Abstract
Burkitt lymphoma (BL) is a unique B-cell non-Hodgkin lymphoma with three established clinical-epidemiological variants: endemic, sporadic, and AIDS-related BL shows characteristic dysregulation of MYC gene, but the causes of MYC dysregulation or BL arising at different ages are poorly understood. Therefore, we examined population-based BL incidence patterns in the United States to determine age-related risk. BL case and population data were obtained from the NCI’s Surveillance, Epidemiology, and End Results Databases (1973–2005). Standard cross-sectional, age-standardized, and age-specific incidence rates were stratified by sex and race and supplemented with age-period-cohort (APC) models. We analyzed 3058 BL cases diagnosed during 1,160,300,297 person-years of observation. Age-standardized incidence rates rose 6.8% per year (95% CI 4.5–9.1) for males and 7.1% (95% CI 3.2–11.1) for females during the study period. The rate among males was 3.2 times that among females, and among Whites 1.3 times that among Blacks. Male-to-female incidence rate ratios did not differ by race, but were 4.2 for pediatric (0–19 years), 4.1 for adult (20–59 years) and 2.0 for geriatric (≥ 60 years) BL. Cross-sectional age-specific rates showed two separate peaks among males and females, near ages 10 and 75 years, and a third peak near age 40 years among males. The tri/bimodal incidence pattern was present in sensitivity analyses excluding registries with many HIV/AIDS cases and in period-specific, cohort-specific analyses. To our knowledge tri/bimodal incidence patterns have not previously been reported for BL. Trimodal/bimodal BL suggests heterogeneity in etiology or biology of BL diagnosed at different ages in males and females.
INTRODUCTION
Burkitt lymphoma (BL) is a rare aggressive B cell non-Hodgkin lymphoma (NHL), first reported in African children fifty-years ago. 1 Description of BL histopathology2 and subsequent diagnosis of cases throughout the world revealed notable differences in incidence in different populations,3 and as well as some differences in the morphology of BL.4 Today, three clinical-epidemiological variants are recognized, i.e., endemic, sporadic, and AIDS-associated BL. These variants are indistinguishable by conventional diagnostic evaluation of morphology and immunostaining, 5–7 but subtle morphological variation broadly classified as typical and atypical BL are recognized among them all.7 All BL show characteristic dysregulation of the MYC gene,8 mostly due to chromosomal translocation involving MYC and immunoglobulin (Ig) genes,9 but also mutations in MYC or associated genes.10
The risk factors for sporadic BL are poorly understood and incompletely understood for endemic and AIDS-related BL11. For endemic BL, Epstein-Barr virus (EBV), whose genome is detected in nearly 100% of cases, and Plasmodium falciparum malaria, which has overlapping geographic distribution, are considered risk factors. For sporadic and AIDS-related BL, malaria is not relevant and EBV, although ubiquitous, is detected in a minority of cases.11 BL is characterized by a very short doubling time (1–2 days),12 and the interval from initiating or promoting events to diagnosis may be comparatively short. Thus, study of age-incidence patterns may provide clues about etiology. Therefore, we conducted a population-based analysis of age-standardized and age-specific incidence patterns for BL by gender using data collected in the National Cancer Institute’s Surveillance Epidemiology and End Results (SEER) program for cases diagnosed during the years 1973 through 2005.
METHODS
STUDY POPULATION
Incident BL case and population data were obtained from the SEER 9 (1973–1991), 13 (1992–1999), and 17 (2000–2005) Registries Databases (November 2007 submission, http://seer.cancer.gov)13–15 using SEER*Stat version 6.5.2 (http://seer.cancer.gov). BL was defined as SEER site recode NHL or leukemia and International Classification of Diseases for Oncology (ICD-O-3) morphology code 9687 for BL16 and 9826 for Burkitt cell leukemia. The ICD-O morphology code for BL has not changed with successive revisions of ICD-O, but acute lymphocytic leukemia L3-type (L3-ALL) has been coded as Burkitt cell leukemia since 1992.7
The age-standardized BL rates in the SEER 9 during recent years were very similar to those in the SEER 13 and SEER 17, supporting the notion that data could be combined geographically to boost statistical power. Thus, data from SEER’s 9, 13, and 17 Registries Databases, covering 10%, 14%, and 26% of the U.S. population, respectively, 17 were combined.
STATISTICAL METHODS
Standard descriptive statistics were calculated for overall rates and for rates stratified by sex, race, age at diagnosis, and calendar-year of diagnosis. To evaluate the pattern and magnitude of BL risk across age-groups, age at diagnosis was categorized into three age-groups: <20 years or “pediatric”, 20–59 years or “adult”, and ≥60 years or “geriatric”. To evaluate time trends, calendar-years of diagnosis were categorized into eight 4-year calendar-periods (1974–1977, 1978–1981, …, 2002–2005). Age-standardized (2000 US standard population) incidence rates were calculated as cases per 100,000 person-years by sex, race, and age-group. Relative risks were expressed as incidence rate ratios (IRR).
