Abstract
Enteropathogenic Escherichia coli (EPEC) is a common cause of watery diarrhea in children in developing countries. After adhering intimately to small intestinal cells, EPEC secretes effector proteins into host cells, altering host cell functions and causing cell damage and death. We previously showed that EPEC infection triggers the release of adenosine triphosphate (ATP) from host cells and that ATP is broken down to ADP, AMP, and adenosine. Adenosine produced from the breakdown of extracellular ATP triggers fluid secretion in cultured intestinal monolayers and may be an important mediator of EPEC-induced diarrhea.
In this study we examined whether adenosine has any effects on EPEC bacteria themselves. Adenosine stimulated EPEC growth in several types of media in vitro. Adenosine also altered the pattern of EPEC adherence to cultured cells from a classic localized adherence pattern to a more diffuse adherence pattern. Adenosine changed the pattern of expression of virulence factors in EPEC, inhibiting the expression of the bundle-forming pilus (BFP) and enhancing expression of the EPEC secreted proteins (Esps). The ability of adenosine to inhibit BFP was dependent on the Plasmid-encoded Regulator (Per).
In vivo, experimental manipulations of adenosine levels had strong effects on the outcome of EPEC infection in rabbit intestinal loops. Reduction in adenosine levels by addition of exogenous adenosine deaminase (ADA) reduced numbers of EPEC bacteria recovered by over 10-fold in rabbit intestine in vivo. Conversely, inhibitors of ADA increased EPEC-induced fluid secretion, the number of EPEC bacteria recovered from intestinal fluid, and increased the in vivo expression of espA and espB.
In addition to its previously reported effects on host cells and tissues, adenosine also has strong effects on EPEC bacteria, stimulating EPEC growth, altering its adherence pattern, and changing the expression of several important virulence genes. Adenosine is released from host cells in response to EPEC infection, and it appears that EPEC has the ability to sense and respond to the adenosine produced.
Keywords: Microbial endocrinology, Diarrheal disease, Adenosine NOT triphosphate, Inter-kingdom signaling
Introduction
Unlike several other types of diarrhea-producing E. coli, EPEC produces no toxins, so the way that it causes watery diarrhea has been a mystery. We previously showed that EPEC infection triggers the release of adenosine triphosphate (ATP) from host cells and that ATP is broken down to ADP, AMP, and adenosine (Crane, et al., 2002). Work done by others (Barrett, et al., 1989, Strohmeier, et al., 1995) and in our own laboratory (Crane, et al., 2007) has shown that adenosine is a potent secretagogue in the intestinal tract, and we hypothesized that adenosine produced from the breakdown of extracellular ATP is an important mediator of EPEC-induced diarrhea.
Adenosine has been recognized as in important signaling molecule in the mammalian cardiovascular system for over 40 years (Linden, 2001, Koszalka, et al., 2004, Olsson, 2004) and in the gastrointestinal tract for about 15 years(Roman & Fitz, 1999). In addition to stimulating fluid and electrolyte secretion, adenosine has other biological functions including protection against ischemia (Synnestvedt, et al., 2002) and down-regulation of inflammation (Haskó & Cronstein, 2004, Cavalcante, et al., 2006, Linden, 2006), especially inhibition of neutrophil chemotaxis (Linden, 2006). Despite this large volume of literature on signaling roles of adenosine, little research has been done on the possible role of adenosine in microbial infections.
In this study we examined whether adenosine has feedback effects on EPEC bacteria, as opposed to the better-studied effects of adenosine on the host. EPEC, like other E. coli, has several membrane transporters capable of taking up adenosine and other nucleosides from the environment (Ye & van den Berg, 2004), so one parameter we examined was the effect of adenosine on EPEC growth. While investigating the effects of adenosine on growth, we noticed that adenosine also altered the pattern of adherence of EPEC to cultured cells, and pursued those findings to produce a better understanding of how adenosine affects EPEC.
Materials and Methods
Bacterial strains used in this report are shown in Table 1.
Table 1.
