Abstract
BACKGROUND
The etiology of accessory pathway formation (AP) is generally unknown.
OBJECTIVE
Using sex and race as proxies to distinguish genetically different individuals, we sought to test the hypothesis that AP formation is genetically mediated by examining whether AP location differs by sex and/or race.
METHODS
This was a single center, retrospective cohort study of 282 consecutive patients undergoing their first electrophysiology study that revealed at least one AP between 2004–2008. Sex and race were compared to AP location determined by the invasive electrophysiology study.
RESULTS
Eighty-nine males (52%) and 40 (36%) females had a left posterior AP (p=0.006). Sixty-four females (57%) had a right annular AP compared to 55 (32%) males (p<0.001). After adjusting for age and race, females had a 2.8 fold greater odds of having a right annular AP compared to males (95% confidence interval [CI] 1.70–4.65 greater odds, p<0.001). While right anterior (free wall) pathways were rare in all other races (12%), a significantly larger proportion of Asians (n=10, 26%) had a right anterior AP (p=0.017). After adjusting for sex and age, Asians had a 3.8 fold greater odds of having a right anterior accessory pathway compared to other races (95% CI 1.5–9.4 greater odds, p=0.004).
CONCLUSIONS
Females more commonly had right annular APs, and Asians had right anterior APs substantially more frequently than other races. These findings suggest that the pathogenesis of AP formation may have a genetic component.
Keywords: Accessory pathway, Bypass tract, Wolff-Parkinson-White Syndrome, Atrioventricular reciprocating tachycardia, Race, Gender, Sex
Introduction
Atrioventricular reciprocating tachycardia (AVRT) is the second most common cause of paroxysmal supraventricular tachycardia (SVT)1, 2 but the etiology of accessory pathway (AP) formation remains largely unknown. Mutations in the PRKAG2 gene can result in a heritable cardiomyopathy that may accompany atrial-ventricular connections,3 and family studies and molecular genetic investigations have alluded to a genetic component in Wolff-Parkinson-White (WPW) syndrome and associated pre-excitation disorders.4, 5 However, it is not known if the development of the more common sporadic form of accessory pathways is genetically determined, due to some environmental exposure, or due to random chance.
Previous investigations have described the prevalence of different AP locations.6, 7 However, a large proportion of the subjects involved in these studies were males and of the same race.6–8 Potential differences by sex and race have not previously been described.
With the hypothesis that AP formation is genetically mediated, we sought to test the hypothesis that AP location differs between patients with genetically distinct backgrounds.
Methods
Study Design and Subjects
This was a single center, retrospective cohort study of 282 consecutive patients undergoing their first electrophysiology study that revealed at least one AP between 2004–2008. Both adult and pediatric populations were included in the study. Only patients with atrial-ventricular accessory pathways were included (e.g., patients with only atrial-fascicular and/or nodal-fascicular pathways were excluded).
Data Collection
Medical history information was obtained from chart review. Self-identified sex and race was obtained from the University of California electronic admissions database. Race was categorized as White, Black, Asian/Pacific Islander, Latino, Native American or Other. Individuals that chose “Other” race and “Latino” ethnicity were categorized as Latino. All those that chose “White” race, regardless of ethnicity, were categorized as “White.” Country of birthplace was identified as North America (United States/Canada), Europe, Latin America, East Asia, South Asia, South East Asia, Middle East, South Africa and Fiji. Foreign born individuals were defined as those whose country of birthplace was outside North America.
Intra-procedural characteristics were extracted from the electrophysiology report obtained at the time the index electrophysiology study was performed. AP location was characterized based on the anatomic nomenclature defined by the European Society of Cardiology and North American Society of Pacing and Electrophysiology.9 Using this nomenclature (with the previous nomenclature in parentheses): left or mitral annulus AP locations were categorized as posterior (lateral), inferoparaseptal (posterolateral), inferior (mid-posterior) and superior (anterior). Right or tricuspid annulus AP locations were categorized as anterior (lateral), inferior (mid-posterior), inferoparaseptal (posteroseptal), septal (mid-septal), superoparaseptal (anteroseptal) and superior (anterior). APs that were successfully ablated via the coronary sinus were categorized depending on their annular location (e.g., as left inferoparaseptal if near the os, as left inferior if in the mid-coronary sinus).
