Abstract
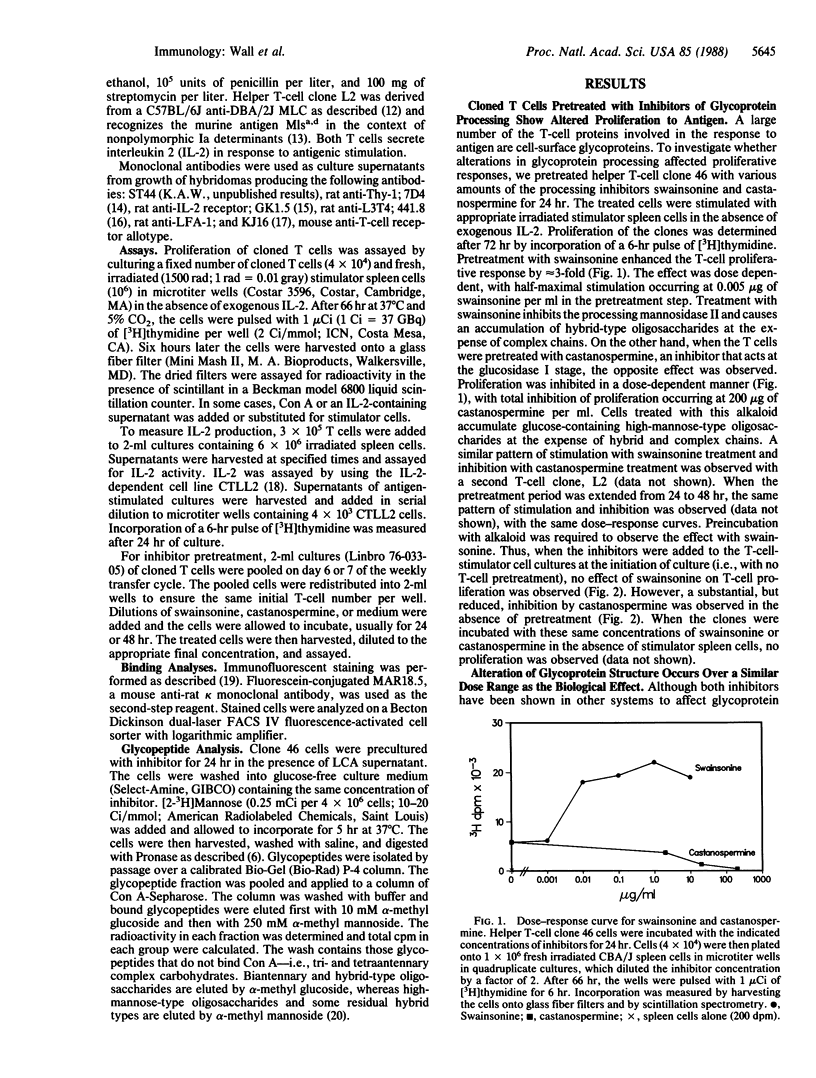
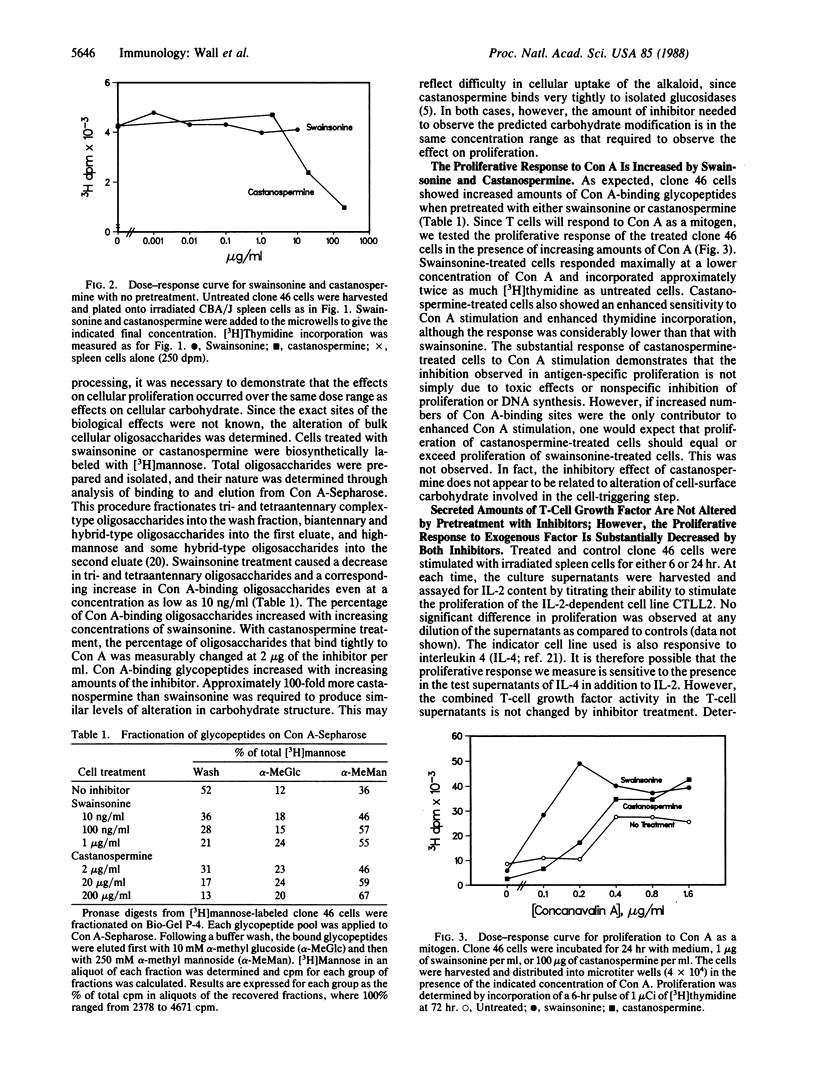
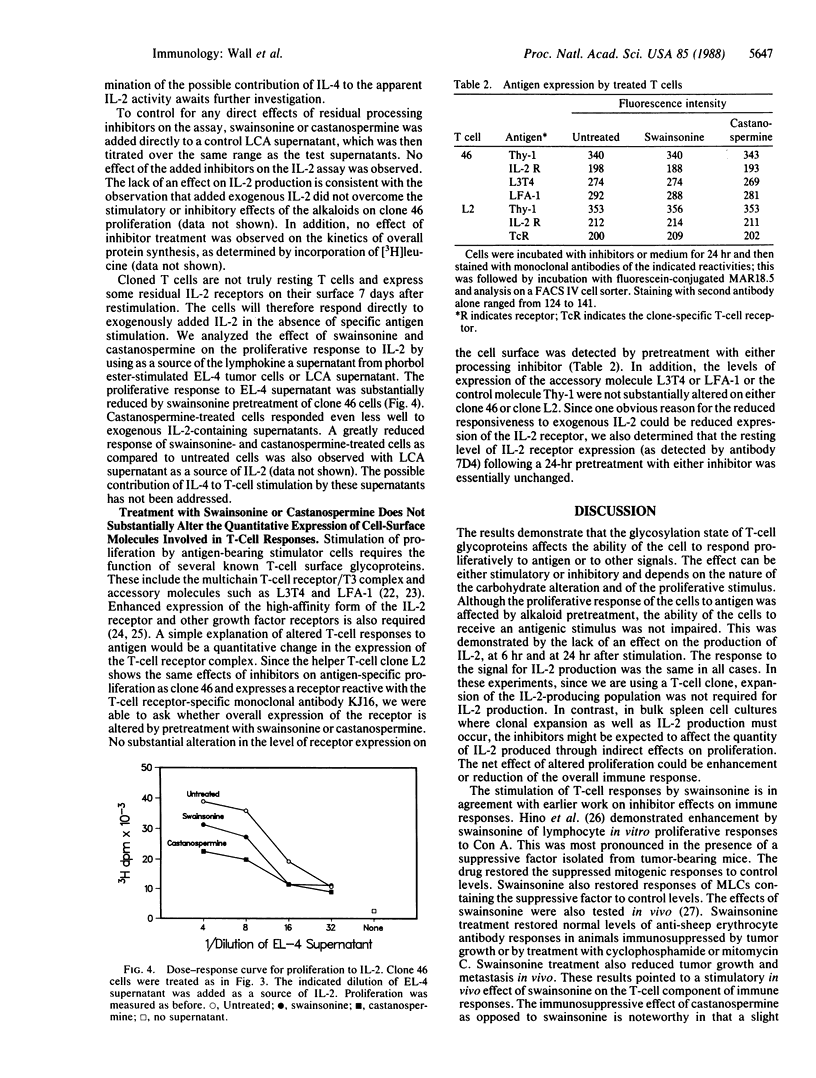
Most of the cell-surface molecules involved in T-cell immune responses are N-linked glycoproteins. We have investigated the effects of inhibitors of glycoprotein processing on specific T-cell functions, with the dual aims of examining the functional role of carbohydrate and of testing the usefulness of such compounds as immunomodulators. Treatment of a cloned murine helper T-cell line with these inhibitors differentially affects the proliferative response of the cell, depending upon the nature of the stimulus. Treatment with the plant alkaloid swainsonine, which inhibits the processing mannosidase II and causes the accumulation of glycoproteins bearing hybrid-type oligosaccharide structures, enhances the proliferative response of the T-cell clone to antigen and to the mitogen concanavalin A. Treatment with another plant alkaloid, castanospermine, which inhibits glucosidase I and causes the accumulation of glucose-containing high-mannose structures, has the opposite effect and inhibits the proliferative response of the T cell to antigen. Cell-surface oligosaccharide alteration does not affect antigen recognition, as judged by the lack of effect of either drug on interleukin 2 production following antigen stimulation. Cells treated with either alkaloid proliferate poorly to exogenous interleukin 2 and may have defective interleukin 2 receptor function. Swainsonine-treated cells apparently have compensatory alterations that can overcome the reduced responsiveness to interleukin 2. Antibody-binding studies indicate that normal quantities of many cell-surface molecules, including the T-cell receptor for antigen, are expressed by the treated cells.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bekoff M., Kubo R., Grey H. M. Activation requirements for normal T cells: accessory cell-dependent and -independent stimulation by anti-receptor antibodies. J Immunol. 1986 Sep 1;137(5):1411–1419. [PubMed] [Google Scholar]
