Abstract
Nearly, one-fifth of childhood cancer survivors (CCSs) smoke cigarettes. Because CCSs are already at greater medical smoking-related risks, targeting them for smoking cessation efforts is a high priority. One of the major challenges with smoking cessation in CCSs is how to reach such a geographically dispersed population. This study aims to demonstrate that these challenges can be overcome through the use of telephone-based tobacco Quit Lines (QLs). This report describes the design of the St. Jude Cancer Survivor Tobacco QL study, which is a randomized controlled clinical trial that will examine the long-term (1-year) efficacy of a counselor initiated versus participant initiated tobacco QL with adjunctive nicotine replacement therapy (NRT) in both groups. Participants (N = 950) will be recruited nationally and randomly assigned to one of the two interventions. The counselor initiated intervention includes six scheduled telephone sessions of a behavioral intervention and provision of 8 weeks of NRT. The participant initiated intervention allows the participant to call the QL at their convenience, but includes the same six telephone sessions and provision of 2 weeks of NRT. Both groups will receive two follow-up phone calls at 8 weeks and 1 year after enrollment to assess their smoking status. The primary outcome measure is cotinine-validated self-reported smoking abstinence at 1-year follow-up. Results from this study will provide the first evidence about the efficacy of intensive QL cessation intervention in this high risk population. Such evidence can lead as well to the dissemination of this intervention to other medically compromised populations.
INTRODUCTION
There are approximately 270,000 adult survivors of childhood cancer in the US [1]. In 2000, the National Cancer Institute estimated that there were approximately 10 million cancer survivors (adult and child survivors) in the U.S. [2]. As cancer therapies improve, the cure rate for pediatric cancers now exceeds 80% and the number of childhood cancer survivors is dramatically increasing [3]. Surprisingly, estimates place the prevalence of smoking among childhood cancer survivors close to that of the general adult population (18% vs. 19.8%, respectively) [4,5]. Pediatric cancer patients are already at risk for developing secondary cancers due to late effects of childhood cancer treatment and genetic factors [6–13]. Relative to their siblings, childhood cancer survivors are 8.2 times more likely to have severe or life-threatening health conditions [14–16]. Exposure to tobacco is known to influence an individual’s risk for disease [17] and, thus, may further increase the risk of adverse health effects among cancer survivors [18, 19]. As such, childhood cancer survivors are likely to be at high risk for health problems when they smoke and targeting them with smoking cessation efforts is a high priority.
One of the major challenges with smoking cessation in CCSs is how to reach such a geographically dispersed population. One method of overcoming the geographic diversity is through the use of technologies such as telephone-based smoking cessation QLs. QLs are increasingly being recognized as a key component of many comprehensive tobacco control programs [20, 21]. QLs are now available throughout most of North America [22], Europe, Australia [23], and many other locations around the world. A key factor in the worldwide adoption of QLs is the solid evidence of their efficacy based on several meta-analytical reviews [24–27].
Pooled data from eight clinical trials with large community samples concluded that counselor initiated QLs (where the counselors proactively contact participants at a predetermined time using a standardized intervention protocol) are associated with increased chances of quitting smoking relative to minimal interventions [28–35]. On the other hand, participant initiated QLs (where participants can call any time during the hours of operation and receive as much or as little intervention as they need), also have been widely implemented, but there have been only two controlled trials that test and support the efficacy of this approach [36,37]. However, most of the studies that have directly compared the effectiveness of counselor initiated QLs versus participant initiated QLs in a “real world setting” showed higher quit rates for counselor initiated compared to participant initiated intervention [28, 30, 38–40].
Research also demonstrates a positive dose-response relationship between number of telephone counseling sessions and long term abstinence. According to a recent Cochrane review [27], three studies compared different schedules and numbers of call-backs to test a dose-response effect [31, 33, 34]. In the first study, no difference was reported between two and six additional calls following an initial 50 minute session [33]. In the second study, six calls increased rates by a further 2% over a single pre-quit call-back [31]. In the third study, an initial extended counseling call with the offer of four further calls increased quit rates by about 1% over an extended counseling call and a brief reminder call [34].
In terms of the intervention content, a recent Cochrane review concluded that the best quit rates are achieved when smokers receive both pharmacological and behavioral treatment [41]. Therefore, an increasing number of QLs have added medication to their cessation service. Eighteen QLs in the United States currently provide free quit medications and five provide discounted quit medications to at least some of their customers [42]. An additional advantage of NRT is that it increases utilization of QLs and adds to their popularity. Lawrence and colleagues recently reported that the addition of free NRT to the Minnesota QL resulted in an eightfold increase in the number of new ex-smokers among registered callers [43]. A similar experience has been reported in the states of New York [44–46], and Oregon [47]. In conclusion, the strongest evidence to date has been for counselor initiated, multi-session counseling with adjunct use of medications [26, 27, 45].
The only known smoking cessation study in childhood cancer survivors demonstrated efficacy [48, 49]. In this study, Emmons and colleagues randomized 796 smokers from the Childhood Cancer Survivor Study (CCSS) to either self-help or peer-counseling tobacco QLs. Results of this study indicate that those in the peer-counseling condition were significantly more likely to self report cessation than the comparison group at 1-year follow-up (15% vs. 9%, p < .01) [48], and at long term (2 to 6 years) follow-up (20.6% vs. 17.6%; P<.0003) [49]. While a seminal study in the field, there are several issues with this particular investigation. First, cessation rates were not biochemically validated. While the majority of smoking cessation studies can rely on self reports of smoking because they are sufficiently reliable and valid, certain subgroups have shown high rates of falsification, one of which is medical patients [50]. In the case of the CCSs, who represent high risk medical patients, determining smoking status by self-report may underestimate the prevalence of smoking. Therefore, biochemical verification of smoking status is especially warranted in this population. Second, in the Emmons’ study, the intervention was delivered by a peer counselor (i.e., the counselors were also childhood cancer survivors). Although peer counseling has been demonstrated to be effective in producing abstinence among CCSs in this study [48], it is more likely that smoking cessation interventions delivered by trained experienced counselors in tobacco dependence will be more effective, less costly, easier to staff, and more amenable to dissemination. Therefore, given that QLs have enormous dissemination potential for CCSs, the main objective of this study is to examine the efficacy of the most intensive QL that has been disseminated to date, counselor initiated QL with an optimal number of sessions, delivered by experienced counselors in tobacco dependence, and with adjunct NRT. To avoid possible falsification of self reports of smoking status, we are validating self-reported point prevalence abstinence at 1-year assessment using salivary cotinine analysis. This report describes the study design, procedures, intervention format and analytic methods of the ongoing St. Jude Cancer Survivors Tobacco QL trial.