The study was approved by the University of California, San Francisco Committee on Human Research.
Statistical Analysis
Continuous variables that are not normally distributed (i.e., age) are expressed as medians and interquartile ranges (IQR) and were compared using the Wilcoxon rank sum test. Categorical variables were compared using the χ2 test. Multivariable analysis was performed using logistic regression analysis; covariates/potential confounders were selected based on both important demographics (e.g., age and sex) and those covariates significantly associated with both the predictors and outcomes of interest with p values <0.10. Two-tailed p values < 0.05 were considered statistically significant. Stata version9.2 (College Station, Texas) was used for statistical analysis.
Results
A total of 282 patients with 300 APs (16 patients with two pathways and 1 patient with three pathways) were included.
Baseline historical characteristics and intra-procedural findings (apart from AP location) divided by sex and race are shown in Table 1. Whites were less commonly foreign-born and more commonly had a history of atrial fibrillation, but neither group otherwise differed by demographics or medical histories. Of the 39 foreign born patients, 15 were born in Latin America, 11 were born in East Asia, 8 were born in South and Southeast Asia, 2 were born in Europe, 1 was born in the Middle East, 1 was born in Africa and 1 was born in Fiji.
Table 1.
Historical characteristics and intra-procedural findings divided by sex (left) and by White versus not White (right). Age is displayed as median and (interquartile range).
| Historical characteristics | Male n=170 | Female n=112 | p value | White n=157 | Not White n=124 | p value |
|---|---|---|---|---|---|---|
| Age (years) | 16 (11–35) | 17 (13–36) | 0.34 | 17 (12–37) | 16 (11–34) | 0.59 |
| Race | 0.21 | --- | --- | --- | ||
| White | 100 (59%) | 57 (51%) | --- | --- | --- | |
| Black | 5 (3%) | 6 (5%) | --- | --- | --- | |
| Asian | 17 (10%) | 21 (19%) | --- | --- | --- | |
| Latino | 28 (17%) | 13 (12%) | --- | --- | --- | |
| Native-American | 2 (1%) | 1 (1%) | --- | --- | --- | |
| Other | 17 (10%) | 14 (13%) | --- | --- | --- | |
| Foreign-born* | 23 (15%) | 16 (16%) | 0.95 | 4 (3%) | 35 (31%) | <0.001 |
| History of Syncope | 16 (9%) | 10 (9%) | 0.89 | 11 (7%) | 15 (12%) | 0.16 |
| History of cardiac arrest | 2 (1%) | 0 (0%) | 0.25 | 2 (1%) | 0 (0%) | 0.20 |
| History of atrial fibrillation | 12 (7%) | 3 (3%) | 0.11 | 12 (8%) | 3 (2%) | 0.048 |
| Intra-procedural characteristics | ||||||
| Dual node physiology | 30 (18%) | 15 (14%) | 0.34 | 29 (19%) | 16 (13%) | 0.18 |
| AVNRT elicited during the case | 3 (2%) | 3 (3%) | 0.60 | 3 (2%) | 3 (2%) | 0.79 |
| Any anterograde conducting accessory pathway | 102 (60%) | 75 (67%) | 0.24 | 99 (63%) | 78 (62%) | 0.79 |
| Any dangerous accessory pathway † | 8 (5%) | 3 (3%) | 0.39 | 6 (4%) | 5 (4%) | 0.96 |
Birth outside of North America
A pathway was considered “dangerous” if anterograde conduction demonstrated an R-R interval during AF < 250 ms, the anterograde block cycle length was < 250 ms, or the anterograde refractory period (at any drive cycle length) was < 250 ms.
There were no significant differences in intra-procedural findings by sex or race (Table 1). In addition, there were no differences by sex or race in antidromic or orthodromic AVRT cycle lengths.
Of the 17 patients with more than one AP, only one patient had pathways on different annuli. He was an Asian male and was the only patient with three APs (found in the left posterior, left inferoparaseptal, and right anterior locations). No patient had more than three APs.
