Abstract
Lysophosphatidic acid (LPA) is a cell membrane phospholipid metabolite that can act as an extracellular signal. Its effects are mediated through at least five G protein-coupled receptors (GPCRs), LPA1-5, and likely others as well. Studies in multiple species including LPA receptor-deficient mice and humans have identified or implicated important roles for receptor-mediated LPA signaling in multiple aspects of vertebrate reproduction. These include ovarian function, spermatogenesis, fertilization, early embryo development, embryo implantation, embryo spacing, decidualization, pregnancy maintenance, and parturition. LPA signaling may also have pathological consequences, influencing aspects of endometriosis and ovarian cancer. Here we review recent progress in LPA signaling research relevant to female and male reproduction.
Overview
Lysophosphatidic acid (LPA) is an extracellular lipid signaling molecule that produces a broad range of cellular influences including survival, differentiation, proliferation, morphological changes, migration, and others. Within vertebrates, LPA signaling has been implicated in numerous physiological and pathological processes affecting most, if not all, organ systems [1-4]. One key area LPA influences is reproduction, both male and female. This review briefly introduces LPA signaling and reviews its effects on reproduction. Additional details on LPA signaling can be found in recent reviews that also note a related lysophospholipid, sphingosine 1-phosphate (S1P) that has other influences on reproduction but will not be discussed here [5-12].
LPA production and metabolism
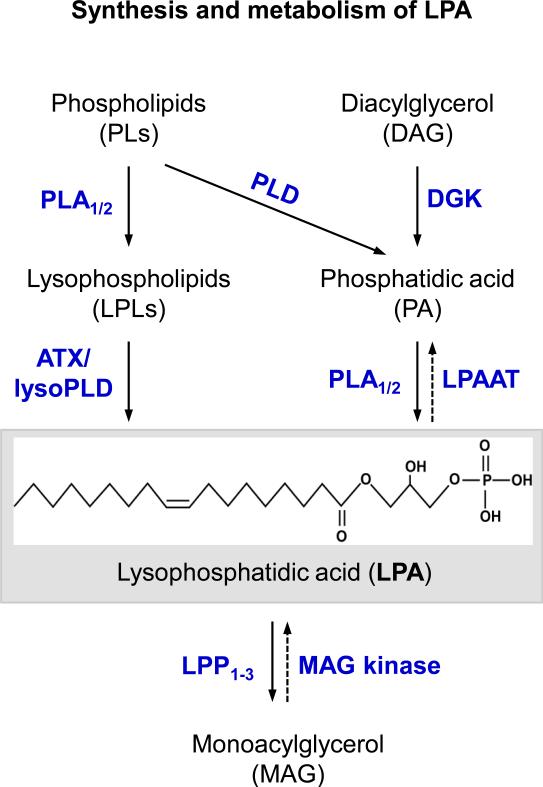
LPA is present in many biological fluids like serum (up to micromolar concentration), plasma, saliva, blister fluids, tears, chicken egg whites, follicular fluid, seminal plasma, and ascites fluids. LPA can be produced by myriad different cell types that include postmitotic neurons, adipocytes, mast cells, other lymphoid cells, endometrial cells, erythrocytes and cancer cells as well as activated platelets (reviewed in [2, 13, 14]). There are different species of LPA because of various acyl chain lengths and degrees of saturation, and position on the glycerophosphate backbone. A commonly used form in the laboratory is 18:1 or oleyl-LPA [5]. While precise and accurate mechanisms accounting for LPA metabolism within most cell types are still unclear, two general pathways of LPA production have been demonstrated. One pathway involves the generation of precursor lysophospholipids (LPLs) from various membrane phospholipids (PLs) by phospholipase A 1 and 2 (PLA1/2), followed by the action of autotaxin (ATX) that is a lysophospholipase D. A second pathway involves formation of phosphatidic acids (PAs) from PL cleavage by phospholipase D (PLD) or from diacylglycerol by diacylglycerol kinase (DGK), and deacylation of PA by PLA-type enzymes. LPA can be metabolized to monoacylglycerol (MAG) by lipid phosphate phosphatases 1-3 (LPP1-3) and lysophospholipases. This process can be reversed by MAG kinase, while the actions of a variety of acyl transferases can also remove LPA from signaling or structural pools [12, 14] (Figure 1). It has been reported that lysophospholipids are the main precursors for LPA production in serum, plasma, and adipocytes, whereas in the platelets and some cancer cells, LPA is mainly derived from phosphatidic acids (PAs) [14]. These differing routes of LPA biosynthesis likely reflect multiple levels of regulation – or disregulation in cancers – available to cells having different lineages or functions. These distinctions are reflected not only in the penultimate precursor molecule, but also in the employed enzymes that produce LPA, such as the lysophospholipase D called autotaxin or ATX that can also be produced locally and plays a key role in determining circulating LPA levels in adult animals [15, 16] by acting on lysophosphatidyl choline (LPC) to produce LPA, vs. cytoplasmic phospholipase A2 (cPLA2) that acts on PAs. These respective enzymes are in turn regulated in different ways, e.g. through transcriptional or post-translational processes that are themselves influenced by both cell autonomous and non-cell autonomous signals. In addition to its biological significance, these different signaling pathways have clear therapeutic repercussions since compounds targeting ATX for pharmaceutical uses [3, 17] would differ from those targeting other LPA biosynthetic pathways. Extracellular LPA is normally protein-bound to albumin, fatty acid binding proteins, other lipoproteins and gelsolin, all of which increase LPA's stability and aid in its transport [18-21], representing yet another level of control.
Figure 1.
Synthesis and metabolism of LPA. LPA can be generated via two general pathways. (i) One involves the generation of LPLs from PLs by PLA1/2, and transformation of LPLs to LPA by ATX/lysoPLD. (ii) The other involves the formation of PAs from PLs by PLD or from DAG by DGK, and transformation of PA to LPA by PLA1/2. (iii) LPA can be metabolized to MAG by LPP1-3 and lysophospholipases, a process that can be reversed by MAG kinase. LPA can also be removed by a variety of LPAATs. ATX/lysoPLD, Autotaxin/lysophospholipase D; LPA, lysophosphatidic acid (shown for the 18:1 chemical species of LPA); LPAAT, LPA acyltransferases; LPPs, lipid phosphate phosphatases; LPLs, lysophospholipids; MAG, monoacylglycerol; PA, phosphatidic acid; PLD, phospholipase D; PLA1/2, phospholipase A1 and A2; PLs, phospholipids.
Receptor-mediated LPA signaling
The study of receptor-mediated LPA signaling mechanisms began with the cloning of LPA receptors and the establishment of receptor-mediated functions. So far five bona fide LPA receptors, LPA1-5, have been identified [1, 5, 9, 12, 22]. Their human genes are designated LPARx with x=1-5 (human genome organization (HUGO)), while mouse names are Lparx, with x also =1-5 (Genome Informatix (MGI)) [5]. P2Y5 has been confirmed to be a new LPA receptor, LPA6 [23, 24]. Two additional possible LPA receptors, GPR87 and P2Y10, have also been reported [25, 26], but await validation. LPA receptors are all cell-surface, seven transmembrane spanning G protein-coupled receptors (GPCRs). They can differentially couple with Gα12/13, Gαq, Gαi/o, or in one instance, Gαs to activate downstream signaling pathways leading to gene regulation and LPA-induced cellular functions (Table 1) [1, 12, 24, 27]. In addition, LPA receptors may have preferences for certain chemical ligand structures. For example, LPA3 has a relatively high affinity for 2-acyl-LPA containing unsaturated fatty acids (with the ester-linked fatty acid in the 2 position) whereas other receptors such as LPA1 and LPA2 do not discriminate 1-acyl- and 2-acyl-LPA [28-30]. LPA receptors also have overlapping and differential gene expression patterns [2]. These LPA receptor characteristics contribute to receptor activities and functions [10, 31]. A critical, strategic approach in determining the biological roles for LPA signaling has been via the creation and study of mice null for one or more LPA receptors [5-12]. In this way, roles for receptor-mediated signaling in reproduction were identified, whereby LPA3 was found to affect embryo spacing and embryo implantation [32, 33], and three receptors, LPA1, LPA2, and LPA3 combined to affect spermatogenesis [34] (Table 2).
Table 1.
LPA receptors and their coupling to G proteins.
Table 2.
