Abstract
A procedure for the coupling of aliphatic imines with allylic and allenic alkoxides is described. The success of these studies was enabled by a unique reactivity profile of Ti(IV) isopropoxide/n-BuLi compared to well-known Ti(IV) isopropoxide/R-MgX systems.
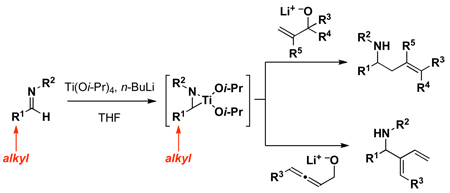
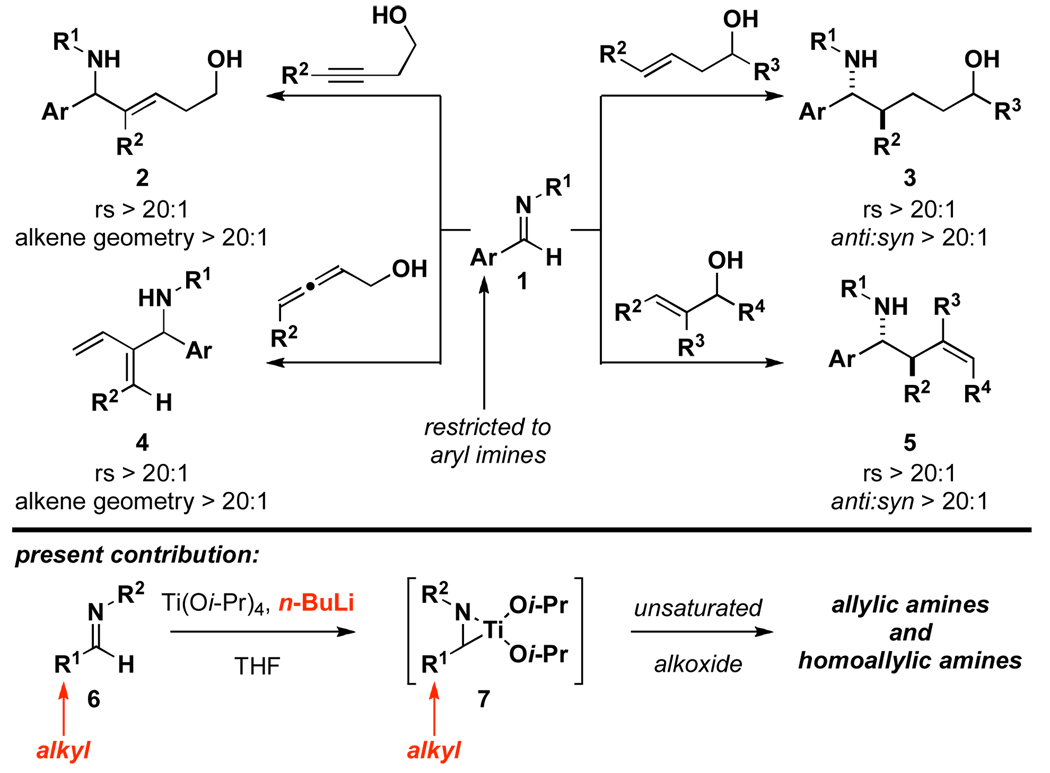
Nitrogen-containing small molecules have emerged as a subset of organic structures that display a range of pharmaceutically relevant properties.1 As such, chemical methods that provide new approaches to the stereoselective assembly of diverse nitrogen-containing small molecules have great potential to affect the pace of discovery in medicine. Of the many robust methods available for the convergent synthesis of such molecules, embracing the reactivity of imines as electrophiles has proven to be a productive pursuit.2 By a mechanistically distinct process, the reductive cross-coupling of imines with alkenes, alkynes and allenes has great potential to unlock unique and powerful convergent coupling reactions for the synthesis of stereodefined nitrogen-containing products. Until recently, many of these bond constructions were ill-defined, with only a handful of processes available for the regioselective coupling of highly activated imine-based systems with a small subset of alkynes.3 In our efforts to discover new cross-coupling reactions capable of forging C–C bonds between unactivated imines and unsymmetrical and electronically unactivated alkynes, alkenes and allenes, we have described a series of highly regio- and stereoselective metal-mediated coupling reactions for the synthesis of unsaturated 1,5-aminoalcohols,4 allylic amines,5 saturated 1,5-aminoalcohols6 and homoallylic amines7 (1 → 2–5; Figure 1). While these advances demonstrated coupling processes of potential significance in chemical synthesis, like other contributions in this general area of reaction methodology (reductive coupling chemistry), they were uniformly limited to a subset of imines – in this case aromatic imines. In an effort to increase the significance of these alkoxide-directed titanium-mediated reductive cross-coupling reactions of imines, we sought to identify a method suitable for the functionalization of aliphatic imines. Here, we describe a useful method for the activation of aliphatic imines via epititanation (6 → 7), and the application of these activated complexes in reductive cross-coupling reactions with allylic- and allenic alcohols.