Accessory Pathway Location by Sex
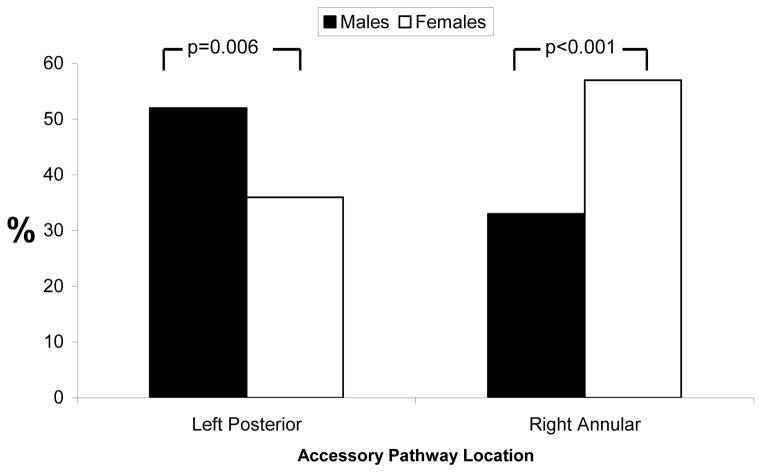
Table 2 displays AP location by sex, with an overall statistically significant chi squared p value of 0.003. This overall statistically significant p value implies that at least one comparison was significant. Subsequent dichotomous comparisons revealed that this significant difference was driven primarily by males having a greater proportion of APs on the mitral annulus and females having a greater proportion on the tricuspid annulus. While the majority of all APs in males were in the left posterior location, the large majority of APs in females were on the tricuspid annulus (Figure 1).
Table 2.
Proportions of accessory pathways in males and females in each location.
| Accessory Pathway Location | Accessory pathways in males n=185 | Accessory pathways in females n=115 |
|---|---|---|
| Left Posterior | 87 (47%) | 42 (37%) |
| Right Inferoparaseptal | 18 (10%) | 26 (23%) |
| Left Inferoparaseptal | 25 (14%) | 3 (3%) |
| Right Anterior | 20 (11%) | 19 (17%) |
| Right Septal | 3 (2%) | 6 (5%) |
| Right Superoparaseptal | 11 (6%) | 10 (9%) |
| Right Inferior | 6 (3%) | 3 (3%) |
| Left Inferior | 11 (6%) | 4 (3%) |
| Right Superior | 2 (1%) | 1 (1%) |
| Left Superior | 2 (1%) | 1 (1%) |
Numbers represent total number of accessory pathways (all accessory pathways in patients with more than one pathway were included). The overall chi squared p value=0.003
Figure 1.
Proportions of males and females with at least one accessory pathway in a left posterior location or right annular location. P values represent comparisons denoted by brackets.
After adjusting for age and race, females had a 2.8 fold greater odds of having at least one right annular AP compared to males (95% confidence interval [CI] 1.70–4.65, p<0.001)
Accessory Pathway Location by Race
AP location by race is shown in Table 3. Among all races combined, the most common AP location was left posterior (129, or 43%, of 302 pathways). Although the overall chi squared p value was not significant across the individual comparisons, the most evident differences involved the proportions with right anterior APs. While right anterior pathways were rare in Whites, they were the second most common pathway in Asians. Although the number of Black subjects was small, the most common pathway in Blacks was a right anterior AP.
Table 3.
Proportions of accessory pathways by race in each location.
| Accessory Pathway Location | White n=169 | Black n=11 | Asian n=40 | Latino n=41 | Native American n=3 | Other n=35 |
|---|---|---|---|---|---|---|
| Left Posterior | 75 (44%) | 2 (18%) | 16 (40%) | 20 (49%) | 1 (33%) | 14 (40%) |
| Right Inferoparaseptal | 27 (16%) | 2 (18%) | 4 (10%) | 7 (17%) | 0 (0%) | 4 (11%) |
| Left Inferioparaseptal | 16 (9%) | 2 (18%) | 5 (13%) | 3 (7%) | 0 (0%) | 2 (6%) |
| Right Anterior | 17 (10%) | 3 (27%) | 11(28%) | 4 (10%) | 0 (0%) | 4 (11%) |
| Right Septal | 5 (3%) | 0 (0%) | 0 (0%) | 1 (2%) | 0 (0%) | 3 (9%) |
| Right Superoparaseptal | 14 (8%) | 1 (9%) | 0 (0%) | 2 (5%) | 1 (33%) | 3 (9%) |
| Right Inferior | 5 (3%) | 1 (9%) | 1 (3%) | 2 (5%) | 0 (0%) | 0 (0%) |
| Left Inferior | 8 (5%) | 0 (0%) | 3 (8%) | 0 (0%) | 1 (33%) | 3 (9%) |
| Right Superior | 2 (1%) | 0 (0%) | 0 (0%) | 1 (2%) | 0 (0%) | 0 (0%) |
| Left Superior | 0 (0%) | 0 (0%) | 0 (0%) | 1 (2%) | 0 (0%) | 2 (6%) |
Numbers represent total number of accessory pathways (all accessory pathways in patients with more than one pathway were included). The overall chi squared p value=0.39.