Description of Bacterial Strains Used in this Study
| Strain Name | General Description | Mutations | Serotype, Comments, Reference(s) |
|---|---|---|---|
| Human Isolates | |||
| E2348/69 | Wild-type, classic human EPEC | -- | O127:H6; Taunton, England; (Levine, et al., 1978) |
| JPN15 | EAF- plasmid-cured derivative of E2348/69 | (Jerse, et al., 1990) | |
| JPN15(pJLM161) | perA,B,C restored on plasmid pJLM161 | (Mellies, et al., 1999) | |
| OG127 | Per mutant of E2348/69 | Δper | (Gomez-Duarte & Kaper, 1995) |
| SE796 | Ler mutant ot E2348/69 | Δler | (Elliott, et al., 2000) |
| B171-8 | Wild-type classic human EPEC | - | O111: NM; Mexico; (Bieber, et al., 1998) |
| JCP88 | Wild-type classic human EPEC | - | O119:B14; Ohio, USA; (Rothbaum, et al., 1983) |
| Rabbit Isolates | |||
| RDEC-1 | Wild-type but less virulent rabbit EPEC | - | O15: H -; Diarrheal disease in rabbits; (Cantey & Inman, 1981) |
| E22 | Wild-type, virulent | O103: H2; Severe diarrheal disease in rabbits; (Milon, et al., 1999) | |
Materials
Adenosine, inosine, N-ethylcarboxamido-adenosine, guanosine, hypoxanthine, thymidine, uridine, 5′-AMP, and adenosine deaminase were purchased from Sigma-Aldrich (St. Louis, Mo.). Erythro-9-(2-hydroxy-3-nonyl)adenine (EHNA) was from Biomol (Plymouth Meeting, Pa.; Biomol is now part of ENZO Life Sciences).
Bacterial culture media used
Minimal medium consisted of 1X M9 salts with 2 % amino acids derived from hydrolysis of casein (casamino acids) plus either 2 mM glucose or 2 mM sodium succinate as the carbon source. The medium referred to in the text and figures as Dulbecco’s modified Eagle’s medium (DMEM) was actually DMEM-F12 medium plus 18 mM NaHCO3 plus 25 mM extra HEPES buffer, pH 7.4, yielding 40 mM HEPES total. DMEM-F12 was prepared from packets of powder (Gibco Division of Invitrogen Corp., Grand Island, NY). No serum was used in the bacterial media. Bacteria were grown overnight in Luria broth (LB) at 37 °C with 300 rpm shaking, then diluted into minimal medium or DMEM for the experiments shown.
Ussing chamber methods
T84 cell monolayers were grown on collagen-coated Snap-Well inserts (Corning-Costar, Corning, NY) of 1.13 cm2 surface area, then mounted in the Ussing chamber for electrophysiology measurements. Short-circuit current was measured exactly as previously described (Crane, et al., 2006, Crane, et al., 2007). With the methods used, an upward deflection in short-circuit current represents chloride secretion toward the apical or lumenal side of the monolayer.
Bacterial Adherence assays
EPEC adherence was assessed visually by allowing EPEC bacteria to adhere to HeLa cells grown in Lab-Tek chamber slides for 2 hours, then rinsing, fixing with glutaraldehyde, and staining with Giemsa stain, as described (Crane, et al., 1999). Quantitative adherence was measured as previously described (Donnenberg & Nataro, 1995, Crane, et al., 2007) except that EPEC was subcultured for 2 h in minimal medium + glucose instead of DMEM before infecting the HeLa monolayers. In the quantitative adherence method HeLa cells are lysed in 0.1% Triton X-100 detergent, and the room temperature incubation steps allows disaggregation of EPEC bacterial clumps, permitting accurate quantitation.
Quantitative Real-time Polymerase Chain Reaction (qRT-PCR)
0.5 ml aliquots of bacterial cultures, or 0.5 ml of infected rabbit loop fluids, were lysed using 40 μg of lysozyme for 5 min, then RNA was extracted and purified using RNeasy kits (Qiagen, Inc., Valencia, Calif.). according to the manufacturer’s instructions. RNA was subjected to reverse transcription using Invitrogen Superscript III reverse transcriptase; 5 μl of purified RNA was used per 50 μl reaction. For bacterial cultures random DNA hexanucleotide primers at 3 μM were used as reverse transcription primers, but for intestinal loops fluids gene-specific primers at 0.2 μM were used. Reverse transcription reaction was at 55° for 1 h. Copy DNA from reverse transcription was diluted 100-fold, then analyzed by quantitative real-time polymerase chain reaction (PCR). Oligonucleotide primers for bfpA, espA, and rrsB were those reported by Leverton & Kaper (Leverton & kaper, 2005). Oligonucleotide primers for perA and espB were as described (Crane, et al., 2007). Primers for EPEC espC were as follows: EspC-forward 5′-TCGCCTGATTCTGGACGGTAATGT-3′; EspC-reverse 5′-TAACTCCTGGCAGCATGCAGGTAA-3′. PCR done on rabbit EPEC strain E22 using primers based on the human strain E2348/69 gave poor results, so we redesigned PCR primers for the REPEC E22 espA gene based on the published sequence of the RDEC-1 LEE (Zhu, et al., 2001). The REPEC espA primers were: forward 5′-GAGTACTTCGACATCGACAG-3′ and reverse 5′-ATCACCAGCGCCTAATTCAG-3′ and PCR was carried out using a MyiQ Single-Color qRT-PCR machine from Bio-Rad (Hercules, Calif.) using SYBR-Green as the dye to monitor the amplification. PCR reactions were performed in triplicate. Relative expression was calculated by the ΔΔCt (“Livak”) method using rrsB as the normalizing gene as described (Crane, et al., 2007).