METHODS
Design
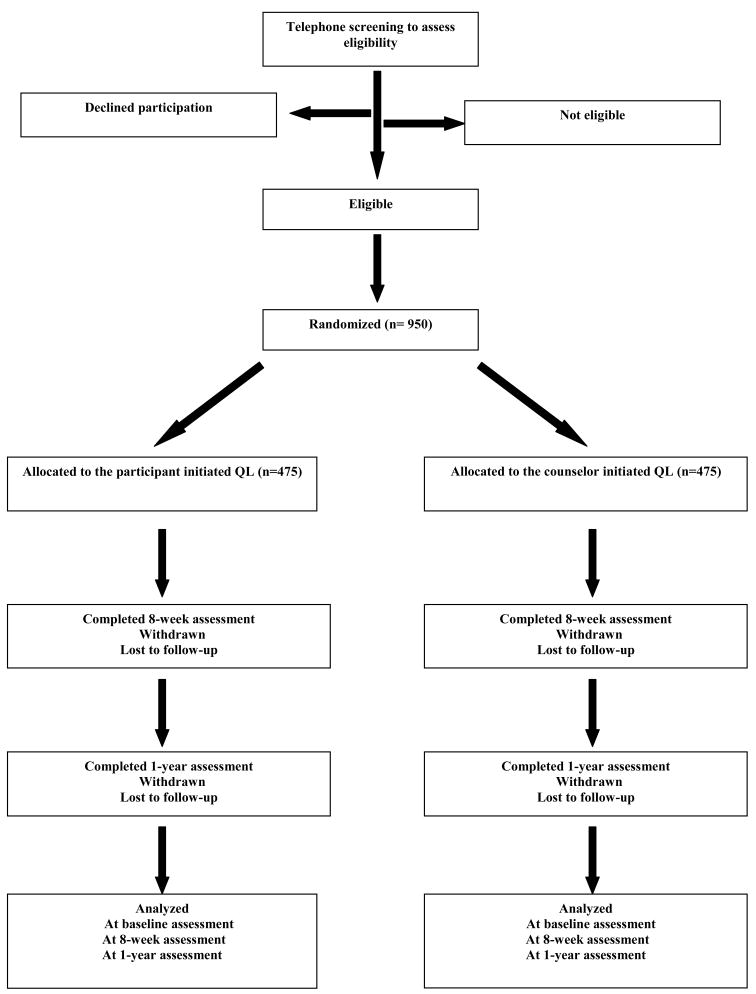
The present study is a two-arm, randomized controlled clinical trial designed to evaluate the long-term (1-year) efficacy of a counselor initiated QL versus a participant initiated QL with adjunctive NRT in both groups. Participants are randomly assigned to one of two interventions. Participants in the counselor initiated intervention receive six scheduled telephone-based counseling sessions and eight weeks of NRT. Participants in the participant initiated intervention who make all six telephone calls receive the same behavioral intervention as in the counselor initiated intervention and 2 weeks of NRT, and are encouraged to obtain additional NRT to complete their treatment course (8 weeks of NRT). Both groups receive two follow-up phone calls at 8 weeks (post treatment) and 1 year after enrollment to assess their smoking status. The main outcome measures are self-reported prolonged and cotinine-validated point-prevalence abstinence at 1-year follow-up. A graph depicting the study flow is presented in Figure (1). The protocol and consent form have been approved by the Institutional Review Board at St. Jude Children’s Research Hospital and the study is registered by the Clinical Trials Registry (#NCT00827866).
Fig. (1).
Study Flow.
It is important to note that we make NRT available to participants in the comparison group for three reasons. First, in designing the study, we opted for a real-world comparison group, in which participants in this group will have information about NRT and access to it as an over-the-counter therapy. Second, we wanted to ensure adherence to the Clinical Practice Guidelines about providing behavioral counseling with medication as a minimum intervention for treating smoking cessation [25]. Third, providing NRT in the comparison group was strongly urged by a panel of quit line experts. There is ample evidence that providing NRT increases both quit line utilization and outcome [43–47]. In addition, it was believed that the most minimal quit lines in the future will include access to counselors plus some provision of NRT. As such, not providing at least some NRT to the comparison group would be an unfair comparison and not in line with what quit lines are, and what they will be providing in the future.
Study Participants
We are nationally recruiting 950 adult smokers (≥18 years old) who are childhood cancer survivors (cancer diagnosis before the age of 21), and are at least one year out of active cancer treatment. They must have been smoking continuously for the last year, and be willing to make a serious quit attempt in the next 30 days. Any histological subtype of childhood cancer qualifies for entry into this study. Participants must speak English and have access to a telephone for participation. It is important to mention that individuals who have contraindications for using NRT, and those who smoke less than 5 cigarettes per day, have allergy to nicotine, or women who are pregnant, breastfeeding or planning to become pregnant within the next 2 months are eligible to participate in this study, but do not receive NRT. This decision was made to reflect the “real world” nature of tobacco QLs in which QLs will take “all comers” for cessation. Table 1 shows detailed inclusion and exclusion criteria.
Table 1.
Inclusion and Exclusion Criteria.
| Inclusion criteria for participating in the study | Exclusion criteria for using NRT |
|---|---|
| Cancer diagnosis before age 21 | Smoke fewer than 5 cigarettes per day |
| At least one year from active cancer treatment | Women who are pregnant, breastfeeding, or planning to become pregnant within the next two months. |
| Age 18 or older | Allergy to nicotine |
| Speaks English | History of: |
| Has telephone access | Myocardial infarction |
| Ability to understand consent procedures | Unstable angina |
| Cerebrovascular incident | |
| Severe arrhythmias | |
| Vascular disease | |
| Phaeochromocytoma | |
| Diabetes | |
| Hyperthyroidism | |
| Abnormal kidney or liver function | |
| Gastritis or peptic ulcers |
PROCEDURES
Recruitment and screening
To overcome the difficulties of reaching potential participants in this highly mobile and geographically dispersed population, we are adopting a combination of proactive (defined as strategies involving real-time interpersonal contact with the researcher) and reactive (defined as strategies without real-time interpersonal contact with the researcher) recruitment techniques. These strategies have been shown consistently to enhance recruitment in smoking studies [51–58]. The proactive approach includes recruitment from three very large data sets of CCSs. First, we are proactively recruiting through the Childhood Cancer Survivors Study (CCSS), which is a multi-institutional collaborative project and the single most comprehensive body of information ever assembled on the long-term health status of childhood cancer survivors. Second, we are proactively recruiting through the St. Jude Lifetime Cohort Study (SJLIFE), which is a lifetime cohort of approximately 3000 childhood cancer survivors used for the investigation of the multi-factorial etiology of adverse health outcomes in aging adults surviving pediatric cancer. Third, we are also proactively recruiting through the St. Jude After Completion of Therapy Clinic (ACT), which provides comprehensive medical services to evaluate late treatment toxicities for over 12,000 pediatric cancer survivors. The reactive recruitment approach includes advertising through television, radio, newspaper, press conferences, internet, and mailings. However, telephone recruitment through the CCSS is serving as the primary method of recruiting participants into the study where the CCSS Coordinating Center and Survey Research Facility are assisting study administration.