- Cantrell D. A., Smith K. A. Transient expression of interleukin 2 receptors. Consequences for T cell growth. J Exp Med. 1983 Dec 1;158(6):1895–1911. doi: 10.1084/jem.158.6.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings R. D., Kornfeld S. Fractionation of asparagine-linked oligosaccharides by serial lectin-Agarose affinity chromatography. A rapid, sensitive, and specific technique. J Biol Chem. 1982 Oct 10;257(19):11235–11240. [PubMed] [Google Scholar]
- Dialynas D. P., Quan Z. S., Wall K. A., Pierres A., Quintáns J., Loken M. R., Pierres M., Fitch F. W. Characterization of the murine T cell surface molecule, designated L3T4, identified by monoclonal antibody GK1.5: similarity of L3T4 to the human Leu-3/T4 molecule. J Immunol. 1983 Nov;131(5):2445–2451. [PubMed] [Google Scholar]
- Elbein A. D., Dorling P. R., Vosbeck K., Horisberger M. Swainsonine prevents the processing of the oligosaccharide chains of influenza virus hemagglutinin. J Biol Chem. 1982 Feb 25;257(4):1573–1576. [PubMed] [Google Scholar]
- Elbein A. D. Inhibitors of the biosynthesis and processing of N-linked oligosaccharides. CRC Crit Rev Biochem. 1984;16(1):21–49. doi: 10.3109/10409238409102805. [DOI] [PubMed] [Google Scholar]
- Elbein A. D., Solf R., Dorling P. R., Vosbeck K. Swainsonine: an inhibitor of glycoprotein processing. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7393–7397. doi: 10.1073/pnas.78.12.7393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis S., Ferm M. M., Ou W., Smith K. A. T cell growth factor: parameters of production and a quantitative microassay for activity. J Immunol. 1978 Jun;120(6):2027–2032. [PubMed] [Google Scholar]
- Glasebrook A. L., Fitch F. W. Alloreactive cloned T cell lines. I. Interactions between cloned amplifier and cytolytic T cell lines. J Exp Med. 1980 Apr 1;151(4):876–895. doi: 10.1084/jem.151.4.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hino M., Nakayama O., Tsurumi Y., Adachi K., Shibata T., Terano H., Kohsaka M., Aoki H., Imanaka H. Studies of an immunomodulator, swainsonine. I. Enhancement of immune response by swainsonine in vitro. J Antibiot (Tokyo) 1985 Jul;38(7):926–935. doi: 10.7164/antibiotics.38.926. [DOI] [PubMed] [Google Scholar]
