectal polyps: an immunohistochemical study. Human Path. 2007;38:710–717. doi: 10.1016/j.humpath.2006.12.004. [DOI] [PubMed] [Google Scholar]