Overall and estimated annual percentage change (EAPC) in age-standardized BL incidence rates and 95% confidence intervals across the study period were calculated by sex. Age-standardized incidence rates for eight 4-year calendar-year periods were plotted on a log-linear scale for males and females, such that a slope of 10 degrees represented a percentage change in rates of 1% per year 18 (see Figure 1A). Using a similar aspect ratio, cross-sectional age-specific incidence rates were plotted for 21 4-year age groups (ages 0–3, 4–7, …, 80–83 years) for males and females (as shown in Figure 1B). Because observed age-specific incidence rates by period or cohort were subject to uncertainty due to small sample sizes, we used age-period-cohort (APC) models to plot “expected” period-specific and cohort-specific age-specific rates (see Figure 2A–D).
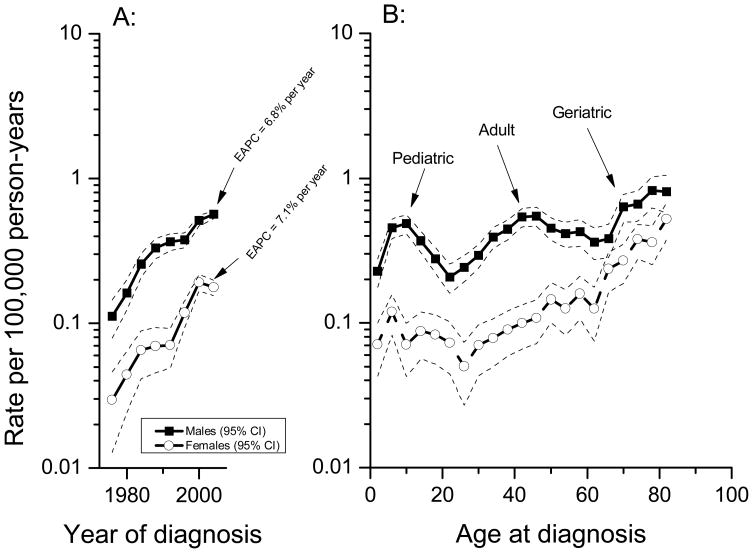
Figure 1.
Burkitt lymphoma male and female age-standardized incidence rates for males (solid squares) and females (open circles), also showing the estimated annual percentage change (EAPC) of the age-standardized incidence rate, (A) and age-specific incidence rates (B) in the United States by sex, SEER 9, 13, and 17 registries, 1974 through 2005. EAPC=estimated annual percentage change of the age-standardized incidence rate.
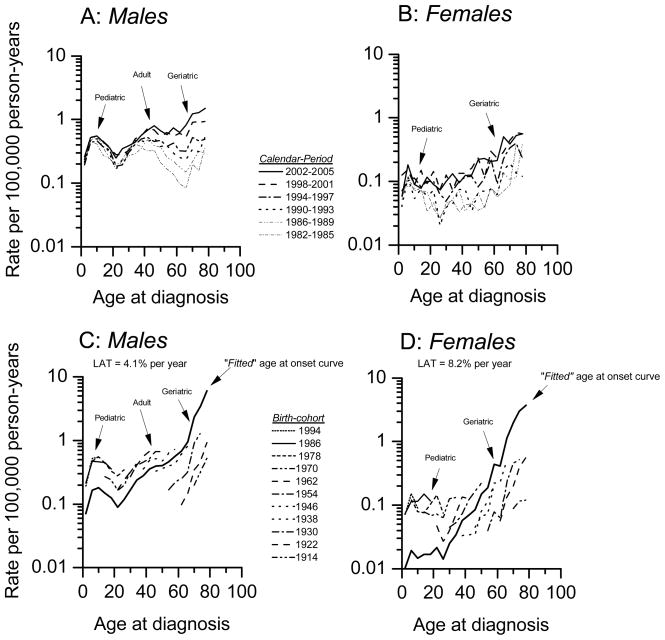
Figure 2.
Burkitt lymphoma APC model-based expected period- and cohort-specific age-specific incidence rates by sex, SEER 9, 13, and17 registries, 1982 through 2005. Panels C and D included “fitted” age-at-onset curves (see methods). Cases diagnosed during 1973–1981 were excluded because of sparse numbers.
AGE-PERIOD-COHORT (APC) ANALYSIS
APC models were used to assess secular and age-specific trends, simultaneously adjusted for age, calendar-period, and birth-cohort effects. 19, 20 Given the relationship [birth-cohort] = [calendar year of diagnosis] minus [age at diagnosis], we had a maximum of 28 4-year birth-cohorts (1894, 1898, …, 2002; referred to by midyear of birth) calculated from eight 4-year calendar-periods (1974–1977, 1978–1981, …, 2002–2005) and 21 4-year age-groups (ages 0–3, 4–7, …, 80–83 years).
Since the APC variables are collinear, the independent linear trends associated with age, calendar-period, and birth-cohort cannot be estimated 19, 20. Nonetheless, useful quantities can be estimated via the APC models 20; including the “drifts” (or linear trends), 21, 22 “deviations” (or nonlinear departures from linear trend) 19, and “slope-contrasts” (or differences between log-linear trends over adjacent blocks of periods, cohorts, or ages).23
Two useful APC quantities for this study were the “fitted” age at onset curve and the longitudinal age trend (or LAT).17, 24 The “fitted” age-at-onset curve provides an extrapolation of the cohort-specific age-specific rates for the mid birth-cohort, based upon the age-specific rates for all cohorts included in the analysis (Figure 2C and D). By construction, the fitted age-at-onset curve is adjusted for period and cohort deviations that might differ by sex. The LAT is a drift parameter that estimates the average annual percentage change of the “fitted” age at onset curve.