- Kang M. S., Elbein A. D. Alterations in the structure of the oligosaccharide of vesicular stomatitis virus G protein by swainsonine. J Virol. 1983 Apr;46(1):60–69. doi: 10.1128/jvi.46.1.60-69.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kino T., Inamura N., Nakahara K., Kiyoto S., Goto T., Terano H., Kohsaka M., Aoki H., Imanaka H. Studies of an immunomodulator, swainsonine. II. Effect of swainsonine on mouse immunodeficient system and experimental murine tumor. J Antibiot (Tokyo) 1985 Jul;38(7):936–940. doi: 10.7164/antibiotics.38.936. [DOI] [PubMed] [Google Scholar]
- Kornbluth J. Human natural killer cells and cytotoxic T lymphocytes require cell surface carbohydrate determinants for lytic function. Cell Immunol. 1985 Oct 15;95(2):276–287. doi: 10.1016/0008-8749(85)90315-6. [DOI] [PubMed] [Google Scholar]
- Lorber M. I., Loken M. R., Stall A. M., Fitch F. W. I-A antigens on cloned alloreactive murine T lymphocytes are acquired passively. J Immunol. 1982 Jun;128(6):2798–2803. [PubMed] [Google Scholar]
- Malek T. R., Robb R. J., Shevach E. M. Identification and initial characterization of a rat monoclonal antibody reactive with the murine interleukin 2 receptor-ligand complex. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5694–5698. doi: 10.1073/pnas.80.18.5694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neckers L. M., Yenokida G., James S. P. The role of the transferrin receptor in human B lymphocyte activation. J Immunol. 1984 Nov;133(5):2437–2441. [PubMed] [Google Scholar]
- Oettgen H. C., Terhorst C. A review of the structure and function of the T-cell receptor-T3 complex. Crit Rev Immunol. 1987;7(2):131–167. [PubMed] [Google Scholar]