Rabbit surgery
Rabbit surgery to create ligated ileal loops was carried out as described in the online Supplement to a recent publication (Crane, et al., 2007). Animal use was approved by the Institutional Animal Care and Use Committee of the University at Buffalo. Preparation of rabbit intestinal loop fluids for growth experiments ex vivo. Loop fluids left over from rabbit ileal loop surgeries were kept frozen at −20 °C. Loop fluids were thawed, centrifuged at 4000 rpm for 10 min to remove as much cellular and particulate matter as possible, then clarified by passage through syringe tip filters with 0.8 μm pore size. Then this filtrate was sterilized by passage through filters with 0.45 μm pore size. Bottle-top filters adapted to 50 ml conical tubes (Nunc Nalgene, Rochester, NY) were used for the final filtration step since they were less prone to clogging than small syringe-tip filters. Loop fluids that were blood-tinged due to surgical trauma were excluded. The sterile filtrates of loop fluids showed a significant light absorbance at 600 nm, and to account for this the baseline OD600 was subtracted from the OD600 measured after inoculation and growth of bacteria. EPEC bacteria were grown overnight in LB broth, then inoculated at a dilution of 1: 100 into the sterile-filtered loop fluids. Note that the small volume of control, uninfected loop fluid available was the limiting factor in the number and size of these experiments that could be performed. In contrast there was often plenty of loop fluid from infected loops available since EPEC infection causes fluid secretion into the loops.
Data analysis
Error bars shown in graphs are standard deviations. Statistical analysis was by ANOVA using InStat 3.0 software (GraphPad Software, San Diego, Calif.) with the Tukey-Kramer post-test for multiple comparisons. Significance was at p ≤ 0.05, or as stated in Legends or Figure panels.
Results
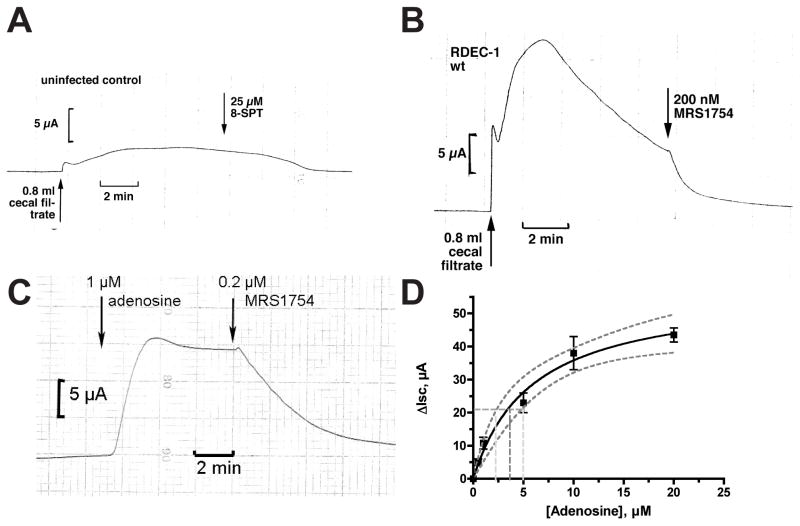
Figure 1 provides evidence that adenosine is released in vivo in a rabbit model of EPEC infection in sufficient amounts to have biological effects. Fig. 1 shows electrophysiologic tracings produced when sterile filtrates of rabbit cecum were applied to naïve (i.e, uninfected) T84 cell monolayers studied in the Ussing chamber, an apparatus for measuring electrical responses in epithelia. In T84 cells, short-circuit current (Isc) represents chloride secretion toward the apical or mucosal side of the tissue. Fig. 1A shows the tracing produced when a filtrate of cecal contents from uninfected control rabbits was applied to the monolayer, producing a very small short-circuit current that is not inhibited by an pan-adenosine receptor blocker, 8-sulfophenyltheophylline. Fig. 1B shows that cecal fluid from rabbits infected with EPEC strain RDEC-1 produced a brisk and very large Isc which was completely blocked by MRS1754, an antagonist selective for adenosine A2b receptors (Crane, et al., 2002). Authentic adenosine triggered short-circuit current which was also blocked by MRS1754 (Fig. 1C) and by also by 8-SPT (data not shown). Using adenosine to produce a standard curve for short-circuit current response (Fig. 1D), we can estimate that the amount of adenosine in the cecal fluid tested in Fig. 1B was 30.8 μM (95 % confidence limits, 17 to 40 μM). Fig. 1 shows that adenosine is released into the intestinal lumen in EPEC infection, and that it accumulates in concentrations sufficient to produce biological effects in mammalian tissues. The lack of detectable adenosine in intestinal fluids of uninfected animals implies that EPEC must be competent to initiate infection in the absence of this nucleoside. Later on, however, EPEC is capable of taking advantage of the adenosine produced by the damage it has inflicted on the host.
