Abstract
Teriparatide (parathyroid hormone, [PTH]) is the only FDA-approved drug that replaces bone lost to osteoporosis. Enhancing PTH efficacy will improve cost-effectiveness and ameliorate contraindications. Combining this hormone with load-bearing exercise may enhance therapeutic potential consistent with a growing body of evidence that these agonists are synergistic and share common signaling pathways. Additionally, neutralizing molecules that naturally suppress the anabolic response to PTH may also improve the efficacy of treatment with this hormone. Nmp4/CIZ (nuclear matrix protein 4/cas interacting zinc finger)-null mice have enhanced responses to intermittent PTH with respect to increasing trabecular bone mass and are also immune to disuse-induced bone loss likely by the removal of Nmp4/CIZ suppressive action on osteoblast function. Nmp4/CIZ activity may be sensitive to changes in the mechanical environment of the bone cell brought about by hormone- or mechanical load-induced changes in cell shape and adhesion. Nmp4 was identified in a screen for PTH-responsive nuclear matrix architectural transcription factors (ATFs) that we proposed translate hormone-induced changes in cell shape and adhesion into changes in target gene DNA conformation. CIZ was independently identified as a nucleocytoplasmic shuttling transcription factor associating with the mechano-sensitive focal adhesion proteins p130Cas and zxyin. The p130Cas/zyxin/Nmp4/CIZ pathway resembles the β-catenin/TCF/LEF1 mechanotransduction response limb and both share features with the HMGB1 (high mobility group box 1)/RAGE (receptor for advanced glycation end products) signaling axis. Here we describe Nmp4/CIZ within the context of the PTH-induced anabolic response and consider the place of this molecule in the hierarchy of the PTH-load response network.
Keywords: architectural transcription factor, mechanosome, nuclear matrix, osteoblast, osteoporosis, teriparatide
I. Teriparatide And What Turns It Off
The FDA-approved armamentarium for osteoporosis treatment comprises drugs that slow bone loss (antiresorptives/anticatabolics) with the exception of teriparatide (parathyroid hormone [PTH]), the only therapeutic that replaces lost bone. Once-daily injections of PTH stimulate new bone formation in the senescent skeleton. The anticatabolic bisphosphonates may compromise some aspects of bone’s mechanical and material properties through the accumulation of microdamage or from alterations to collagen structure and the accumulation of advanced glycation end-products (AGEs) (Allen & Burr, 2007). Nevertheless cost and limitations on the length of treatment restrict teriparatide use to osteoporosis patients with high fracture risk or who have inadequate responses to bisphosphonates (Blick et al., 2008). Thus boosting the efficacy of teriparatide might eliminate these impediments.
A key mechanism underlying intermittent PTH escalation of osteoblast function and formation of mineralized bone is by enhancing the survival and differentiation of the osteoblast sufficiently to increase their number and lifespan beyond that required to refill the resorption pit excavated by the osteoclast (reviewed in Jilka, 2007). Therefore boosting the activities of signaling pathways regulating survival or differentiation might improve treatment efficacy.
The combination of intermittent PTH and load-bearing exercise may boost hormone efficacy since these agonists have a synergistic effect on bone gain in rodents (Sugiyama et al., 2008; and reviewed in Fuchs & Warden, 2008). Molecules common to the convergent hormone and load anabolic pathways likely play key regulatory roles in augmenting osteoid synthesis (Zhang et al., 2006).
Disabling pathways that inhibit the action of anabolic PTH should also enhance hormone efficacy. For example, PTH enhances osteoblast expression of Runx2, which supports the anti-apoptotic phenotype while concomitantly inducing Smurf1-mediated Runx2 proteasomal degradation thus providing a self-limiting response to the anabolic action of PTH (Bellido et al., 2003). Nevertheless, the consequence of neutralizing this pathway in vivo has not been reported.
PTH activation of the osteoblast cAMP/protein kinase A pathway drives bone gain (Jilka, 2007 and references therein) yet knocking out β-arrestin2, an inhibitor of cAMP signaling had unexpected consequences on the mouse skeleton (Bouxsein et al., 2005; Ferrari et al., 2005). Male and female mice lacking β-arrestin2 showed a 5–10% decrease in total body bone mass and cortical and trabecular bone parameters as compared to wild-type (WT) mice (Bouxsein et al., 2005; Ferrari et al., 2005). Male β-arrestin2-null mice exhibited little PTH-induced gain in bone due to enhanced osteoclast activity (Ferrari et al., 2005). In contrast, PTH similarly enhanced total body BMD and trabecular architecture in the estrogen-replete female β-arrestin2-null and WT mice (Bouxsein et al., 2005). However, high concentrations of hormone stimulated periosteal bone formation 2-fold higher in the null mice. Additionally the β-arrestin2-null mice exhibited an increase in endocortical bone resorption leading to an increase in midfemoral cross-sectional and medullary area not observed in the WT mice (Bouxsein et al., 2005).
Sclerostin (SOST) is a potent inhibitor of Wnt-mediated bone formation via its interference with LRP5/LRP6 receptor signaling and PTH regulates SOST expression (reviewed in Jilka, 2007). However PTH-induced bone gain was blunted in mice over-expressing SOST as well as in mice deficient in SOST expression. This blunted PTH response was due to attenuated bone formation rates in both models (Kramer et al., 2009). Therefore the role of SOST in the PTH anabolic response is unclear.
II. Nmp4/CIZ Turns Down Teriparatide And Turns On Disuse-Induced Bone Loss
Nmp4/CIZ (nuclear matrix protein 4/cas interacting zinc finger) appears to couple the skeleton’s response to PTH and mechanical load. Deficiency in Nmp4/CIZ in mice enhances PTH-induced gain in trabecular bone (Robling et al., 2009), augments BMP2-mediated orthotopic bone formation (Morinobu et al., 2005), and abrogates bone loss induced by tail suspension (Hino et al., 2007).
Both the independently prepared CIZ-and Nmp4-knockout (KO) mice exhibited a significant increase in baseline skeletal mass. Nmp4-KO estrogen-replete female mice showed 8–10% increases in baseline bone mineral density (BMD) and bone mineral content (BMC) throughout the skeleton (8–17wks of age) as compared to WT mice (Robling et al., 2009). Female (8wks) and male (12wks) CIZ-KO mice showed a significant increase in baseline femoral trabecular bone volume as compared to WT mice (Hino et al., 2007; Morinobu et al., 2005). Female Nmp4-KO mice exhibited no differences in baseline femoral trabecular architecture at 17wks of age but this was not examined in younger mice (Robling et al., 2009). Examination of the cortical bone geometry of the midshaft femur at 17wks showed that the Nmp4-null mice exhibited a moderate trend toward an increase in cortical area along with enhanced bending and torsional properties (Robling et al., 2009). The expression of genes encoding the proteins Col1a1, alkaline phosphatase and osteopontin as well as the transcription factor, osterix, was increased in the humerus of 8 wk-old female CIZ-KO (Morinobu et al., 2005) mice whereas only Col1a1 RNA expression was modestly elevated in the femur of 8wk-old Nmp4-KO mice of Robling et al., 2009.
Treating estrogen-replete female mice with recombinant human PTH(1-34) (30μg/kg/day s.c.) for 7wks (10wk–17wks of age) significantly enhanced whole body, femur, tibia, and spine BMD and BMC in both the Nmp4-KO and WT mice (Robling et al., 2009). Interestingly, although hormone equivalently augmented the cortical bone geometry of the midshaft femur in both genotypes these parameters for the PTH-treated WT mice were not significantly different from those values observed for the untreated Nmp4-KO mice.