CCSS participants identified as smokers are mailed study invitation letters. This letter introduces them to the study and notifies them that a member of the QL team will contact them to provide additional information and assess their interest in participation. After a period of approximately two weeks, an initial telephone screening interview is conducted to give potential participants a detailed description of the study and to assess their interest in participating. Individuals declining participation are encouraged to quit smoking, counseled regarding the benefits of quitting and given a list of nationally available cessation resources. For those who are interested, eligibility is confirmed, and then verbal consent, baseline measures, and contact information are obtained.
Randomization
Randomization is performed in blocks of size six to ensure that treatment assignment is balanced across groups over the enrollment period. After randomization, participants are contacted by phone and notified of their study group assignment. During this call, the informed consent and contact information are reviewed again. Participants in both interventions are provided the opportunity to begin the smoking cessation program immediately during this call. Should they choose to postpone it, they are encouraged to begin the program within the next 7 days.
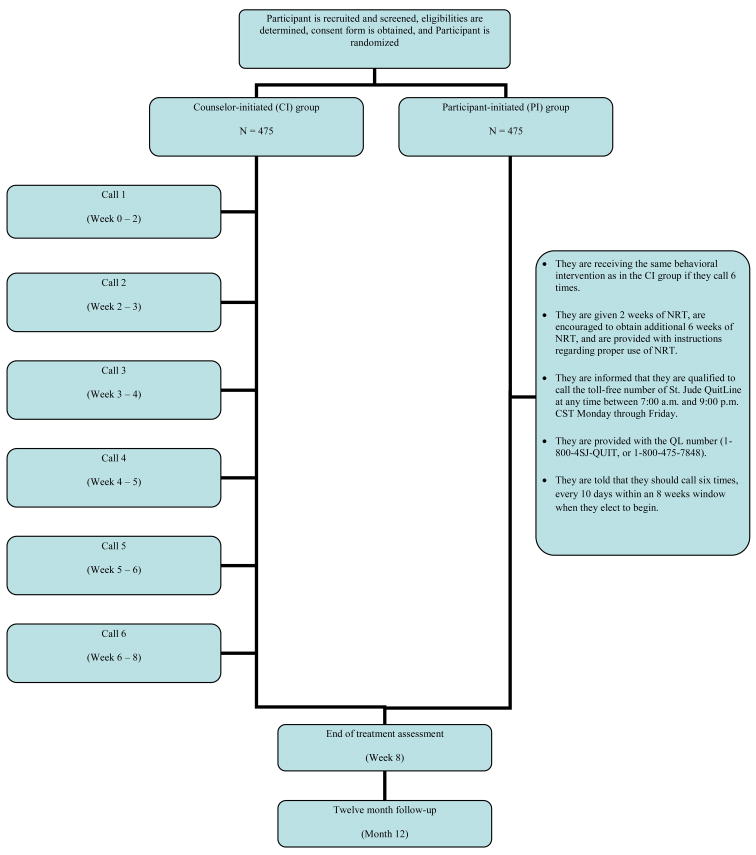
After the phone call, participants assigned to the counselor initiated intervention are mailed; (1) a summary letter that contains an overview of the study, a written version of the informed consent, group assignment, an explanation of risks associated with participation, and assurance of confidentiality, (2) “Curbing your Cravings” brochure to provide them with detailed instructions regarding the proper use of NRT (patches & gum) and their side effects, and (3) a 4-week supply of nicotine patches followed by a second mailing of four additional weeks if they have successfully quit or have reset their quit date after one failure. Participants assigned to the participant initiated intervention receive the same information but a 2-week starter pack of nicotine patches, and are encouraged to purchase the patch for six additional weeks. They are also informed that they are qualified to call the toll-free telephone number of St. Jude Childhood Cancer Survivors Tobacco Quit Line at anytime between 7 a.m. and 9 p.m. Central Standard Time, Monday through Friday and they are provided with the QL number (1-800-4SJ-QUIT, or 1-800-475-7848). Finally, they are told that they have an eight week “window” and should call six times within that window (approximately one call every 10 days). Initial patch dose is tailored to participants’ smoking rates based on the following algorithm, > 20 cigarettes/d (21 mg), 10–19 cigarettes/d (14 mg), and 5–9 cigarettes/d (7 mg). If a participant is initially placed on the 21 mg patch, the following dosing schedule is being used: 21 mg patch/day for 4 weeks, 14 mg patch/day for 2 weeks, 7 mg patch/day for 2 weeks, and then off. If a participant is initially placed on the 14 mg patch, the following dosing schedule is being used: 14 mg patch/day for 4 weeks, 7 mg patch/day for 4 weeks, and then off. If a participant is initially placed on the 7 mg patch, the following dosing schedule is being used: 7 mg patch/day for 8 weeks and then off. This dosing schedule provides eight weeks of exposure to the patch. Patch use begins at the day of the quit date.
Intervention format
The intervention integrates principles from two theories of behavior change, Social Cognitive Theory [59] and Social Determination Theory [60]. It reflects a strong focus on enhancing self-efficacy, modifying outcome expectancies, and developing effective self-regulatory skills. The intervention utilizes strategies to enhance self-efficacy for behavior change by providing opportunities for mastery experiences through short-term goal setting and helping participants alter cognitions associated with their perceived ability to successfully execute behaviors associated with cessation [61–63]. In addition, the intervention involves modifying outcome expectancies related to the targeted behavior changes in an effort to provide more accurate expectations regarding what they can expect to experience after quitting smoking. These include both positive (e.g., improved stamina, improved social image) and negative (e.g., nicotine withdrawal symptoms, weight gain) changes associated with abstinence. Finally, the intervention focuses on developing effective self-regulatory skills through strategies such as self-monitoring, goal setting, skills training, modeling, and self-reinforcement [64, 65].