Figure 1.
Reductive cross-coupling reactions of imines with unsaturated alkoxides: A new procedure for the coupling of aliphatic imines.
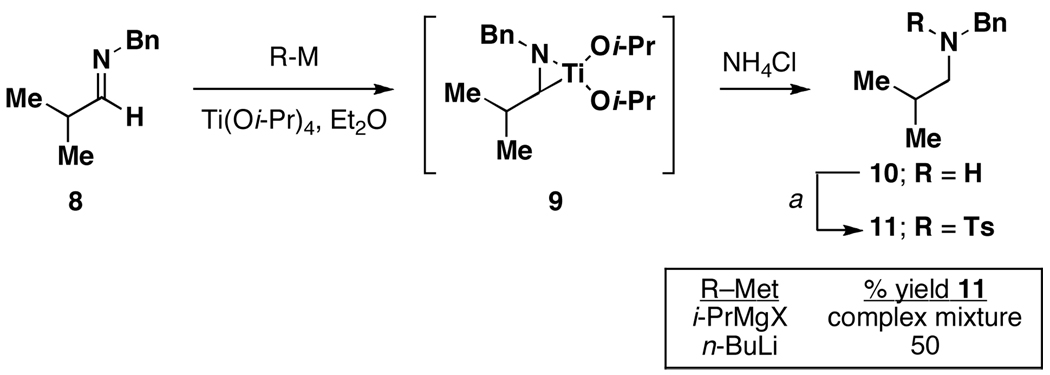
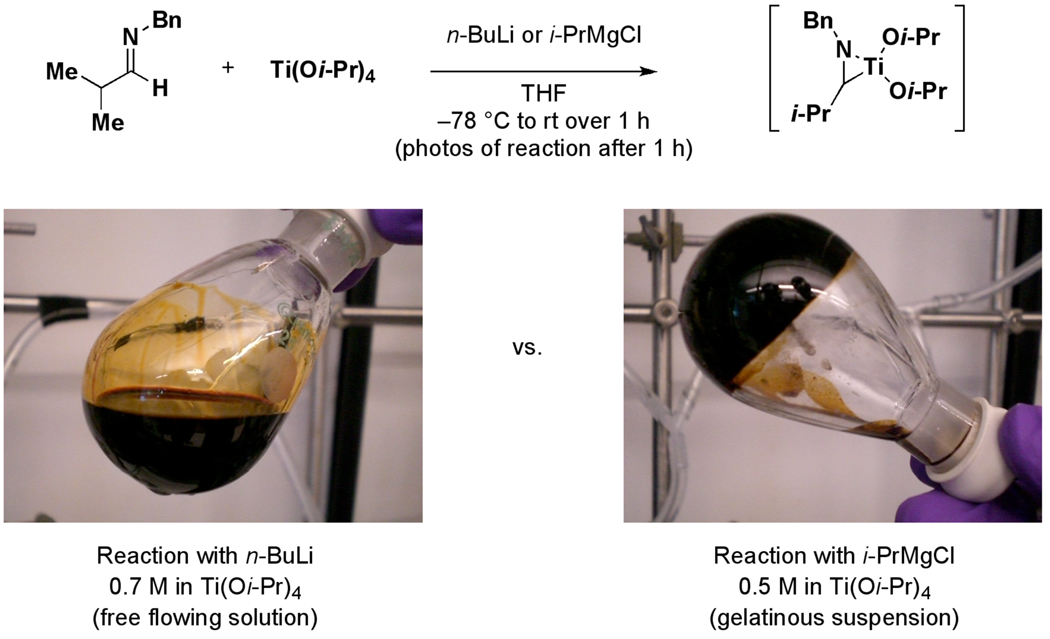
The reduction of an aromatic imine by formation of an azametallacyclopropane, and subsequent hydrolysis, is a well-known process.8 To date, azametallacyclopropane formation has generally been accomplished in one of two ways: 1) by exposure of preformed aromatic imines to the combination of a titanium(IV) alkoxide and a reactive organometallic reagent (i.e. RMgX), or 2) by a metal-mediated redox process with suitably functionalized amines.9 For the former case, it has been documented that aliphatic imines are poor substrates for the process10 although the mechanistic details that lead to their poor behavior remain poorly understood. Deleterious side reactions, including pinacol-type coupling (homocoupling) and addition of the organometallic reducing agent to the imine have been observed. In studies aimed at overcoming these significant limitations, we explored the titanium-mediated reduction of imine 8. As depicted in Figure 2, our preliminary study confirmed the poor reactivity of the Kulinkovich/Sato system for this process (Ti(Oi-Pr)4, i-PrMgCl), but demonstrated a significant dependence of this reaction on the nature of the organometallic reducing agent employed. In this case, employing n-BuLi as the reductant11 led to superior results. Aside from the differences in reactivity of the Ti(Oi-Pr)4/n-BuLi system in the reduction of aliphatic imine 8, we observed a significant change in physical properties associated with the reaction media at elevated concentrations. As depicted in Figure 3, exposure of imine 8 to Ti(Oi-Pr)4 / n-BuLi in THF (0.7 M in titanium) led to the formation of a free flowing solution. In contrast, treatment of imine 8 with Ti(Oi-Pr)4/i-PrMgCl in THF (0.5 M in titanium) led to the formation of a gelatinous suspension. While the unique reactivity of the n-BuLi-based system is of central interest to the studies presented here, the ability to maintain a free flowing solution at elevated concentrations of Ti(Oi-Pr)4 is a notable property of the system.
Figure 2.
Reactivity differences between RMgX and RLi in the reduction of aliphatic imines.
a The crude 2° amine was dissolved in CH2Cl2 and treated with TsCl (1.2 equiv) and 2 M NaOH (See Supporting Information for details).
Figure 3.
Solution characteristics as a function of the nature of the reducing metal employed.