The Nmp4-KO mice exhibited a strikingly enhanced PTH-induced gain in femoral trabecular bone. Vehicle-treated WT mice and vehicle-treated Nmp4-KO mice displayed comparable bone volume/total volume (BV/TV), connectivity density (Conn D) and structure model index (SMI) of the distal femur. However, while hormone treatment significantly altered these parameters in both genotypes, the magnitude of the hormone-induced changes in the Nmp4-null mice ranged from 2.3–3.5-fold greater than responses observed in the WT animals (Robling et al., 2009). Likewise, both the vehicle-treated WT and Nmp4-KO mice exhibited equivalent femoral trabecular number (Tb.N) and trabecular thickness (Tb.Th) indices but hormone-mediated enhancement of these parameters was significantly greater in the Nmp4-null mice (Robling et al., 2009).
CIZ deficiency augmented BMP2-induced orthotopic bone formation on adult mouse calvariae in vivo (Morinobu et al., 2005). BMP2 was injected onto the calvariae of WT and CIZ-KO mice every other day for 10 days. The calvariae were removed and the area of de novo bone formation was assessed using soft X-ray images. The CIZ-KO mice showed a two-fold greater area of newly formed bone than their WT counterparts (Morinobu et al., 2005).
Remarkably, CIZ-KO mice were refractive to unloading-induced bone loss after two weeks of tail suspension (Hino et al., 2007). CIZ-KO mice showed no significant decrease in BV/TV, no reduction of cortical bone area and thickness, and no attenuation of matrix apposition rate (MAR), mineralizing surface/bone surface (MS/BS) or bone formation rate (BFR) compared to the significant decreases in all of these parameters in WT mice. Interestingly, bone histomorphometry indicated no significant difference in osteoclast parameters regardless of genotype or loading condition (Hino et al., 2007).
III. Nmp4/CIZ Is A Transcription Factor That Associates With The Integrin Signaling Machinery
Nmp4/CIZ was independently discovered and cloned as an osteoblast nuclear matrix transcription factor (Alvarez et al., 1997; Alvarez et al., 1998; Thunyakitpisal et al., 2001) and as a nucleocytoplasmic shuttling binding partner to the integrin signaling molecule p130Cas (Nakamoto et al., 2000). We postulated that nuclear matrix architectural transcription factors (ATFs) capable of bending DNA convert changes in osteoblast adhesion and shape into changes in DNA conformation thus altering gene expression (Bidwell et al., 1998). Nuclear matrix proteins often serve as scaffolding for macro-protein complexes involved in transcription and RNA processing. Subsequently we recovered Nmp4 from the osteoblast nuclear matrix and this protein exhibited both DNA-binding and -bending activity along the regulatory regions of rat Col1a1 (Alvarez et al., 1998), whose expression is extraordinarily sensitive to alterations in cell and nuclear morphology (Li et al., 2008; Thomas et al., 2002).
Nmp4/CIZ is a Cys2His2 zinc finger transcription factor expressed in species ranging from humans to yeast and the rodent and human protein sequences are highly conserved (~96%). Several rat isoforms have been cloned from the single copy gene and in the mouse Nmp4/CIZ is located on chromosome 6 band F1 and ZNF384 on human chromosome 12p12 (Alvarez et al., 2001; Thunyakitpisal et al., 2001; Nakamoto et al., 2000). Nmp4/CIZ is expressed in all tissues examined and in bone is expressed in osteoblasts, osteocytes, and chondrocytes (Thunyakitpisal et al., 2001).
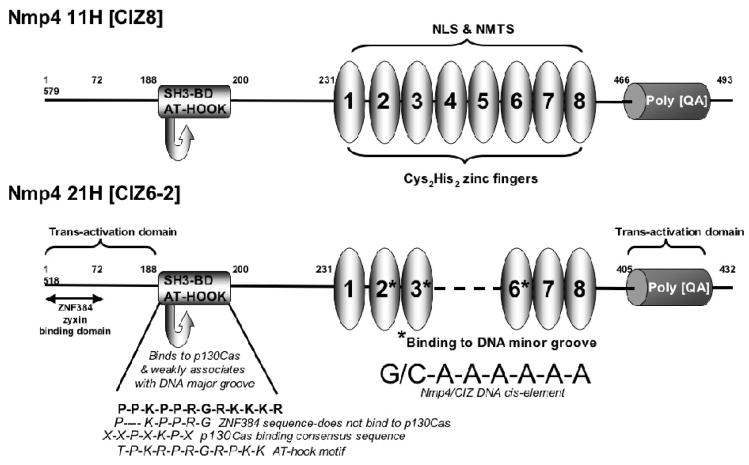
The Nmp4-21H/CIZ6-2 isoform is comprised of six zinc fingers and #2, #3, and #6 mediate DNA-binding via the minor groove of the unusual homopolymeric (dA•dT) consensus sequence (Torrungruang et al., 2002). The zinc fingers also act as a nuclear localization signal and target Nmp4/CIZ to the nuclear matrix (Figure 1, Feister et al., 2000a).
FIGURE 1. Functional domains of Nmp4/CIZ.
Nmp4/CIZ is an HMG-motif Cys2His2 zinc finger ATF. Rat isoforms Nmp4 11H [CIZ8] and Nmp4 21H [CIZ6-2] are shown (Thunyakitpisal et al., 2001; Nakamoto et al., 2000). The zinc fingers act as a nuclear localization signal (NLS) and nuclear matrix-targeting signal (NMTS) for the Nmp4/CIZ proteins and zinc fingers #4 through #8 are minimally required for the NLS and NMTS of Nmp4 11H (Feister et al., 2000a). The Nmp4/CIZ SH3-binding domain (SH3-BD) conforms to the p130Cas SH3-binding consensus sequence XXPXKPX, however one P residue is absent in the human ortholog ZNF384 which precludes p130Cas binding. ZNF384 binds to zyxin via the first 72 amino acids of the N-terminus (Janssen & Marynen, 2006). Nmp4 21H was used to map DNA-binding and trans-activation domains (Torrungruang et al., 2002). Zinc fingers #2, #3, and #6 mediate DNA-binding to the minor groove of the homopolymeric (dA•dT) cis-element and the AT-Hook motif, which overlaps with the p130Cas SH3-binding sequence, weakly associates with the major groove. The polyglutamine/alanine repeat (poly QA) domain exhibits trans-activation capacity but tethering the Nmp4 21H to the GAL4-DNA-binding domain, thus neutralizing the effects of zinc finger association with the homopolymeric (dA•dT) cis-element, revealed that the N terminus (1-186aa) had a strong trans-activation capacity that was masked by the full-length protein. Thus the zinc finger binding to the AT-rich DNA has profound effects on the activity of these modular domains (Torrungruang et al., 2002).
An AT-hook domain associates weakly with the DNA major groove and likely confers ATF functionality (Figure 1; Torrungruang et al., 2002; Thunyakitpisal et al., 2001). The AT-hook motif was first described in the high mobility group (HMG) nonhistone chromosomal ATF HMGA proteins (reviewed in Alvarez et al., 2003). The AT-hook facilitates changes in local DNA structure typically by tethering to the minor groove of AT-rich DNA, although some association with the major groove has been reported (Alvarez et al., 2003 and references therein).
Canonical ATFs stabilize multi-protein transcriptional units along the regulatory region of the gene by bending, looping, unwinding or straightening DNA (Alvarez et al., 2003 and references therein). Typically ATFs recognize a unique structural aspect of DNA instead of a specific sequence, e.g. the narrow minor groove of AT-rich DNA via the AT-hook or a motif known as the HMG box (Alvarez et al., 2003).