LPA receptor-mediated signaling in reproduction.
| Site | Effect(s) | Identified receptor(s) | Reference(s) |
|---|---|---|---|
| Testis | Germ cell survival | LPA1 LPA2 LPA3 |
[34] |
| Oocyte | Maturation | LPA1? LPA2? |
[106] |
| Xenopus oocyte | Early embryo shape maintenance | XLPA1-2 | [101] |
| Ovary | IL-6, IL-8, and growth factor induction | LPA1 LPA2 |
[45] |
| Cancer cell growth, survival, migration, and invasion | LPA2 LPA3 |
[107-110] | |
| Blastocyst | Differentiation | LPA1? LPA2? |
[99] |
| Uterus | Uterine receptivity | LPA3 | [34] |
| Embryo spacing | LPA3 | [32, 33] | |
| Uterine contraction | LPA1? LPA2? LPA3 |
[33, 82, 87] | |
| Angiogenesis of endometrium | LPA1? | [72] | |
| Placenta | Angiogenesis of placenta | LPA1? | [72] |
| Pregnancy hypertension | LPA2? LPA3? |
[73] |
Indicating proposed but not fully confirmed receptor(s).
LPA signaling in ovary
LPA signaling has been extensively studied in ovarian cells and the ovary. In one study, mRNA expression of three LPA receptors, LPA1, LPA2, and LPA3 was detected in the granulosa-lutein cells from women undergoing in vitro fertilization (IVF) [35]. LPA4 is highly expressed in human and mouse ovary [36, 37]. LPA itself is present at significant levels in follicular fluid of the human preovulatory follicle [38]. Serum ATX/lysoPLD activity from patients receiving ovarian stimulation was higher than in women with natural cycles [35], and LPA was induced in incubated human follicular fluid by ATX/lysoPLD [38], suggesting that ovarian stimulation in women may increase LPA levels. High amounts of LPA are also known to be present in chicken eggs [39, 40].
LPA signaling plays multiple roles in ovarian function (reviewed in [2]). LPA induces Ca2+-activated Cl− current in naked Xenopus laevis oocytes via Gαi. Also, LPA receptor(s), Gαi, and ERK (extracellular signal-regulated kinase)/p38 signaling pathways were implicated in mouse oocyte maturation in vitro. LPA induced expression of angiogenic cytokines, interleukin-6 (IL-6) and IL-8, in granulosa-lutein cells from women undergoing IVF. LPA1, Gαi, MAPK (mitogen-activated protein kinase)/p38, PI3K (phosphoinositol 3-kinase)/Akt, and NF-κB signaling pathways, and LPA2, Gαi, MAPK/p38, and NF-κB signaling pathways were shown to be involved in the LPA-induced IL-8 and IL-6 expression, respectively. Excessive induction of these angiogenic cytokines by LPA from multiple corpora luteae of stimulated ovaries may pathophysiologically contribute to ovarian hyperstimulation syndrome, a complication from some fertility medication with symptoms of abdominal bloating, nausea, to name a few [35]. LPA also induced Chinese hamster ovary (CHO) cell growth through Gαi [41], suggesting its physiological and pathological roles in CHO cells. LPA signaling also affects bovine ovarian theca cells and luteal cells, inducing ERK phosphorylation through the LPA1-Gα12/13 signaling pathway; redistribution of protein kinase C delta (PKCδ) from the cytosol to the perinuclear area; morphological changes and steroid synthesis [42-44].
Available data from LPA receptor-null mice indicate that LPA signaling may not be critical for ovulation in mice. LPA1, LPA2, and LPA4 are expressed in mouse ovary [37, 45], while expression data for LPA3 in mouse ovary are inconsistent [32, 45]. However, deletion of any of these receptors as well as LPA1/2/3 does not cause any obvious defect in ovulation [32, 37, 46]. LPA signaling in human ovulation has yet to be determined.
LPA signaling may play a significant role in ovarian pathology, especially relevant to ovarian cancer, through both LPA levels and LPA receptor upregulation (reviewed in [2, 20]). LPA levels are elevated in the plasma and ascites of ovarian cancer patients. LPA2 and LPA3, but not LPA1, were upregulated in ovarian cancer tissues. LPA promoted ovarian cancer cell proliferation and migration, and was suggested as a potential biomarker for ovarian cancers [5, 47], although this relationship remains controversial [48]. A complex interplay of many other molecular factors modulate LPA signaling in ovarian cancer, examples of which include glycodelin, TRIP6 (thyroid receptor interacting protein 6), telomerase, granulin-epithelin precursor, Fas (which internalizes from the cell membrane to the cytosol), IL-6, IL-8, cyclooxygenase-2 (COX-2), growth-regulated oncogene alpha (GROα), and urokinase plasminogen activator (uPA) [49]. Several LPA-induced downstream signaling pathways have been identified in ovarian cancer cells. For example, uPA upregulation by LPA was mainly mediated through the Gαi-Ras-PKCα-CARMA3-NF-κB signaling pathway [50, 51]. LPA-induced IL-6 expression was via a Gαi/PI3K-Akt/NF-κB pathway. Both Gαi-Ras-MEKK1 (MAPK kinase kinase 1) and Gα12/13-RhoA-ROCK (Rho-associated kinase) signaling pathways contributed to LPA-stimulated ovarian cancer cell migration.
In addition, LPA signaling induces breast cancer cell proliferation and chemotaxis. LPA2 is upregulated in mammary gland carcinoma tissue, and it has been suggested that LPA signaling may also play a role in breast cancer progression (reviewed in [2]). The involvement of LPA signaling in female reproductive organ function and dysfunction indicate common mechanistic threads that should provide insights into normal function, disease, and future therapeutic strategies. LPA signaling in both ovarian and breast cancers may allow the development of novel treatments, particularly in cases where these cancers are co-morbid.
LPA signaling in the oviduct
It was suggested that the receptor-mediated Gαi-Ca2+ signaling pathway was involved in LPA-induced mouse ovum transport [52]. However, in mice, deletion of LPA1, LPA2, or LPA3, the three Gαi-coupled LPA receptors expressed in the oviduct, did not grossly appear to affect embryo transport to the uterus when the blastocysts were examined in embryonic day 3.5 (E3.5) mouse uterus (E0 is defined as the mating night) [32, 46]. This suggests that 1) receptor-mediated LPA signaling is involved in embryo transport in the oviduct and the effect was not detectable at E3.5 but at an earlier time point not been determined by the authors; 2) LPA signaling is not critical for embryo transport in the oviduct under physiological conditions, and/or 3) if LPA signaling is indeed important for ovum transport, LPA4 and/or other yet-to-be identified Gαi-coupled LPA receptor(s) in the mouse oviduct can compensate for null mutations of normally expressed LPA1, LPA2, or LPA3, a possibility that remains to be examined.
LPA signaling in uterus and pregnancy maintenance
LPA can be locally produced and released in the bovine endometrium during estrous and early pregnancy [53]. It is also detected in the ovine uterus at early pregnancy stages [54]. LPA containing varied fatty acid acyl chains was also detected in the porcine uterine lumen on day 12 of both the estrous cycle and pregnancy [55]. The production of LPA in mouse uterus has not been specifically determined; however, LPA-producing enzymes, PLD1 and PLD2, are expressed in young adult mouse uterus (unknown stage of estrous cycle) [56].
Gene expression patterns of LPA3 in porcine uterus suggest that this isoform plays a role during early pregnancy, as the predominant LPA receptor subtype of four LPA receptors examined (LPA1-4) and regulated by pregnancy and estrous cycle [55]. LPA3 gene expression peaked on day 12 of pregnancy, when an embryo undergoes a dramatic elongation process prior to implantation [57], localized in the luminal and glandular epithelium, and induced by estrogen in the endometrium [55]. By comparison, studies in the mouse uterus indicate that LPA3 is mainly localized in the luminal epithelium, upregulated by progesterone and unlike the porcine uterus, is downregulated by estrogens [32, 58]. In addition, the presence of embryos induces porcine uterine LPA3 expression [59], contrasting with that in mice, wherein LPA3 had similar gene expression patterns in uteri from early pregnant and pseudopregnant (mating with a vasectomized male) females [32, 58].
A dramatic effect of LPA signaling in the uterus was identified in LPA3-deficient mice, where loss of LPA3 influenced embryo implantation and spacing [32, 33]. Embryo implantation involves a competent embryo, a receptive uterus, and their reciprocal interaction [60]. Embryo spacing refers to the regular and approximately equidistant implantation of embryos along the uterus of polytocous (multiple offspring in a single birth) species. Deletion of LPA3 in mice led to uneven embryo spacing and delayed embryo implantation [32]. This was associated with delayed embryonic development, prolonged pregnancy duration, and ~50% embryonic lethality. Decidualization was not adversely affected in LPA3-deficient uterus. Embryo crowding and delayed implantation seem to be two segregated events, in that restoration of on-time implantation by exogenous prostaglandins PGE2 and PGI2 failed to correct embryo crowding in LPA3-deficient females while transferring a single embryo, which does not pose embryo crowding, to pseudopregnant LPA3-deficient uterus does not restore delayed embryo implantation [32, 33].