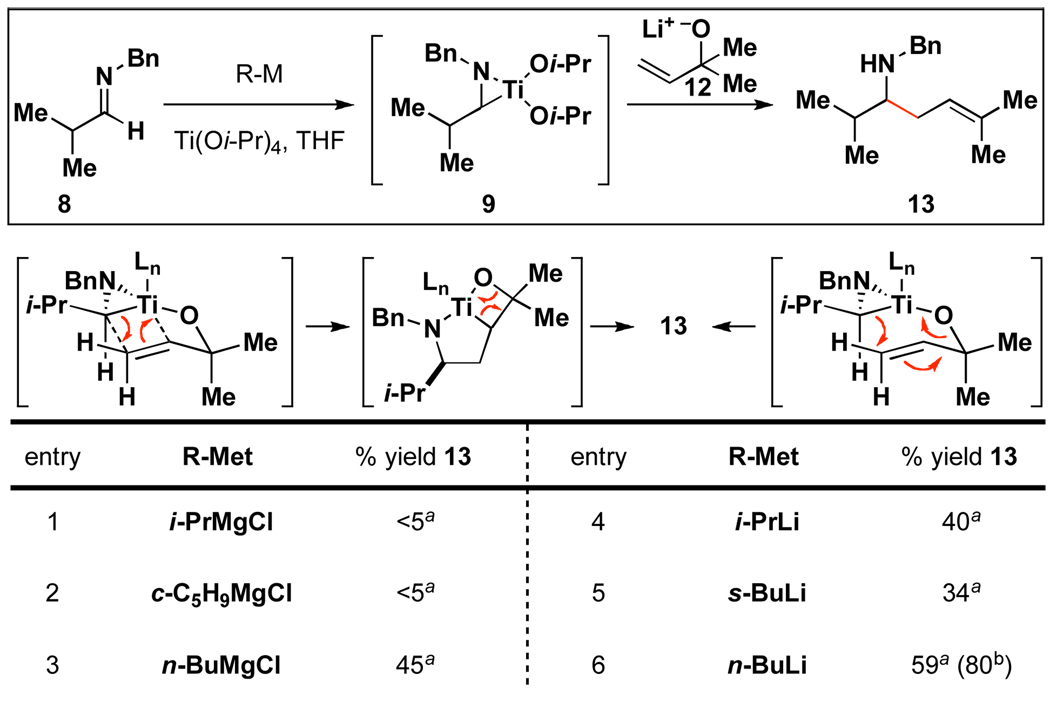
Moving on to explore C–C bond forming processes with aliphatic imines, success in titanium-mediated prenylation of 8 was similarly dependent on the nature of the reducing metal used (Figure 4).12 While the secondary Grignard reagents investigated led to little product formation (entries 1–2), n-BuMgCl was marginally effective (entry 3). Interestingly, organolithium reagents were uniformly more effective for this transformation, with n-BuLi providing the prenylated product 13 in 59% yield (entry 6). Due to the enhanced efficiency observed in this preliminary screen, and the practical advantages of n-BuLi, further study was dedicated to this reducing metal. Optimization of the prenylation was relatively straightforward, where simply employing two equivalents of aliphatic imine in the coupling reaction with 12 led to the formation of the homoallylic amine 13 in 80% yield.13
Figure 4.
Differences in reactivity of reducing agents for imine prenylation.
Reaction conditions: a Imine (1 equiv), Ti(Oi-Pr)4 (1.5 equiv), reducing agent (3.0 equiv), alkoxide 12 (1.5 equiv) THF. b Imine (2 equiv), Ti(Oi-Pr)4 (2.0 equiv), n-BuLi (4.0 equiv), alkoxide 12 (1.0 equiv) Et2O / THF (6:1).