ATFs typically lack a trans-acting domain, i.e. their function is strictly architectural (Alvarez et al., 2003) although there are numerous conventional transcription factors that bend DNA as part of their regulatory function. For example, TCF/LEF-1 and Nmp4/CIZ are both HMG-motif ATFs but have context-dependent transactivation domains, i.e. the activities of these domains are dependent on the organization of the regulatory elements surrounding their respective DNA-binding sites (Torrungruang et al., 2002; Carlsson et al., 1993).
The presence of Nmp4/CIZ in the cytoplasm was revealed when Hisamaru Hirai and colleagues cloned CIZ during a search for the ligands of the p130Cas SH3 domain (Nakamoto et al., 2000). Hirai had previously cloned p130Cas as a docking protein of the focal adhesions and it is now appreciated that this ubiquitously expressed adaptor protein is part of the integrin signaling machinery involved in mediating cell proliferation, survival, and motility (Defilippi et al., 2006; Nakamoto et al., 2000 and references therein). The rat Nmp4/CIZ sequence APPKPPR from the 186th amino acid residue conforms to the p130Cas SH3-binding consensus XXPXKPX and resembles the p130Cas SH3-binding site of FAK (APPKPSR) and that of the guanine exchange factor C3G (see Figure 1, Nakamoto et al., 2000 and references therein). Interestingly, this motif overlaps with the AT-hook domain (Figure 1). Far-Western screening demonstrated that Nmp4/CIZ discriminates between the SH3 domains of p130Cas, Src, Crk, Ash/Grb2, Fyn, and Abl (Nakamoto et al., 2000). Although the rat Nmp4/CIZ and the human ortholog ZNF384 are highly conserved one P residue in the p130Cas SH3-binding consensus sequence is absent in the human protein which precludes ZNF384 binding to p130Cas but instead mediates its binding to zyxin, another adaptor protein that associates with focal adhesions (Figure 1, Janssen & Marynen, 2006). Therefore, within the context of the human cell, ZNF384 binds to zyxin and zyxin binds to p130Cas (Janssen & Marynen, 2006). In this review we use the designation Nmp4/CIZ, instead of CIZ/Nmp4, to de-emphasize an exclusive association with p130Cas.
Nmp4/CIZ suppresses BMP2-induced transcriptional activity of the receptor regulated SMADS (R-SMADs), SMAD1 and SMAD5 (Shen et al., 2002) but whether this occurs by interfering with R-SMAD DNA-binding activity, R-SMAD trans-activation, or by R-SMAD sequestration in the cytoplasm by a p130Cas/zyxin/Nmp4/CIZ complex remains to be determined. A recent study using fibroblasts and epithelial cells has demonstrated that integrin-mediated p130Cas phosphorylation promotes its interaction with phosphorylated SMAD3 preventing SMAD3 nuclear translocation and activation of target genes (Kim et al., 2008).
IV. Nmp4/CIZ Suppresses Osteoid Synthesis
Nmp4/CIZ antagonizes the transcription of bone matrix genes. Introduction of null-binding mutations in Nmp4/CIZ cis-elements within rat Col1a1 enhanced the activity of this promoter in osteoblast-like cells; conversely over-expression of Nmp4/CIZ repressed this activity (Thunyakitpisal et al., 2001). Nmp4/CIZ suppressed PTH-mediated transcriptional induction of rat matrix metalloproteinase-13 (Mmp-13) in osteoblast-like UMR 106-01 cells (Shah et al., 2004). The human, rat, and mouse MMP-13 genes have a conserved PTH response region containing two cis elements for Runx2, cis-elements for AP-1, PEA3/Ets-1, p53, and an Nmp4/CIZ element (Shah et al., 2004 and references therein). We introduced null-binding mutations in the Nmp4/CIZ element and/or the proximal Runx2 element in a series of promoter-reporter constructs containing the first 1329 nucleotides of the rat 5′ regulatory region. The UMR-106-01 cells were transfected with these constructs and treated with rat PTH(1-34). Hormone treatment (6hrs) induced a significant 2-fold increase in the wild-type construct activity, a 5-fold increase in the activity of the construct bearing the Nmp4/CIZ null-binding mutation, and a synergistic 11-fold increase in the activity of the construct containing both the Nmp4/CIZ and Runx2 null-binding mutations (Shah et al, 2004). Finally, both the BMP2-induced orthotopic bone formation and abrogated bone loss in hind limb-suspended CIZ-KO mice can be explained by Nmp4/CIZ functioning as a suppressor of bone matrix synthesis (Hino et al., 2007; Morinobu et al., 2005). Interestingly, unloading appears to enhance this suppressive activity of Nmp4/CIZ (Hino et al., 2007).
V. Nmp4/CIZ Is Part Of A Mechano-sensitive Pathway Mediating Response to Both PTH and Load
PTH and load alter bone cell shape and adhesion, which in turn affects type I collagen expression
PTH sensitizes bone to mechanical signals; the anabolic effect of mechanical loading is lost in rats with the removal of the parathyroid gland (see Turner, 2006 and references therein). In addition to the synergistic effect on bone gain in rodents (Sugiyama et al., 2008), intermittent PTH treatment abrogates hindlimb-induced bone loss in rats by preventing disuse-mediated decreases in bone formation and matrix apposition rates (Turner et al., 2007; 2006).
Mechanical loading bends bone, which in turn generates mechanical stretch and pressure gradients in the canaliculi of the mineralized tissue. These gradients drive the extracellular fluid through the lacuno-canalicular network and this load-derived fluid shear stress (FSS) initiates mechano-sensitive signaling pathways in osteocytes and other osteogenic cells (Rubin et al., 2006 and references therein). Unloading or micro-gravity has the opposite effects on bone via common signaling pathways however signaling pathways unique to unloading has not been fully discounted. For the purpose of describing the response of the bone cell to non-hormonal changes in the mechanical environment we will use the broad term “load” for designating steady or dynamic FSS, strain, and unloading, unless otherwise noted.
Both PTH and load physically impact the bone cell. These agonists alter cell shape (Guignandon et al., 2001; Horikawa et al., 2000; Egan et al., 1991; Matthews and Talmadge, 1981), modulate the organization of the cytoskeleton (Rubin et al., 2006 and references therein; Guignandon et al., 2001; Egan et al., 1991), alter nuclear organization (Hughes-Fulford et al., 2006; Feister et al., 2000b; Torrungruang et al., 1998), and change the activity of focal adhesions (Rubin et al., 2006 and references therein; Davies & Chambers, 2004).
Type I collagen synthesis, including alterations in transcription and mRNA stability, in the cultured Swiss 3T3 fibroblast line is influenced by alterations in cell shape and adherence. Adherence is associated with a flat spread out shape and increase expression of Col1a1 whereas suspended cells round up decreasing expression of Col1a1 (Dhawan et al., 1991). Studies using micropatterned adhesive islands have demonstrated that expression of type I collagen is sensitive to the actual shape of a tendon cell, not to total cell area (Li et al., 2008). Similarly, Healy and colleagues seeded osteoblasts on adhesive islands to control cell and nuclear shape and determined that type I collagen protein synthesis was significantly influenced by the nuclear space index, defined as the maximal nuclear area in the x-y plane divided by nuclear height (Thomas et al., 2002).