Prostaglandins (PGs) were identified to be at least partially responsible for the phenotypes of LPA3–deficient females. Expression of COX-2, important in PG synthesis, as well as levels of the PG forms PGE2 and PGI2 were suppressed in pre-implantation E3.5 LPA3-deficient uteri. Exogenous PGE2 and PGI2 rescued delayed implantation in the LPA3-deficient females, reinforcing the importance of PGs in embryo implantation [61-63]. However, PGE2 and PGI2 failed to correct embryo spacing, suggesting that other PGs that control uterine contraction or possibly non-PG mechanisms may be responsible for embryo spacing [33].
LPA signaling, COX-2, and prostaglandins have also been examined in porcine and bovine uterus. LPA can induce COX-2 expression in porcine uterine endometrium, suggesting that LPA produced in the uterine endometrium may play a role in porcine uterine endometrial function [55]. In bovine uterus, LPA is locally produced and released from the endometrium. LPA can induce progesterone and PGE2 secretion and increase the PGE2/PGF2α ratio. These effects can be inhibited by a dual LPA1 and LPA3 antagonist (Ki16425) [53, 64]. Further study indicates that LPA induces PGE2 production in endometrial stromal cells but not in epithelial cells. The effect is through upregulation of PGE2 synthesis enzymes, COX-2 and PGE2 synthase. LPA1 is suggested to be the main receptor in mediating this process [65]. It is suggested then that LPA may play autocrine and/or paracrine roles in the maintenance of early bovine pregnancy [53, 64, 65].
In addition to data from non-human species, a recent report indicates that LPA3 may also have function in human reproduction. This study demonstrates that LPA3 levels decrease in the middle and later secretory endometrium (implantation occurs in the secretory phase) of patients with endometriosis [66], a medical condition in which the endometrial tissue grows outside of the uterus and that is associated with increased rate of subfertility, affecting 5 to 10% of women of reproductive age in the United States [67]. LPA3 and other putative uterine receptivity biomarkers (glycodelin A, osteopontin, and HOXA10) examined in this study are all regulated by progesterone. Reduced expression of these genes may explain the progesterone resistance associated with endometriosis [66]. On the other hand, increased levels of COX-2-derived PGE2 are detected in the endometrium, especially in ectopic endometriotic tissue from women with endometriosis [67]. In addition to the evidence that LPA signaling stimulates COX-2 expression in endometrial and endothelial cells [32, 65, 68], it is intriguing to speculate that LPA signaling may play a role in endometriosis as well as endometriosis-associated subfertility.
In human decidual cells, LPA can increase embryo outgrowth and induce actin stress fiber formation [69], a result consistent with the earliest known effects of receptor-mediated LPA signaling [70, 71]. RhoA signaling mediates these LPA effects in decidual cells that may contribute to embryo development and differentiation after attachment [69]. LPA can induce IL-8 enhanced migration, permeability, capillary tube formation, and proliferation of human endometrial microvascular endothelial cells. Of the three LPA receptors examined, LPA1 but not LPA2 or LPA3 are highly expressed in human endometrial stromal cells. LPA1 mediates LPA-induced IL-8 expression via a NFκB-dependent pathway. A role of LPA in angiogenesis of endometrium and placenta through induction of IL-8 in endometrial stromal cells during pregnancy has thus been suggested [72].
LPA signaling has also been suggested to have pathological roles during pregnancy. Although LPA2 and LPA3 are not detectable in human endometrial stromal cells, high levels of LPA2 and LPA3 gene expression were detected in the placentas of patients with gestational hypertension and preeclampsia (pregnancy-induced hypertension in association with significant amounts of protein in the urine) [73]. In addition, LPA can potentiate endothelin-1 induced vasoconstriction [74]. These data suggest that LPA signaling might contribute to gestational hypertension and preeclampsia. These hypertensive disorders can worsen with pregnancy progression, and LPA levels are known to increase during pregnancy [75]. It is unknown whether even higher levels of LPA are present in pregnant patients with complicated hypertensive disorders. LPA levels increase during blood clotting that can promote wound healing processes. However, aberrant accumulation of LPA in blood may lead to adverse effects such as endothelial barrier dysfunction [76] and platelet aggregation[77], which can contribute to thrombosis during pregnancy. LPA signaling also may be associated with preterm labor or pre-eclampsia [78] and influence the growth of uterine tumors such as leiomyomas or fibroids (tumors of uterine smooth muscle) [79]. LPA signaling may also play a role in endometrial cancer. A recent study indicates that LPA can promote endometrial cancer invasion. LPA2 and matrix metalloproteinase-7 (MMP-7) are implicated in this process [80]. The functions of LPA signaling in these processes await further exploration.
LPA signaling has also been suggested in maintenance of human pregnancy. Serum ATX/lysoPLD activity, a key enzyme for LPA production, and LPA levels were shown to increase during pregnancy [75]. High lysophospholipase activity was present in human placental tissues, with the highest in the amnion [81]. Although the amnion has been heavily implicated in the initiation of labor, presumably through the release of arachidonic acid, the high lysophospholipase activity in the amnion suggests that lysophospholipid substrates, including LPA, might also be involved in the regulation of labor. These issues deserve further examination.
LPA signaling could potentially regulate uterine contractility as well as load-bearing during pregnancy and labor. An early study indicated that LPA had similar effects as PGF2α on rat smooth muscle contraction and intrauterine pressure [82]. Although the effect of LPA signaling in parturition per se has not been established in vivo, deletion of FP, the GPCR for PGF2α, led to parturition failure [83]. The potential roles of LPA signaling in uterine smooth muscles can be further demonstrated by the following studies. LPA stimulated myosin light chain phosphorylation through RhoA signaling in myometrial tissue from pregnant women [84], specifically through Gα12/13-Rho kinase signaling [85]. In addition, the Gαi/o signaling pathway involving [Ca2+]i regulation regulates LPA-induced cell proliferation of human myometrial smooth muscle cells [86]. A follow-up study identified the critical role of Ca2+/calmodulin-dependent protein kinase in this effect as well as the detection of LPA1, LPA2, and LPA3 in the human myometrial smooth muscle cells [87]. The involvement of LPA3 in uterine smooth muscle contraction has been demonstrated in mice using LPA3 specific agonist T13 and LPA3-deficient uterus [33]. These data indicate that LPA signaling can regulate uterine contractility, which may render its roles not only in embryo spacing in mice but also in load-bearing during pregnancy and eventually labor in humans.
LPA might also have relevance to infection-related preterm labor. Significantly higher levels of lysophosphatidylcholine (LPC), the substrate for ATX to produce LPA, was detected in human uterine endometrial cells upon exposure to extracts from common anaerobes involved in intrauterine infection, accompanied with elevated arachidonic acid, a key precursor for PG synthesis in regulating labor. PLA2 activity was involved in the reported lipid metabolism [88, 89]. Since PLA2 and LPC are important components in the LPA metabolism pathway [19], it is reasonable to expect that LPA signaling might also be involved in infection-related preterm labor.
In the bovine reproductive tract, LPA has been demonstrated to stimulate progesterone and PGE2 secretion, which may indicate a supporting role for LPA in the corpus luteum. Hence, it was suggested that LPA may be involved in the maintenance of early bovine pregnancy [53]. LPA signaling has also been implicated in the establishment and maintenance of porcine pregnancy [90].
LPA signaling in testis and prostate
Three lines of evidence suggested that LPA signaling may have potential roles in male reproduction (reviewed in [2]). First, LPA biosynthetic enzymes, including PLA1, PLA2, and autotaxin/lysoPLD are present in the testis. Second, Lpar1-3 are highly expressed in the mouse testis [34, 91] while LPAR1-4 are detected in human testis [5, 36]. Third, transgenic mice overexpressing LPP1, which degrades LPA, show impaired spermatogenesis [92]. In situ hybridization demonstrated Lpar1-3 expression in male germ cells. Deletion of these receptors in mice led to a testosterone-independent reduction of mating activity and sperm count, with an increased prevalence of azoospermia in aging animals. Increased germ cell apoptosis was responsible for the consequent reduction of germ cell proliferation and diminished sperm count, implicating LPA signaling as a germ cell survival factor in spermatogenesis [34].