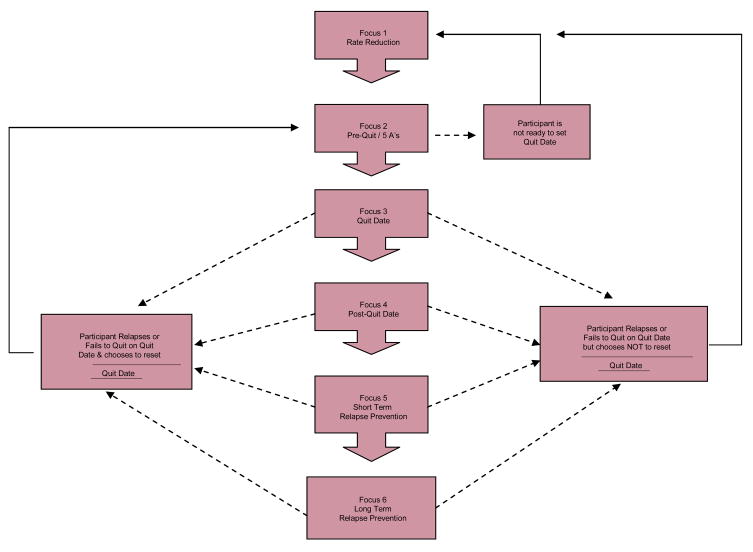
The behavioral smoking cessation intervention consists of six weekly, 15–30 minute individualized telephone counseling sessions that lead participants through three primary phases: (1) preparing to quit, (2) going through the quitting process, and (3) relapse prevention and maintaining short and long-term smoking abstinence. The “preparing to quit” phase includes implementing smoking rate reduction strategies, breaking the cues related to smoking, engaging in a stop smoking "ritual" prior to quitting, and enhancing social support. The quitting phase includes setting a quit date and observing careful instructions regarding NRT use. Finally, the “relapse prevention” phase includes identifying high-risk situations and devising an individual relapse prevention plan to deal with slips and lapses (Fig. 2; Intervention Format). Participants in both interventions receive a maximum of 6 telephone counseling sessions and the intervention is discontinued at this point regardless of where they are in the cessation process (Fig. 3; Study Time-line and Procedures by Conditions).
Fig. 2.
Intervention Format.
Figure (3).
The study time-line and procedures.
Rate reduction intervention
Participants who slip or relapse are encouraged to set a new quit date as soon as possible. Participants who fail to quit smoking and decline to reset a quit date are given a rate reduction intervention. There is empirical evidence to suggest that rate reduction serves as a springboard to later cessation for individuals who are not ready to quit [66–69]. Research has shown that even in participants who are unable or not ready to quit, rate reduction may enhances the probability of cessation [70–71]. Therefore, supplemental rate reduction materials as well as nicotine gum are provided to these participants in hopes of increasing their ability to quit smoking in the future. Rate reduction strategies for these select participants include; switching to a low tar/low nicotine cigarette, reducing number of daily cigarettes by 60%, delaying the first cigarette of the day, monitoring their daily intake in comparison to their daily goal, carrying only the number of goal cigarettes each day, limiting smoking to only certain situations and certain times, and limiting access to cigarettes.
Follow-up and retention
All participants receive two follow-up calls at 8 weeks (post treatment) and 1 year after enrollment to assess their smoking status. Biochemical validation of smoking status via salivary cotinine analysis is obtained for those who report prolonged abstinence at 1 year. Biochemical measurement of smoking abstinence (e.g. salivary cotinine) in population-based studies is problematic as these studies enroll a large number of participants that are often widely dispersed in geography. Some studies have tried to validate cessation through the mail; unfortunately, this method has proven to be limited by high refusal and non-adherence rates in spite of monetary incentives (e.g., 44.3% adherence rate) [30]. A second limitation is uncertainly about the source of the returned saliva samples as one could argue that falsifiers might provide saliva from a nonsmoking family member or friend. To address this issue, we are using a strategy employed by the CCSS, which involves contracting with a nationwide reach private paramedical company, Examination Management Services, Inc. (EMSI) [www.emsinet.com/research.aspx], to obtain saliva samples from participants anywhere in the country at a convenient time and place (e.g., their home, workplace, or public place). This approach eliminates the inconvenience of having to travel to a clinic to provide a sample, and guarantees the source of the obtained sample. It also lessens the number of circumstances that may result in participants not responding to mailed sampling such as loss of test kits, misunderstanding directions, and general apathy.
To maintain active participation for the entire year of the study, extensive retention measures are being taken. These include; collecting names, addresses, and phone numbers for relatives/friends who would know the participant’s whereabouts; contacting participants with personalized letters and cards and remembering them at special occasions (e.g., birthdays, holidays); setting up a central file with names, addresses, phone numbers, and time windows of all participants; sending out a newsletter with project-relevant information twice a year; and taking an individual case management approach for handling difficult cases.
Interventionist training
All interventionists underwent an intensive full time three-week training period. This involved training in human subjects protection, motivational interviewing, behavioral theories, pharmacotherapies used in smoking cessation, health conditions in childhood cancer survivors, counseling protocol, quality control procedures, adverse events, and role playing of project procedures. The counselors meet twice a week for quality control, supervision, and case review.
Data collection, measures and outcomes
Data are collected at baseline, 8 weeks (post treatment) and 1 year after enrollment. At baseline, several demographic variables are collected, including age, height/weight, gender, marital status, income, occupation, years of education, and ethnicity. Several tobacco use/quitting variables are also collected at baseline including the number of years as a cigarette smoker, current amount smoked, attitudes toward quitting, prior cessation experience, and the Fagerström Test for Nicotine Dependence (FTND) [72].
NRT side effects and adverse events are monitored throughout the study. Concomitant medication use is monitored as it can affect cessation rates. For this same reason, participation in additional stop-smoking behavioral programs is carefully tracked. We also carefully track participant adherence to treatment sessions as well as patch use. This information will be used in secondary analyses to explore whether there is a relationship between adherence and cessation outcomes and whether differences in “intervention dose” account for some or all of the proposed increased efficacy of the counselor initiated condition.
Smoking status is evaluated at 8 weeks (post treatment) and 1 year after enrollment in both intervention conditions using both prolonged and 7-day point-prevalence abstinence criteria [73]. Prolonged abstinence refers to sustained abstinence following an initial grace period defined for purposes of this study as the two weeks immediately following a participants' quit date [73]. Point-prevalence abstinence refers to self-reported smoking status (yes vs. no) at a particular point in time, with no correction for previous or subsequent smoking status. Given that medical patients are at risk for underreporting their smoking status [48, 50], we are validating self-reported abstinence at 1-year assessment using salivary cotinine analysis <15ng/ml [74]. Participants who report abstinence but refuse to provide a saliva sample, will be coded as smokers.
Sample size
Power estimates are based on detecting a difference in cotinine-validated self-reported smoking abstinence at 1-year follow-up using unadjusted proportions, which is a conservative approach to powering cessation trials. As the only published study of smoking cessation in childhood cancer survivors yielded a 6% difference between treatment and control [48], we believe that we can replicate this 6% difference with a well-validated counselor initiated QL, NRT supplementation, and a motivated medically at risk population. Still, given the paucity of data on childhood cancer patients from randomized trials, we chose to be conservative and powered the study to detect a difference of 5% in cotinine-validated self-reported smoking abstinence between the counselor initiated and participant initiated conditions. Based on these assumptions and using a two-sided significance level of α=0.05 and 80% power, we calculated sample sizes assuming a 5% quit rate in the participant initiated group and a 10% quit rate in the counselor initiated group, we would need approximately 474 participants per intervention arm or about 950 total.