Finally, age, period, and cohort deviations from the APC models were plotted separately (Figure 3A–C). Wald tests were used to compare two or more APC parameters for the null hypothesis of no difference. All tests were two-sided and α < 0.05 was considered to be statistically significant. All statistical analyses were implemented using Matlab version 2009a (MathWorks, Inc., Natick, MA). More details about how the APC models were fitted and the p-values were calculated can be found in an online appendix.
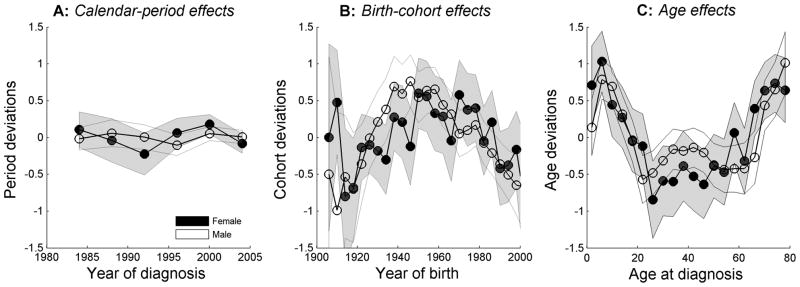
Figure 3.
Burkitt lymphoma age, calendar-period, and birth-cohort deviations for males (solid squares) and females (open circles), based on age-period-cohort (APC) models, SEER 9, 13, and 17 registries, 1982 through 2005.
ANALYSES TO ASSESS IMPACT OF THE HIV/AIDS EPIDEMIC
The potential impact of HIV/AIDS on cross-sectional age-specific BL rates, especially among males, who are disproportionately affected by the epidemic (73% of all cases),25 was assessed by repeating analyses restricted to: a) registries with low HIV/AIDS risk, 25 i.e., exclusive of registries in California and New Jersey (Supplemental Figure 1); and b) excluding cases among males age ≥20 years whose marital status was recorded in SEER as never-married, which identifies a group with a comparatively high prevalence of homosexual men, the largest risk group for HIV/AIDS 26 (Supplemental Figure 2). Data on marital status was lacking for the population-at-risk, so the person-years of never-married men could not be excluded from the denominator for this assessment. Data for BL before the AIDS epidemic were too sparse to be adequately evaluated.
BL-SPECIFIC MORTALITY AND HAZARD RATES
Cumulative mortality percentages among BL patients during the 15 years following diagnosis and associated 95% confidence intervals were calculated for males and females in three age-groups (Figure 4A–C) using the Kaplan-Meier (KM) method27. BL-related deaths were defined as deaths from all NHL or from diseases related to HIV infection (ICD-10 codes B20–24).28 Deaths from other conditions or subjects lost to follow up were treated as independent censoring events.
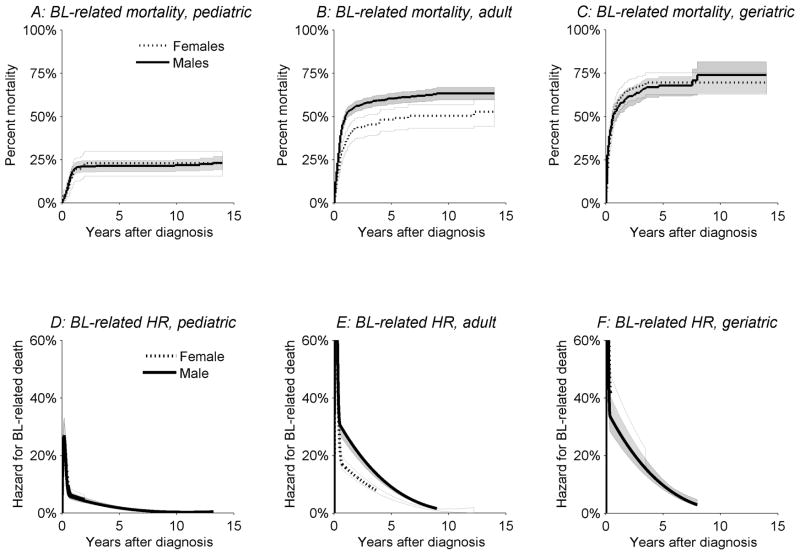
Figure 4.
Kaplan-Meier BL-related mortality (Panels A–C) and hazard rates for BL-related deaths for males (solid lines) and females (dotted lines) (Panels D–F). BL related deaths included deaths from non-Hodgkin lymphomas and deaths from other infections and parasitic diseases related to HIV, SEER 9, 13, and 17 registries, 1974 through 2005.
The annual hazard rates for BL-related death were also estimated for males and females by age-group29 (Figure 4D-F). The annual hazard rate was a conditional rate of BL death in a specified time interval following BL diagnosis given that the subject was alive at the beginning of that time interval. The relative rate of failure was plotted on the y-axis, and the time interval following BL diagnosis was plotted on the x-axis.