LPA signaling may have functions in the pathology of the prostate. Lpar1-3 were detected in the prostate, and significantly higher expression levels were reported in human prostate malignancies compared with benign tissues [93, 94]. LPA signaling may have multiple roles in prostate cancer by facilitating early prostate cancer development, inducing prostate cancer cell proliferation, survival, morphological changes, migration, and invasion. LPA1 seems to be the key receptor in mediating LPA-induced prostate cancer cell proliferation and migration because the expression of this receptor is correlated with these LPA-induced cellular events in cultured prostate cancer cells [94]. NF-κB is constitutively activated in prostate cancer but not in benign prostate tissues. LPA can activate Akt-NF-κB pathway in cultured prostate cancer cells. It is suggested that LPA receptor-Akt-NF-κB signaling axis may mediate LPA-induced prostate cell survival [95]. LPA may also play roles in prostate cells via secondary factors, such as cytokines, CYR61, RhoA, etc (reviewed in [2]).
LPA signaling in fertilization
The spermatic acrosome reaction is a main step in fertilization, involving the binding and fusion of sperm and egg. LPA can activate spermatic PKCα, implicated in the acrosome reaction, and could promote actin polymerization, a process necessary for spermatozoa penetration into the egg cytoplasm (reviewed in [2]). LPA and Rho GTPases are involved in the latter process consistent with receptor-mediated phenomena [96, 97]. However, the LPA receptor(s) mediating this process is (are) unknown. LPA signaling does not appear to alter sperm motility in either mice deficient for three LPA receptors, LPA1/2/3 or in studies of bovine motility. No obvious deficiencies in fertilization were observed in LPA3-deficient mice [32]. To determine the potential function of LPA signaling in fertilization, especially the acrosome reaction, systematic study needs to be carried out. Currently, expression levels of different LPA receptors in the sperm remain unknown, although several are detected in the testis [5, 34, 36].
LPA signaling in preimplantation embryo development
LPA signaling has been implicated in post-implantation embryo developmental processes such as vascular formation, vascular maturation and maintenance, heart development, and brain formation (references in [2]). In a study preceding LPA receptor identification, culturing of embryos from the pronuclear stage in the presence of LPA significantly increased the success rate of the development of 2-cell and 4-cell stage embryos to blastocysts via a Gαi-protein receptor mechanism [98]. A more recent study reported Lpar1 mRNA expression in differentiating mouse blastocysts, expression of Lpar2 in late-stage blastocysts and no expression of Lpar3 at any of the examined stages [99]. One potential mechanism could be that LPA elevates [Ca2+]i levels to accelerate murine blastocyst differentiation. LPA induces the transient accumulation of heparin-binding EGF-like growth factor (HB-EGF) on the embryo surface, and interfering with HB-EGF signaling through EGF receptors ErbB1 or ErbB4 could attenuate LPA-stimulated blastocyst differentiation [99]. However, there is no obvious defect in blastocyst development in LPA3-deficient or LPA1/LPA2-double deficient mice [32]. Delayed post-implantation embryo development in LPA3-deficient uteri reflects delayed embryo implantation that is maternal in origin [32]. Nevertheless, LPA signaling can influence post-implantation embryo development. Deletion of ATX, a key enzyme in LPA production, leads to embryonic lethality due to defects in blood vessel formation, brain development, etc [15, 16], suggesting roles for LPA signaling in embryo development. Expression patterns of LPA receptors in embryo suggest potential functions of LPA signaling in organogenesis [31]. We have observed embryonic hematoma and embryonic lethality with incomplete but increased penetrance in LPA1, LPA1/2 double knockout, and LPA1/2/3 triple knockout mice [34, 46, 100]. However, these phenotypes can't represent what are observed in ATX knockout mice. Considering the fact that more LPA receptors are being identified, any single or a few LPA receptors may not be able to represent the function of ATX.
LPA signaling may play a role in reproduction for other species as well. XLPA1 and XLPA2 receptor-mediated LPA signaling is important for early embryo development in Xenopus via the maintenance of overall rigidity and shape of the embryo [101, 102]. LPA3 was detected in porcine concepti of day 12 and 15, and it was suggested that LPA produced in porcine uterine endometrium influences conceptus development during implantation and establishment of porcine pregnancy [55]. A recent study shows multiple effects of LPA on ovine trophectoderm cells, such as activation of MAPK ERK1/2 phosphorylation, promotion of proliferation and cytoskeletal rearrangement, as well as release of PGF2α and PGE2, suggesting a potential role of LPA signaling in the ovine conceptus at the time of implantation [54].
Summary.
Progress over the past decade has revealed the importance of LPA signaling in female and male reproduction, as well as fertilization and pre-implantation embryo development. All examined vertebrate species utilize LPA receptor-mediated mechanisms, and this form of lysophospholipid signaling influences most elements of reproduction directly or indirectly. Numerous issues remain to be addressed in the future (Box 1), a partial list that underscores both the multitude of pathway elements to consider, along with selected questions within each group. The therapeutic potential of LPA signaling represents an area of opportunity that awaits future investigations.
Box 1. Outstanding Questions.
LPA ligand-related issues
What chemical forms of LPA exist within the reproductive system?
What are local LPA concentrations?
Do LPA concentrations vary with reproductive stage or age?
Are there LPA concentration gradients?
Which cells produce LPA?
What is most critical for LPA signaling with respect to ligand: synthesis or degradation?
Are there physiological or disease conditions that significantly alter LPA levels or gradients (e.g., obesity)?
LPA biosynthetic and degradative enzyme-related issues
Which enzymes are most important in the reproductive system?
How is their expression and activity controlled?
Do LPA precursors and/or products create positive and negative feedback loops affecting activity?
Are there unidentified enzymes that contribute to LPA enzymatic pathways?
LPA receptor-related issues
Have all LPA receptors involved in reproduction been identified?
What are rate-limiting mechanisms in controlling LPA receptor activity?
Which downstream signaling pathways are dominant for LPA-mediated reproductive effects?
What is the relationship between LPA signaling and other lysophospholipid pathways?
What is the relationship between LPA signaling and other signaling pathways involved in reproduction?
LPA-based therapeutic issues
Which molecular targets are tractable for intervention?
What physiological or disease indications could be therapeutically and safely accessed?