DATA ANALYSES
Two preliminary analyses will be conducted. First, to enhance participation and reach of our intervention in the future based on CONSORT-RE-AIM approach [75], we will initially compare differences in the characteristics (e.g., demographics, smoking history, nicotine dependence) of smokers who agree to participate in our study with those who decline participation using descriptive statistics for the nominal variables [chi-square (X2) or Fisher’s exact test] and t-test or a Mann-Whitney test for continuous variables, as appropriate. Second, using logistic regression and classifying all study participants as completers or lost to follow-up, we will explore which variables, if any, are associated with missing data. Most important is to investigate whether there is an association between dropout rate and the two study interventions (counselor initiated and participant initiated).
The primary analysis for which the study is powered involves comparing the difference between the two interventions in the self-reported prolonged abstinence and cotinine-validated point-prevalence abstinence at 1-year follow-up. Consistent with the intent-to-treat analyses, all eligible randomized participants will be included in the primary analysis, and thus, any participant who fails to be evaluated one year after enrollment will be included as a smoker. Another analysis will be conducted to assess the difference in point prevalence of abstinence at two time points of interest (8 weeks and 1 year). Two-way categorical analyses using X2 statistics for categorical variables and analysis of variance for continuous variables will be used to assess the relationships between smoking cessation and the potential independent predictors, including demographic and medical history factors, smoking history, nicotine dependence (FTND), and intervention conditions. A logistic regression model predicting cessation will be developed using all variables from the bivariate analyses with statistical significance at the P<0.05 level. A final parsimonious model will include only significant variables and effect modifiers. All two-way interactions will be examined for significance in this final model.
We will also conduct secondary analyses to account for dose of treatment (NRT use plus number of QL contact) vs. success (quit for one year response) independent of treatment assignment. For this analysis we will ignore intervention assignment and look at two factors that might influence the two primary outcomes (self reported prolonged abstinence and cotinine-validated point-prevalence abstinence). The two factors, regardless of treatment assignment, are use of NRT during the eight weeks post randomization (yes vs. no) and number of QL contacts during the 8 weeks treatment period. For this latter variable, a participant will score from 0 to 6, with six meaning at least one contact with the QL in each of the weekly sessions. Ideally, a participant assigned to the counselor initiated intervention would get a “yes” for NRT and a score of six for QL contacts. A participant assigned to the participant initiated group could also have a yes response for NRT use and a score of six for QL contacts if they purchased and used NRT beyond the 2 week supply, and contacted the QL six times during their treatment period. We will also track how much NRT is used by participants to determine if there is a “dose” effect between degree of use and outcome.
Discussion
This paper describes the design of the St. Jude Cancer Survivors Quit Line (SJCSQL) study, which aims to evaluate the long-term (1-year) efficacy of a counselor initiated QL versus a participant initiated QL with adjunctive NRT in both groups among smokers who had cancer as children. Given the scarcity of evidence about the efficacy of QLs among childhood cancer survivors and that QLs have enormous dissemination potential in this geographically dispersed population, we believe that this randomized controlled trial will be the first to provide evidence for the efficacy of an intensive counselor initiated QL with provided NRT in childhood cancer survivors.
There are several important strengths of the SJCSQL study. In designing the study, we opted for a real-world comparison group, in which participants in the participant initiated intervention will have information about NRT and access to it as an over-the-counter medication. This study also involves a large sample size that will be recruited nationally, and thus will be sufficiently powered to detect the study outcomes, as well as be conducive to generalization. In addition, the outcome data will be analyzed using the intention-to-treat principle so that the study findings will be conservative.
As previously mentioned, to overcome the difficulties in reaching potential participants in this highly mobile and dispersed population, we are utilizing a combination of proactive and reactive recruitment strategies. This will allow us to test the effectiveness of each recruitment strategy, identify the characteristics of CCS smokers that affect their likelihood of participation, and determine whether tobacco QLs are as effective for directly enrolled participants as for those who call on their own. As such, this study will guide future work on the best way to approach and reach this population in order to improve the intervention dissemination and success.
The issue of biochemical validation in smoking cessation studies is still an important one. This is due to the difficulty and cost of obtaining and analyzing biological samples, and the presence of different views on the necessity of obtaining biochemical validation [50]. Some researchers believe that participants will typically underreport their level of cigarette consumption and recommend reliance on biochemical measures [76,77] while others favor the use of self-report and trust its validity [78,79]. Still, studies have demonstrated that certain subgroups have shown high rates of falsification, one of which is high risk medical patients [50]. In the only known smoking cessation study in childhood cancer survivors [48], abstinence rates were not biochemically validated. In order to overcome this shortcoming, we opted to obtain biomedical validation of smoking status in this study. This will guide future studies among CCSs and other high risk medical groups in terms of whether it is necessary to use biochemical validation, a procedure with great cost and labor implications.
Finally, it is important to note that the present study has the potential for immediate dissemination. Following the completion of this study, if the program is proven efficacious, St. Jude Children’s Research Hospital is committed to disseminating this program as part of its outreach activities. In conclusion, we believe that the present study will address several key questions and provide important insights on the future of smoking cessation QL studies in CCSs and other medically compromised populations.
Acknowledgments
This study described in this manuscript is supported by research grants (R01CA127964-01A1) from the National Cancer Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hewitt M, Weiner SL, Simone JV. Childhood Cancer Survivorship: Improving Care and Quality of Life. Washington, DC: National Cancer Policy Board; 2003. [PubMed] [Google Scholar]
- 2.Reis LAG, Eisner MP, Kosary CL, Hankey BF, Miller BA, Clegg L, et al. SEER cancer statistics review, 1973–1999. National Cancer Institute; Bethesda, MD: 2002. http://seer.cancer.gov/csr/1973_1999/ [Google Scholar]
- 3.Ries LAG, Melbert D, Krapcho M, Mariotto A, Miller BA, Feuer EJ, et al. SEER Cancer Statistics Review, 1975–2004. National Cancer Institute; Bethesda, MD: 2007. http://seer.cancer.gov/csr/1975_2004/ [Google Scholar]
- 4.Emmons KM, Butterfield RM, Puleo E, Park ER, Mertens A, Gritz ER, et al. Smoking among participants in the childhood cancer survivors cohort: the Partnership for Health study. J Clin Oncol. 2003;21:189–196. doi: 10.1200/JCO.2003.06.130. [DOI] [PubMed] [Google Scholar]
- 5.CDC. Cigarette Smoking Among Adults - United States, 2006. MMWR. 2007;56:1157–1161. [PubMed] [Google Scholar]
- 6.Eiser C. Beyond Survival: Quality of Life and Follow-up After Childhood Cancer. Journal of Pediatric Psychology. 2007;32(9):1140–1150. doi: 10.1093/jpepsy/jsm052. [DOI] [PubMed] [Google Scholar]
- 7.Hudson MM, Oeffinger KC. Future Health of Survivors of Adolescent and Young Adult Cancer. Pediatric Oncology. 2007:451–467. [Google Scholar]
- 8.Tercyak KP, Britto MT, Hanna KM, Hollen PJ, Hudson MM. Prevention of tobacco use among medically at-risk children and adolescents: clinical and research opportunities in the interest of public health. J Pediatr Psychology. 2008;33(2):119–32. doi: 10.1093/jpepsy/jsm132. [DOI] [PubMed] [Google Scholar]
- 9.Children’s Oncology Group. [Accessed on February 2, 2007];Long-term follow-up guidelines for survivors of childhood, adolescent, and young adult cancers. Available at URL: http://www.survivorshipguidelines.org.