RESULTS
DEMOGRAPHIC CHARACTERISTICS OF BL
We analyzed the 3058 cases diagnosed during 1,160,300,297 person-years of observation, including 228 with Burkitt cell leukemia (7.5% of all BL cases). Most cases occurred among Whites (84% of cases) and males (75% of cases) (Table 1). The mean age at diagnosis was 40.3 years. Males were on average 10.7 years younger than females (p ≈ 0 for the null hypothesis of equality of means). BL was extremely rare before one year of age; only 2 cases (both males) were diagnosed before their first birthday. The pediatric, adult, and geriatric groups accounted for 30%, 50%, and 20% of the cases, respectively.
Table 1.
Burkitt lymphoma incidence in the United States, SEER 9, 13, and 17 registries, 1973 through 2005
| All cases | Males | Females | Males to females p≈0 for difference | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean age at diagnosis (SE), years | 40.3 (0.4) | 35.3 (0.6) | 46.0 (1.3) | |||||||||
| Median age at diagnosis, years | 40 | 37.4 | 48.9 | |||||||||
| age range, years | 0 to 101 | 0 to 97 years | 0 to 101 | |||||||||
| Variable | N | Rate | SE | N | Rate | SE | N | Rate | SE | IRRMF | 95% CI | |
| All cases | 3058 | 0.27 | 0.005 | 2300 | 0.42 | 0.009 | 758 | 0.13 | 0.005 | 3.2 | 3.1–3.4 | |
| Race | ||||||||||||
| White | 2,566 | 0.28 | 0.006 | 1,942 | 0.44 | 0.010 | 624 | 0.13 | 0.005 | 3.4 | 3.1, 3.7 | |
| Black | 244 | 0.21 | 0.014 | 186 | 0.34 | 0.026 | 58 | 0.10 | 0.013 | 3.5 | 2.5, 4.6 | |
| Other or unknown | 248 | ~ | ~ | 172 | ~ | ~ | 76 | ~ | ~ | ~ | ~ | |
| Age | ||||||||||||
| <20 years (Pediatric) | 788 | 0.23 | 0.002 | 642 | 0.36 | 0.014 | 146 | 0.09 | 0.007 | 4.2 | 3.4, 4.7 | |
| 20–59 years (Adult) | 1,547 | 0.25 | 0.006 | 1,241 | 0.40 | 0.012 | 306 | 0.10 | 0.006 | 4.1 | 3.5, 4.6 | |
| ≥60 years (Geriatric) | 723 | 0.42 | 0.016 | 417 | 0.58 | 0.029 | 306 | 0.30 | 0.018 | 2.0 | 1.7, 2.3 | |
| Time period | ||||||||||||
| 1973 | 3 | ~ | ~ | 0 | ~ | ~ | 3 | ~ | ~ | ~ | ~ | |
| 1974–1977 | 56 | 0.07 | 0.009 | 44 | 0.10 | 0.015 | 12 | 0.03 | 0.009 | 3.2 | 1.7, 6.4 | |
| 1978–1981 | 87 | 0.10 | 0.011 | 67 | 0.16 | 0.020 | 20 | 0.05 | 0.011 | 3.4 | 1.9, 5.3 | |
| 1982–1985 | 140 | 0.16 | 0.014 | 110 | 0.25 | 0.025 | 30 | 0.07 | 0.013 | 3.7 | 2.4, 5.4 | |
| 1986–1989 | 181 | 0.20 | 0.015 | 149 | 0.34 | 0.029 | 32 | 0.07 | 0.013 | 4.9 | 3.3, 7.3 | |
| 1990–1993 | 258 | 0.22 | 0.014 | 215 | 0.38 | 0.026 | 43 | 0.07 | 0.011 | 5.3 | 3.9, 7.6 | |
| 1994–1997 | 370 | 0.25 | 0.013 | 276 | 0.38 | 0.023 | 94 | 0.12 | 0.013 | 3.2 | 2.5, 4.0 | |
| 1998–2001 | 809 | 0.36 | 0.013 | 576 | 0.53 | 0.023 | 233 | 0.20 | 0.013 | 2.7 | 2.3, 3.1 | |
| 2002–2005 | 1154 | 0.38 | 0.011 | 863 | 0.59 | 0.020 | 291 | 0.18 | 0.011 | 3.3 | 2.6, 3.8 | |
| % Change in rates | ~ | 481.1% | ~ | ~ | 495.3% | ~ | ~ | 474.1% | ~ | ~ | ~ | |
| EAPC | 6.8% | 0.020 | 6.8% | 0.010 | 7.1% | 0.180 | ||||||
Rate per 100,000 person-years, (2000 US standard population); SE standard error; N, number of cases; IRRMF, Male to female incidence rate ratio; 95% CI, 95% confidence intervals; %Change, percentage change in rates from 1974–1977 to 2002–2005; EAPC, estimated annual percentage change.
AGE-STANDARDIZED INCIDENCE RATES AND CROSS-SECIONAL AGE-SPECIFIC INCIDENCE RATES
Overall, the age-standardized BL rate was 0.27 per 100,000 person-years (Table 1). The rate among males was 3.2 times that among females (0.42 versus 0.13 per 100,000), and among Whites 1.3 times that among Blacks (0.28 versus 0.21 per 100,000). The age-standardized BL rates were similar for pediatric and adult groups (0.23 and 0.25 per 100,000 person-years), but about twice as high in the geriatric group (0.42 per 100,000 person-years). The male-to-female age-standardized rate ratio (IRRMF) did not differ by race, but varied with age. The IRRMF was 4.2 for pediatric, 4.1 for adult BL and 2.0 for geriatric BL. The IRRMF fluctuated across calendar-year periods from 3.2 in 1974–1977 to 5.3 in 1990–1993 and then fell to 3.3 in 2002–2005, but these temporal variations were not statistically significant from each other. Age-standardized BL rates rose 481% during the study period (495% among males and 474% among females).