Acknowledgements
We thank Ms. Danielle Letourneau for editorial assistance. This work was supported by funding from the Office of the Vice President for Research at The University of Georgia (XY) and the NIH (HD050685 to JC).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ishii I, Fukushima N, Ye X, Chun J. LYSOPHOSPHOLIPID RECEPTORS: Signaling and Biology. Annu Rev Biochem. 2004;73:321–354. doi: 10.1146/annurev.biochem.73.011303.073731. [DOI] [PubMed] [Google Scholar]
- 2.Ye X. Lysophospholipid signaling in the function and pathology of the reproductive system. Hum Reprod Update. 2008;14:519–536. doi: 10.1093/humupd/dmn023. [DOI] [PubMed] [Google Scholar]
- 3.Kano K, Arima N, Ohgami M, Aoki J. LPA and its analogs-attractive tools for elucidation of LPA biology and drug development. Curr Med Chem. 2008;15:2122–2131. doi: 10.2174/092986708785747562. [DOI] [PubMed] [Google Scholar]
- 4.Skoura A, Hla T. Lysophospholipid receptors in vertebrate development, physiology, and pathology. J Lipid Res. 2009;50(Suppl):S293–298. doi: 10.1194/jlr.R800047-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choi JW, Herr DR, Noguchi K, Yung YC, Lee C, Mutoh T, Lin M, Teo ST, Park KE, Mosley AN, Chun J. LPA Receptors: Subtypes and Biological Actions. Annu Rev Pharmacol. 2009 doi: 10.1146/annurev.pharmtox.010909.105753. In press. [DOI] [PubMed] [Google Scholar]
- 6.Mutoh T, Chun J. Lysophospholipid activation of G protein-coupled receptors. Subcell Biochem. 2008;49:269–297. doi: 10.1007/978-1-4020-8831-5_10. [DOI] [PubMed] [Google Scholar]
- 7.Rivera R, Chun J. Biological effects of lysophospholipids. Rev Physiol Biochem Pharmacol. 2008;160:25–46. doi: 10.1007/112_0507. [DOI] [PubMed] [Google Scholar]
- 8.Birgbauer E, Chun J. New developments in the biological functions of lysophospholipids. Cell Mol Life Sci. 2006;63:2695–2701. doi: 10.1007/s00018-006-6155-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Herr DR, Chun J. Effects of LPA and S1P on the nervous system and implications for their involvement in disease. Curr Drug Targets. 2007;8:155–167. doi: 10.2174/138945007779315669. [DOI] [PubMed] [Google Scholar]
- 10.Choi JW, Lee CW, Chun J. Biological roles of lysophospholipid receptors revealed by genetic null mice: an update. Biochim Biophys Acta. 2008;1781:531–539. doi: 10.1016/j.bbalip.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chun J, Rosen H. Lysophospholipid receptors as potential drug targets in tissue transplantation and autoimmune diseases. Curr Pharm Des. 2006;12:161–171. doi: 10.2174/138161206775193109. [DOI] [PubMed] [Google Scholar]
- 12.Gardell SE, Dubin AE, Chun J. Emerging medicinal roles for lysophospholipid signaling. Trends Mol Med. 2006;12:65–75. doi: 10.1016/j.molmed.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 13.Smyth SS, Cheng HY, Miriyala S, Panchatcharam M, Morris AJ. Roles of lysophosphatidic acid in cardiovascular physiology and disease. Biochim Biophys Acta. 2008;1781:563–570. doi: 10.1016/j.bbalip.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aoki J, Inoue A, Okudaira S. Two pathways for lysophosphatidic acid production. Biochim Biophys Acta. 2008;1781:513–518. doi: 10.1016/j.bbalip.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 15.Tanaka M, Okudaira S, Kishi Y, Ohkawa R, Iseki S, Ota M, Noji S, Yatomi Y, Aoki J, Arai H. Autotaxin stabilizes blood vessels and is required for embryonic vasculature by producing lysophosphatidic acid. J Biol Chem. 2006;281:25822–25830. doi: 10.1074/jbc.M605142200. [DOI] [PubMed] [Google Scholar]
- 16.van Meeteren LA, Ruurs P, Stortelers C, Bouwman P, van Rooijen MA, Pradere JP, Pettit TR, Wakelam MJ, Saulnier-Blache JS, Mummery CL, Moolenaar WH, Jonkers J. Autotaxin, a secreted lysophospholipase D, is essential for blood vessel formation during development. Mol Cell Biol. 2006;26:5015–5022. doi: 10.1128/MCB.02419-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Federico L, Pamuklar Z, Smyth SS, Morris AJ. Therapeutic potential of autotaxin/lysophospholipase d inhibitors. Curr Drug Targets. 2008;9:698–708. doi: 10.2174/138945008785132439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gaits F, Fourcade O, Le Balle F, Gueguen G, Gaige B, Gassama-Diagne A, Fauvel J, Salles JP, Mauco G, Simon MF, Chap H. Lysophosphatidic acid as a phospholipid mediator: pathways of synthesis. FEBS Lett. 1997;410:54–58. doi: 10.1016/s0014-5793(97)00411-0. [DOI] [PubMed] [Google Scholar]
- 19.Aoki J. Mechanisms of lysophosphatidic acid production. Semin Cell Dev Biol. 2004;15:477–489. doi: 10.1016/j.semcdb.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 20.Mills GB, Moolenaar WH. The emerging role of lysophosphatidic acid in cancer. Nat Rev Cancer. 2003;3:582–591. doi: 10.1038/nrc1143. [DOI] [PubMed] [Google Scholar]
- 21.Pages C, Simon M, Valet P, Saulnier-Blache JS. Lysophosphatidic acid synthesis and release(1). Prostaglandins. 2001;64:1–10. doi: 10.1016/s0090-6980(01)00110-1. [DOI] [PubMed] [Google Scholar]
- 22.Chun J. How the lysophospholipid got its receptor. The Scientist. 2007:48–54. [Google Scholar]
- 23.Pasternack SM, von Kugelgen I, Aboud KA, Lee YA, Ruschendorf F, Voss K, Hillmer AM, Molderings GJ, Franz T, Ramirez A, Nurnberg P, Nothen MM, Betz RC. G protein-coupled receptor P2Y5 and its ligand LPA are involved in maintenance of human hair growth. Nat Genet. 2008;40:329–334. doi: 10.1038/ng.84. [DOI] [PubMed] [Google Scholar]
- 24.Yanagida K, Masago K, Nakanishi H, Kihara Y, Hamano F, Tajima Y, Taguchi R, Shimizu T, Ishii S. Identification and characterization of a novel lysophosphatidic acid receptor, p2y5/LPA6. J Biol Chem. 2009;284:17731–17741. doi: 10.1074/jbc.M808506200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tabata K, Baba K, Shiraishi A, Ito M, Fujita N. The orphan GPCR GPR87 was deorphanized and shown to be a lysophosphatidic acid receptor. Biochem Biophys Res Commun. 2007;363:861–866. doi: 10.1016/j.bbrc.2007.09.063. [DOI] [PubMed] [Google Scholar]
- 26.Murakami M, Shiraishi A, Tabata K, Fujita N. Identification of the orphan GPCR, P2Y(10) receptor as the sphingosine-1-phosphate and lysophosphatidic acid receptor. Biochem Biophys Res Commun. 2008;371:707–712. doi: 10.1016/j.bbrc.2008.04.145. [DOI] [PubMed] [Google Scholar]
- 27.Stortelers C, Kerkhoven R, Moolenaar WH. Multiple actions of lysophosphatidic acid on fibroblasts revealed by transcriptional profiling. BMC Genomics. 2008;9:387. doi: 10.1186/1471-2164-9-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bandoh K, Aoki J, Hosono H, Kobayashi S, Kobayashi T, Murakami-Murofushi K, Tsujimoto M, Arai H, Inoue K. Molecular cloning and characterization of a novel human G-protein-coupled receptor, EDG7, for lysophosphatidic acid. J Biol Chem. 1999;274:27776–27785. doi: 10.1074/jbc.274.39.27776. [DOI] [PubMed] [Google Scholar]
- 29.Sonoda H, Aoki J, Hiramatsu T, Ishida M, Bandoh K, Nagai Y, Taguchi R, Inoue K, Arai H. A novel phosphatidic acid-selective phospholipase A1 that produces lysophosphatidic acid. J Biol Chem. 2002;277:34254–34263. doi: 10.1074/jbc.M201659200. [DOI] [PubMed] [Google Scholar]
- 30.Fischer DJ, Nusser N, Virag T, Yokoyama K, Wang D, Baker DL, Bautista D, Parrill AL, Tigyi G. Short-chain phosphatidates are subtype-selective antagonists of lysophosphatidic acid receptors. Mol Pharmacol. 2001;60:776–784. [PubMed] [Google Scholar]