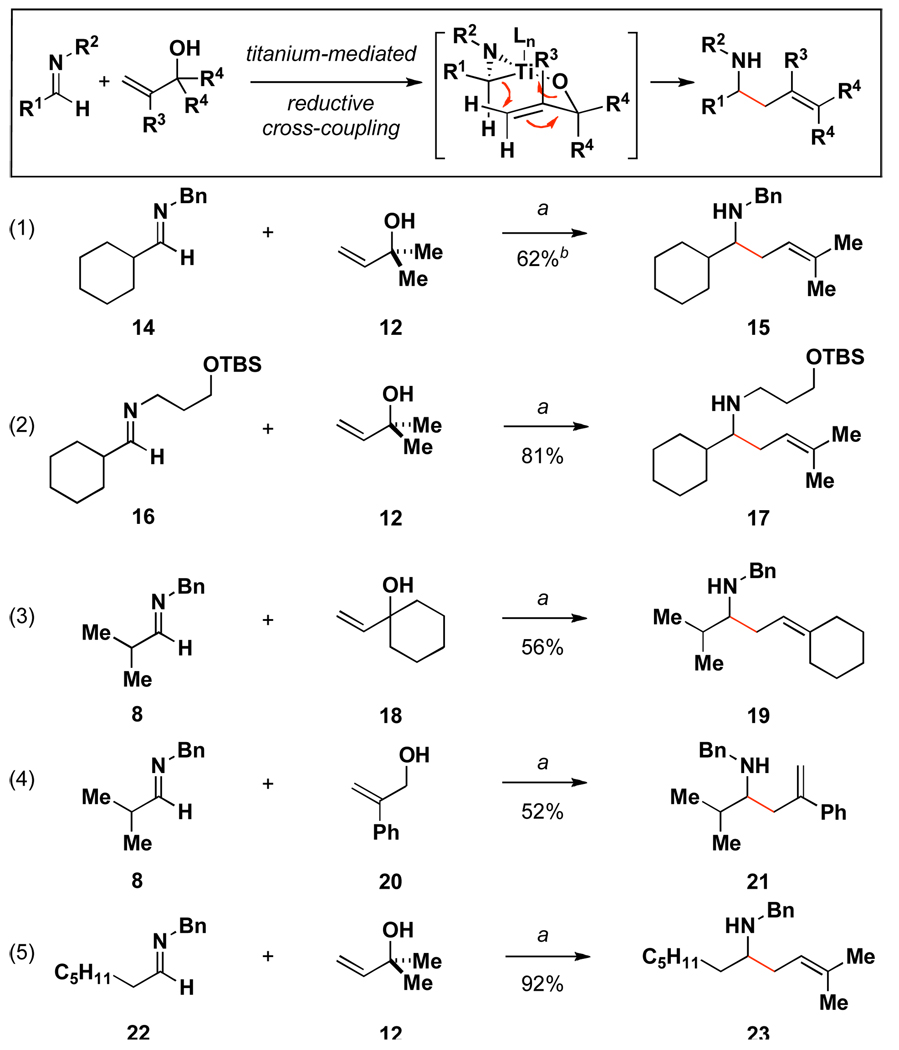
As illustrated in Figure 5 and Figure 6, this preparatively simple protocol to produce aliphatic imine-derived azametallacyclopropanes is useful for coupling reactions that extend beyond prenylation. Figure 5 highlights the compatibility of this process for the coupling of aliphatic imines with a variety of allylic alcohols. Eqs 1–4 describe coupling reactions of aliphatic imines that bear α-branching (eqs 1–4). Finally, aliphatic imines that lack α-branching are also competent reaction partners for coupling with allylic alcohols. As demonstrated in eq 5, the heptylimine 22 was converted to the prenylation product 23 in 92% yield.14
Figure 5.
Reductive cross-coupling reactions of aliphatic imines with allylic alcohols.
a Reaction conditions: Imine (2.0 equiv), Ti(Oi-Pr)4 (2.0 equiv), n-BuLi (4.0 equiv), Et2O, −78 °C - rt 1 h; recool to −78 °C, add Li-alkoxide of allylic alcohol (1.0 equiv) in THF (0.5 mL) −78 °C to rt. b This product was isolated as an inseparable mixture containing an additional 10% of the product derived from butyl addition to the imine.