Fig. 1.
Ussing chamber tracings showing a chloride secretory response when a sterile filtrate of cecal contents from rabbits infected with rabbit EPEC strain RDEC-1 was applied to uninfected T84 cell monolayers. T84 cells were grown on Snap-Well inserts as described in Materials and Methods and chloride secretion is measured as short-circuit current (Isc). Both panels show responses to pooled cecal filtrates from two rabbits; test fluids were added to the apical side of the monolayer. Panel A, a sterile filtrate of cecal fluids from uninfected control rabbits produced only a small secretory response and the response was not affected by 8-sulfophenyltheophylline (8-SPT), an adenosine receptor antagonist. Panel B, 0.8 ml of a sterile filtrate from cecal contents of rabbits infected for 7 days with RDEC-1 triggered a large and brisk short-circuit current in the T84 cell monolayer and the response was abolished by MRS1754, an adenosine antagonist selective for adenosine A2b receptors. The total volume of buffer in the apical chamber in these experiments was 6.5 ml. Panel C, Isc response to 1 μM adenosine and reversal by MRS1754. Panel D, standard curve of Isc vs. adenosine in the Ussing chamber. The solid curve indicates the best-fit to the data, while the dotted lines indicate upper and lower 95% confidence limits.
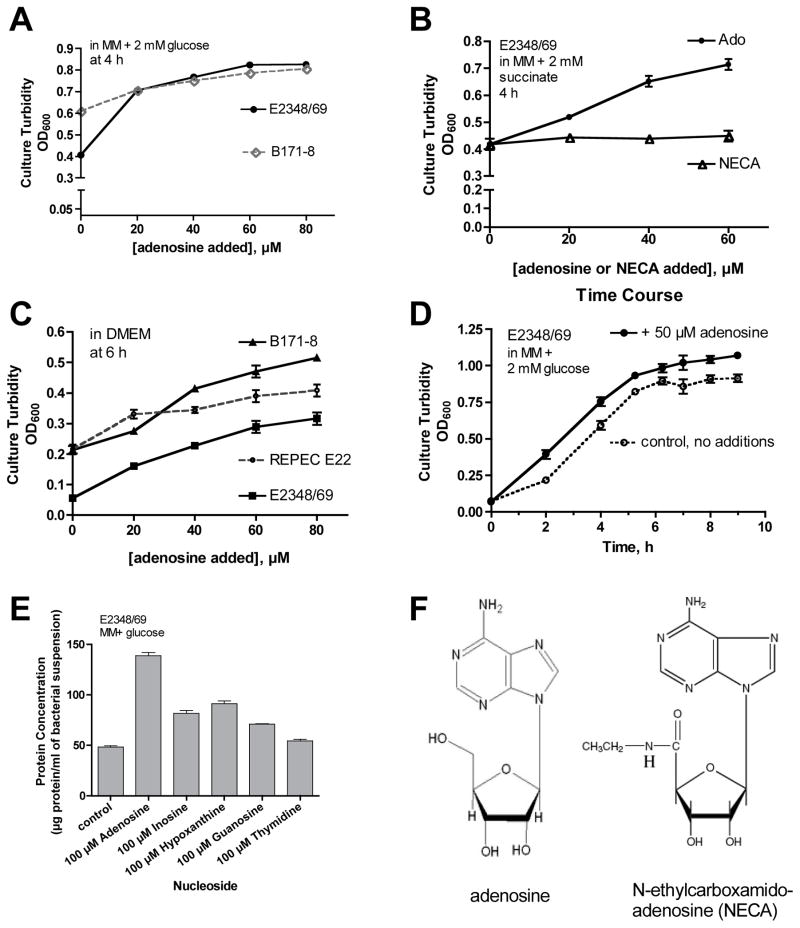
Fig. 2 shows results of experiments to determine if adenosine had effects on growth of EPEC strains. Adenosine did not increase growth of any EPEC or E. coli strains tested when grown in LB medium (data not shown). However, adenosine did stimulate growth of the EPEC strains tested in minimal medium with either glucose (Fig. 2A) or succinate (Fig. 2B) as the carbon and energy source. Interestingly, in some EPEC strains the growth stimulation by adenosine was most obvious in DMEM (Dulbecco’s modified Eagle’s medium, a mammalian tissue culture medium, Fig. 2C), which is considered a rich medium. In contrast to adenosine, the poorly metabolizable analog of adenosine, N-ethylcarboxamido-adenosine (NECA) produced no stimulation of growth of EPEC E2348/69 (Fig. 2B) or of EPEC strains B171-8 or E22 (data not shown for latter two). Fig. 2D shows the time course of adenosine effects on growth of strain E2348/69, showing that the growth stimulation produced by adenosine was greatest in the first 2–4 hours portion of the growth curve. Fig. 2E shows a comparison of the growth effects of adenosine with those of other nucleosides and the purine hypoxanthine. Adenosine stimulated EPEC growth significantly more than did the other nucleosides tested, and also more than AMP, ADP, or ATP (data for the latter 3 nucleotides not shown). The results of Fig. 2 showed that adenosine did have strong effects on EPEC growth, a finding which might be relevant to EPEC growth and survival in the intestinal lumen, which is a purine-limited environment.
