Abstract
Background & Aims
Data are conflicting on the benefit of selective serotonin reuptake inhibitors (SSRIs) for patients with irritable bowel syndrome (IBS); the role of visceral sensitivity in IBS pathophysiology is unclear. We assessed the effects of citalopram and the relationships between, symptoms, and quality of life (QOL), and rectal sensitivity in non-depressed patients with IBS.
Methods
Patients from primary, secondary and tertiary care centers were randomly assigned to groups given citalopram (20 mg/day for 4 weeks, then 40 mg/day for 4 weeks) or placebo. The study was double masked with concealed allocation. Symptoms were assessed weekly; IBS-QOL and rectal sensation were determined from barostat measurements made at the beginning and end of the study.
Results
Patients that received citalopram did not have a higher rate of adequate relief from IBS symptoms than subjects that received placebo (12/27, 44% vs 15/27, 56% respectively; P=0.59), regardless of IBS subtype. The odds ratio for weekly response to citalopram vs placebo was 0.80 (95% confidence interval [CI] 0.61–1.04). Citalopram did not reduce specific symptoms or increase IBS-QOL scores; it had no effect on rectal compliance and a minimal effect on sensation. Changes in IBS-QOL score and pressure-eliciting pain were correlated (r=0.33, 95% CI 0.03–0.57); changes in symptoms and rectal sensitivity or IBS-QOL scores were not correlated.
Conclusions
Citalopram was not superior to placebo in treating non-depressed IBS patients. Changes in symptoms were not correlated with changes in rectal sensation assessed by barostat; Any benefit of citalopram in non-depressed IBS patients is likely to be modest.
INTRODUCTION
Irritable bowel syndrome (IBS) is a classic functional gastrointestinal disorder characterized by abdominal pain and altered defecation that is responsible for significant morbidity, decrement in quality of life, and burden of disease.1–5 No therapy for IBS has an excellent response rate.1 The pathophysiology of IBS is believed to involve alterations in gastrointestinal motility and sensation, and brain-gut interactions.2
Antidepressants are often used to treat functional gastrointestinal disorders and other chronic pain syndromes.1, 6–9 Tricyclic antidepressants have been studied more thoroughly than the selective serotonin reuptake inhibitors (SSRIs) for the treatment of IBS.8, 10 Data on the effect of SSRIs in IBS are mixed.11–15 Depression, anxiety and other psychiatric diagnoses are prevalent in persons with functional gastrointestinal disorders.16 The effect of antidepressants in functional gastrointestinal disorders does not appear to be explained by treatment of depression.
Visceral hypersensitivity can be demonstrated in laboratory studies in a significant fraction of patients with IBS and other functional gastrointestinal disorders.17–20 While abnormalities in visceral sensation have been proposed as contributors to symptoms, the relevance of sensitivity during experimental distension remains controversial.18–20 The scant published data on the correlation between symptoms and visceral sensitivity suggest weak if any correlation,17, 21–24 and there have been no detailed examinations of the longitudinal relationship between changes in symptoms and sensitivity to barostat-mediated distension.20
We designed this study to examine the effect of the SSRI citalopram on symptoms and quality of life in non-depressed patients with IBS. We also explored the longitudinal relationships between symptoms, quality of life, and sensitivity to barostat-mediated distension.
MATERIALS AND METHODS
General Study Design
This prospective, randomized, placebo-controlled trial with double-masking and concealed allocation was approved by the Committee of Human Research of the University of California, San Francisco (UCSF), and the Institutional Review Board of Kaiser Permanent Northern California (KPNC). The trial design was guided by published recommendations25 and the CONSORT statement.26 Enrollment was open from 2001 to 2008.
Hypotheses and Study Outcomes
We hypothesized that citalopram treatment improves IBS symptoms in non-depressed patients with IBS more than placebo, and that changes in symptoms quality of life, and rectal sensitivity assessed by barostat are correlated significantly.
The primary measure of response was achieving self-reported weekly “adequate relief” of IBS symptoms.27–29 Overall response was defined as achieving “adequate relief” on at least 3 of the last 6 weeks. The primary measure of quality of life was the change in IBS-QOL score from baseline to study end.30 Rectal sensitivity was measured as symptom level as a function of distending pressure. “Sensation” was scored on a 0–10 scale, where 0=no inflation sensation, 1–5=increasing painless sensation, and 6–10=increasing pain, with 6=threshold pain and 10=worst imaginable pain. “Urgency” was scored on a 0–5 scale, where 0=no urgency, and 1–5=increasing urgency, with 1=threshold urgency and 5=worst imaginable urgency.
Secondary outcomes included changes in overall IBS symptom score, pain/discomfort score, number and consistency of daily bowel movements, urgency score, number of days per week with adequate relief, and satisfaction with these parameters.
Study Participants
Potentially eligible subjects were adult men or women of age 18–75 years who fulfilled the Rome II IBS criteria,25 were in good general health without conditions to explain abdominal pain and altered defecation, were not depressed, and could provide written informed consent. Exclusion criteria included a diagnosis of depression, taking antidepressant medication, or scoring 17 (borderline clinical depression) or higher on the Beck Depression Inventory; pregnancy; use of IBS medications including alosetron, tegaserod, antispasmodics or anticholinergics, or chronic pain medications including opiates within 4 weeks of entry; prior colon or rectal surgery; and major organ disease including diabetes. Fiber or loperamide use as needed was allowed.