BL rates rose steeply during the study period (Figure 1A), with the EAPC equal to 6.8% per calendar-year (95% CI: 4.5–9.1) for males and 7.1% (95% CI: 3.2–11.1) for females. A notable finding was distinct trimodal age-specific BL incidence patterns among males, with three separate incidence peaks near ages 10, 40, and 75 years, respectively (Figure 1B). Among females, the pediatric and geriatric, but not adult, peaks were notable. BL incidence rates were significantly higher among males for pediatric and adult BL, but only marginally so for geriatric BL (Figure 1B).
Notably, the trimodal peaks were present among the males and, possibly, among females, from low HIV/AIDS-risk cancer registries (i.e., exclusive of California and New Jersey) (Supplemental figure 1), as well as among the males, when never-married male cases aged ≥20 years were excluded from the numerator (Supplemental figure 2).
AGE PERIOD COHORT MODELS
Distinct trimodal incidence patterns were apparent in both the period- and cohort-specific age-specific incidence rates among males (Figure 2A and C) with bimodal patterns among females (Figure 2B and D). Similar age-specific patterns were observed among Whites and Blacks (p = 0.88 for the null hypothesis of no difference), although there were small numbers and unstable patterns in Blacks, albeit similar, to the patterns in Whites (data not shown). Age-specific rates rose over time both for males (Figure 2A) and females (Figure 2B). For example, among males (Figure 2A), from 1982–1985 to 2002–2005, age-specific incidence rates rose 34% for pediatric BL (from 0.41 to 0.55 per 100,000), 113% for adult BL (from 0.32 to 0.68 per 100,000), and 713% for geriatric BL (from 0.16 to 1.3 per 100,000).
Similar to the cross-sectional age-specific incidence trends (Figure 1B), the APC fitted-age-at-onset curve demonstrated trimodal incidence peaks among males (Figure 2C), which were less pronounced among the females (Figure 2D). In contrast to the relatively flat (but trimodal/bimodal) cross-sectional rate increase with age (Figure 1B), the age-at-onset curves that were adjusted for period and cohort effects rose steeply with age for males (Figure 2C) and females (Figure 2D), with LAT per year of attained age equal to 4.1% (95% CI 3.6–6.0) for males and 8.2% (95% 6.1–10.3) for females.
Period deviations showed that BL rates were steady over time (Figure 3A, p = 0.37 for males and p = 0.30 for females), confirming continuously rising long-term temporal trends (Figure 1A). Statistically significant cohort deviations were observed after the 1950 birth-cohort for both males (Figure 3B, p = 0.001 and females (p = 0.007), consistent with declining cohort trends for the more recent birth cohorts. Age deviations (i.e., nonlinear departure from linear age trend) confirming trimodal BL rate slope changes (or valleys) near age 22 (Figure 3C, p = 5.74 × 10−6) years and 58 years (p = 8.6 × 10−4) for males. Among females, the age deviations showed a statistically significant slope contrast near age 26 years (p = 5.3 × 10−4) and a trend towards significance at age 58 years (p = 0.08).
BL-SPECIFIC MORTALITY AND ANNUAL HAZARD RATES
Most deaths among BL patients that were due to BL or HIV occurred within the first year of diagnosis (Figure 4A–C). Mortality among both male and female BL patients related to their disease was lowest for pediatric BL (Figure 4A, approximately 25% 5-years after diagnosis), intermediate for adult BL (Figure 4B, about 50% by 5 years), and highest for geriatric BL (Figure 4C, about 70% by 5 years). BL-related mortality did not differ by sex for pediatric or geriatric BL, whereas mortality was higher among males than females with adult BL (Figure 4B). Corresponding patterns of the annual hazard of death from BL were observed for pediatric, adult, and geriatric BL (Figure D–F).
DISCUSSION
The novel findings of our study were trimodal incidence patterns for BL among males and bimodal among females for both Whites and Blacks. The bimodal incidence peaks were robust in APC models24 and in analyses excluding registries with many cases of HIV/AIDS, and were supported by significant BL rate slope contrasts near age 22 years and 58 years. The middle-aged peak was observed among both White and Black males, but not among females, suggesting that the HIV/AIDS epidemic among males 25 may explain the excess in the middle aged men compared with females.
Bimodality is not uncommon in other cancers,17, 30 but BL is currently not considered to be bimodal and, to our knowledge, trimodality has never been reported for BL. Tri/bimodality suggests heterogeneity in a disease due two or more distinct cancer populations for a condition coded a one disease.30 BL is a unique B-cell lymphoproliferative malignancy that is currently considered to be a single entity with isomorphic clinical-epidemiological variants.6 Tri/bimodality suggests that the underlying risk of BL does not increase monotonically log-linearly with age. Therefore, linear summary measures for the age effect on risk, such as the LAT, may obscure fluctuation in age-specific risk of BL and the insights, about exposure or the underlying biology, such fluctuation may provide. Confirmation of bi/trimodality of BL in the established BL clinical-epidemiological variants, i.e., endemic, sporadic, and AIDS-related,5, 6 will justify conducting hypothesis-driven studies to investigate the biological basis for peak risks of BL at different ages in different populations.