The physical connection between the cell adhesion complexes and the interior of the nucleus is vital to mechanotransduction (Gieni & Hendzel, 2008 and references therein). Ingber and colleagues demonstrated this connection by tugging on integrin receptors with glass micropipettes causing a spatial shift in chromatin (Maniotis et al., 1997). The LINC (linker of nucleus and cytoskeleton) complex physically bridges this gulf between adhesion complex and gene and is comprised of actin filaments, intermediate filaments, plectin, SUN proteins, nesprins, the lamins, and other nuclear envelop proteins (Gieni & Hendzel, 2008). On the nucleoplasmic side, SUN proteins form macromolecular complexes comprised of lamins, proteins of the nuclear pore complex, and other lamin-associated proteins (Gieni & Hendzel, 2008). These nuclear envelop-lamina-spanning complexes (NELSC) directly interact with chromatin, DNA, and transcription factors (Gieni & Hendzel, 2008). Whether there is a karyoskeleton (deterministic model) or instead large and transiently immobile nuclear structures housing transcription factories (self-organization model) is the subject of some recent debate (Gieni & Hendzel, 2008, and references therein; Misteli, 2007) but nuclear matrix ATFs, like Nmp4/CIZ, could be operative in either model. For example, the LINC proteins may pull a target gene into a transcription factory and Nmp4/CIZ, informed by its interaction with the focal adhesion protein p130Cas (or zyxin), may change target DNA conformation and gene activity (Bidwell et al., 1998).
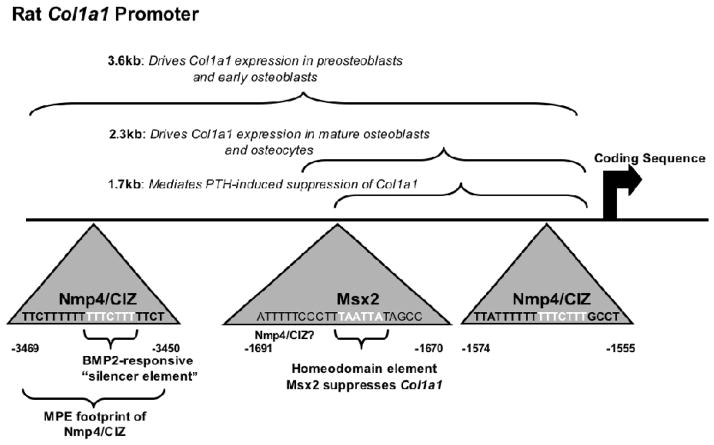
Bone cell shape and adhesion may dictate which regions of the COL1A1 promoter control expression of this gene. The rat Col1a1 promoter is organized as functional modules that drive expression during distinct periods of osteogenic cell differentiation (see Figure 2; Rowe, 2005). Whether these shifts in the control regions of the Col1a1 promoter reflect changes in the developing osteoblast’s shape and adhesion as suggested in an early study (Krebsbach et al., 1993) remains to be determined but Nmp4/CIZ binds to these distinct promoter regions (Alvarez et al., 1998 Figure 2).
FIGURE 2. Nmp4/CIZ binding to the 5′ regulatory region of rat Col1a1.
Nmp4/CIZ binds to the Col1a1 regulatory sequences. The rat Col1a1 promoter is organized as functional modules that drive expression during distinct periods of osteogenic cell differentiation (Rowe, 2005). The region within the first 1700 bp mediates suppression by chronic PTH in calvariae organ culture (Bogdanovic et al., 2000). Methidium propyl EDTA (MPE) footprinting mapped the boundaries of Nmp4/CIZ binding between −3469 bp/−3450 bp. A very similar site was characterized between −1574 bp/−1555 bp (Alvarez et al., 1998). Introduction of null-binding mutations in either of these sites enhanced Col1a1 promoter activity in osteoblast-like cells (Thunyakitpisal et al., 2001). Both of these sites contain a BMP2-responsive osteoblastic silencer element characterized in the osteocalcin gene but present in numerous genes that support the mature osteoblast phenotype (Goto et al., 1996). A homeodomain element is present between −1691 bp/−1670 bp. Msx2 binds to this element in undifferentiated osteoblasts and suppresses transcription (Dodig et al., 1996).
Nmp4/CIZ, p130Cas and zyxin are mechano-sensitive
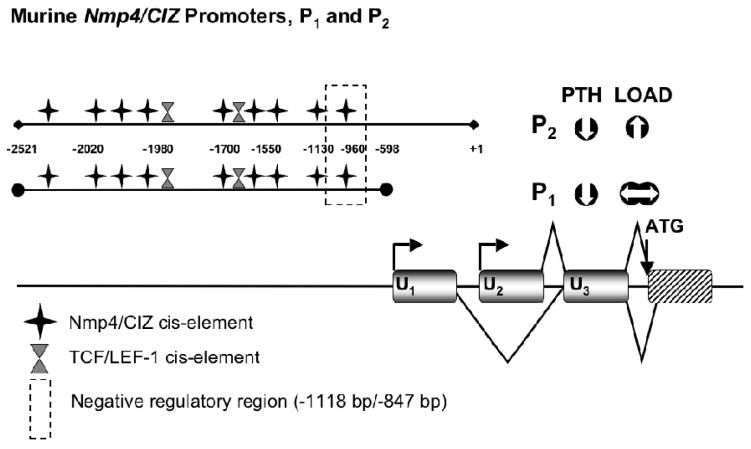
Nmp4/CIZ regulates FSS-driven induction of rat Mmp-13 in bone cells and the expression of Nmp4/CIZ itself is governed by FSS (Charoonpatrapong-Panyayong et al., 2007). Murine Nmp4/CIZ has two promoters, P1 and P2, both of which are positively auto-regulated; additionally PTH negatively regulates both of these promoters (Figure 3; Alvarez et al., 2005). Interestingly, only P2 activity was upregulated in MC3T3-E1 cells exposed to FSS (Charoonpatrapong-Panyayong et al., 2007).
FIGURE 3.
Murine Nmp4/CIZ has two promoters. The two overlapping promoters P1 (−2521 bp/−597 bp) and P2 (−2521 bp/+1 bp) initiate transcription of alternative first exons (U1 and U2; exons 1–3 are shown as gray boxes and the first coding exon is represented as a striped box). The splice patterns up to the start of translation are indicated with straight lines. Both promoters lack TATA and CCAAT boxes but contain CpG islands and initiator sites. The Nmp4/CIZ promoters are autoregulated and sequence analysis identifies potential Nmp4/CIZ cis-elements and two TCF/LEF-1 binding sites. Deletion analysis identified a region containing negative regulatory element(s) suppressing basal transcription (dashed box). The Nmp4/CIZ promoters comprise a genomic regulatory organization that supports constitutive expression as well as cell- and tissue-specific regulation. PTH attenuated P1 and P2 activity in MC3T3-E1 osteoblast-like cells, and FSS enhanced P2 activity but had no significant impact on P1 activity in these same cells (Charoonpatrapong-Panyayong et al., 2007; Alvarez et al., 2005).
As a primary force sensor p130Cas transduces force into the mechanical extension of the molecule itself, making it more susceptible to phosphorylation and thus triggering its activation of downstream mechanotransduction signaling cascades (Sawada et al., 2006). p130Cas has various functional domains that serve as substrates for a variety of kinases and/or mediate protein-protein interactions. Upon phosphorylation by tyrosine kinases, the tyrosine residues within the central substrate domain provide binding sites for the Src homology 2 (SH2) and phosphotyrosine-binding (PTB) domains of effector proteins (Defilippi et al., 2006). Sawada and colleagues attached recombinant p130Cas to a latex membrane and demonstrated that the ability of c-Src to phosphorylate this molecule was significantly enhanced upon the stretching of the membrane (Sawada et al., 2006). Stretching results in the unfolding of the p130Cas central substrate domain enhancing the accessibility of target tyrosine residues and their phosphorylation by FAK or Src-family kinases (Sawada et al., 2006). Stretched-p130Cas localizes to sites of high traction force in cells perhaps as a first-responder to changes in cell shape and cytoskeletal stretch (Sawada et al., 2006).