- 31.Ohuchi H, Hamada A, Matsuda H, Takagi A, Tanaka M, Aoki J, Arai H, Noji S. Expression patterns of the lysophospholipid receptor genes during mouse early development. Dev Dyn. 2008;237:3280–3294. doi: 10.1002/dvdy.21736. [DOI] [PubMed] [Google Scholar]
- 32.Ye X, Hama K, Contos JJ, Anliker B, Inoue A, Skinner MK, Suzuki H, Amano T, Kennedy G, Arai H, Aoki J, Chun J. LPA3-mediated lysophosphatidic acid signalling in embryo implantation and spacing. Nature. 2005;435:104–108. doi: 10.1038/nature03505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hama K, Aoki J, Inoue A, Endo T, Amano T, Motoki R, Kanai M, Ye X, Chun J, Matsuki N, Suzuki H, Shibasaki M, Arai H. Embryo Spacing and Implantation Timing Are Differentially Regulated by LPA3-Mediated Lysophosphatidic Acid Signaling in Mice. Biol Reprod. 2007;77:954–959. doi: 10.1095/biolreprod.107.060293. [DOI] [PubMed] [Google Scholar]
- 34.Ye X, Skinner MK, Kennedy G, Chun J. Age-dependent loss of sperm production in mice via impaired lysophosphatidic acid signaling. Biol Reprod. 2008;79:328–336. doi: 10.1095/biolreprod.108.068783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen SU, Chou CH, Lee H, Ho CH, Lin CW, Yang YS. Lysophosphatidic acid up-regulates expression of interleukin-8 and -6 in granulosa-lutein cells through its receptors and nuclear factor-kappaB dependent pathways: implications for angiogenesis of corpus luteum and ovarian hyperstimulation syndrome. J Clin Endocrinol Metab. 2008;93:935–943. doi: 10.1210/jc.2007-1512. [DOI] [PubMed] [Google Scholar]
- 36.Noguchi K, Ishii S, Shimizu T. Identification of p2y9/GPR23 as a novel G protein-coupled receptor for lysophosphatidic acid, structurally distant from the Edg family. J Biol Chem. 2003;278:25600–25606. doi: 10.1074/jbc.M302648200. [DOI] [PubMed] [Google Scholar]
- 37.Lee Z, Cheng CT, Zhang H, Subler MA, Wu J, Mukherjee A, Windle JJ, Chen CK, Fang X. Role of LPA4/p2y9/GPR23 in negative regulation of cell motility. Mol Biol Cell. 2008;19:5435–5445. doi: 10.1091/mbc.E08-03-0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tokumura A, Miyake M, Nishioka Y, Yamano S, Aono T, Fukuzawa K. Production of lysophosphatidic acids by lysophospholipase D in human follicular fluids of In vitro fertilization patients. Biol Reprod. 1999;61:195–199. doi: 10.1095/biolreprod61.1.195. [DOI] [PubMed] [Google Scholar]
- 39.Nakane S, Tokumura A, Waku K, Sugiura T. Hen egg yolk and white contain high amounts of lysophosphatidic acids, growth factor-like lipids: distinct molecular species compositions. Lipids. 2001;36:413–419. doi: 10.1007/s11745-001-0737-1. [DOI] [PubMed] [Google Scholar]
- 40.Morishige J, Touchika K, Tanaka T, Satouchi K, Fukuzawa K, Tokumura A. Production of bioactive lysophosphatidic acid by lysophospholipase D in hen egg white. Biochim Biophys Acta. 2007;1771:491–499. doi: 10.1016/j.bbalip.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 41.Yamaji H, Sakai K, Joho T, Izumoto E, Fukuda H. Cell cycle analysis of Chinese hamster ovary cells stimulated by phosphatidic acid in serum-free culture. J Biosci Bioeng. 2004;98:487–489. doi: 10.1016/S1389-1723(05)00317-8. [DOI] [PubMed] [Google Scholar]
- 42.Budnik LT, Brunswig-Spickenheier B, Mukhopadhyay AK. Lysophosphatidic acid signals through mitogen-activated protein kinase-extracellular signal regulated kinase in ovarian theca cells expressing the LPA1/edg2-receptor: involvement of a nonclassical pathway? Mol Endocrinol. 2003;17:1593–1606. doi: 10.1210/me.2002-0371. [DOI] [PubMed] [Google Scholar]
- 43.Budnik LT, Mukhopadhyay AK. Lysophosphatidic acid-induced nuclear localization of protein kinase C delta in bovine theca cells stimulated with luteinizing hormone. Biol Reprod. 2002;67:935–944. doi: 10.1095/biolreprod.101.003087. [DOI] [PubMed] [Google Scholar]
- 44.Budnik LT, Brunswig-Spickenheier B. Differential effects of lysolipids on steroid synthesis in cells expressing endogenous LPA2 receptor. J Lipid Res. 2005;46:930–941. doi: 10.1194/jlr.M400423-JLR200. [DOI] [PubMed] [Google Scholar]
- 45.Huang MC, Lee HY, Yeh CC, Kong Y, Zaloudek CJ, Goetzl EJ. Induction of protein growth factor systems in the ovaries of transgenic mice overexpressing human type 2 lysophosphatidic acid G protein-coupled receptor (LPA2). Oncogene. 2004;23:122–129. doi: 10.1038/sj.onc.1206986. [DOI] [PubMed] [Google Scholar]
- 46.Contos JJ, Ishii I, Fukushima N, Kingsbury MA, Ye X, Kawamura S, Brown JH, Chun J. Characterization of lpa(2) (Edg4) and lpa(1)/lpa(2) (Edg2/Edg4) lysophosphatidic acid receptor knockout mice: signaling deficits without obvious phenotypic abnormality attributable to lpa(2). Mol Cell Biol. 2002;22:6921–6929. doi: 10.1128/MCB.22.19.6921-6929.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sutphen R, Xu Y, Wilbanks GD, Fiorica J, Grendys EC, Jr., LaPolla JP, Arango H, Hoffman MS, Martino M, Wakeley K, Griffin D, Blanco RW, Cantor AB, Xiao YJ, Krischer JP. Lysophospholipids are potential biomarkers of ovarian cancer. Cancer Epidemiol Biomarkers Prev. 2004;13:1185–1191. [PubMed] [Google Scholar]
- 48.Moolenaar WH, van Meeteren LA, Giepmans BN. The ins and outs of lysophosphatidic acid signaling. Bioessays. 2004;26:870–881. doi: 10.1002/bies.20081. [DOI] [PubMed] [Google Scholar]
- 49.Gil OD, Lee C, Ariztia EV, Wang FQ, Smith PJ, Hope JM, Fishman DA. Lysophosphatidic acid (LPA) promotes E-cadherin ectodomain shedding and OVCA429 cell invasion in an uPA-dependent manner. Gynecol Oncol. 2008;108:361–369. doi: 10.1016/j.ygyno.2007.10.027. [DOI] [PubMed] [Google Scholar]
- 50.Li H, Ye X, Mahanivong C, Bian D, Chun J, Huang S. Signaling mechanisms responsible for lysophosphatidic acid-induced urokinase plasminogen activator expression in ovarian cancer cells. J Biol Chem. 2005;280:10564–10571. doi: 10.1074/jbc.M412152200. [DOI] [PubMed] [Google Scholar]
- 51.Mahanivong C, Chen HM, Yee SW, Pan ZK, Dong Z, Huang S. Protein kinase C alpha-CARMA3 signaling axis links Ras to NF-kappa B for lysophosphatidic acid-induced urokinase plasminogen activator expression in ovarian cancer cells. Oncogene. 2008;27:1273–1280. doi: 10.1038/sj.onc.1210746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kunikata K, Yamano S, Tokumura A, Aono T. Effect of lysophosphatidic acid on the ovum transport in mouse oviducts. Life Sci. 1999;65:833–840. doi: 10.1016/s0024-3205(99)00310-0. [DOI] [PubMed] [Google Scholar]
- 53.Woclawek-Potocka I, Komiyama J, Saulnier-Blache JS, Brzezicka E, Bah MM, Okuda K, Skarzynski DJ. Lysophosphatic acid modulates prostaglandin secretion in the bovine uterus. Reproduction. 2009;137:95–105. doi: 10.1530/REP-08-0209. [DOI] [PubMed] [Google Scholar]
- 54.Liszewska E, Reinaud P, Billon-Denis E, Dubois O, Robin P, Charpigny G. Lysophosphatidic acid signaling during embryo development in sheep: involvement in prostaglandin synthesis. Endocrinology. 2009;150:422–434. doi: 10.1210/en.2008-0749. [DOI] [PubMed] [Google Scholar]
- 55.Seo H, Kim M, Choi Y, Lee CK, Ka H. Analysis of lysophosphatidic acid (LPA) receptor and LPA-induced endometrial prostaglandin-endoperoxide synthase 2 expression in the porcine uterus. Endocrinology. 2008;149:6166–6175. doi: 10.1210/en.2008-0354. [DOI] [PubMed] [Google Scholar]