Eligible subjects had to have a normal sigmoidoscopy or colonoscopy within 5 years of enrollment, normal complete blood count and thyroid function studies, and subjects with diarrhea had to have negative stool studies for ova and parasites and normal colon biopsies. Women of child-bearing age required a negative urine pregnancy test. Subjects were counseled to avoid pregnancy during the study and to use contraception.
Subjects were drawn from primary, secondary and tertiary care settings, including General Medicine and Gastroenterology clinics at UCSF and KPNC, and community practices. At UCSF, recruitment efforts included fliers, letters to providers, on-site recruitment, and invitation letters approved by patients' physicians sent to patients with electronic entry diagnoses of “IBS” or “abdominal pain” with either “diarrhea” or “constipation.” For KPNC, recruitment was based on invitation letters sent to patients with at least two electronic diagnostic entries for “irritable bowel syndrome,” at least 1 visit within the last year, a colonoscopy or sigmoidoscopy within 5 years, and no entries for a psychiatric diagnosis or antidepressant prescription within 1 year. Community recruitment included letters to providers and posting of study information on the General Clinical Research Center website and ClinicalTrials.gov (NCT00477165).
Potentially eligible subjects were screened over the phone. Up to three attempts were made to phone patients who were sent letters and did not return a refusal-to-participate postcard.
Study Protocol
After screening, during the initial study visit, written informed consent was obtained. Subjects completed the 21-item Beck's Depression Inventory. All enrolled subjects scored ≤16 (1–10=normal, 11–16 mild mood disturbance). Subjects completed a questionnaire with the Rome II criteria and Supportive Symptoms of IBS,25 and were stratified into Diarrhea-predominant, Constipation-predominant or Alternating IBS.25 Urine pregnancy tests were obtained on women of child-bearing age. Screening daily symptom diaries were dispensed to score the secondary symptom outcomes on 0–10 scales.
A second study visit occurred 1 week later (week 0). To continue participation, subjects had to score an average of pain/discomfort ≥3 during the screening week. All subjects who enrolled met this criterion. Subjects completed the IBS-QOL and the first barostat study. Questionnaires to be filled out on a weekly basis were dispensed to score whether weekly “adequate relief” was achieved, and the secondary symptom outcomes on 0–10 scales.
Study medication or placebo was dispensed. The Investigational Drug Pharmacy prepared identical citalopram 20 mg and placebo capsules and generated 3 block-randomization lists, stratified by IBS-subtype. For the first 4 weeks, 30 tablets were dispensed with instructions to take 1 capsule/day.
At a third, interim visit 4 weeks later (week 4), questionnaires and remaining capsules were collected, questionnaires were dispensed and 60 tablets were dispensed with instructions to take 2 capsules/day.
At a final visit 4 weeks later (week 8), questionnaires and capsules were collected. Subjects completed a second IBS-QOL and barostat study.
During the study, subjects were asked to call a research coordinator weekly to report completion of questionnaires. If the phone call was not received, a research coordinator contacted the subject. Subjects were asked to call if they experienced side effects, which could lead to dose reduction to 1 capsule per day.
Medication adherence was determined by counting remaining capsules and is reported as % of capsules taken as directed.
Barostat Study
Polyethylene barostat bags of 500 mL were affixed to 18 French nasogastric tubes. A bag was passed into the rectum, with tubing connected to the barostat (Distender Series II, G&J Electronics Inc., Willowdale, Ontario, Canada), which was controlled by a computer that recorded bag pressure, volume and corrected volume every second. After 5 minutes, the bag was unfurled with 100 mL of air and deflated. A distension sequence using the ascending method of limits was performed, with inflations lasting 45 seconds from 0 to up to 60 mm Hg, increasing by 3 mm Hg and separated by 45-second deflations. Subjects rated sensation and urgency 30 seconds into each inflation. The study was stopped when subjects reported sensation ≥8/10.
Sample Size and Statistical Analysis
The standardized mean improvement in pain with antidepressants in functional gastrointestinal disorders has been reported as 0.9 SD units (95% CI: 0.6–1.2 SD units), with a summary odds ratio for improvement of 4.2 (95% CI: 2.3–7.9).7 We estimated that to detect a standardized effect size of 0.9 in global symptoms with 2-sided α=0.05 and β=0.1, 54 subjects were needed; and that to detect an overall response to citalopram of 66% vs. 30% with placebo (odds ratio for response of 4.5) with 2-sided α=0.05 and β=0.2, 60 subjects were needed.