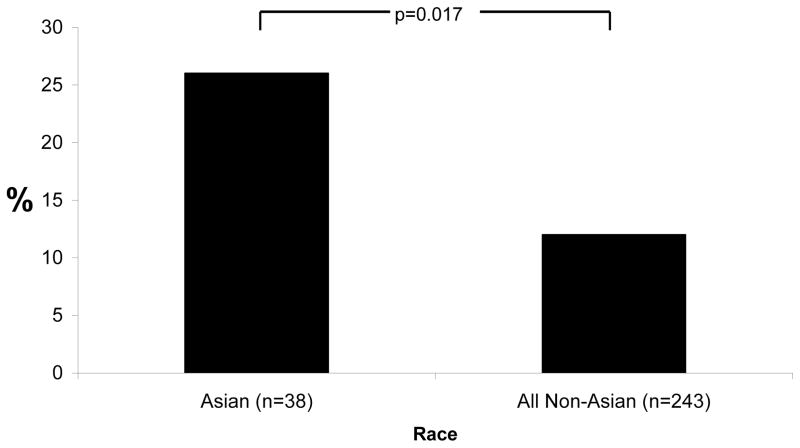
When AP location was dichotomized to right anterior versus all other locations, a significantly larger proportion of Asians had at least one right anterior AP compared to all other races combined (Figure 2). Of note, consistent with the right versus left annular AP locations by sex, 11 out of 21 (52%) of Asian women had a right annular AP.
Figure 2.
Proportions of Asians and all non-Asians with at least one right anterior accessory pathway. P values represent comparisons denoted by brackets.
Restricting the analysis to only those with data regarding self-identified race (excluding Latino ethnicity without a race designation and “Other” race), Whites were compared to Asians and Blacks: whereas 17 (10%) of Whites had a right anterior AP, 13 (27%) of Asians and Blacks combined had a pathway in this location (p=0.007).
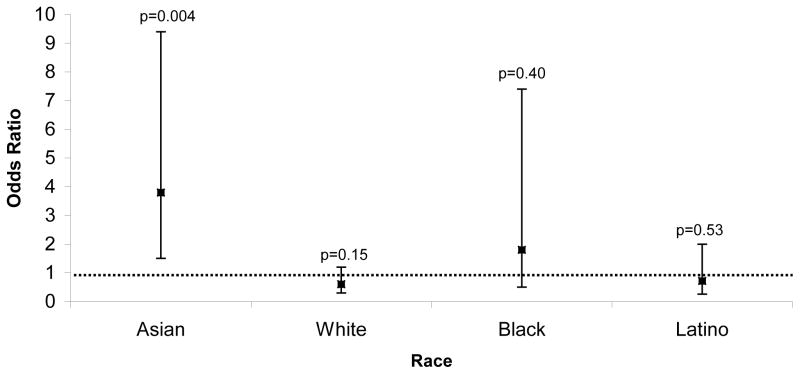
After adjusting for sex and age, Asians continued to more commonly exhibit a right anterior AP (Figure 3). Of note, after adjusting for the same potential confounders, the point estimate odds ratio for Blacks favored a right anterior pathway being more common than other races, but the 95% confidence interval crossed one (Figure 3). In contrast, the point estimate odds ratio for Whites and Latinos were each less than 1.
Figure 3.
Odds ratios of having at least one right anterior accessory pathway in Asian, White, Black and Latino patients compared to the rest of the population after adjusting for age and sex. Error bars denote 95% confidence intervals.