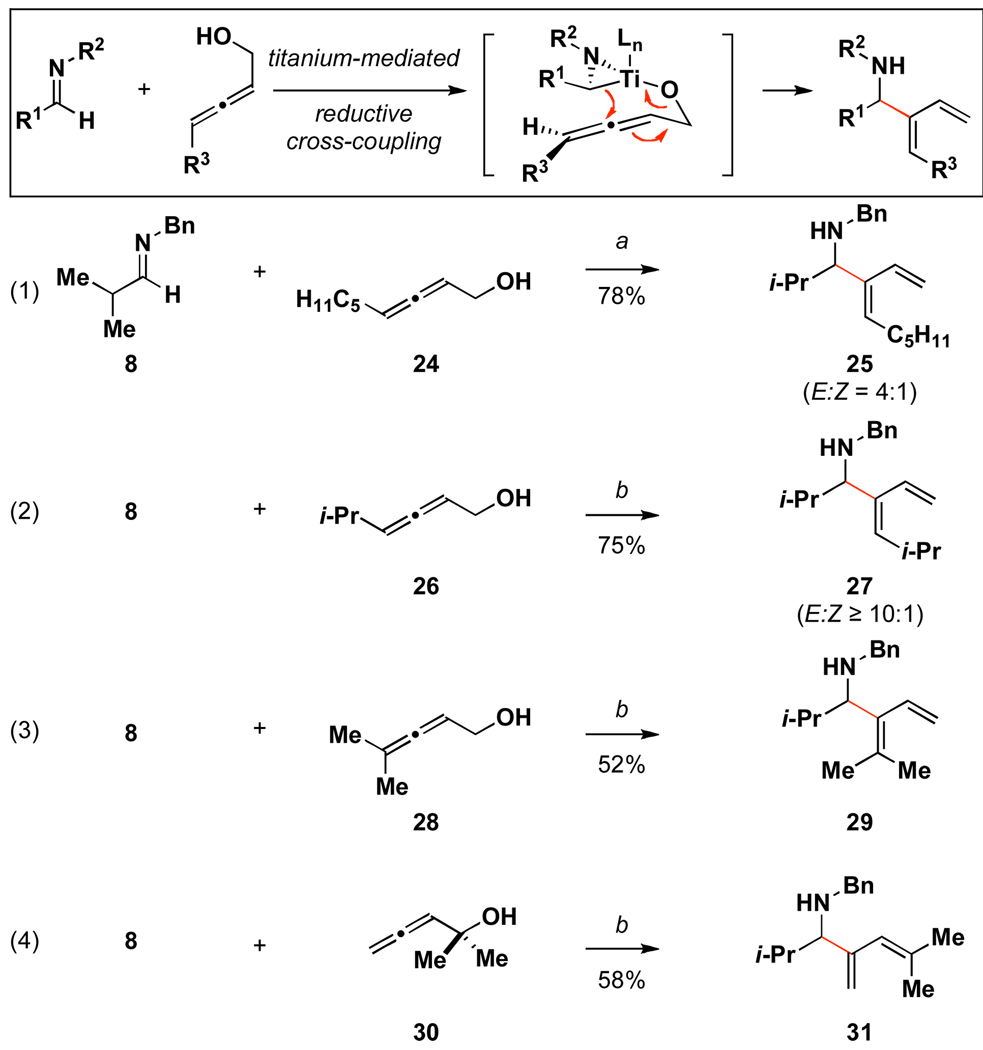
Figure 6.
Reductive cross-coupling reactions of aliphatic imines with allenic alcohols.
Reaction conditions: a Imine (2.0 equiv), Ti(Oi-Pr)4 (2.0 equiv), n-BuLi (4.0 equiv), THF, −78 °C - rt 1 h; recool to −78 °C, add Li-alkoxide of allenyl alcohol (1.0 equiv) in THF, −78 °C to rt (10 min), then warm to 60 °C. b Imine (2.0 equiv), Ti(Oi-Pr)4 (2.0 equiv), n-BuLi (4.0 equiv), THF, −78 °C - rt 1 h; recool to −78 °C and add Li-alkoxide of allenyl alcohol (1.0 equiv) in THF, −78 °C to rt.