P-values for comparing continuous variables were obtained by the Mann-Whitney test, and for dichotomous outcomes by Fisher's exact test. Mixed effect models accounting for repeated measures within subjects were used to compare curves of symptom scores vs. week and vs. barostat pressure. The effect of treatment on adequate relief at weeks 3–8 was modeled by logistic regression, with a random subject effect included to account for non-independence of responses from the same patient at different weeks. Volume vs. distending pressure was defined as rectal compliance. The last observation was carried forward if distensions stopped at a given pressure for a given subject. Pearson's correlation coefficient was used to examine pre-specified correlations between symptoms, IBS-QOL scores and barostat parameters. The primary analyses were based on the intention to treat, with patients who withdrew being considered non-responders. Exploratory per-protocol analyses were performed.
RESULTS
Study Subjects and Adherence to Treatment
A total of 234 potentially eligible subjects were identified. Of these, 180 were excluded and 54 (44 women) enrolled in the study, with 27 each randomized to citalopram and placebo (See CONSORT flow chart). Demographic characteristics did not differ substantially between groups, and stratification at randomization yielded a balance of IBS subtypes between groups (Table 1). During the screening week, the median number of days with any pain and with pain score ≥3 were 7.0 (interquartile range 7.0–7.0) and 5.0 (interquartile range 3.3–7.0), respectively. Nine subjects (6 women) withdrew from the study due to side effects, 7 in the citalopram group and 2 in the placebo group (P=0.14) (Table 1).
Table 1.
Demographic characteristics of study subjects.
| PLACEBO | CITALOPRAM | Total | P-Value | ||
|---|---|---|---|---|---|
| N=27 | N=27 | ||||
|
Age in years Mean (SD) [range] |
51 (14) [30-75] |
53 (15) [27-75] |
-- | 0.60 | |
| Gender | Women | 23 (85%) | 21 (78%) | 44 (81%) | 0.73 |
| Men | 4 (15%) | 6 (22%) | 10 (19%) | ||
| Race | Caucasian | 23 (85%) | 22 (82%) | 45 (83%) | 0.083 |
|
African American |
4 (15%) | 1 (4%) | 5 (9%) | ||
| Asian | 0 (0%) | 4 (15%) | 4 (7%) | ||
| Ethnicity |
Hispanic or Latino |
3 (11%) | 6 (22%) | 9 (17%) | 0.47 |
|
Not Hispanic or Latino |
24 (89%) | 21 (78%) | 45 (83%) | ||
| IBS Subgroup | Constipation | 11 (41%) | 10 (37%) | 21 (39%) | 1.00 |
| Diarrhea | 11 (41%) | 12 (44%) | 23 (43%) | ||
| Alternator | 5 (19%) | 5 (19%) | 10 (19%) | ||
|
Beck Depression Inventory Score Mean (SD) |
5.9 (5.4) | 6.6 (5.1) | -- | 0.47 | |
|
Discontinued Treatment |
No | 25 (93%) | 20 (74%) | 45 (83%) | 0.14 |
| Yes | 2 (7%) | 7 (26%) | 9 (17%) |
During weeks 1 through 4, mean adherence to treatment was 97% (range 86–100%) for citalopram and 97% (range 79–100%) for placebo. During weeks 5 through 8, 1 patient in the citalopram group and 2 patients in the placebo group required dose reduction to 1 capsule per day. During weeks 5 through 8, mean adherence to treatment was 96% (range 86–100%) for citalopram and 91% (range 73–100%) for placebo.
Symptoms
The overall response rate was 12/27 (44%) in the citalopram group and 15/27 (56%) in the placebo group (P=0.59). The response rate was not superior for citalopram compared with placebo for any of the IBS subgroups (constipation-predominant 5/10 vs. 6/11; diarrhea predominant 6/12 vs. 6/11; alternators 1/5 vs. 3/5).
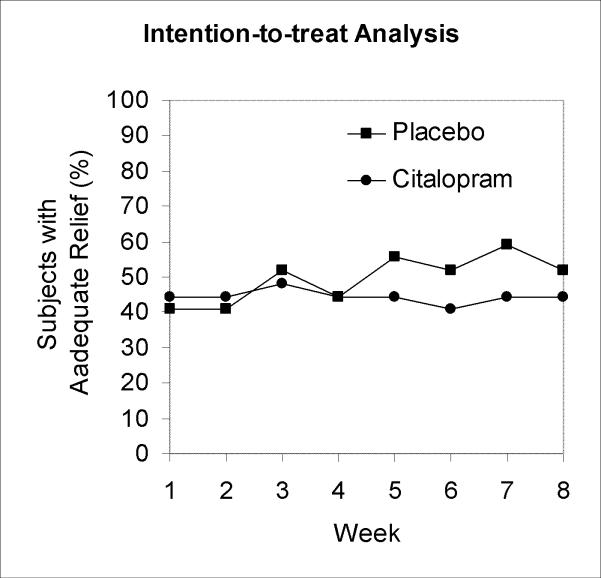
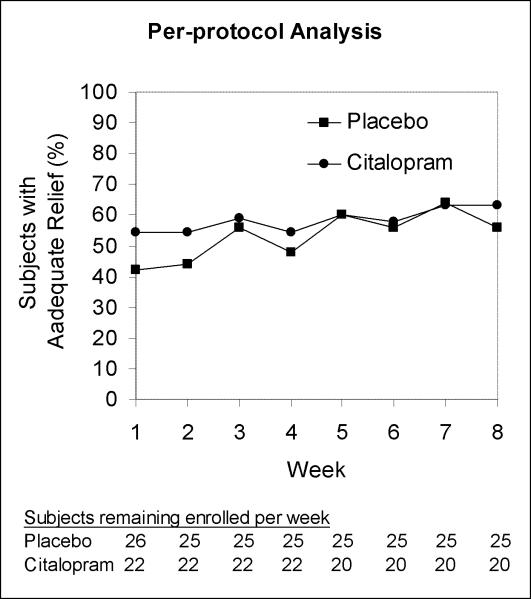
There were no statistically significant differences between groups in the rates of adequate relief during any week by intention-to-treat analysis (Figure 1A). In a repeated measures logistic regression model of adequate relief as a function of study week, assuming the citalopram effect builds linearly over time starting at week 3, the odds ratio for weekly response with citalopram vs. placebo was 0.80 (95% CI, 0.614 to 1.035). The upper bound on the possible benefit of citalopram by study end was an odds ratio for response of (1.035)6, or 1.23. In per-protocol analysis with a logistic regression model of the same form, the odds ratio for weekly response with citalopram vs. placebo was 0.91 (95% CI, 0.687 to 1.196) (Figure 1B).