Fig. 2.
Growth promoting effects of adenosine on EPEC strains. Adenosine at various concentrations was added to minimal medium (MM) or DMEM medium and growth was measured by culture turbidity (optical density at 600 nm, Panels A–D) or bacterial protein content (Panel E) at different times. Panel A, effect of adenosine on EPEC strains E2348/69 and B171-8 in MM with 2 mM glucose as the carbon source. Panel B, effect of adenosine and N-ethylcarboxamidoadenosine (NECA) on EPEC strain E2348/69 in MM with 2 mM succinate as carbon source. Panel C, growth promotion by adenosine in DMEM. In Panels A– C concentrations of adenosine of 40 μM or greater produced statistically significant increases in growth for strains B171 and E22, and for strain E2348/69 adenosine at ≥20 μM significantly stimulated growth (p < 0.05). Panel D, time course of adenosine effects on E2348/69 growth. Panel E, comparison of growth effects of adenosine with other nucleosides. Panel F, structure of N-ethylcarboxamidoadenosine (NECA) in comparison with adenosine.
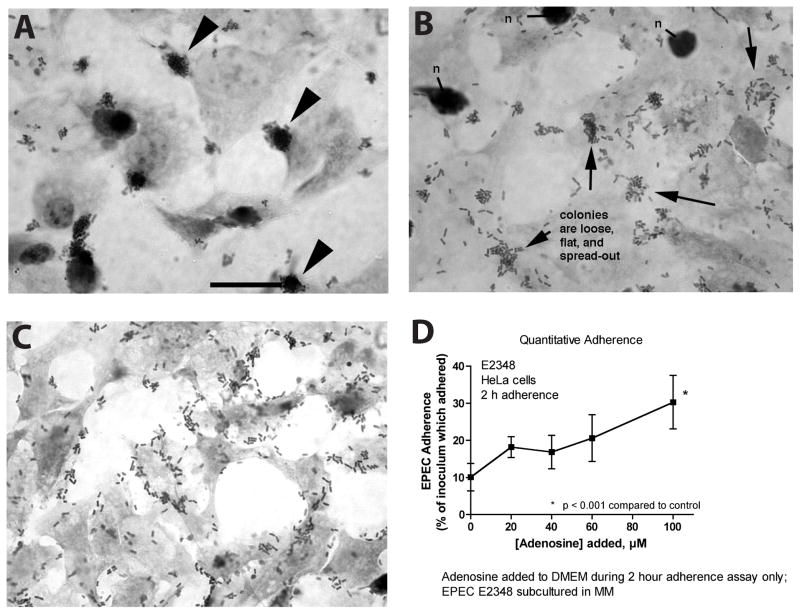
We attempted to determine if adenosine had any effects on EPEC other than growth stimulation. We assessed EPEC adherence to cultured HeLa cells quantitatively and qualitatively, using a visual microscopic examination to determine the pattern of adherence. Fig. 3A shows that in the absence of added adenosine EPEC E2348/69 adheres in the localized adherence pattern typical of classic EPEC (Cravioto, et al., 1991). In localized adherence EPEC adheres in large three-dimensional clumps or microcolonies consisting of hundreds of EPEC bacteria (Fig. 3A, arrowheads). In the presence of 40 μM exogenous adenosine, the localized adherence pattern was altered to one of looser adherence with smaller, flatter microcolonies (Fig. 3B, arrows). In the presence of 80 μM exogenous adenosine, the localized adherence pattern was completely abolished and replaced by a pattern of adherence resembling enteroaggregative adherence (Nataro, et al., 1998) or even diffuse adherence (Giron, et al., 1991). Despite the fact that adenosine abolished the localized adherence phenotype, adenosine did not decrease EPEC adherence to HeLa cells in a quantitative adherence assay (Fig. 3D), and in fact adherence even increased at the highest concentration of adenosine tested.