- 56.Kim H, Lee J, Kim S, Shin MK, Min do S, Shin T. Differential expression of phospholipases D1 and D2 in mouse tissues. Cell Biol Int. 2007;31:148–155. doi: 10.1016/j.cellbi.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 57.Waclawik A, Ziecik AJ. Differential expression of prostaglandin (PG) synthesis enzymes in conceptus during peri-implantation period and endometrial expression of carbonyl reductase/PG 9-ketoreductase in the pig. J Endocrinol. 2007;194:499–510. doi: 10.1677/JOE-07-0155. [DOI] [PubMed] [Google Scholar]
- 58.Hama K, Aoki J, Bandoh K, Inoue A, Endo T, Amano T, Suzuki H, Arai H. Lysophosphatidic receptor, LPA3, is positively and negatively regulated by progesterone and estrogen in the mouse uterus. Life Sci. 2006;79:1736–1740. doi: 10.1016/j.lfs.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 59.Kaminska K, Wasielak M, Bogacka I, Blitek M, Bogacki M. Quantitative expression of lysophosphatidic acid receptor 3 gene in porcine endometrium during the periimplantation period and estrous cycle. Prostaglandins Other Lipid Mediat. 2008;85:26–32. doi: 10.1016/j.prostaglandins.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 60.Paria BC, Reese J, Das SK, Dey SK. Deciphering the cross-talk of implantation: advances and challenges. Science. 2002;296:2185–2188. doi: 10.1126/science.1071601. [DOI] [PubMed] [Google Scholar]
- 61.Song H, Lim H, Paria BC, Matsumoto H, Swift LL, Morrow J, Bonventre JV, Dey SK. Cytosolic phospholipase A2alpha is crucial [correction of A2alpha deficiency is crucial] for ‘on-time’ embryo implantation that directs subsequent development. Development. 2002;129:2879–2889. doi: 10.1242/dev.129.12.2879. [DOI] [PubMed] [Google Scholar]
- 62.Kennedy TG. Evidence for a role for prosaglandins in the initiation of blastocyst implantation in the rat. Biol Reprod. 1977;16:286–291. doi: 10.1095/biolreprod16.3.286. [DOI] [PubMed] [Google Scholar]
- 63.Kinoshita K, Satoh K, Ishihara O, Tsutsumi O, Nakayama M, Kashimura F, Mizuno M. Involvement of prostaglandins in implantation in the pregnant mouse. Adv Prostaglandin Thromboxane Leukot Res. 1985;15:605–607. [PubMed] [Google Scholar]
- 64.Woclawek-Potocka I, Brzezicka E, Skarzynski DJ. Lysophosphatic Acid Modulates Prostaglandin Secretion in the Bovine Endometrial Cells Differently on Days 8-10 of the Estrous Cycle and Early Pregnancy. J Reprod Dev. 2009 doi: 10.1262/jrd.20206. In press. [DOI] [PubMed] [Google Scholar]
- 65.Woclawek-Potocka I, Kondraciuk K, Skarzynski DJ. Lysophosphatidic Acid Stimulates Prostaglandin E2 Production in Cultured Stromal Endometrial Cells through LPA1 Receptor. Exp Biol Med (Maywood) 2009;234:986–93. doi: 10.3181/0901-RM-36. [DOI] [PubMed] [Google Scholar]
- 66.Wei Q, St Clair JB, Fu T, Stratton P, Nieman LK. Reduced expression of biomarkers associated with the implantation window in women with endometriosis. Fertil Steril. 2009;91:1686–1691. doi: 10.1016/j.fertnstert.2008.02.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bulun SE. Endometriosis. N Engl J Med. 2009;360:268–279. doi: 10.1056/NEJMra0804690. [DOI] [PubMed] [Google Scholar]
- 68.Lin CI, Chen CN, Huang MT, Lee SJ, Lin CH, Chang CC, Lee H. Lysophosphatidic acid upregulates vascular endothelial growth factor-C and tube formation in human endothelial cells through LPA(1/3), COX-2, and NF-kappaB activation- and EGFR transactivation-dependent mechanisms. Cell Signal. 2008;20:1804–1814. doi: 10.1016/j.cellsig.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 69.Shiokawa S, Sakai K, Akimoto Y, Suzuki N, Hanashi H, Nagamatsu S, Iwashita M, Nakamura Y, Hirano H, Yoshimura Y. Function of the small guanosine triphosphate-binding protein RhoA in the process of implantation. J Clin Endocrinol Metab. 2000;85:4742–4749. doi: 10.1210/jcem.85.12.7054. [DOI] [PubMed] [Google Scholar]
- 70.Hecht JH, Weiner JA, Post SR, Chun J. Ventricular zone gene-1 (vzg-1) encodes a lysophosphatidic acid receptor expressed in neurogenic regions of the developing cerebral cortex. J Cell Biol. 1996;135:1071–1083. doi: 10.1083/jcb.135.4.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fukushima N, Kimura Y, Chun J. A single receptor encoded by vzg-1/lpA1/edg-2 couples to G proteins and mediates multiple cellular responses to lysophosphatidic acid. Proc Natl Acad Sci U S A. 1998;95:6151–6156. doi: 10.1073/pnas.95.11.6151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chen SU, Lee H, Chang DY, Chou CH, Chang CY, Chao KH, Lin CW, Yang YS. Lysophosphatidic acid mediates interleukin-8 expression in human endometrial stromal cells through its receptor and nuclear factor-kappaB-dependent pathway: a possible role in angiogenesis of endometrium and placenta. Endocrinology. 2008;149:5888–5896. doi: 10.1210/en.2008-0314. [DOI] [PubMed] [Google Scholar]
- 73.Li LX, Zhou W, Qiao YH, Wang M, Zhang JH. [Expression and significance of Edg4 and Edg7 in the placentas of patients with hypertensive disorder complicating pregnancy]. Zhonghua Fu Chan Ke Za Zhi. 2007;42:386–389. [PubMed] [Google Scholar]
- 74.Yakubu MA, Leffler CW. Enhanced pial arteriolar sensitivity to bioactive agents following exposure to endothelin-1. Life Sci. 2000;66:307–316. doi: 10.1016/s0024-3205(99)00592-5. [DOI] [PubMed] [Google Scholar]
- 75.Tokumura A, Kanaya Y, Miyake M, Yamano S, Irahara M, Fukuzawa K. Increased production of bioactive lysophosphatidic acid by serum lysophospholipase D in human pregnancy. Biol Reprod. 2002;67:1386–1392. doi: 10.1095/biolreprod.102.004051. [DOI] [PubMed] [Google Scholar]
- 76.van Nieuw Amerongen GP, Vermeer MA, van Hinsbergh VW. Role of RhoA and Rho kinase in lysophosphatidic acid-induced endothelial barrier dysfunction. Arterioscler Thromb Vasc Biol. 2000;20:E127–133. doi: 10.1161/01.atv.20.12.e127. [DOI] [PubMed] [Google Scholar]
- 77.Haseruck N, Erl W, Pandey D, Tigyi G, Ohlmann P, Ravanat C, Gachet C, Siess W. The plaque lipid lysophosphatidic acid stimulates platelet activation and platelet-monocyte aggregate formation in whole blood: involvement of P2Y1 and P2Y12 receptors. Blood. 2004;103:2585–2592. doi: 10.1182/blood-2003-04-1127. [DOI] [PubMed] [Google Scholar]
- 78.Tokumura A, Kume T, Taira S, Yasuda K, Kanzaki H. Altered activity of lysophospholipase D, which produces bioactive lysophosphatidic acid and choline, in serum from women with pathological pregnancy. Mol Hum Reprod. 2009;15:301–310. doi: 10.1093/molehr/gap017. [DOI] [PubMed] [Google Scholar]
- 79.Billon-Denis E, Tanfin Z, Robin P. Role of lysophosphatidic acid in the regulation of uterine leiomyoma cell proliferation by phospholipase D and autotaxin. J Lipid Res. 2008;49:295–307. doi: 10.1194/jlr.M700171-JLR200. [DOI] [PubMed] [Google Scholar]
- 80.Hope JM, Wang FQ, Whyte JS, Ariztia EV, Abdalla W, Long K, Fishman DA. LPA receptor 2 mediates LPA-induced endometrial cancer invasion. Gynecol Oncol. 2009;112:215–223. doi: 10.1016/j.ygyno.2008.09.019. [DOI] [PubMed] [Google Scholar]
- 81.Jarvis AA, Cain C, Dennis EA. Purification and characterization of a lysophospholipase from human amnionic membranes. J Biol Chem. 1984;259:15188–15195. [PubMed] [Google Scholar]
- 82.Tokumura A, Fukuzawa K, Yamada S, Tsukatani H. Stimulatory effect of lysophosphatidic acids on uterine smooth muscles of non-pregant rats. Arch Int Pharmacodyn Ther. 1980;245:74–83. [PubMed] [Google Scholar]
- 83.Sugimoto Y, Yamasaki A, Segi E, Tsuboi K, Aze Y, Nishimura T, Oida H, Yoshida N, Tanaka T, Katsuyama M, Hasumoto K, Murata T, Hirata M, Ushikubi F, Negishi M, Ichikawa A, Narumiya S. Failure of parturition in mice lacking the prostaglandin F receptor. Science. 1997;277:681–683. doi: 10.1126/science.277.5326.681. [DOI] [PubMed] [Google Scholar]