Figure 1.
Weekly rates of adequate relief by intention-to-treat analysis (A) and per-protocol analysis (B).
Weekly symptom scores, satisfaction scores, and number of days with adequate relief did not differ substantially between groups during any week, as illustrated for weeks 4 and 8 in Table 2.
Table 2.
Symptom and satisfaction scores at weeks 4 and 8 of treatment.
| PLACEBO | CITALOPRAM | P-Value | PLACEBO | CITALOPRAM | P-Value | |
|---|---|---|---|---|---|---|
| N=25 | N=22 | N=25 | N=20 | |||
| Week 4 Mean (SD) | Week 8 Mean (SD) | |||||
| Overall IBS symptoms | 4.4 (2.4) | 4.0 (2.2) | 0.48 | 4.4 (3.0) | 3.3 (2.5) | 0.24 |
| Satisfaction with IBS symptoms | 4.6 (3.0) | 5.4 (2.7) | 0.42 | 5.4 (3.4) | 5.9 (3.4) | 0.71 |
| Days with adequate relief per week | 3.4 (2.1) | 3.6 (2.1) | 0.76 | 4.0 (2.3) | 4.0 (2.3) | 0.88 |
| Abdominal pain | 4.4 (2.5) | 4.2 (2.6) | 0.80 | 4.3 (3.0) | 3.7 (2.6) | 0.39 |
| Urgency | 3.3 (2.6) | 3.7 (2.6) | 0.60 | 3.2 (2.7) | 3.6 (2.5) | 0.56 |
| Number of bowel movements per week | 2.2 (3.2) | 2.2 (1.1) | 0.09 | 1.9 (1.6) | 2.3 (1.4) | 0.29 |
| Stool consistency* | 6.0 (1.9) | 6.5 (2.0) | 0.38 | 6.1 (1.8) | 6.2 (2.0) | 0.79 |
0=solid and hard, 10=completely watery
Quality of Life
The IBS-QOL overall score and subscores improved slightly over 8 weeks for both groups. However, there were no statistically significant differences in scores between groups at either week 0 or 8, or in the magnitude of the changes from week 0 to 8 (Table 3). For instance, the changes in overall IBS-QOL score for citalopram (6.3; 95% CI, −0.07 to 12.7) and placebo (7.6; 95% CI, 2.4 to 12.9) were not significantly different (P=0.47)
Table 3.
Quality of life scores at weeks 0 and 8 of treatment.