- Pan Y. T., Hori H., Saul R., Sanford B. A., Molyneux R. J., Elbein A. D. Castanospermine inhibits the processing of the oligosaccharide portion of the influenza viral hemagglutinin. Biochemistry. 1983 Aug 2;22(16):3975–3984. doi: 10.1021/bi00285a038. [DOI] [PubMed] [Google Scholar]
- Sarmiento M., Dialynas D. P., Lancki D. W., Wall K. A., Lorber M. I., Loken M. R., Fitch F. W. Cloned T lymphocytes and monoclonal antibodies as probes for cell surface molecules active in T cell-mediated cytolysis. Immunol Rev. 1982;68:135–169. doi: 10.1111/j.1600-065x.1982.tb01063.x. [DOI] [PubMed] [Google Scholar]
- Saul R., Chambers J. P., Molyneux R. J., Elbein A. D. Castanospermine, a tetrahydroxylated alkaloid that inhibits beta-glucosidase and beta-glucocerebrosidase. Arch Biochem Biophys. 1983 Mar;221(2):593–597. doi: 10.1016/0003-9861(83)90181-9. [DOI] [PubMed] [Google Scholar]
- Tkacz J. S., Lampen O. Tunicamycin inhibition of polyisoprenyl N-acetylglucosaminyl pyrophosphate formation in calf-liver microsomes. Biochem Biophys Res Commun. 1975 Jul 8;65(1):248–257. doi: 10.1016/s0006-291x(75)80086-6. [DOI] [PubMed] [Google Scholar]
- Wagh P. V., Bahl O. P. Sugar residues on proteins. CRC Crit Rev Biochem. 1981;10(4):307–377. doi: 10.3109/10409238109113602. [DOI] [PubMed] [Google Scholar]
- Walker B. D., Kowalski M., Goh W. C., Kozarsky K., Krieger M., Rosen C., Rohrschneider L., Haseltine W. A., Sodroski J. Inhibition of human immunodeficiency virus syncytium formation and virus replication by castanospermine. Proc Natl Acad Sci U S A. 1987 Nov;84(22):8120–8124. doi: 10.1073/pnas.84.22.8120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall K. A., Lorber M. I., Loken M. R., McClatchey S., Fitch F. W. Inhibition of proliferation of MIs- and Ia-reactive cloned T cells by a monoclonal antibody against a determinant shared by I-A and I-E. J Immunol. 1983 Sep;131(3):1056–1064. [PubMed] [Google Scholar]
- Weiss A., Imboden J., Hardy K., Manger B., Terhorst C., Stobo J. The role of the T3/antigen receptor complex in T-cell activation. Annu Rev Immunol. 1986;4:593–619. doi: 10.1146/annurev.iy.04.040186.003113. [DOI] [PubMed] [Google Scholar]