- 84.Moore F, Da Silva C, Wilde JI, Smarason A, Watson SP, Lopez Bernal A. Up-regulation of p21- and RhoA-activated protein kinases in human pregnant myometrium. Biochem Biophys Res Commun. 2000;269:322–326. doi: 10.1006/bbrc.2000.2290. [DOI] [PubMed] [Google Scholar]
- 85.Gogarten W, Emala CW, Lindeman KS, Hirshman CA. Oxytocin and lysophosphatidic acid induce stress fiber formation in human myometrial cells via a pathway involving Rho-kinase. Biol Reprod. 2001;65:401–406. doi: 10.1095/biolreprod65.2.401. [DOI] [PubMed] [Google Scholar]
- 86.Nilsson UK, Grenegard M, Berg G, Svensson SP. Different proliferative responses of Gi/o-protein-coupled receptors in human myometrial smooth muscle cells. A possible role of calcium. J Mol Neurosci. 1998;11:11–21. doi: 10.1385/JMN:11:1:11. [DOI] [PubMed] [Google Scholar]
- 87.Nilsson UK, Svensson SP. Inhibition of Ca2+/calmodulin-dependent protein kinase or epidermal growth factor receptor tyrosine kinase abolishes lysophosphatidic acid-mediated DNA-synthesis in human myometrial smooth muscle cells. Cell Biol Int. 2003;27:341–347. doi: 10.1016/s1065-6995(02)00352-9. [DOI] [PubMed] [Google Scholar]
- 88.Mikamo H, Kawazoe K, Sato Y, Imai A, Tamaya T. Preterm labor and bacterial intra-amniotic infection: arachidonic acid liberation by phospholipase A2 of Prevotella bivia. Anaerobe. 1998;4:209–212. doi: 10.1006/anae.1998.0165. [DOI] [PubMed] [Google Scholar]
- 89.Mikamo H, Kawazoe K, Sato Y, Imai A, Tamaya T. Preterm labor and bacterial intraamniotic infection: arachidonic acid liberation by phospholipase A2 of Fusobacterium nucleatum. Am J Obstet Gynecol. 1998;179:1579–1582. doi: 10.1016/s0002-9378(98)70028-6. [DOI] [PubMed] [Google Scholar]
- 90.Ziecik AJ, Waclawik A, Bogacki M. Conceptus signals for establishment and maintenance of pregnancy in pigs - lipid signaling system. Exp Clin Endocrinol Diabetes. 2008;116:443–449. doi: 10.1055/s-2008-1042405. [DOI] [PubMed] [Google Scholar]
- 91.Contos JJ, Ishii I, Chun J. Lysophosphatidic acid receptors. Mol Pharmacol. 2000;58:1188–1196. doi: 10.1124/mol.58.6.1188. [DOI] [PubMed] [Google Scholar]
- 92.Yue J, Yokoyama K, Balazs L, Baker DL, Smalley D, Pilquil C, Brindley DN, Tigyi G. Mice with transgenic overexpression of lipid phosphate phosphatase-1 display multiple organotypic deficits without alteration in circulating lysophosphatidate level. Cell Signal. 2004;16:385–399. doi: 10.1016/j.cellsig.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 93.Im DS, Heise CE, Harding MA, George SR, O'Dowd BF, Theodorescu D, Lynch KR. Molecular cloning and characterization of a lysophosphatidic acid receptor, Edg-7, expressed in prostate. Mol Pharmacol. 2000;57:753–759. [PubMed] [Google Scholar]
- 94.Guo R, Kasbohm EA, Arora P, Sample CJ, Baban B, Sud N, Sivashanmugam P, Moniri NH, Daaka Y. Expression and function of lysophosphatidic acid LPA1 receptor in prostate cancer cells. Endocrinology. 2006;147:4883–4892. doi: 10.1210/en.2005-1635. [DOI] [PubMed] [Google Scholar]
- 95.Raj GV, Sekula JA, Guo R, Madden JF, Daaka Y. Lysophosphatidic acid promotes survival of androgen-insensitive prostate cancer PC3 cells via activation of NF-kappaB. Prostate. 2004;61:105–113. doi: 10.1002/pros.20083. [DOI] [PubMed] [Google Scholar]
- 96.Garbi M, Rubinstein S, Lax Y, Breitbart H. Activation of protein kinase calpha in the lysophosphatidic acid-induced bovine sperm acrosome reaction and phospholipase D1 regulation. Biol Reprod. 2000;63:1271–1277. doi: 10.1095/biolreprod63.5.1271. [DOI] [PubMed] [Google Scholar]
- 97.Delgado-Buenrostro NL, Hernandez-Gonzalez EO, Segura-Nieto M, Mujica A. Actin polymerization in the equatorial and postacrosomal regions of guinea pig spermatozoa during the acrosome reaction is regulated by G proteins. Mol Reprod Dev. 2005;70:198–210. doi: 10.1002/mrd.20192. [DOI] [PubMed] [Google Scholar]
- 98.Kobayashi T, Yamano S, Murayama S, Ishikawa H, Tokumura A, Aono T. Effect of lysophosphatidic acid on the preimplantation development of mouse embryos. FEBS Lett. 1994;351:38–40. doi: 10.1016/0014-5793(94)00815-9. [DOI] [PubMed] [Google Scholar]
- 99.Liu Z, Armant DR. Lysophosphatidic acid regulates murine blastocyst development by transactivation of receptors for heparin-binding EGF-like growth factor. Exp Cell Res. 2004;296:317–326. doi: 10.1016/j.yexcr.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 100.Contos JJ, Fukushima N, Weiner JA, Kaushal D, Chun J. Requirement for the lpA1 lysophosphatidic acid receptor gene in normal suckling behavior. Proc Natl Acad Sci U S A. 2000;97:13384–13389. doi: 10.1073/pnas.97.24.13384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lloyd B, Tao Q, Lang S, Wylie C. Lysophosphatidic acid signaling controls cortical actin assembly and cytoarchitecture in Xenopus embryos. Development. 2005;132:805–816. doi: 10.1242/dev.01618. [DOI] [PubMed] [Google Scholar]
- 102.Kimura Y, Schmitt A, Fukushima N, Ishii I, Kimura H, Nebreda AR, Chun J. Two novel Xenopus homologs of mammalian LP(A1)/EDG-2 function as lysophosphatidic acid receptors in Xenopus oocytes and mammalian cells. J Biol Chem. 2001;276:15208–15215. doi: 10.1074/jbc.M011588200. [DOI] [PubMed] [Google Scholar]
- 103.An S, Bleu T, Hallmark OG, Goetzl EJ. Characterization of a novel subtype of human G protein-coupled receptor for lysophosphatidic acid. J Biol Chem. 1998;273:7906–7910. doi: 10.1074/jbc.273.14.7906. [DOI] [PubMed] [Google Scholar]
- 104.Lee CW, Rivera R, Gardell S, Dubin AE, Chun J. GPR92 as a new G12/13- and Gq-coupled lysophosphatidic acid receptor that increases cAMP, LPA5. J Biol Chem. 2006;281:23589–23597. doi: 10.1074/jbc.M603670200. [DOI] [PubMed] [Google Scholar]
- 105.Kotarsky K, Boketoft A, Bristulf J, Nilsson NE, Norberg A, Hansson S, Owman C, Sillard R, Leeb-Lundberg LM, Olde B. Lysophosphatidic acid binds to and activates GPR92, a G protein-coupled receptor highly expressed in gastrointestinal lymphocytes. J Pharmacol Exp Ther. 2006;318:619–628. doi: 10.1124/jpet.105.098848. [DOI] [PubMed] [Google Scholar]
- 106.Komatsu J, Yamano S, Kuwahara A, Tokumura A, Irahara M. The signaling pathways linking to lysophosphatidic acid-promoted meiotic maturation in mice. Life Sci. 2006;79:506–511. doi: 10.1016/j.lfs.2006.01.028. [DOI] [PubMed] [Google Scholar]
- 107.Pustilnik TB, Estrella V, Wiener JR, Mao M, Eder A, Watt MA, Bast RC, Jr., Mills GB. Lysophosphatidic acid induces urokinase secretion by ovarian cancer cells. Clin Cancer Res. 1999;5:3704–3710. [PubMed] [Google Scholar]
- 108.Estrella VC, Eder AM, Liu S, Pustilnik TB, Tabassam FH, Claret FX, Gallick GE, Mills GB, Wiener JR. Lysophosphatidic acid induction of urokinase plasminogen activator secretion requires activation of the p38MAPK pathway. Int J Oncol. 2007;31:441–449. [PubMed] [Google Scholar]
- 109.Sengupta S, Xiao YJ, Xu Y. A novel laminin-induced LPA autocrine loop in the migration of ovarian cancer cells. Faseb J. 2003;17:1570–1572. doi: 10.1096/fj.02-1145fje. [DOI] [PubMed] [Google Scholar]
- 110.Park KS, Kim MK, Lee HY, Kim SD, Lee SY, Kim JM, Ryu SH, Bae YS. S1P stimulates chemotactic migration and invasion in OVCAR3 ovarian cancer cells. Biochem Biophys Res Commun. 2007;356:239–244. doi: 10.1016/j.bbrc.2007.02.112. [DOI] [PubMed] [Google Scholar]