| PLACEBO | CITALOPRAM | P-Value | PLACEBO | CITALOPRAM | P-Value | |
|---|---|---|---|---|---|---|
| N=27 | N=27 | N=25 | N=20 | |||
| Week 0 Mean (SD) | Week 8 Mean (SD) | |||||
| Overall | 67 (23) | 71 (6) | 0.85 | 74 (24) | 74 (18) | 0.85 |
| Body image | 70 (21) | 71 (20) | 0.82 | 79 (22) | 75 (18) | 0.26 |
| Dysphoria | 65 (27) | 69 (21) | 0.73 | 72 (29) | 73 (24) | 0.64 |
| Food avoidance | 56 (29) | 61 (23) | 0.62 | 66 (27) | 60 (30) | 0.38 |
| Health worry | 58 (29) | 68 (21) | 0.24 | 68 (27) | 74 (21) | 0.58 |
| Interference with activity | 67 (25) | 67 (20) | 0.83 | 76 (27) | 68 (22) | 0.16 |
| Relationships | 72 (32) | 77 (17) | 0.78 | 78 (26) | 83 (18) | 0.89 |
| Social reaction | 73 (26) | 79 (17) | 0.77 | 79 (26) | 83 (21) | 0.73 |
| Sexual | 74 (32) | 77 (32) | 0.71 | 77 (31) | 83 (28) | 0.62 |
Rectal Sensitivity to Barostat-Mediated Distension
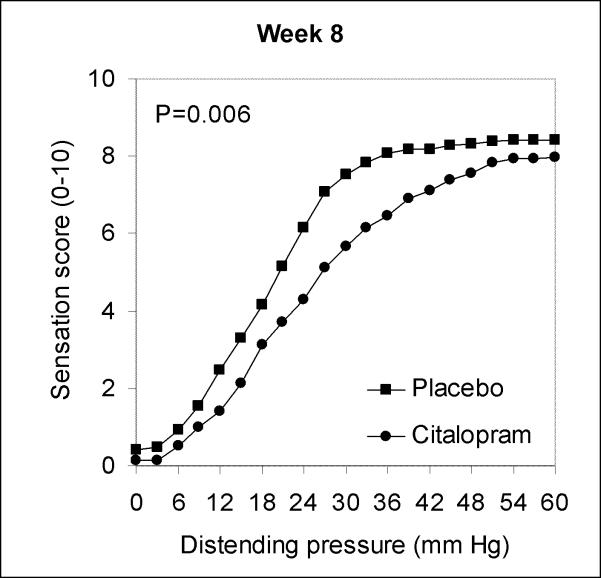
There were no substantial differences in rectal compliance between groups at week 0 (P=0.16) or week 8 (P=0.24). At week 8 but not week 0, sensation but not urgency scores were slightly lower for the citalopram group than the placebo group (Figure 2).
Figure 2.
Mean rectal sensation as a function of distending pressure at end of study.
Correlations between Symptoms, Quality of Life, and Barostat Results
The change in weekly overall IBS symptom score was not statistically significantly correlated with the change in the overall IBS-QOL score (r=−0.23, 95% CI −0.50 to 0.07) There was a modest correlation between improvement in overall IBS-QOL score and increase in pressure eliciting threshold pain (r=0.33, 95% CI, 0.03 to 0.57).
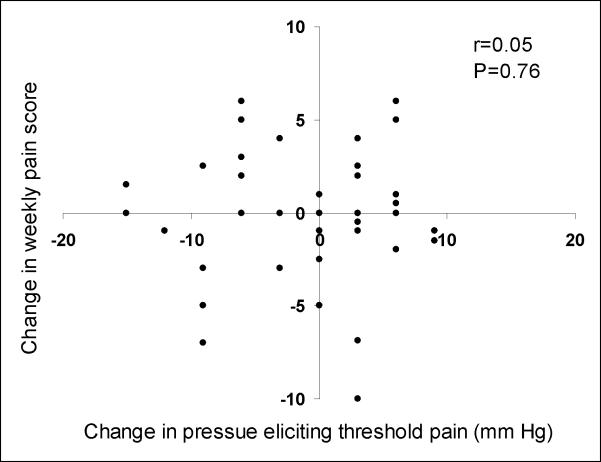
No statistically significant correlation was observed between the absolute change in weekly pain score on a 0–10 scale and the absolute change in pressures eliciting threshold pain measured in mm Hg (r=0.05, 95% CI, −0.26 to 0.34) (Figure 3), or between absolute change in the weekly urgency score and absolute change in pressures eliciting threshold urgency (r=0.01, 95% CI, −0.30 to 0.32). There were no statistically significant correlations between the absolute change in overall IBS symptom score and the absolute changes in pressures eliciting threshold pain (r=0.12, 95% CI, −0.18 to 0.41) or urgency (r=−0.05, 95% CI, −0.34 to 0.26).
Figure 3.
Correlation between absolute changes in abdominal pain scores on a 0–10 scale and absolute changes in rectal distension pressure threshold for pain measured in mm Hg.
DISCUSSION
In this study, citalopram was not superior to placebo in achieving adequate relief of IBS symptoms, improving individual symptom scores, or improving quality of life in non-depressed patients with IBS. Citalopram treatment had minimal impact on rectal sensitivity. In a comprehensive exploration of the longitudinal relationship between symptoms and sensitivity to barostat-mediated rectal distension, there were no statistically significant correlations between the changes in overall IBS symptoms and quality of life, or IBS symptoms and rectal sensation assessed by barostat.
Five previous studies have examined the effect of SSRIs on IBS symptoms, with conflicting results. Three studies of paroxetine,12 fluoxetine,13 and citalopram14 reported a modest benefit, while two studies of fluoxetine11 and citalopram15 did not. A recent meta-analysis of these studies found a relative risk of persistent or unimproved IBS symptoms or abdominal pain of 0.62 (95% CI, 0.45 to 0.87) for SSRIs vs. placebo, with significant heterogeneity between studies.8 The meta-analysis used data from 113 SSRI-treated subjects and 117 controls. Ours is the second largest study to address the effect of an SSRI in IBS, and we found evidence against a substantial benefit of citalopram, contrary to the findings of the meta-analysis.