Fig. 3.
Effect of adenosine on the pattern of adherence of EPEC E2348/69 to HeLa cells. HeLa cells were grown in 4-chamber Permanox plastic Lab-Tek chamber slides or in 24-well plates (Panel D) and infected with E2348/69 for 2 hours. To some wells adenosine was added to 40 μM (Panel B) or 80 μM (Panel C). Panel A, in the absence of exogenous adenosine EPEC adheres in tight clumps or microcolonies (arrowheads), the localized adherence pattern typical of classic EPEC strains; size bar represents 25 μm and also applies to Panels B and C. Panel B, in the presence of 40 μM adenosine EPEC microcolonies are smaller, looser, and more spread out. Panel C, in the presence of 80 μM adenosine localized adherence is abolished and replaced by an adherence pattern resembling enteroaggregative or diffuse adherence. Panel D, quantitative adherence assay showing that addition of adenosine does not inhibit the total number of EPEC bacteria adhering to the HeLa cells.
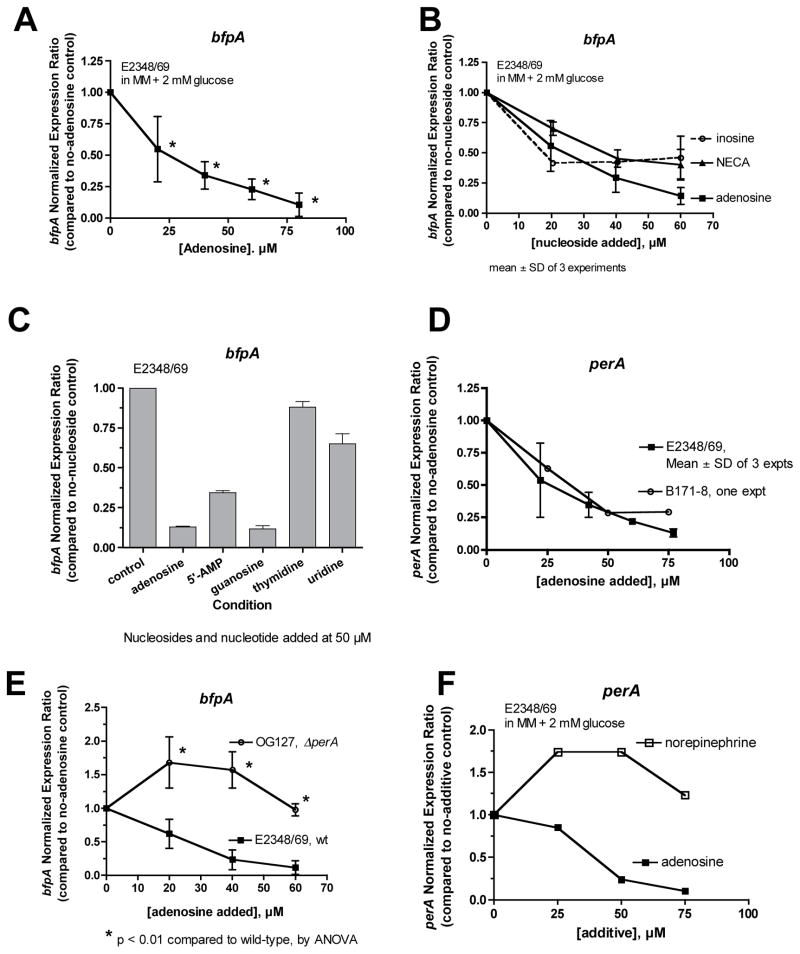
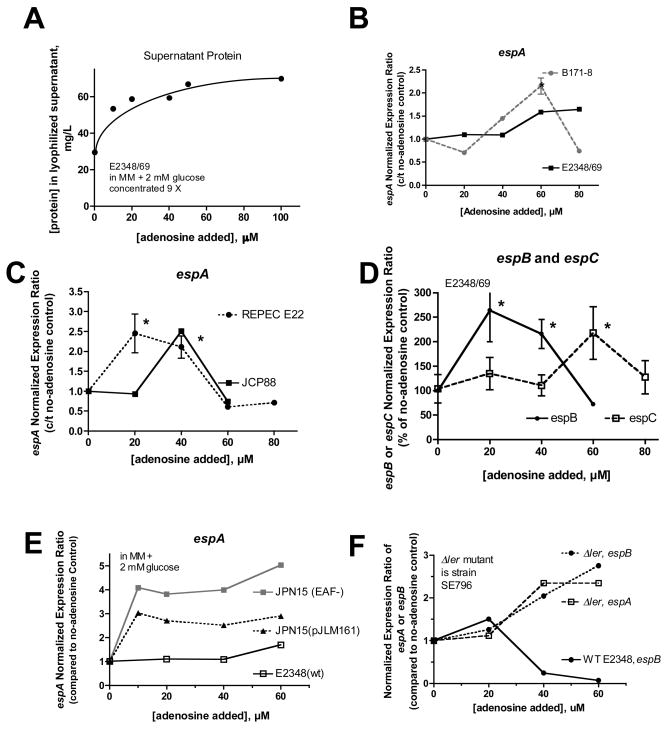
The localized adherence pattern of EPEC adherence is highly dependent on expression of the EPEC bundle-forming pilus (BFP, Vuopio-Varkila & Schoolnik, 1991, Knutton, et al., 1999). Therefore the inhibition of localized adherence by adenosine suggested that adenosine might be inhibiting the expression of the BFP. We examined the effect of adenosine on the expression of RNA transcribed from the bfpA gene by qRT-PCR (Fig. 4). Fig. 4A shows that bfpA RNA is reduced in EPEC bacteria grown in the presence of adenosine. In addition to adenosine, inosine and NECA also reduced the abundance of bfpA RNA (Fig. 4B). The results with NECA are notable because NECA did not stimulate EPEC growth (Fig. 2B) and these data demonstrate that the growth-promoting effect of adenosine can be separated from its effects on gene expression. The inhibitory effect of adenosine was also observed at the level of BFP protein measured by Western immunoblotting (Supporting Figure 1).
Fig. 4.
Effect of adenosine on the abundance of RNA transcribed from bfpA, by quantititative real-time PCR. Panel A, effect of adenosine on expression of bfpA in vitro in EPEC strain E2348/69; *, significantly decreased compared to control. Panel B, comparison of the inhibitory effect of adenosine with that of inosine and NECA on bfpA. Panel C, comparison of adenosine with other nucleosides and 5′-AMP on bfpA expression. Panel D, effect of adenosine on the expression of RNA from the Plasmid-Encoded Regulator, perA, in two EPEC strains. In Panels B–D, asterisks were omitted to avoid confusing clutter, but