When foreign birth (birth outside North America) was added to the regression model, the association between Asian race and right anterior AP location was attenuated (OR 1.73, CI 0.44–6.77, p=0.43). This was explored by adjusting for specific region of birth, and the attenuation was found to be driven by a strong association between birth in East Asia (including China, n= 11, or Korea, n=1): those born in East Asia had an 18 fold greater odds of having at least one right anterior AP (95% CI 1.22–248.69, p=0.030). No other regions of birth were independently associated with a right anterior AP location.
Discussion
This is the first study to demonstrate a relationship between sex or racial background and AP location. We discovered that females more commonly have right annular APs compared to males and that Asians have right anterior APs (previously referred to as the right lateral or free wall APs) substantially more frequently than other races. Both these relationships suggest a potential inherited component to AP development.
AVRT is a common clinical entity with manifestations including palpitations, syncope and sudden cardiac death.10, 11 When counseling patients regarding potential invasive electrophysiology and ablation procedures, it is useful to be informed regarding the epidemiology of AP location by sex and race. Perhaps more importantly, as these patient characteristics can serve as proxies for genetic differences, sex and race may help reveal whether AP formation is genetically mediated.
Previous studies have identified electrophysiologic characteristics in patients with the WPW syndrome, but these involved relatively homogenous populations.6, 7, 10 Although a previous study examined sex differences in AP conduction properties by electrophysiology study, 12 none have identified AP location as a specific characteristic. One study involving only native Taiwanese patients described AP findings nearly identical to those observed in the Asians in our cohort: the right anterior position was found in 30% of patients and was the second most common location.8 To our knowledge, no previous study has specifically investigated the relationship between sex or race and AP location.
Despite no significant differences in intra-procedural characteristics other than location between sex or race, females more frequently had right annular pathways and Asians more often had right anterior APs. Both of these findings persisted after adjusting for potential confounders. Of interest, the right anterior position was the most common AP location in Blacks. Although the number of Blacks was likely too small to detect a statistically significant difference, this finding is consistent with the general concept that individuals of non-White races (excluding those who self-identified as “Other” race and either no or Latino ethnicity) more commonly exhibited right anterior APs.
After adjusting for country of birth, the relationship between Asian race and right anterior AP location was lost. This attenuation was driven by the fact that right anterior AP pathways were particularly common in those born in East Asia (China or Korea). This finding can be interpreted in two ways. First, if AP formation is indeed genetically determined, it would appear that the association between Asian race and right anterior AP formation is strongest in those of East Asian descent. Alternatively, this finding may suggest that some environmental factor more common in East Asia is important in determining AP location and/or formation. Regardless, either explanation would argue against the notion that AP formation is entirely random.
Our study has several limitations. First, determination of sex and race relied on self-identified report. While this is the standard in the majority of publications on the subject, it is clear that the genetic origins of an individual may not be accurately reflected by this method. In addition, such simple categorizations of race do not take complex genetically mixed backgrounds into account. However, both of these limitations would, if anything, likely create more “noise” in our analysis and reduce our power to detect a difference. It is unlikely that these limitations would result in false positive associations. In addition, our finding related to country of birth (that right anterior APs were most common in those born in East Asia) suggests that the Asian race designation was likely accurate. “Latino” is technically an ethnicity, rather than a race. However, as many patients selected “Other” race and “Latino” ethnicity, we categorized this group as a mutually exclusive category. Again, this method should only have reduced our power and would not have created any of the positive findings we describe. In addition, excluding this group from our analyses did not meaningfully change any of our results. Due to the relatively small numbers, we were unable to confidently comment on AP locations in Blacks and/or Native Americans. Finally, significant differences by sex and/or race do not prove a genetic origin and might instead represent some common environmental exposure. However, this is the first investigation into this possibility, and our findings at a minimum demonstrate that AP formation does not appear to be random—if the causal factors involved in the strong associations we describe can be identified, preventative therapies can theoretically be developed.
Conclusion
We found that females more frequently have right annular APs and that individuals of Asian race more commonly have right anterior (previously named right lateral or free wall) APs. These findings suggest that the pathogenesis of AP formation may have a genetic component that has yet to be elucidated.