Differences in study designs and patient populations may account in part for the different results among the six trials. Kuiken et al. enrolled 40 non-depressed IBS patients of all subtypes from a tertiary center in a 6-week study of fluoxetine or placebo.11 Tabas et al. randomized 81 patients with IBS of all subtypes and without exclusion for depression to a high fiber diet with paroxetine or placebo for 12 weeks.12 Vahedi et al. randomized 44 patients with constipation-predominant IBS to fluoxetine or placebo for 12 weeks, and excluded patients with severe depression.13 Tack et al. recruited 23 non-depressed IBS patients from a tertiary care center for a crossover trial comparing citalopram vs. placebo.14 Talley et al. randomized 51 patients with IBS of all types without psychiatric illness to citalopram (17 subjects), imipramine, or placebo (16 subjects) for 12 weeks.15 Features of the trials in which some benefit was detected include the lack of exclusion for depression,12 inclusion of only constipation-predominant patients,13 and a crossover design with patients from tertiary care.14 The specific drug, dose and duration used in each trial could be important factors, but no clear pattern emerges. The study by Tack et al. is the only one that showed benefit for citalopram, while the study by Talley et al. reported identical response rates for citalopram and placebo, and our study found evidence against any substantial benefit of citalopram.
The trials used different definitions for clinical response, and this may explain much of the conflict in the conclusions. We used adequate relief of IBS symptoms as the primary outcome. The same outcome was assessed by Talley et al. In contrast, the other trials assessed global symptom response, overall well-being, or the effect on specific symptoms. However, in our trial we also assessed overall symptom response, change in specific symptoms, and quality of life, and we saw little benefit of citalopram.
Based on all available data, we conclude that there is only weak evidence that citalopram may be superior to placebo in achieving a stringent criterion of response in non-depressed patients with IBS. We recognize that our trial has the limitation of relatively small sample size, as do all previous studies in this area, but in our study citalopram was not even numerically superior to placebo and the upper confidence bound provides evidence against any substantial benefit. Thus, the lack of significant benefit of citalopram cannot be explained exclusively by a type II error. A much larger trial with hundreds of patients per group would be needed to detect a relatively small difference in overall response,31 as other investigators have also pointed out.11 Such a trial is challenging to perform. The formal rate of exclusion from our trial due to depression or antidepressant use underestimates greatly the number of patients who were actually excluded from our trial for these reasons, because patients “screened informally” in clinics were never formally screened for inclusion. While we cannot quantitate this effect, this presented a substantial recruitment challenge. Similar challenges have been recognized by other investigators.15 A potential limitation is that we do not know whether patients could have been unblinded based on side effects.
The relationship between clinical symptoms and sensitivity to barostat-mediated rectal distension has not been studied thoroughly.20 Rectal sensitivity has been proposed as a potential biological marker for IBS,17 and effects on visceral sensitivity have been considered as ways to screen for promising pharmaceuticals.20 We found no statistically significant correlations between changes in IBS symptoms and changes in barostat parameters. While we acknowledge controversies pertaining to the optimal barostat protocol and the relevance of barostat data to clinical symptoms, our results suggest that changes in rectal sensitivity to barostat-mediated distension may not lead to improvements in clinical symptoms.
We urge caution against over-interpreting the modest correlation between the changes in IBS-QOL score and the pressure eliciting threshold pain. This correlation is plausible if decreased rectal sensitivity were associated with decreased clinical symptoms, and in turn improved quality of life. But we found evidence against substantial positive values for these other correlations.
In conclusion, in this study citalopram was not superior to placebo in treating non-depressed patients with IBS, and changes in clinical symptoms were not substantially correlated with changes in rectal sensation. Considering all available evidence, any benefit of SSRIs compared to placebo in non-depressed patients with IBS is likely to be modest at best.
Supplementary Material
Acknowledgments
Grant Support: NIH Grant M01-RR00079, including a Clinical Associate Physician Award to Dr. Ladabaum, NIH/NCRR UCSF-CTSI Grant Number UL1 RR024131, and AGA/Solvay Award for Clinical Research in Irritable Bowel Syndrome/Motility
Abbreviations
- IBS
irritable bowel syndrome
- SSRI
selective serotonin reuptake inhibitor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented in part at Digestive Disease Week, San Diego, California, May 2008
Finanacial Disclosures: Dr. Ladabaum: Speaker Bureaus of Novartis, Sucampo and Takeda.
Conflict of Interest: No conflicts of interest exist for any authors.
1) The statistical analysis of the entire data sets pertaining to efficacy (specifically primary and major secondary efficacy endpoints) and safety (specifically, serious adverse events as defined in federal guidelines) have been independently confirmed by a biostatistician who is not employed by the corporate entity; and 2) The corresponding author had full access to all of the data and takes full responsibility for the veracity of the data and analysis.