adenosine, inosine, and NECA all produced statistically significant inhibition of bfpA and perA at concentrations of 20 μM and higher. Panel E, role of Per in the inhibitory effect of adenosine on bfpA; expression is normalized to the no-adenosine control separately for each strain. Results shown are the average of two separate experiments; *, significantly increased compared to the same concentration of adenosine in the wild-type. Panel F, opposite effects of norepinephrine and adenosine on the expression of perA in EPEC.
Fig. 4C compares the ability of various nucleosides to inhibit bfpA expression. The purine nucleosides adenosine and guanosine were the most efficacious, followed by 5′-AMP, but the pyrimidine nucleosides thymidine and uridine were less active.
In EPEC, expression of BFP is upregulated by the Plasmid-encoded Regulator (Per), a transcription factor located on the large EAF plasmid. Per activates BFP expression and also activates Ler, the LEE- encoded Regulator (Mellies, et al., 1999, Elliott, et al., 2000) and thereby affects many virulence genes. Therefore we wondered if adenosine affected the expression of per. Fig. 4D shows that adenosine inhibited the expression of perA with a dose-response relationship closely resembling adenosine’s effects on bfpA (Fig. 4A). The strong similarity in the effects of adenosine on per and bfpA suggested that adenosine might act through per to inhibit expression of bfp. This idea was borne out because, as shown in Fig. 4E, the inhibitory effect of adenosine on bfpA was completely abolished in the ΔperA mutant (OG127). In the perA mutant, adenosine instead triggered a modest, paradoxical increase in bfpA expression. The results of Fig. 4, Panels D and E, support the idea that adenosine exerts its inhibitory effects on bfpA via per.
Virulence gene expression in EPEC can be influenced by quorum-sensing signals, especially autoinducer-3 (AI-3), although EHEC strains (such as O157:H7) are more sensitive than EPEC in this regard (Sircili, et al., 2004, Spears, et al., 2006, Walters & Sperandio, 2006). It seemed unlikely that adenosine was acting via quorum signaling pathways, but to be sure we compared the effects of adenosine and norepinephrine (noradrenaline) on perA expression. Norepinephrine was used because it mimicks the actions of AI-3 in EPEC and EHEC (Walters & Sperandio, 2006). Fig. 4F shows that norepinephrine stimulates perA expression while adenosine inhibits it. The opposite effects of adenosine and norepinephrine on perA, which were also observed in EPEC strain B171-8, show that adenosine is not acting as an AI-3 agonist.