- 10.O’Driscoll BR, Hasleton PS, Taylor PM, Poulter LW, Gattamaneni HR, Woodcock AH. Active lung fibrosis up to 17 years after chemotherapy with carmustine (BCNU) in childhood. N Engl J Med. 1990;323:378–382. doi: 10.1056/NEJM199008093230604. [DOI] [PubMed] [Google Scholar]
- 11.Kaste SC, Rai SN, Fleming K, McCammon EA, Tylavsky FA, Danish RK, et al. Changes in bone mineral density in survivors of childhood acute lymphoblastic leukemia. Pediatr Blood Cancer. 2006;46:77–87. doi: 10.1002/pbc.20553. [DOI] [PubMed] [Google Scholar]
- 12.Benoist MR, Lemerle J, Jean R. Effects on pulmonary function of whole lung irradiation for Wilm’s tumor in children. Thorax. 1982;37:175–180. doi: 10.1136/thx.37.3.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lipshultz SE, Colan SD, Gelbar RD, Perez-Atayde AR, Sallan SE, Sanders SP. Late cardiac effects of doxorubicin therapy for acute lymphoblastic leukemia in childhood. N Engl J Med. 1991;324:808–815. doi: 10.1056/NEJM199103213241205. [DOI] [PubMed] [Google Scholar]
- 14.Mulrooney DA, Dover DC, Li S, Yasui Y, Ness KK, Mertens AC, et al. Childhood Cancer Survivor Study. Twenty years of follow-up among survivors of childhood and young adult acute myeloid leukemia: a report from the Childhood Cancer Survivor Study. Cancer. 2008;112(9):2071–9. doi: 10.1002/cncr.23405. [DOI] [PubMed] [Google Scholar]
- 15.Oeffinger KC, Mertens AC, Sklar CA, Kawashima T, Hudson MM, Meadows AT, et al. Chronic health conditions in adult survivors of childhood cancer. The New England Journal of Medicine. 2006;355:1572–1582. doi: 10.1056/NEJMsa060185. [DOI] [PubMed] [Google Scholar]
- 16.Hudson MM, Mertens AC, Yasui Y, Hobbie W, Chen H, Gurney JG, et al. Health Status of Adult Long-term Survivors of Childhood Cancer. A Report From the Childhood Cancer Survivor Study. JAMA. 2003;290:1583–1592. doi: 10.1001/jama.290.12.1583. [DOI] [PubMed] [Google Scholar]
- 17.U. S. Department of Health and Human Services. The tobacco use and dependence clinical practice guideline panel, staff and consortium representatives: A clinical practice guideline for treating tobacco use and dependence: A US Public Health Service Report. J Am Med Assoc. 2000;283:2344–2354. [PubMed] [Google Scholar]
- 18.Forrest CB, Riley AW. Childhood origins of adult health: A basis for life-course health policy. Health Affairs. 2004;23:155–164. doi: 10.1377/hlthaff.23.5.155. [DOI] [PubMed] [Google Scholar]
- 19.Mackenbach JP, Borsboom GJ, Nusselder WJ, Looman CW, Schrijvers CT. Determinants of levels and changes of physical functioning in chronically ill persons: Results from the GLOBE Study. Journal of Epidemiology and Community Health. 2001;55:631–638. doi: 10.1136/jech.55.9.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fiore M, Croyle R, Curry S, Cutler CM, Davis RM, Gordon C, et al. Preventing 3 million premature deaths and helping 5 million smokers quit: a national action plan for tobacco cessation. Am J Public Health. 2004;94:205–10. doi: 10.2105/ajph.94.2.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Centers for Disease Control and Prevention. Telephone quitlines: a resource for development, implementation, and evaluation. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, Final Edition; Sep, 2004. [Google Scholar]
- 22.North American Quitline Consortium. Quitlines of North America and Europe 2006. Phoenix, AZ: NAQC; 2006. [Google Scholar]
- 23.MCDS. Australian national tobacco strategy, 2004–2009. Canberra: Department of Health and Ageing; 2005. [Google Scholar]
- 24.Lichtenstein E, Glasgow RE, Lando HA, Ossip-Klein DJ, Boles SM. Telephone counseling for smoking cessation: rationales and meta-analytic review of evidence. Health Educ Res. 1996;11:243–257. doi: 10.1093/her/11.2.243. [DOI] [PubMed] [Google Scholar]
- 25.Treating tobacco use and dependence: 2008 update. Rockville (MD): U.S. Department of Health and Human Services, Public Health Service; 2008. May, p. 257. [Google Scholar]
- 26.Stead LF, Lancaster T, Perera R. Telephone counseling for smoking cessation. The Cochrane DB Syst Rev. 2004;4 doi: 10.1002/14651858.CD002850. [DOI] [PubMed] [Google Scholar]
- 27.Stead LF, Perera R, Lancaster T. A systematic review of interventions for smokers who contact quitlines. Tob Control. 2007;16:i3–i8. doi: 10.1136/tc.2006.019737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Borland R, Segan CJ, Livingston PM, Owen N. The effectiveness of callback counseling for smoking cessation: a randomized trial. Addiction. 2001;96:881–889. doi: 10.1046/j.1360-0443.2001.9668819.x. [DOI] [PubMed] [Google Scholar]
- 29.Borland R, Balmford J, Segan C, Livingston P, Owen N. The effectiveness of personalized smoking cessation strategies for callers to a Quitline service. Addiction. 2003;98(6):837–846. doi: 10.1046/j.1360-0443.2003.00389.x. [DOI] [PubMed] [Google Scholar]
- 30.Zhu SH, Anderson CM, Tedeschi GJ, Rosbrook B, Johnson CE, Byrd M, et al. Evidence of real-world effectiveness of a telephone quitline for smokers. N Engl J Med. 2002;347(14):1087–93. doi: 10.1056/NEJMsa020660. [DOI] [PubMed] [Google Scholar]
- 31.Zhu S-H, Stretch V, Balabanis M, Rosbrook B, Sadler G, Pierce JP. Telephone Counseling for Smoking Cessation: Effects of Single-Session and Multiple-Session Interventions. Journal of Consulting and Clinical Psychology. 1996;64:202–211. doi: 10.1037//0022-006x.64.1.202. [DOI] [PubMed] [Google Scholar]