Zyxin accumulates at high traction force focal adhesions and disassociates from these sites with a relaxation in stretch (Hirata et al., 2008; Cattaruzza et al., 2004). Zyxin is targeted to focal adhesions via its LIM domains and is required for stretch-induced actin polymerization at these sites of cell attachment (Hirata et al., 2008). In smooth muscle cells cyclic stretch ultimately induced zyxin translocation to the nucleus accompanied by changes in the expression of endothelin B receptor, tenascin-C, and plasminogen-activator inhibitor-1 (Cattaruzza et al., 2004). Abolishing zyxin expression attenuated stretch-induced induction of endothelin B receptor, enhanced the load-mediated induction of tenascin-C, and had no effect on the load induction of plasminogen-activator inhibitor-1 (Cattaruzza et al., 2004).
The p130Cas/zyxin/Nmp4/CIZ association resembles the β-catenin/TCF/LEF-1 mechanotransduction pathway
We have proposed that the p130Cas/zyxin/Nmp4/CIZ and the β-catenin/TCF/LEF-1 complexes form “mechanosomes” that carry information from adhesion complexes to the nucleus and translate this information into conformational changes in the promoters of target genes (Pavalko et al., 2003). Altering osteoblast β-catenin activity alters bone phenotype via changes in osteoprotegerin expression and osteoclast activity, but bone formation is not modified (Glass et al., 2005). However, β-catenin translocation to the nucleus is triggered by PTH and is necessary to some load-induced changes in osteoblast gene expression (Case et al., 2008; Kulkarni et al., 2005) and therefore may mobilize the bone-formation activity of the Wnt pathway in response to anabolic signals. The similarities between these pathways are striking. As shuttling HMG-motif ATFs both Nmp4/CIZ and TCF/LEF1 associate with mechano-sensitive adhesion proteins but whether the p130Cas/zyxin/Nmp4/CIZ complex translocates to the nucleus (see Charoonpatrapong-Panyayong et al., 2007 and references therein) or whether p130Cas sequesters Nmp4/CIZ in the cytoplasm remains to be fully established.
A molecular convergence point for PTH and load may be the regulation of R-SMAD activity by the p130Cas/zyxin/Nmp4/CIZ and β-catenin/TCF/LEF-1 pathways. R-SMAD activity is upregulated by both PTH and load (Ho et al., 2008; Sowa et al., 2003). R-SMADs enhance β-catenin/TCF/LEF-1 activation of bone genes (Sato et al., 2009) and PTH-induced upregulation of β-catenin activity via SMAD3 contributes to the hormone’s anti-apoptotic action in MC3T3-E1 cells (Sowa et al., 2003). Nmp4/CIZ suppresses the activation of R-SMADs (Shen et al., 2002), as does p130Cas (Kim et al., 2008). Perhaps p130Cas/zyxin/Nmp4/CIZ antagonizes β-catenin/TCF/LEF-1/R-SMAD-mediated bone-formation by suppressing R-SMAD activity.
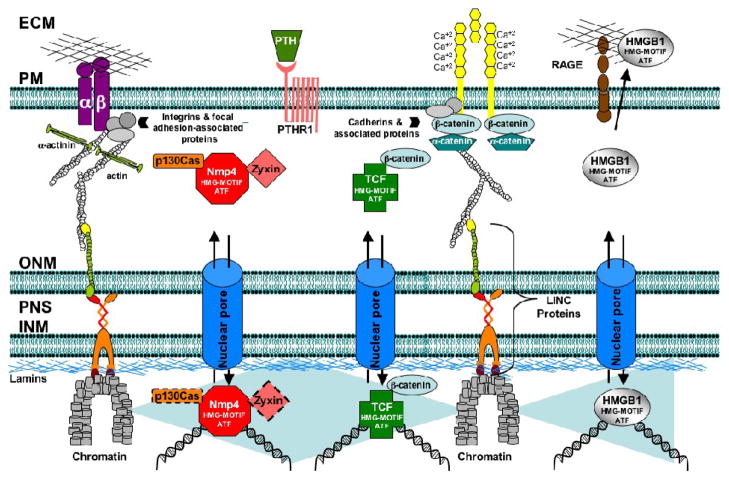
HMGB1 (high mobility group box 1 protein) is another nucleocytoplasmic shuttling HMG-motif ATF with a connection to PTH and perhaps to load. Hormone regulates its release from osteoblasts and in turn extracellular HMGB1 appears to regulate some aspects of osteoclastogenesis (Bidwell et al., 2008 and references therein). HMGB1 is a ligand for RAGE (receptor for advanced glycation end products) and we have determined that RAGE-KO mice exhibit deficits in PTH-induced gains in trabecular bone (Philip et al., 2009). The HMGB1/RAGE signaling axis is linked to the mechanotransduction machinery. RAGE can act as an adhesion receptor and binds to type I collagen, it mediates cell spreading in alveolar epithelial cells, and AGEs, a ligand of RAGE, inhibit FSS-induced ERK5 transcription in endothelial cells (reviewed in Sparvero et al., 2009; Woo et al., 2008). Finally, HMGB1 localizes with focal adhesions in myofibroblasts (Lenga et al., 2008). Therefore p130Cas/zyxin/Nmp4/CIZ, β-catenin/TCF/LEF-1, and HMGB1/RAGE may comprise part of a mechanosome network that mediates the convergence of PTH and load response programs (Figure 4).
FIGURE 4.
The proposed mechanosome network mediates physical and biochemical links between adhesion receptors and target genes and underlies the convergence of PTH and load signaling. The actin cytoskeleton (and the intermediate filament network, not shown) physically couple focal and cell-cell adhesion complexes with chromatin and DNA via the LINC complex proteins (Gieni & Hendzel, 2008; Stewart et al., 2007). RAGE is a multiligand receptor of the immunoglobulin superfamily, and can mediate cell adhesion and activate Nf-Kb signaling (Sparvero et al., 2009) thus acting as an adhesion-coupled mechanosensor. The p130Cas/zyxin/Nmp4/CIZ, β-catenin/TCF/LEF-1, and HMGB1/RAGE signaling axes comprise the biochemical link that carries mechanical information from the adhesion receptors to the target genes. These shuttling HMG-motif ATFs alter gene expression in part by bending DNA. The dashed outline of nuclear p130Cas and zyxin represent the question as to whether both proteins accompany Nmp4/CIZ to the target gene or instead sequester this ATF in the cytoplasm. Abbreviations: ECM, extracellular matrix; INM, inner nuclear membrane; ONM, outer nuclear membrane; PM, plasma membrane; PNS, perinuclear space.
VI. Summary And Conclusions
Enhancing the efficacy of teriparatide will alleviate some of its current limitations as an osteoporosis drug. This might be accomplished by disabling self-limiting pathways regulating PTH anabolic action and/or by boosting the activity of pathways that drive bone gain e.g. combining teriparatide treatment with exercise. Nmp4/CIZ suppresses PTH-induced anabolic gains in skeletal mass and drives disuse-associated bone loss by suppressing osteoblast function and synthesis and deposition of a mineralized bone matrix. An Nmp4/CIZ binding element in the 5′ regulatory region of human COL1A1 has been identified as a polymorphism associated with osteoporosis (Jin et al., 2009 and references therein). Thus, inhibiting Nmp4/CIZ in patients may abbreviate and/or enhance teriparatide therapy, provide a prophylactic to disuse osteoporosis, and enhance the anabolic tonic derived from exercise. This raises the interesting question as to whether neutralizing Nmp4/CIZ function would ameliorate bone loss in primary hyperparathyroidism (PHPT). Decreased BMD in PHPT appears to result from an expansion of the remodeling space and increased endocortical resorption (Mosekilde, 2008 and references therein). Therefore, if Nmp4/CIZ primarily acts to limit bone formation by attenuating osteoblast function then inhibiting its activity may have little influence on the enhanced osteoclast activity in these patients.