Trimodality among adult males was due to a third peak during the adult years and may be mostly explained by the U.S. HIV/AIDS epidemic, given the well-established association of BL with HIV/AIDS31 and that males are disproportionately affected by the HIV/AIDS epidemic25. The lack of a peak among middle-aged females may be due to a lower prevalence of HIV/AIDS in this group25 and possibly to other factors that may be responsible for to gender disparity in the risk for BL in middle-aged adults as has been shown for childhood BL.32 In sensitivity analyses excluding registries with many AIDS cases, the age-incidence peak in male middle-aged adults was blunted, but not eliminated. The blunted adult peak may be due to residual HIV/AIDS-related BL cases or may imply that the adult peak is a mixture of AIDS- and non-AIDS-related BL. The attributable fraction (AF) of BL cases due to HIV/AIDS can be estimated mathematically using the formula AF=p(RR-1)/1+(RR-1), 33 where p=prevalence of HIV/AIDS in the general population and RR=relative risk for BL in persons with HIV/AIDS. We estimated that 71% (or 1099 of adult cases) of adult BL would be AIDS-related and 29% (448 of adult cases) non-AIDS related, assuming that the average HIV/AIDS prevalence was 0.05% 25 and the RR for BL given AIDS is 50 among U.S. adults with AIDS as compared to the general adult population 34. Our results suggest that there are adult and geriatric onset BL cases that appear different from childhood and AIDS-related BL that need to be studied further. The possibilities include an AIDS-related adult BL peak that may be due to a confluence, i.e., mixture, of distinct late-onset pediatric-type and early-onset geriatric-type BL rates, which are shifted right and left, respectively, or due to an infectious agent other than HIV.
Geriatric BL among both males and females accounted for one-fifth of BL and rates increased rapidly in contrast to the stable childhood rates. Because HIV-related BL occurs relatively early during HIV disease and is often AIDS-defining, 28 and trends in geriatric BL were increasing before the mid-1980s when the impact of the HIV epidemic was minimal, geriatric BL is unlikely to be due to HIV/AIDS. However, it is possible that a small fraction of BL associated with HIV/AIDS may have a long latency. Trends in geriatric BL most likely have been influenced by improvements in diagnosis or access to care 35 or by improvements in treatment of more indolent forms of lymphoma in older persons thus allowing for increased transformation of indolent lymphomas to BL.36 Our finding of substantial differences in BL mortality by age group is consistent with heterogeneity in BL suggested by tri/bimodal incidence peaks, but differences in the treatments routinely used, or tolerance to them, at different ages may also contribute.
The major strengths of our study were its large size, population-based sample, and the use of APC models to simultaneously adjust for potential confounding by calendar-period and birth-cohort factors. APC models allowed us to confirm, as demonstrated in analysis of cross-sectional age-specific rates (Figure 1B and Figures 2A–D), that the underlying risk of BL is non-linear, consisting of a tri/bimodal pattern comprising a well established pediatric peak, a novel geriatric peak, and a peak in middle-aged adults, which is explained, in part, by the HIV/AIDS. Even so, large numbers and modeling cannot overcome some limitations. Lack of centralized, standardized expert pathology review of cases was a limitation, 8 but any resulting diagnostic misclassification should be random. SEER cancer registry data does not include important individual clinical data including HIV serology, EBV serology, or EBV or molecular phenotype.
To summarize, we found novel tri/bimodal incidence patterns for BL, which showed disparities by gender but not race. Future research should investigate factors contributing to occurrence of BL at different ages, which may involve different behavior of B-cells in immune function at different ages, different infectious organisms associated with risk at different ages, or variation in the interaction of genetic and infectious factors at different ages, particularly in relation to MYC translocation and/or dysfunction.
Supplementary Material
Supplemental figure 1: Burkitt lymphoma age-specific incidence rates among males and females after excluding tumor registries in with many HIV/AIDS cases (i.e., California and New Jersey), SEER 9, 13, and 17 registries, 1974 through 2005.
Supplemental figure 2: Burkitt lymphoma age-specific incidence rates among males after excluding cases among never married men aged ≥20 years, SEER 9, 13, and 17 registries, 1974 through 2005 (n=1,569). Females were not included because marital status is not a strong marker of HIV/AIDS risk behavior
Acknowledgments
We are grateful to Drs. John Ziegler at the University of California and San Francisco (San Francisco, California), Elaine Jaffe at the National Cancer Institute (Bethesda, Maryland), and Ian Magrath at the International Network for Cancer Treatment and Research (Brussels, Belgium) for their comments on the manuscript. This work was supported by the Intramural Research Program of the Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health, Department of Health and Human Services. The content is the responsibility of the authors alone and does not necessarily reflect the views or policies of the United States Department of Health and Human Services.