The presumed azametallacyclopropanes prepared by exposure of aliphatic imines to the combination of BuLi and Ti(Oi-Pr)4 are also effective in cross-coupling reactions with allenic alcohols. As depicted in Figure 6, mono-, di- and trisubstituted allenes are all compatible with the process and deliver allylic amines containing a valuable 1,3-diene motif. Consistent with the trends in stereoselectivity observed in the related coupling reaction of aromatic imines, the present process was generally E-selective, with highest levels of stereoselection observed in coupling reactions of allenes bearing branched terminal substitution (i.e. 24 vs 26; eqs 1–2). Finally isomeric allenes 28 and 30 are viable partners with imine 8 (eqs 3–4). In these cases, the production of dienes 29 and 31 occurred in a regiospecific fashion in 52 and 58% yield.
In conclusion, it has long been accepted that alkyl imines are particularly challanging substrates in reductive cross-coupling chemistry. In titanium alkoxide-mediated processes, alkylation of aliphatic imines with the reducing Grignard reagent typically employed in these processes has been cited as the root cause of this limitation. In an attempt to overcome this limitation, we initiated empirical studies to probe the structure/activity relationships of a range of reducing organometallic reagents in titanium alkoxide-mediated reduction of aliphatic imines. Initial studies led to the identification of n-BuLi as a particularly effective reagent in combination with Ti(Oi-Pr)4 for the net reduction of aliphatic imines. Subsequent to this observation, the reductive cross-coupling of a variety of aliphatic imines with allylic and allenic alcohols was accomplished. While the mechanistic underpinnings that have resulted in empirical success remain undefined, the current findings greatly impact the potential utility of metal-mediated reductive cross-coupling reactions for the assembly of stereodefined nitrogen-containing small molecules. Due to the significance of such molecules in biology and medicine, and the potential impact of stereoselective convergent coupling processes well-suited for their synthesis, we look forward to future developments that emerge from these findings.
Supplementary Material
Supporting Information Available Experimental procedures and tabulated spectroscopic data for new compounds (PDF). This material is available free of charge via the Internet at http://pubs.acs.org.
Acknowledgment
We gratefully acknowledge financial support of this work by the American Cancer Society (RSG-06-117-01), Boehringer Ingelheim, Eli Lilly & Co. and the National Institutes of Health – NIGMS (GM80266).
References
- 1.Fischer J, Ganellin CRc, editors. Analogue-based Drug Discovery. John Wiley & Sons; 2006. (b) Four of the top ten best-selling drugs currently marketed contain nitrogen heterocycles (Lipitor, Plavix, Prevacid, and Nexium) – Forbes.com.
- 2.(a) Martin SF. Pure Appl. Chem. 2009;81:195–204. doi: 10.1351/PAC-CON-08-07-03. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Ferraris D. Tetrahedron. 2007;63:9581–9597. [Google Scholar]; (c) Kobayashi S, Ishitani H. Chem. Rev. 1999;99:1069–1094. doi: 10.1021/cr980414z. [DOI] [PubMed] [Google Scholar]
- 3.(a) Patel SJ, Jamison TF. Angew. Chem. Int. Ed. 2003;42:1364–1367. doi: 10.1002/anie.200390349. [DOI] [PubMed] [Google Scholar]; (b) Kong J-R, Cho C-W, Krische MJ. J. Am. Chem. Soc. 2005;127:11269–11276. doi: 10.1021/ja051104i. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Barchuk A, Ngai M-Y, Krische MJ. J. Am. Chem. Soc. 2007;129:8432–8433. doi: 10.1021/ja073018j. [DOI] [PubMed] [Google Scholar]; (d) Ngai M-Y, Barchuk A, Krische MJ. J. Am. Chem. Soc. 2007;129:12644–12645. doi: 10.1021/ja075438e. [DOI] [PubMed] [Google Scholar]; (e) Skucas E, Zbieg JR, Krische MJ. J. Am. Chem. Soc. 2007;129:7242–7243. doi: 10.1021/ja0715896. [DOI] [PubMed] [Google Scholar]
- 4.McLaughlin M, Takahashi M, Micalizio GC. Angew. Chem. Int. Ed. 2007;46:3912–3914. doi: 10.1002/anie.200605060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McLaughlin M, Shimp HL, Navarro R, Micalizio GC. Synlett. 2008:735–738. doi: 10.1055/s-2008-1042808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takahashi M, Micalizio GC. J. Am. Chem. Soc. 2007;129:7514–7416. doi: 10.1021/ja071974v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takahashi M, McLaughlin M, Micalizio GC. Angew. Chem. Int. Ed. 2009;48:3648–3652. doi: 10.1002/anie.200900236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.For a review, see: Guan H. Curr. Org. Chem. 2008;12:1406–1430.Uchikawa W, Matsuno C, Okamoto S. Tetrahedron Lett. 2004;45:9037–9045.Fukuhara K, Okamoto S, Sato F. Org. Lett. 2003;5:2145–2148. doi: 10.1021/ol034599u.Gao Y, Yoshida Y, Sato F. Synlett. 1997:1353–1354.