REFERENCES
- 1.Brandt LJ, Chey WD, Foxx-Orenstein AE, Schiller LR, Schoenfeld PS, Spiegel BM, Talley NJ, Quigley EM, Moayyedi P. An evidence-based systematic review on the management of irritable bowel syndrome. Am J Gastroenterol. 2009;104:s1–s35. doi: 10.1038/ajg.2008.122. [DOI] [PubMed] [Google Scholar]
- 2.Drossman DA, Camilleri M, Mayer EA, Whitehead WE. AGA technical review on irritable bowel syndrome. Gastroenterology. 2002;123:2108–2131. doi: 10.1053/gast.2002.37095. [DOI] [PubMed] [Google Scholar]
- 3.Sandler RS, Everhart JE, Donowitz M, Adams E, Cronin K, Goodman C, Gemmen E, Shah S, Avdic A, Rubin R. The burden of selected digestive diseases in the United States. Gastroenterology. 2002;122:1500–11. doi: 10.1053/gast.2002.32978. [DOI] [PubMed] [Google Scholar]
- 4.Gralnek IM, Hays RD, Kilbourne A, Naliboff B, Mayer EA. The impact of irritable bowel syndrome on health-related quality of life. Gastroenterology. 2000;119:654–60. doi: 10.1053/gast.2000.16484. [DOI] [PubMed] [Google Scholar]
- 5.Hahn BA, Yan S, Strassels S. Impact of irritable bowel syndrome on quality of life and resource use in the United States and United Kingdom. Digestion. 1999;60:77–81. doi: 10.1159/000007593. [DOI] [PubMed] [Google Scholar]
- 6.Clouse RE, Lustman PJ, Geisman RA, Alpers DH. Antidepressant therapy in 138 patients with irritable bowel syndrome: a five-year clinical experience. Alimentary Pharmacology and Therapeutics. 1994;8:409–16. doi: 10.1111/j.1365-2036.1994.tb00308.x. [DOI] [PubMed] [Google Scholar]
- 7.Jackson JL, O'Malley PG, Tomkins G, Balden E, Santoro J, Kroenke K. Treatment of functional gastrointestinal disorders with antidepressant medications: a meta-analysis. American Journal of Medicine. 2000;108:65–72. doi: 10.1016/s0002-9343(99)00299-5. [DOI] [PubMed] [Google Scholar]
- 8.Ford AC, Talley NJ, Schoenfeld PS, Quigley EM, Moayyedi P. Efficacy of antidepressants and psychological therapies in irritable bowel syndrome: systematic review and meta-analysis. Gut. 2009;58:367–78. doi: 10.1136/gut.2008.163162. [DOI] [PubMed] [Google Scholar]
- 9.Verdu B, Decosterd I, Buclin T, Stiefel F, Berney A. Antidepressants for the treatment of chronic pain. Drugs. 2008;68:2611–32. doi: 10.2165/0003495-200868180-00007. [DOI] [PubMed] [Google Scholar]
- 10.Drossman DA, Toner BB, Whitehead WE, Diamant NE, Dalton CB, Duncan S, Emmott S, Proffitt V, Akman D, Frusciante K, Le T, Meyer K, Bradshaw B, Mikula K, Morris CB, Blackman CJ, Hu Y, Jia H, Li JZ, Koch GG, Bangdiwala SI. Cognitive-behavioral therapy versus education and desipramine versus placebo for moderate to severe functional bowel disorders. Gastroenterology. 2003;125:19–31. doi: 10.1016/s0016-5085(03)00669-3. [DOI] [PubMed] [Google Scholar]
- 11.Kuiken SD, Tytgat GN, Boeckxstaens GE. The selective serotonin reuptake inhibitor fluoxetine does not change rectal sensitivity and symptoms in patients with irritable bowel syndrome: a double blind, randomized, placebo-controlled study. Clin Gastroenterol Hepatol. 2003;1:219–28. doi: 10.1053/cgh.2003.50032. [DOI] [PubMed] [Google Scholar]
- 12.Tabas G, Beaves M, Wang J, Friday P, Mardini H, Arnold G. Paroxetine to treat irritable bowel syndrome not responding to high-fiber diet: a double-blind, placebo-controlled trial. Am J Gastroenterol. 2004;99:914–20. doi: 10.1111/j.1572-0241.2004.04127.x. [DOI] [PubMed] [Google Scholar]
- 13.Vahedi H, Merat S, Rashidioon A, Ghoddoosi A, Malekzadeh R. The effect of fluoxetine in patients with pain and constipation-predominant irritable bowel syndrome: a double-blind randomized-controlled study. Aliment Pharmacol Ther. 2005;22:381–5. doi: 10.1111/j.1365-2036.2005.02566.x. [DOI] [PubMed] [Google Scholar]
- 14.Tack J, Broekaert D, Fischler B, Van Oudenhove L, Gevers AM, Janssens J. A controlled crossover study of the selective serotonin reuptake inhibitor citalopram in irritable bowel syndrome. Gut. 2006;55:1095–103. doi: 10.1136/gut.2005.077503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Talley NJ, Kellow JE, Boyce P, Tennant C, Huskic S, Jones M. Antidepressant therapy (imipramine and citalopram) for irritable bowel syndrome: a double-blind, randomized, placebo-controlled trial. Dig Dis Sci. 2008;53:108–15. doi: 10.1007/s10620-007-9830-4. [DOI] [PubMed] [Google Scholar]