References
- 1.Burkitt D. A sarcoma involving the jaws in African children. Br J Surg. 1958;46:218–23. doi: 10.1002/bjs.18004619704. [DOI] [PubMed] [Google Scholar]
- 2.Wright DH. Cytology and histochemistry of the Burkitt lymphoma. Br J Cancer. 1963;17:50–5. doi: 10.1038/bjc.1963.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ziegler JL. Burkitt’s lymphoma. N Engl J Med. 1981;305:735–45. doi: 10.1056/NEJM198109243051305. [DOI] [PubMed] [Google Scholar]
- 4.Carbone PP, Berard CW, Bennett JM, Ziegler JL, Cohen MH, Gerber P. NIH clinical staff conference. Burkitt’s tumor. Ann Intern Med. 1969;70:817–32. doi: 10.7326/0003-4819-70-4-817. [DOI] [PubMed] [Google Scholar]
- 5.Wright DH. What is Burkitt’s lymphoma and when is it endemic? Blood. 1999;93:758. [PubMed] [Google Scholar]
- 6.Jaffe ES, Diebold J, Harris NL, Muller-Hermelink HK, Flandrin G, Vardiman JW. Burkitt’s lymphoma: a single disease with multiple variants. The World Health Organization classification of neoplastic diseases of the hematopoietic and lymphoid tissues. Blood. 1999;93:1124. [PubMed] [Google Scholar]
- 7.Harris NL, Jaffe ES, Diebold J, Flandrin G, Muller-Hermelink HK, Vardiman J. Lymphoma classification--from controversy to consensus: the R.E.A.L. and WHO Classification of lymphoid neoplasms. Ann Oncol. 2000;11 (Suppl 1):3–10. [PubMed] [Google Scholar]
- 8.Hummel M, Bentink S, Berger H, Klapper W, Wessendorf S, Barth TF, Bernd HW, Cogliatti SB, Dierlamm J, Feller AC, Hansmann ML, Haralambieva E, et al. A biologic definition of Burkitt’s lymphoma from transcriptional and genomic profiling. N Engl J Med. 2006;354:2419–30. doi: 10.1056/NEJMoa055351. [DOI] [PubMed] [Google Scholar]
- 9.Manolov G, Manolova Y. Marker band in one chromosome 14 from Burkitt lymphomas. Nature. 1972;237:33–4. doi: 10.1038/237033a0. [DOI] [PubMed] [Google Scholar]
- 10.Leucci E, Cocco M, Onnis A, De Falco G, van Cleef P, Bellan C, van Rijk A, Nyagol J, Byakika B, Lazzi S, Tosi P, van Krieken H, et al. MYC translocation-negative classical Burkitt lymphoma cases: an alternative pathogenetic mechanism involving miRNA deregulation. J Pathol. 2008;216:440–50. doi: 10.1002/path.2410. [DOI] [PubMed] [Google Scholar]
- 11.Thorley-Lawson DA, Allday MJ. The curious case of the tumour virus: 50 years of Burkitt’s lymphoma. Nat Rev Microbiol. 2008;6:913–24. doi: 10.1038/nrmicro2015. [DOI] [PubMed] [Google Scholar]
- 12.Iversen U, Iversen OH, Bluming AZ, Ziegler JL, Kyalwasi S. Cell kinetics of African cases of Burkitt lymphoma. A preliminary report. Eur J Cancer. 1972;8:305–8. doi: 10.1016/0014-2964(72)90025-4. [DOI] [PubMed] [Google Scholar]
- 13.SEER-9. Surveillance, Epidemiology, and End Results (SEER) Program. SEER*Stat Database: Incidence-SEER 9 Regs Limited-Use, Nov 2007 Sub (1973–2005) <Single Ages to 85+, Katrinia/Rita Population Adjustment> -Linked To County Attributes - Total U.S., 1969–2005 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch, released April 2008, based on November 2007 submission. 2008 ( www.seer.cancer.gov)
- 14.SEER-13. Surveillance, Epidemiology, and End Results (SEER) Program. SEER*Stat Database: Incidence-SEER 13 Regs Limited-Use, Nov 2007 Sub (1973–2005) <Single Ages to 85+, Katrinia/Rita Population Adjustment> -Linked To County Attributes - Total U.S., 1969–2005 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch, released April 2008, based on November 2007 submission. 2008 ( www.seer.cancer.gov)
- 15.SEER-17. Surveillance, Epidemiology, and End Results (SEER) Program. SEER*Stat Database: Incidence-SEER 17 Regs Limited-Use, Nov 2007 Sub (1973–2005) <Single Ages to 85+, Katrinia/Rita Population Adjustment> -Linked To County Attributes - Total U.S., 1969–2005 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch, released April 2008, based on November 2007 submission. 2008 ( www.seer.cancer.gov)
- 16.Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Vardiman JW. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4. Geneva, (Switzerland): World Health Organization; 2008. [Google Scholar]
- 17.Grimley PM, Matsumo R, Rosenberg PS, Henson DE, Schwartz AM, Anderson WF. Qualitative age interactions between low and high grade serous ovarian carcinoma. Cancer Epidemiol Biomarkers Prev. 2009 doi: 10.1158/1055-9965.EPI-09-0240. in press. [DOI] [PubMed] [Google Scholar]