Part of Nmp4/CIZ action includes restraining the magnitude of hormone-mediated transcription induction of target genes and neutralizing R-SMAD activity. The inherent mechano-sensitivity of Nmp4/CIZ is reinforced by its interaction with p130Cas and zyxin, which themselves are mechano-sensitive focal adhesion-associated proteins. As an HMG-motif, mechano-sensitive ATF Nmp4/CIZ may regulate transcription in part by altering local DNA structure in response to changes in cell shape and adhesion. The p130Cas/zyxin/Nmp4/CIZ pathway resembles the β-catenin/TCF/LEF1 mechanotransduction response limb and the HMGB1/RAGE pathway and we speculate that these are part of a biochemical mechanosome network reinforcing and informing the physical link between the adhesion receptors and target genes.
Acknowledgments
This work was supported by a grant from NIH NIDDK, contract grant number: DK053796, JPB.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allen MR, Burr DB. Mineralization, microdamage and matrix: how bisphosphonates influence material properties of bone. BoneKEy-Osteovision. 2007;4:49–60. [Google Scholar]
- Alvarez M, Long H, Onyia J, Hock J, Xu W, Bidwell J. Rat osteoblast and osteosarcoma nuclear matrix proteins bind with sequence specificity to the rat type I collagen promoter. Endocrinology. 1997;138:482–489. doi: 10.1210/endo.138.1.4852. [DOI] [PubMed] [Google Scholar]
- Alvarez M, Rhodes SJ, Bidwell JP. Context-dependent transcription: all politics is local. Gene. 2003;313:43–57. doi: 10.1016/s0378-1119(03)00627-9. [DOI] [PubMed] [Google Scholar]
- Alvarez M, Shah R, Rhodes SJ, Bidwell JP. Two promoters control the mouse Nmp4/CIZ transcription factor gene. Gene. 2005;347:43–54. doi: 10.1016/j.gene.2004.10.025. [DOI] [PubMed] [Google Scholar]
- Alvarez M, Thunyakitpisal P, Morrison P, Onyia J, Hock J, Bidwell JP. PTH-responsive osteoblast nuclear matrix architectural transcription factor binds to the rat type I collagen promoter. J Cell Biochem. 1998;69:336–352. doi: 10.1002/(sici)1097-4644(19980601)69:3<336::aid-jcb11>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Alvarez MB, Thunyakitpisal P, Rhodes SJ, Everett ET, Bidwell JP. Assignment of Nmp4 to mouse chromosome 6 band F1 flanked by D6Mit134 and D6Mit255 using radiation hybrid mapping and fluorescence in situ hybridization. Cytogenet Cell Genet. 2001;94(3–4):244–245. doi: 10.1159/000048824. [DOI] [PubMed] [Google Scholar]
- Bellido T, Ali AA, Plotkin LI, Fu Q, Gubrij I, Roberson PK, Weinstein RS, O’Brien CA, Manolagas SC, Jilka RL. Proteasomal degradation of Runx2 shortens parathyroid hormone-induced anti-apoptotic signaling in osteoblasts. A putative explanation for why intermittent administration is needed for bone anabolism. J Biol Chem. 2003;278:50259–50272. doi: 10.1074/jbc.M307444200. [DOI] [PubMed] [Google Scholar]
- Bidwell JP, Alvarez M, Feister H, Onyia J, Hock J. Nuclear matrix proteins and osteoblast gene expression. J Bone Miner Res. 1998;13:155–167. doi: 10.1359/jbmr.1998.13.2.155. [DOI] [PubMed] [Google Scholar]
- Bidwell JP, Yang J, Robling AG. Is HMGB1 an osteocyte alarmin? J Cell Biochem. 2008;103:1671–1680. doi: 10.1002/jcb.21572. [DOI] [PubMed] [Google Scholar]
- Blick SK, Dhillon S, Keam SJ. Teriparatide: a review of its use in osteoporosis. Drugs. 2008;68:2709–2737. doi: 10.2165/0003495-200868180-00012. [DOI] [PubMed] [Google Scholar]
- Bogdanovic Z, Huang YF, Dodig M, Clark SH, Lichtler AC, Kream BE. Parathyroid hormone inhibits collagen synthesis and the activity of rat col1a1 transgenes mainly by a cAMP-mediated pathway in mouse calvariae. J Cell Biochem. 2000;77:149–158. doi: 10.1002/(sici)1097-4644(20000401)77:1<149::aid-jcb15>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Bouxsein ML, Pierroz DD, Glatt V, Goddard DS, Cavat F, Rizzoli R, Ferrari SL. beta-Arrestin2 regulates the differential response of cortical and trabecular bone to intermittent PTH in female mice. J Bone Miner Res. 2005;20(4):635–643. doi: 10.1359/JBMR.041204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson P, Waterman ML, Jones KA. The hLEF/TCF-1 alpha HMG protein contains a context-dependent transcriptional activation domain that induces the TCR alpha enhancer in T cells. Genes Dev. 1993;7:2418–2430. doi: 10.1101/gad.7.12a.2418. [DOI] [PubMed] [Google Scholar]
- Case N, Ma M, Sen B, Xie Z, Gross TS, Rubin J. Beta-catenin levels influence rapid mechanical responses in osteoblasts. J Biol Chem. 2008;283:29196–29205. doi: 10.1074/jbc.M801907200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattaruzza M, Lattrich C, Hecker M. Focal adhesion protein zyxin is a mechanosensitive modulator of gene expression in vascular smooth muscle cells. Hypertension. 2004;43:726–730. doi: 10.1161/01.HYP.0000119189.82659.52. [DOI] [PubMed] [Google Scholar]
- Charoonpatrapong-Panyayong K, Shah R, Yang J, Alvarez M, Pavalko FM, Gerard-O’Riley R, Robling AG, Templeton E, Bidwell JP. Nmp4/CIZ contributes to fluid shear stress induced MMP-13 gene induction in osteoblasts. J Cell Biochem. 2007;102:1202–1213. doi: 10.1002/jcb.21349. [DOI] [PubMed] [Google Scholar]
- Davies J, Chambers TJ. Parathyroid hormone activates adhesion in bone marrow stromal precursor cells. Journal of Endocrinology. 2004;180:505–513. doi: 10.1677/joe.0.1800505. [DOI] [PubMed] [Google Scholar]
- Defilippi P, Di Stefano P, Cabodi S. p130Cas: a versatile scaffold in signaling networks. Trends Cell Biol. 2006;16:257–263. doi: 10.1016/j.tcb.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Dhawan J, Lichtler AC, Rowe DW, Farmer SR. Cell adhesion regulates pro-alpha 1(I) collagen mRNA stability and transcription in mouse fibroblasts. J Biol Chem. 1991;266:8470–8475. [PubMed] [Google Scholar]
- Dodig M, Kronenberg MS, Bedalov A, Kream BE, Gronowicz G, Clark SH, Mack K, Liu YH, Maxon R, Pan ZZ, Upholt WB, Rowe DW, Lichtler AC. Identification of a TAAT-containing motif required for high level expression of the COL1A1 promoter in differentiated osteoblasts of transgenic mice. J Biol Chem. 1996;271:16422–16429. doi: 10.1074/jbc.271.27.16422. [DOI] [PubMed] [Google Scholar]
- Egan JJ, Gronowicz G, Rodan GA. Parathyroid hormone promotes the disassembly of cytoskeletal actin and myosin in cultured osteoblastic cells: mediation by cyclic AMP. J Cell Biochem. 1991;45:101–111. doi: 10.1002/jcb.240450117. [DOI] [PubMed] [Google Scholar]