- 16.Whitehead WE, Palsson O, Jones KR. Systematic review of the comorbidity of irritable bowel syndrome with other disorders: what are the causes and implications? Gastroenterology. 2002;122:1140–56. doi: 10.1053/gast.2002.32392. [DOI] [PubMed] [Google Scholar]
- 17.Mertz H, Naliboff B, Munakata J, Niazi N, Mayer EA. Altered rectal perception is a biological marker of patients with irritable bowel syndrome. Gastroenterology. 1995;109:40–52. doi: 10.1016/0016-5085(95)90267-8. [DOI] [PubMed] [Google Scholar]
- 18.Azpiroz F, Bouin M, Camilleri M, Mayer EA, Poitras P, Serra J, Spiller RC. Mechanisms of hypersensitivity in IBS and functional disorders. Neurogastroenterol Motil. 2007;19:62–88. doi: 10.1111/j.1365-2982.2006.00875.x. [DOI] [PubMed] [Google Scholar]
- 19.Camilleri M. Testing the sensitivity hypothesis in practice: tools and methods, assumptions and pitfalls. Gut. 2002;51(Suppl 1):i34–40. doi: 10.1136/gut.51.suppl_1.i34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mayer EA, Bradesi S, Chang L, Spiegel BM, Bueller JA, Naliboff BD. Functional GI disorders: from animal models to drug development. Gut. 2008;57:384–404. doi: 10.1136/gut.2006.101675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Whitehead WE, Diamant N, Meyer K, Mikulka K, Hu JB, Jia H, Bangdiwala S, Toner B, Drossman D. Pain thresholds measured by the barostat predict the severity of clinical pain in patients with irritable bowel syndrome (Abstract) Gastroenterology. 1998;114:A858. [Google Scholar]
- 22.Kuiken SD, Lindeboom R, Tytgat GN, Boeckxstaens GE. Relationship between symptoms and hypersensitivity to rectal distension in patients with irritable bowel syndrome. Aliment Pharmacol Ther. 2005;22:157–64. doi: 10.1111/j.1365-2036.2005.02524.x. [DOI] [PubMed] [Google Scholar]
- 23.Izquierdo S, Rey E, Garcia Alonso M, Almansa C, Diaz-Rubio M. Has the identification of rectal hypersensitivity any implication in the clinical outcome of irritable bowel syndrome? Rev Esp Enferm Dig. 2005;97:223–8. doi: 10.4321/s1130-01082005000400002. [DOI] [PubMed] [Google Scholar]
- 24.Zar S, Benson MJ, Kumar D. Rectal afferent hypersensitivity and compliance in irritable bowel syndrome: differences between diarrhoea-predominant and constipation-predominant subgroups. Eur J Gastroenterol Hepatol. 2006;18:151–8. doi: 10.1097/00042737-200602000-00007. [DOI] [PubMed] [Google Scholar]
- 25.Drossman DA, Corazziari E, Talley NJ, Thompson WG. Diagnosis, pathophysiology and treatment: a multinational concensus. Degnon Associates; 2000. Rome II. The functional gastrointestinal disorders. [Google Scholar]
- 26.Moher D, Schulz KF, Altman DG. The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomized trials. Ann Intern Med. 2001;134:657–62. doi: 10.7326/0003-4819-134-8-200104170-00011. [DOI] [PubMed] [Google Scholar]
- 27.Mangel AW, Hahn BA, Heath AT, Northcutt AR, Kong S, Dukes GE, McSorley D. Adequate relief as an endpoint in clinical trials in irritable bowel syndrome. Journal of International Medical Research. 1998;26:76–81. doi: 10.1177/030006059802600203. [DOI] [PubMed] [Google Scholar]
- 28.Camilleri M, Chey WY, Mayer EA, Northcutt AR, Heath A, Dukes GE, McSorley D, Mangel AM. A randomized controlled clinical trial of the serotonin type 3 receptor antagonist alosetron in women with diarrhea-predominant irritable bowel syndrome. Arch Intern Med. 2001;161:1733–40. doi: 10.1001/archinte.161.14.1733. [DOI] [PubMed] [Google Scholar]
- 29.Camilleri M, Northcutt AR, Kong S, Dukes GE, McSorley D, Mangel AW. Efficacy and safety of alosetron in women with irritable bowel syndrome: a randomised, placebo-controlled trial. Lancet. 2000;355:1035–40. doi: 10.1016/S0140-6736(00)02033-X. [DOI] [PubMed] [Google Scholar]
- 30.Drossman DA, Patrick DL, Whitehead WE, Toner BB, Diamant NE, Hu Y, Jia H, Bangdiwala SI. Further validation of the IBS-QOL: a disease-specific quality-of-life questionnaire. American Journal of Gastroenterology. 2000;95:999–1007. doi: 10.1111/j.1572-0241.2000.01941.x. [DOI] [PubMed] [Google Scholar]
- 31.Andresen V, Montori VM, Keller J, West CP, Layer P, Camilleri M. Effects of 5-hydroxytryptamine (serotonin) type 3 antagonists on symptom relief and constipation in nonconstipated irritable bowel syndrome: a systematic review and meta-analysis of randomized controlled trials. Clin Gastroenterol Hepatol. 2008;6:545–55. doi: 10.1016/j.cgh.2007.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.