- 18.Devesa SS, Donaldson J, Fears T. Graphical presentation of trends in rates. Am J Epidemiol. 1995;141:300–4. doi: 10.1093/aje/141.4.300. [DOI] [PubMed] [Google Scholar]
- 19.Holford TR. Understanding the effects of age, period, and cohort on incidence and mortality rates. Annu Rev Public Health. 1991;12:425–57. doi: 10.1146/annurev.pu.12.050191.002233. [DOI] [PubMed] [Google Scholar]
- 20.Holford TR. The estimation of age, period and cohort effects for vital rates. Biometrics. 1983;39:311–24. [PubMed] [Google Scholar]
- 21.Clayton D, Schifflers E. Models for temporal variation in cancer rates. II: Age-period-cohort models. Stat Med. 1987;6:469–81. doi: 10.1002/sim.4780060406. [DOI] [PubMed] [Google Scholar]
- 22.Clayton D, Schifflers E. Models for temporal variation in cancer rates. I: Age-period and age-cohort models. Stat Med. 1987;6:449–67. doi: 10.1002/sim.4780060405. [DOI] [PubMed] [Google Scholar]
- 23.Tarone RE, Chu KC. Evaluation of birth cohort patterns in population disease rates. Am J Epidemiol. 1996;143:85–91. doi: 10.1093/oxfordjournals.aje.a008661. [DOI] [PubMed] [Google Scholar]
- 24.Anderson WF, Rosenberg PS, Menashe I, Mitani A, Pfeiffer RM. Age-related crossover in breast cancer incidence rates between black and white ethnic groups. J Natl Cancer Inst. 2008;100:1804–14. doi: 10.1093/jnci/djn411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Epidemiology of HIV/AIDS--United States, 1981–2005. MMWR Morb Mortal Wkly Rep. 2006;55:589–92. [PubMed] [Google Scholar]
- 26.Biggar RJ, Horm J, Goedert JJ, Melbye M. Cancer in a group at risk of acquired immunodeficiency syndrome (AIDS) through 1984. Am J Epidemiol. 1987;126:578–86. doi: 10.1093/oxfordjournals.aje.a114697. [DOI] [PubMed] [Google Scholar]
- 27.Kaplan El, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–81. [Google Scholar]
- 28.Human immunodeficiency virus (HIV) infection codes. Official authorized addendum. ICD-9-CM (Revision No. 1). Effective January 1, 1988. MMWR Morb Mortal Wkly Rep. 1987;36 (Suppl 7):1S–20S. [PubMed] [Google Scholar]
- 29.Rosenberg PS. Hazard function estimation using B-splines. Biometrics. 1995;51:874–87. [PubMed] [Google Scholar]
- 30.Macmahon B. Epidemiological evidence of the nature of Hodgkin’s disease. Cancer. 1957;10:1045–54. doi: 10.1002/1097-0142(195709/10)10:5<1045::aid-cncr2820100527>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 31.Ziegler JL, Drew WL, Miner RC, Mintz L, Rosenbaum E, Gershow J, Lennette ET, Greenspan J, Shillitoe E, Beckstead J, Casavant C, Yamamoto K. Outbreak of Burkitt’s-like lymphoma in homosexual men. Lancet. 1982;2:631–3. doi: 10.1016/s0140-6736(82)92740-4. [DOI] [PubMed] [Google Scholar]
- 32.Mbulaiteye SM, Biggar RJ, Bhatia K, Linet MS, Devesa SS. Sporadic childhood Burkitt lymphoma incidence in the United States during 1992–2005. Pediatr Blood Cancer. 2009;53:366–70. doi: 10.1002/pbc.22047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cole P, MacMahon B. Attributable risk percent in case-control studies. Br J Prev Soc Med. 1971;25:242–4. doi: 10.1136/jech.25.4.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Engels EA, Pfeiffer RM, Goedert JJ, Virgo P, McNeel TS, Scoppa SM, Biggar RJ. Trends in cancer risk among people with AIDS in the United States 1980–2002. AIDS. 2006;20:1645–54. doi: 10.1097/01.aids.0000238411.75324.59. [DOI] [PubMed] [Google Scholar]
- 35.Perkins AS, Friedberg JW. Burkitt lymphoma in adults. Hematology Am Soc Hematol Educ Program. 2008;2008:341–8. doi: 10.1182/asheducation-2008.1.341. [DOI] [PubMed] [Google Scholar]
- 36.Salaverria I, Zettl A, Bea S, Hartmann EM, Dave SS, Wright GW, Boerma EJ, Kluin PM, Ott G, Chan WC, Weisenburger DD, Lopez-Guillermo A, et al. Chromosomal alterations detected by comparative genomic hybridization in subgroups of gene expression-defined Burkitt’s lymphoma. Haematologica. 2008;93:1327–34. doi: 10.3324/haematol.13071. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental figure 1: Burkitt lymphoma age-specific incidence rates among males and females after excluding tumor registries in with many HIV/AIDS cases (i.e., California and New Jersey), SEER 9, 13, and 17 registries, 1974 through 2005.
Supplemental figure 2: Burkitt lymphoma age-specific incidence rates among males after excluding cases among never married men aged ≥20 years, SEER 9, 13, and 17 registries, 1974 through 2005 (n=1,569). Females were not included because marital status is not a strong marker of HIV/AIDS risk behavior