- 32.McAlister AL, Rabius V, Geiger A, Glynn TJ, Huang P, Todd R. Telephone assistance for smoking cessation: one year cost effectiveness estimations. Tob Control. 2004;13(1):85–6. doi: 10.1136/tc.2003.004515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith PM, Cameron R, McDonald PW, Kawash B, Madill C, Brown KS. Telephone counseling for population-based smoking cessation. American Journal of Health Behavior. 2004;28(3):231–41. doi: 10.5993/ajhb.28.3.4. [DOI] [PubMed] [Google Scholar]
- 34.Hollis J, McAfee T, Stark M, Fellows J, Zbikowski S, Riedlinger K. One-year outcomes for six Oregon tobacco Quitline interventions. Annals of Behavioral Medicine. 2005;29 Suppl:S056. [Google Scholar]
- 35.Gilbert H, Sutton S. Evaluating the effectiveness of proactive telephone counselling for smoking cessation in a randomized controlled trial. Addiction. 2006;101:590–8. doi: 10.1111/j.1360-0443.2006.01398.x. [DOI] [PubMed] [Google Scholar]
- 36.Ossip Klein DJ, Giovino GA, Megahed N, Black PM, Emont SL, Stiggins J, et al. Effects of a smoker’s hotline: results of a 10-county self-help trial. Journal of Consulting and Clinical Psychology. 1991;59:325–32. doi: 10.1037//0022-006x.59.2.325. [DOI] [PubMed] [Google Scholar]
- 37.McFall SL, Michener A, Rubin D, Flay BR, Mermelstein RJ, Burton D, et al. The effects and use of maintenance newsletters in a smoking cessation intervention. Addictive Behavior. 1993;18:151–8. doi: 10.1016/0306-4603(93)90045-b. [DOI] [PubMed] [Google Scholar]
- 38.Macleod ZR, Charles MA, Arnaldi VC, Adams IM. Telephone counseling as an adjunct to nicotine patches in smoking cessation: a randomized controlled trial. Med J Aust. 2003;79:349–352. doi: 10.5694/j.1326-5377.2003.tb05590.x. [DOI] [PubMed] [Google Scholar]
- 39.Helgason AR, Tomson T, Lund KE, Galanti R, Ahnve S, Gilljam H. Factors related to abstinence in a telephone helpline for smoking cessation. Eur J Public Health. 2004;14:306–310. doi: 10.1093/eurpub/14.3.306. [DOI] [PubMed] [Google Scholar]
- 40.Holtrop JS, Wadland WC, Vansen S, Weismantel D, Fadel H. Recruiting health plan members receiving pharmacotherapy into smoking cessation counseling. Am J Manage Care. 2005;11:501–507. [PubMed] [Google Scholar]
- 41.Stead LF, Perera R, Lancaster T. Telephone counselling for smoking cessation. Cochrane Database of Systematic Reviews. 2006;(3) doi: 10.1002/14651858.CD002850.pub2. [DOI] [PubMed] [Google Scholar]
- 42.Cummins SE, Bailey L, Campbell S, Koon-Kirby C, Zhu S-H. Tobacco cessation quitlines in North America: a descriptive study. Tob Control. 2007;6:i9–15. doi: 10.1136/tc.2007.020370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.An LC, Zhu S-H, Nelson DB, Arikian NJ, Nugent S, Partin MR, et al. Benefits of telephone care over primary care for smoking cessation. Archives of Internal Medicine. 2006;166:536–42. doi: 10.1001/archinte.166.5.536. [DOI] [PubMed] [Google Scholar]
- 44.Bauer JE, Carlin-Menter SM, Celestino PB, Hyland A, Cummings KM. Giving away free nicotine medications and a cigarette substitute (Better Quit) to promote calls to a quitline. J Public Health Management Practice. 2006;12:60–7. doi: 10.1097/00124784-200601000-00012. [DOI] [PubMed] [Google Scholar]
- 45.Cummins SE, Hebert KK, Anderson CM, Mills JA, Zhu S-H. Reaching Young Adult Smokers Through Quitlines. American Journal of Public Health. 2007;97(8):1402–1405. doi: 10.2105/AJPH.2006.101741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tinkelman D, Wilson SM, Willett J, Sweeney CT. Offering free NRT through a tobacco quitline: impact on utilisation and quit rates. Tob Control. 2007;16:i42–i46. doi: 10.1136/tc.2007.019919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hollis JF, McAfee TA, Fellows JL, Zbikowski SM, Michael SM, Riedlinger K. The effectiveness and cost effectiveness of telephone counselling and the nicotine patch in a state tobacco quitline. Tob Control. 2007;16:i53–i59. doi: 10.1136/tc.2006.019794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Emmons KM, Puleo E, Park E, Gritz ER, Butterfield RM, Weeks JC, Mertens A, Li FP. Peer-delivered smoking counseling for childhood cancer survivors increases the rate of cessation: the Partnership for Health Study. J Clin Oncol. 2005;23:6516–6523. doi: 10.1200/JCO.2005.07.048. [DOI] [PubMed] [Google Scholar]
- 49.Emmons KM, Puleo E, Mertens A, Gritz ER, Diller L, Li FP. Long-Term Smoking Cessation Outcomes Among Childhood Cancer Survivors in the Partnership for Health Study. J Clin Oncol. 2009;27:52–60. doi: 10.1200/JCO.2007.13.0880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Velicer WF, Prochaska JO, Rossi JS, Snow MG. Assessing outcome in smoking cessation studies. Psychol Bull. 1992;11:23–41. doi: 10.1037/0033-2909.111.1.23. [DOI] [PubMed] [Google Scholar]
- 51.Bircan E, Quang B, Huggins R, Harper T, White V. Investigating the relation between placement of quit antismoking advertisements and number of telephone calls to quitline: a semiparametric modelling approach. J Epidemiol Community Health. 2006;60:180–2. doi: 10.1136/jech.2005.038109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wilson N, Grigg M, Graham L, Cameron G. The effectiveness of television advertising campaigns on generating calls to a national Quitline by Maori. Tob Control. 2005;14:284–6. doi: 10.1136/tc.2004.010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pierce JP, Anderson DM, Romano RM, Meissner HI, Odenkirchen JC. Promoting smoking cessation in the United States: effect of public service announcements on the Cancer Information Service telephone line. J Natl Cancer Inst. 1992;84:677–83. doi: 10.1093/jnci/84.9.677. [DOI] [PubMed] [Google Scholar]