- Feister HA, Torrungruang K, Thunyakitpisal P, Parker GE, Rhodes SJ, Bidwell JP. NP/NMP4 transcription factors have distinct osteoblast nuclear matrix subdomains. J Cell Biochem. 2000a;79:506–17. [PubMed] [Google Scholar]
- Feister HA, Onyia JE, Miles RR, Yang X, Galvin R, Hock JM, Bidwell JP. The expression of the nuclear matrix proteins NuMA, topoisomerase II-alpha, and -beta in bone and osseous cell culture: regulation by parathyroid hormone. Bone. 2000b;26:227–234. doi: 10.1016/s8756-3282(99)00269-0. [DOI] [PubMed] [Google Scholar]
- Ferrari SL, Pierroz DD, Glatt V, Goddard DS, Bianchi EN, Lin FT, Manen D, Bouxsein ML. Bone response to intermittent parathyroid hormone is altered in mice null for {beta}-Arrestin2. Endocrinology. 2005;146:1854–1862. doi: 10.1210/en.2004-1282. [DOI] [PubMed] [Google Scholar]
- Fuchs RK, Warden SJ. Combination Therapy Using Exercise and Pharmaceutical Agents to Optimize Bone Health Clinic. Rev Bone Miner Metab. 2008;6:37–45. [Google Scholar]
- Gieni RS, Hendzel MJ. Mechanotransduction from the ECM to the genome: are the pieces now in place? J Cell Biochem. 2008;104:1964–1987. doi: 10.1002/jcb.21364. [DOI] [PubMed] [Google Scholar]
- Glass DA, 2nd, Bialek P, Ahn JD, Starbuck M, Patel MS, Clevers H, Taketo MM, Long F, McMahon AP, Lang RA, Karsenty G. Canonical Wnt signaling in differentiated osteoblasts controls osteoclast differentiation. Dev Cell. 2005;8:751–764. doi: 10.1016/j.devcel.2005.02.017. [DOI] [PubMed] [Google Scholar]
- Goto K, Heymont JL, Klein-Nulend J, Kronenberg HM, Demay MB. Identification of an osteoblastic silencer element in the first intron of the rat osteocalcin gene. Biochemistry. 1996;35:11005–11011. doi: 10.1021/bi960723o. [DOI] [PubMed] [Google Scholar]
- Guignandon A, Lafage-Proust MH, Usson Y, Laroche N, Caillot-Augusseau A, Alexandre C, Vico L. Cell cycling determines integrin-mediated adhesion in osteoblastic ROS 17/2.8 cells exposed to space-related conditions. FASEB J. 2001;15:2036–2061. doi: 10.1096/fj.00-0837fje. [DOI] [PubMed] [Google Scholar]
- Hino K, Nakamoto T, Nifuji A, Morinobu M, Yamamoto H, Ezura Y, Noda M. Deficiency of CIZ, a nucleocytoplasmic shuttling protein, prevents unloading-induced bone loss through the enhancement of osteoblastic bone formation in vivo. Bone. 2007;40:852–860. doi: 10.1016/j.bone.2006.03.019. [DOI] [PubMed] [Google Scholar]
- Hirata H, Tatsumi H, Sokabe M. Mechanical forces facilitate actin polymerization at focal adhesions in a zyxin-dependent manner. J Cell Sci. 2008;121:2795–2804. doi: 10.1242/jcs.030320. [DOI] [PubMed] [Google Scholar]
- Ho AM, Marker PC, Peng H, Quintero AJ, Kingsley DM, Huard J. Dominant negative Bmp5 mutation reveals key role of BMPs in skeletal response to mechanical stimulation. BMC Dev Biol. 2008;8:35. doi: 10.1186/1471-213X-8-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horikawa A, Okada K, Sato K, Sato M. Morphological changes in osteoblastic cells (MC3T3-E1) due to fluid shear stress: cellular damage by prolonged application of fluid shear stress. Tohoku J Exp Med. 2000;191:127–137. doi: 10.1620/tjem.191.127. [DOI] [PubMed] [Google Scholar]
- Hughes-Fulford M, Rodenacker K, Jütting U. Reduction of anabolic signals and alteration of osteoblast nuclear morphology in microgravity. J Cell Biochem. 2006;99:435–449. doi: 10.1002/jcb.20883. [DOI] [PubMed] [Google Scholar]
- Janssen H, Marynen P. Interaction partners for human ZNF384/CIZ/NMP4--zyxin as a mediator for p130CAS signaling? Exp Cell Res. 2006;312:1194–1204. doi: 10.1016/j.yexcr.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Jilka RL. Molecular and cellular mechanisms of the anabolic effect of intermittent PTH. Bone. 2007;40:1434–1446. doi: 10.1016/j.bone.2007.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin H, Van’t Hof RJ, Albagha OM, Ralston SH. Promoter and intron 1 polymorphisms of COL1A1 interact to regulate transcription and susceptibility to osteoporosis. Hum Mol Genet. 2009;18:2729–2738. doi: 10.1093/hmg/ddp205. [DOI] [PubMed] [Google Scholar]
- Kim W, Seok Kang Y, Soo Kim J, Shin NY, Hanks SK, Song WK. The integrin-coupled signaling adaptor p130Cas suppresses Smad3 function in transforming growth factor-beta signaling. Mol Biol Cell. 2008;19:2135–2146. doi: 10.1091/mbc.E07-10-0991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer I, Loots GG, Studer A, Keller H, Kneissel M. Parathyroid Hormone (PTH) Induced Bone Gain is Blunted in SOST Overexpressing and Deficient Mice. J Bone Miner Res. 2009 Jul 13; doi: 10.1359/jbmr.090730. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebsbach PH, Harrison JR, Lichtler AC, Woody CO, Rowe DW, Kream BE. Transgenic expression of COL1A1-chloramphenicol acetyltransferase fusion genes in bone: differential utilization of promoter elements in vivo and in cultured cells. Mol Cell Biol. 1993;13:5168–5174. doi: 10.1128/mcb.13.9.5168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni NH, Halladay DL, Miles RR, Gilbert LM, Frolik CA, Galvin RJ, Martin TJ, Gillespie MT, Onyia JE. Effects of parathyroid hormone on Wnt signaling pathway in bone. J Cell Biochem. 2005;95:1178–1190. doi: 10.1002/jcb.20506. [DOI] [PubMed] [Google Scholar]
- Lenga Y, Koh A, Perera AS, McCulloch CA, Sodek J, Zohar R. Osteopontin expression is required for myofibroblast differentiation. Circ Res. 2007;102:319–327. doi: 10.1161/CIRCRESAHA.107.160408. [DOI] [PubMed] [Google Scholar]
- Li F, Li B, Wang QM, Wang JH. Cell shape regulates collagen type I expression in human tendon fibroblasts. Cell Motil Cytoskeleton. 2008;65:332–341. doi: 10.1002/cm.20263. [DOI] [PubMed] [Google Scholar]
- Maniotis AJ, Chen CS, Ingber DE. Demonstration of mechanical connections between integrins, cytoskeletal filaments, and nucleoplasm that stabilize nuclear structure. Proc Natl Acad Sci U S A. 1997;94:849–854. doi: 10.1073/pnas.94.3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews JL, Talmage RV. Influence of parathyroid hormone on bone cell ultrastructure. Clin Orthop Relat Res. 1981;156:27–38. [PubMed] [Google Scholar]
- Misteli T. Beyond the sequence: cellular organization of genome function. Cell. 2007;128:787–800. doi: 10.1016/j.cell.2007.01.028. [DOI] [PubMed] [Google Scholar]