- 9.Zr: Buchwald SL, Wannamaker MW, Watson BT. J. Am. Chem. Soc. 1989;111:776–777.Buchwald SL, Watson BT, Wannamaker MW, Dewan JC. J. Am. Chem. Soc. 1989;111:4486–4494.Coles N, Whitby RJ, Blagg J. Synlett. 1990:271–272.Ca:Buch F, Harder S. Organometallics. 2007;26:5132–5135.Ta, Nb:Castro A, Galakhov MV, Gomez M, Gomez-Sal P, Martin A, Sanchez F, Velasco P. Eur. J. Inorg. Chem. 2000:2047–2054.
- 10.Propionaldimine results in a 38% yield under Ti(Oi-Pr)4 / i-PrMgCl conditions: Ref 8d. A recent example by Cha and coworkers demonstrated two aliphatic substrates in 40–60% yield using a Ti / RMgX system:Lysenko IL, Lee HG, Cha JK. Org. Lett. 2009;11:3132–3134. doi: 10.1021/ol901006c. In this report, preformation of the presumed Ti–imine complex was not required for allylation to ensue.
- 11.(a) Eisch JJ, Gitua JN, Otieno PO, Shi X. J. Organomet. Chem. 2001;624:229–238. [Google Scholar]; (b) Eisch JJ, Gitua JN. Organometallics. 2003;22:24–26. [Google Scholar]; (c) Eisch JJ, Adeosun AA, Birmingham JM. Eur. J. Inorg. Chem. 2007:39–43. [Google Scholar]
- 12.Amin SR, Crowe WE. Tetrahedron Lett. 1997;38:7487–7490. (see footnote 15) [Google Scholar]
- 13.(a) No evidence was found for the production of regioisomeric products (derived from reverse prenylation) (b) This reaction also produced 15% of the product derived from butyl addition to the imine (c) An interesting Mg-ion effect was observed in this coupling reaction: When one equivalent of MgBr2•OEt2 was added to this reaction (coupling of 8 with 12 under the optimized conditions with n-BuLi) the yield of 13 dropped to 47% (c) 1H NMR spectra of the crude reaction mixtures resulting from attempted prenylation of imine 8 via procedures employing a 2° alkyl Grignard reagent and n-BuLi can be found in the Supporting Information.
- 14.A limitation of this coupling reaction, compared to our previously described allylation of aromatic imines, currently includes 2-halo-substituted allylic alcohols. Employing the reaction conditions used to generate the homoallylic amines in Figure 5 for the coupling of 2-bromo-propen-1-ol with imine 12 did not lead to appreciable quantities of the anticipated product of allyltransfer.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information Available Experimental procedures and tabulated spectroscopic data for new compounds (PDF). This material is available free of charge via the Internet at http://pubs.acs.org.