- 54.Miller CL, Wakefield M, Roberts L. Uptake and effectiveness of the Australian telephone quitline service in the context of a mass media campaign. Tob Control. 2003;12(Suppl II):ii53–8. doi: 10.1136/tc.12.suppl_2.ii53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Carroll T, Rock B. Generating quitline calls during Australia’s national tobacco campaign: effective of television advertisement execution and programme placement. Tob Control. 2003;12(Suppl II):ii40–4. doi: 10.1136/tc.12.suppl_2.ii40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Paul CL, Wiggers J, Daly JB, Green S, Walsh RA, Knight J, Girgis A. Direct telemarketing of smoking cessation interventions: Will smokers take the call? Addiction. 2004;99:907–913. doi: 10.1111/j.1360-0443.2004.00773.x. [DOI] [PubMed] [Google Scholar]
- 57.Prochaska JO, Velicer WF, Fava JL, Rossi JS, Tsoh JY. Evaluating a population-based recruitment approach and a stage-based expert system intervention for smoking cessation. Addictive Behaviors. 2001;26:583–602. doi: 10.1016/s0306-4603(00)00151-9. [DOI] [PubMed] [Google Scholar]
- 58.Velicer WF, Prochaska JO, Fava JL, Laforge RG, Rossi JS. Interactive versus noninteractive interventions and dose-response relationships for stage-matched smoking cessation programs in a managed care setting. Healthy Psychology. 1999;18:21–28. doi: 10.1037//0278-6133.18.1.21. [DOI] [PubMed] [Google Scholar]
- 59.Bandura A. Social Foundations of Thought and Action: A Social Cognitive Theory. Englewood Cliffs, NJ: Prentice-Hall, Inc.; 1986. [Google Scholar]
- 60.Williams GC, Gagne? M, Ryan RM, Deci EL. Facilitating autonomous motivation for smoking cessation. Health Psychology. 2002;21:40–50. [PubMed] [Google Scholar]
- 61.Bandura A. Social Learning Theory. Englewood Cliffs, NJ: Prentice-Hall, Inc.; 1977a. [Google Scholar]
- 62.Bandura A. Self-efficacy: toward a unifying theory of behavioral change. Psychological Review. 1977b;84:191–215. doi: 10.1037//0033-295x.84.2.191. [DOI] [PubMed] [Google Scholar]
- 63.Bandura A. Health promotion from the perspective of social cognitive theory. Psychology and Health. 1998;13:623–649. [Google Scholar]
- 64.Bandura A. Self-Efficacy: The Exercise of Control. New York: W.H. Freeman and Company; 1997. [Google Scholar]
- 65.Elder JP, Ayala GX, Harris S. Theories and intervention approaches to health-behavior change in primary care. Am J Prev Med. 1999;17:275–284. doi: 10.1016/s0749-3797(99)00094-x. [DOI] [PubMed] [Google Scholar]
- 66.Etter JF, Laszlo E, Perneger TV. Postintervention effect of nicotine replacement therapy on smoking reduction in smokers who are unwilling to quit: randomized trial. J Clin Psychopharmacol. 2004;24:174–9. doi: 10.1097/01.jcp.0000115666.45074.d6. [DOI] [PubMed] [Google Scholar]
- 67.Batra A, Klingler K, Landfeldt B, Friederich HM, Westin A, Danielsson T. Smoking reduction treatment with 4-mg nicotine gum: a double-blind, randomized, placebo-controlled study. Clin Pharmacol Ther. 2005;78:689–96. doi: 10.1016/j.clpt.2005.08.019. [DOI] [PubMed] [Google Scholar]
- 68.Etter JF, Laszlo E. Postintervention effect of nicotine replacement therapy for smoking reduction: a randomized trial with a 5-year follow-up. J Clin Psychopharmacol. 2007;27:151–5. doi: 10.1097/JCP.0b013e318033bd72. [DOI] [PubMed] [Google Scholar]
- 69.Carpenter MJ, Hughes JR, Solomon LJ, Callas PW. Both smoking reduction with nicotine replacement therapy and motivational advice increase future cessation among smokers unmotivated to quit. J Consult Clin Psychol. 2004;72:371–81. doi: 10.1037/0022-006X.72.3.371. [DOI] [PubMed] [Google Scholar]
- 70.Hughes JR, Carpenter MJ. The feasibility of smoking reduction: an update. Addiction. 2005;100:1074–89. doi: 10.1111/j.1360-0443.2005.01174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hyland A, Levy DT, Rezaishiraz H, Hughes J, Bauer JE, Giovino GA, et al. Psychol Addict Behav. 2005;19:221–5. doi: 10.1037/0893-164X.19.2.221. [DOI] [PubMed] [Google Scholar]
- 72.Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: a revision of the Fagerström Tolerance Questionnaire. Br J Addict. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 73.Hughes JR, Keely JP, Niaura RS, Ossip-Klein DJ, Richmond RL, Swan GE. Measures of abstinence in clinical trials: issues and recommendations. Nicotine Tob Res. 2003;5:13–25. [PubMed] [Google Scholar]
- 74.Benowitz NL, Jacob P, Ahijevych K, Jarvis MF, Hall S, LeHouezec J, et al. Biochemical verification of tobacco use and cessation. Nicotine Tob Res. 2002;4:149–59. doi: 10.1080/14622200210123581. [DOI] [PubMed] [Google Scholar]
- 75.Klesges LM, Estabrooks PA, Dzewaltowski DA, Bull SS, Glasgow RE. Beginning with the application in mind: designing and planning health behavior change interventions to enhance dissemination. Ann Behav Med. 2005;29:S66–S75. doi: 10.1207/s15324796abm2902s_10. [DOI] [PubMed] [Google Scholar]
- 76.Luepker RV, Pallonen UE, Murray DM, Pirie PL. Validity of telephone surveys in assessing cigarette smoking in young adults. American Journal of Public Health. 1989;79:202–204. doi: 10.2105/ajph.79.2.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Murray DM, Perry CL. The measurement of substance use among adolescents: When is the “bogus pipeline” method needed? Addiction Behaviors. 1987;12:225–233. doi: 10.1016/0306-4603(87)90032-3. [DOI] [PubMed] [Google Scholar]
- 78.Assaf AR, McKenney JL, Banspach SW, Carleton RA. Validation of self-report smoking practices. Paper presented at the 10th annual convention of the Society of Behavioral Medicine; San Francisco. 1989. [Google Scholar]
- 79.Crossen JR, Dougher MJ, Belew J. Comparison of reactive and non-reactive measures of smoking cessation at follow-up. Addictive Behaviors. 1984;9:295–298. doi: 10.1016/0306-4603(84)90023-6. [DOI] [PubMed] [Google Scholar]