Acknowledgments
Funding Sources
This work was made possible by grant number KL2 RR024130 (G.M.M.) from the National Center for Research Resources (NCRR), a component of the NIH, Bethesda, MD
Abbreviations
- AP
Accessory pathway
- SVT
Supraventricular tachycardia
- AVRT
Atrioventricular reciprocating tachycardia
- WPW
Wolff-Parkinson-White
- AVNRT
AV nodal reentrant tachycardia
- CI
Confidence interval
- IQR
Interquartile range
Footnotes
Author disclosures/potential conflicts of interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ganz LI, Friedman PL. Supraventricular tachycardia. N Engl J Med. 1995;332:162–173. doi: 10.1056/NEJM199501193320307. [DOI] [PubMed] [Google Scholar]
- 2.Blomstrom-Lundqvist C, Scheinman MM, Aliot EM, et al. ACC/AHA/ESC guidelines for the management of patients with supraventricular arrhythmias--executive summary. J Am Coll Cardiol. 2003;42:1493–1531. doi: 10.1016/j.jacc.2003.08.013. [DOI] [PubMed] [Google Scholar]
- 3.Gollob MH, Green MS, Tang AS, et al. Identification of a gene responsible for familial Wolff-Parkinson-White syndrome. N Engl J Med. 2001;344:1823–1831. doi: 10.1056/NEJM200106143442403. [DOI] [PubMed] [Google Scholar]
- 4.Scheinman MM. Familial preexcitation and pseudo-preexcitation syndromes. J Cardiovasc Electrophysiol. 2006;17:733–734. doi: 10.1111/j.1540-8167.2006.00474.x. [DOI] [PubMed] [Google Scholar]
- 5.Vidaillet HJ, Jr, Pressley JC, Henke E, Harrell FE, Jr, German LD. Familial occurrence of accessory atrioventricular pathways (preexcitation syndrome) N Engl J Med. 1987;317:65–69. doi: 10.1056/NEJM198707093170201. [DOI] [PubMed] [Google Scholar]
- 6.Fitzsimmons PJ, McWhirter PD, Peterson DW, Kruyer WB. The natural history of Wolff-Parkinson-White syndrome in 228 military aviators: a long-term follow-up of 22 years. Am Heart J. 2001;142:530–536. doi: 10.1067/mhj.2001.117779. [DOI] [PubMed] [Google Scholar]
- 7.Sorbo MD, Buja GF, Miorelli M, et al. The prevalence of the Wolff-Parkinson-White syndrome in a population of 116,542 young males. G Ital Cardiol. 1995;25:681–687. [PubMed] [Google Scholar]
- 8.Lee PC, Hwang B, Chen YJ, Tai CT, Chen SA, Chiang CE. Electrophysiologic characteristics and radiofrequency catheter ablation in children with Wolff-Parkinson-White syndrome. Pacing Clin Electrophysiol. 2006;29:490–495. doi: 10.1111/j.1540-8159.2006.00381.x. [DOI] [PubMed] [Google Scholar]
- 9.Cosio FG, Anderson RH, Kuck KH, et al. Living anatomy of the atrioventricular junctions. A guide to electrophysiologic mapping. A Consensus Statement from the Cardiac Nomenclature Study Group, Working Group of Arrhythmias, European Society of Cardiology, and the Task Force on Cardiac Nomenclature from NASPE. Circulation. 1999;100:e31–37. doi: 10.1161/01.cir.100.5.e31. [DOI] [PubMed] [Google Scholar]
- 10.Munger TM, Packer DL, Hammill SC, et al. A population study of the natural history of Wolff-Parkinson-White syndrome in Olmsted County, Minnesota, 1953–1989. Circulation. 1993;87:866–873. doi: 10.1161/01.cir.87.3.866. [DOI] [PubMed] [Google Scholar]
- 11.Guize L, Soria R, Chaouat JC, Chretien JM, Houe D, Le Heuzey JY. Prevalence and course of Wolf-Parkinson-White syndrome in a population of 138,048 subjects. Ann Med Interne (Paris) 1985;136:474–478. [PubMed] [Google Scholar]
- 12.Liu S, Yuan S, Hertervig E, Kongstad O, Olsson SB. Gender and atrioventricular conduction properties of patients with symptomatic atrioventricular nodal reentrant tachycardia and Wolff-Parkinson-White syndrome. J Electrocardiol. 2001;34:295–301. doi: 10.1054/jelc.2001.26316. [DOI] [PubMed] [Google Scholar]