- Morinobu M, Nakamoto T, Hino K, Tsuji K, Shen ZJ, Nakashima K, Nifuji A, Yamamoto H, Hirai H, Noda M. The nucleocytoplasmic shuttling protein CIZ reduces adult bone mass by inhibiting bone morphogenetic protein-induced bone formation. J Exp Med. 2005;201:961–970. doi: 10.1084/jem.20041097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosekilde L. Primary hyperparathyroidism and the skeleton. Clin Endocrinol (Oxf) 2008;69:1–19. doi: 10.1111/j.1365-2265.2007.03162.x. [DOI] [PubMed] [Google Scholar]
- Nakamoto T, Yamagata T, Sakai R, Ogawa S, Honda H, Ueno H, Hirano N, Yazaki Y, Hirai H. CIZ, a zinc finger protein that interacts with p130(cas) and activates the expression of matrix metalloproteinases. Mol Cell Biol. 2000;20:1649–1658. doi: 10.1128/mcb.20.5.1649-1658.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavalko FM, Norvell SM, Burr DB, Turner CH, Duncan RL, Bidwell JP. A model for mechanotransduction in bone cells: the load-bearing mechanosomes. J Cell Biochem. 2003;88:104–112. doi: 10.1002/jcb.10284. [DOI] [PubMed] [Google Scholar]
- Philip B, Childress P, Robling A, Heller A, Bidwell JP. ASBMR 31st Annual Meeting; September 11–15, 2009; Denver, Colorado, USA. 2009. p. SU0107. [Google Scholar]
- Robling AG, Childress P, Yu J, Cotte J, Heller A, Philip BK, Bidwell JP. Nmp4/CIZ suppresses parathyroid hormone-induced increases in trabecular bone. J Cell Physiol. 2009;219:734–743. doi: 10.1002/jcp.21717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe DW. Viewing problems in bone biology from the perspective of lineage identification. J Musculoskelet Neuronal Interact. 2005;5:350–352. [PubMed] [Google Scholar]
- Rubin J, Rubin C, Jacobs CR. Molecular pathways mediating mechanical signaling in bone. Gene. 2006;367:1–16. doi: 10.1016/j.gene.2005.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato MM, Nakashima A, Nashimoto M, Yawaka Y, Tamura M. Bone morphogenetic protein-2 enhances Wnt/beta-catenin signaling-induced osteoprotegerin expression. Genes Cells. 2009;14:141–53. doi: 10.1111/j.1365-2443.2008.01258.x. [DOI] [PubMed] [Google Scholar]
- Sawada Y, Tamada M, Dubin-Thaler BJ, Cherniavskaya O, Sakai R, Tanaka S, Sheetz MP. Force sensing by mechanical extension of the Src family kinase substrate p130Cas. Cell. 2006;127:1015–1026. doi: 10.1016/j.cell.2006.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah R, Alvarez M, Jones DR, Torrungruang K, Watt AJ, Selvamurugan N, Partridge NC, Quinn CO, Pavalko FM, Rhodes SJ, Bidwell JP. Nmp4/CIZ regulation of matrix metalloproteinase 13 (MMP-13) response to parathyroid hormone in osteoblasts. Am J Physiol Endocrinol Metab. 2004;287:E289–296. doi: 10.1152/ajpendo.00517.2003. [DOI] [PubMed] [Google Scholar]
- Shen ZJ, Nakamoto T, Tsuji K, Nifuji A, Miyazono K, Komori T, Hirai H, Noda M. Negative regulation of bone morphogenetic protein/Smad signaling by Cas-interacting zinc finger protein in osteoblasts. J Biol Chem. 2002;277:29840–29846. doi: 10.1074/jbc.M203157200. [DOI] [PubMed] [Google Scholar]
- Sowa H, Kaji H, Iu MF, Tsukamoto T, Sugimoto T, Chihara K. Parathyroid hormone-Smad3 axis exerts anti-apoptotic action and augments anabolic action of transforming growth factor beta in osteoblasts. J Biol Chem. 2003;278:52240–52252. doi: 10.1074/jbc.M302566200. [DOI] [PubMed] [Google Scholar]
- Sparvero LJ, Asafu-Adjei D, Kang R, Tang D, Amin N, Im J, Rutledge R, Lin B, Amoscato AA, Zeh HJ, Lotze MT. RAGE (Receptor for Advanced Glycation Endproducts), RAGE ligands, and their role in cancer and inflammation. J Transl Med. 2009;7:17. doi: 10.1186/1479-5876-7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama T, Saxon LK, Zaman G, Moustafa A, Sunters A, Price JS, Lanyon LE. Mechanical loading enhances the anabolic effects of intermittent parathyroid hormone (1–34) on trabecular and cortical bone in mice. Bone. 2008;43:238–248. doi: 10.1016/j.bone.2008.04.012. [DOI] [PubMed] [Google Scholar]
- Thomas CH, Collier JH, Sfeir CS, Healy KE. Engineering gene expression and protein synthesis by modulation of nuclear shape. Proc Natl Acad Sci U S A. 2002;99:1972–1977. doi: 10.1073/pnas.032668799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thunyakitpisal P, Alvarez M, Tokunaga K, Onyia JE, Hock J, Ohashi N, Feister H, Rhodes SJ, Bidwell JP. Cloning and functional analysis of a family of nuclear matrix transcription factors (NP/NMP4) that regulate type I collagen expression in osteoblasts. J Bone Miner Res. 2001;16:10–23. doi: 10.1359/jbmr.2001.16.1.10. [DOI] [PubMed] [Google Scholar]
- Torrungruang K, Alvarez M, Shah R, Onyia JE, Rhodes SJ, Bidwell JP. DNA binding and gene activation properties of the Nmp4 nuclear matrix transcription factors. J Biol Chem. 2002;277:16153–16159. doi: 10.1074/jbc.M107496200. [DOI] [PubMed] [Google Scholar]
- Torrungruang K, Feister H, Swartz D, Hancock EB, Hock J, Bidwell JP. Parathyroid hormone regulates the expression of the nuclear mitotic apparatus protein in the osteoblast-like cells, ROS 17/2.8. Bone. 1998;22:317–324. doi: 10.1016/s8756-3282(97)00300-1. [DOI] [PubMed] [Google Scholar]
- Turner CH. Bone strength: current concepts. Ann N Y Acad Sci. 2006 Apr;1068:429–46. doi: 10.1196/annals.1346.039. Review. [DOI] [PubMed] [Google Scholar]
- Turner RT, Evans GL, Lotinun S, Lapke PD, Iwaniec UT, Morey-Holton E. Dose-response effects of intermittent PTH on cancellous bone in hindlimb unloaded rats. J Bone Miner Res. 2007;22:64–71. doi: 10.1359/jbmr.061006. [DOI] [PubMed] [Google Scholar]
- Turner RT, Lotinun S, Hefferan TE, Morey-Holton E. Disuse in adult male rats attenuates the bone anabolic response to a therapeutic dose of parathyroid hormone. J Appl Physiol. 2006;101:881–886. doi: 10.1152/japplphysiol.01622.2005. [DOI] [PubMed] [Google Scholar]
- Woo CH, Shishido T, McClain C, Lim JH, Li JD, Yang J, Yan C, Abe J. Extracellular signal-regulated kinase 5 SUMOylation antagonizes shear stress-induced antiinflammatory response and endothelial nitric oxide synthase expression in endothelial cells. Circ Res. 2008 Mar 14;102(5):538–45. doi: 10.1161/CIRCRESAHA.107.156877. Epub 2008 Jan 24. [DOI] [PubMed] [Google Scholar]
- Zhang J, Ryder KD, Bethel JA, Ramirez R, Duncan RL. PTH-induced actin depolymerization increases mechanosensitive channel activity to enhance mechanically stimulated Ca2+ signaling in osteoblasts. J Bone Miner Res. 2006;21:1729–1737. doi: 10.1359/jbmr.060722. [DOI] [PubMed] [Google Scholar]