We next examined if adenosine had effects on the EPEC secreted proteins (Esps). Addition of adenosine to EPEC cultures increased the amount of protein in EPEC supernatants by 2.3-fold over control (Fig. 5A). When analyzed by gel electrophoresis and silver stain, adenosine increased the amount of EspC in supernatants by ~3-fold, even when samples were adjusted to contain the same amount of total protein (Supporting Figure 2). Adenosine-induced increase in secretion of EspA into EPEC supernatants was also seen by Western immunoblot. When adjusted for equal protein loading, 50 μM adenosine increased EspA by ≥ 3-fold compared to control (data not shown). When equal volumes of supernatant were loaded per lane, 50 μM adenosine increased the amount of EspA increased by nearly 10-fold over control supernatants. The results of Fig. 5A and Supporting Fig. 2 suggested that adenosine increased the expression of the Esps. To test this idea we again used quantitative RT-PCR, and we chose the 5 h time point for most of these analyses because both the no-adenosine control cultures and and adenosine-treated cultures has reached late-logarithmic phase by this time (Fig. 2D). Fig. 5, Panels B–D shows that adenosine had biphasic effects on expression of espA, espB, and espC in several EPEC strains, including 3 human strains and rabbit EPEC strain E22. Lower concentrations of adenosine stimulated esp expression ~2-fold, while at higher concentrations of added adenosine (60– 80 μM) expression of several of the esp genes fell back to that of the control or even below the no-adenosine control.
Fig. 5.
Effect of adenosine on the EPEC secreted proteins (Esps). Panel A, effect of adenosine on the total amount of secreted protein produced by EPEC E2348/69, after removal of bacterial cells and concentration by lyophilization. Panels B and C, effect of adenosine on espA in three human EPEC strains (E2348/69, B171-8, and JCP88) and the rabbit EPEC strain E22 by qRT-PCR, at 5 hours of culture. Panel D, effect of adenosine on espB and espC in strain E2348/69, again showing a biphasic dose-response curve. Note that espC is encoded outside the locus of enterocyte effacement (LEE). Panels B–D, *, statistically significant compared to no-adenosine control by ANOVA. Panel E, role of the EPEC Adherence Factor (EAF) plasmid and the per locus in adenosine regulation of espA. Strain JPN15 is a derivative of E2348/69 lacking the EAF plasmid including the BFP and Per loci. Plasmid pJLM161 encodes the per operon (perA,B, and C). In the absence of the EAF adenosine’s stimulatory effects on espA are enhanced (4- to 5-fold increase); reintroduction of per on plasmid pJLM161 partially restores the effect of adenosine on espA toward the wild-type pattern. Panel F, role of the LEE encoded regulator Ler in expression of espA and espB; in the Δler mutant adenosine’s inhibitory effects on the espA and espB are abolished. In Panel F the normalized expression ratios were calculated separately compared to no-adenosine control for the wild-type and the Δler mutant. In SE796 (Δler), basal expression of espA and espB is about 10% of the wild-type, but in SE796 the esp expression goes up in the presence of adenosine rather than down as in wild-type.
Since the Plasmid-encoded Regulator was involved in the inhibition of bfpA, we once again examined its role in the regulation of the esps. Fig. 5E shows that in strain JPN15, which lacks the entire EAF plasmid including the Per operon, adenosine’s stimulatory effects on espA are enhanced or exaggerated compared to the wild-type E2348/69. Restoring the perA,B,C element on plasmid pJLM161 reduced the expression of espA partially toward that of the wild-type. Expression of espB in JPN15 and JPN15(pJLM161) was similar to that of espA (data not shown). Fig. 5E implicates Per in the regulation of the Esps, at least in the inhibitory limb of the adenosine dose-response relationship. However, the mechanism of the stimulatory effect of adenosine on the esps remained unclear. Although it seemed far-fetched based on the literature on regulation of the Esp genes, we even considered whether adenosine might bypass Per and act directly on Ler to stimulate Esp genes. However, this was ruled out by the results of experiments such as that in Fig. 5F, which shows that the stimulatory effects of adenosine on espA and espB are enhanced rather than abolished in the Δler mutant, SE796. Fig. 5 shows that both Per and Ler are involved in the inhibitory limb of adenosine’s effects on the Esps, while the pathway by which adenosine stimulates the Esps remains unknown.
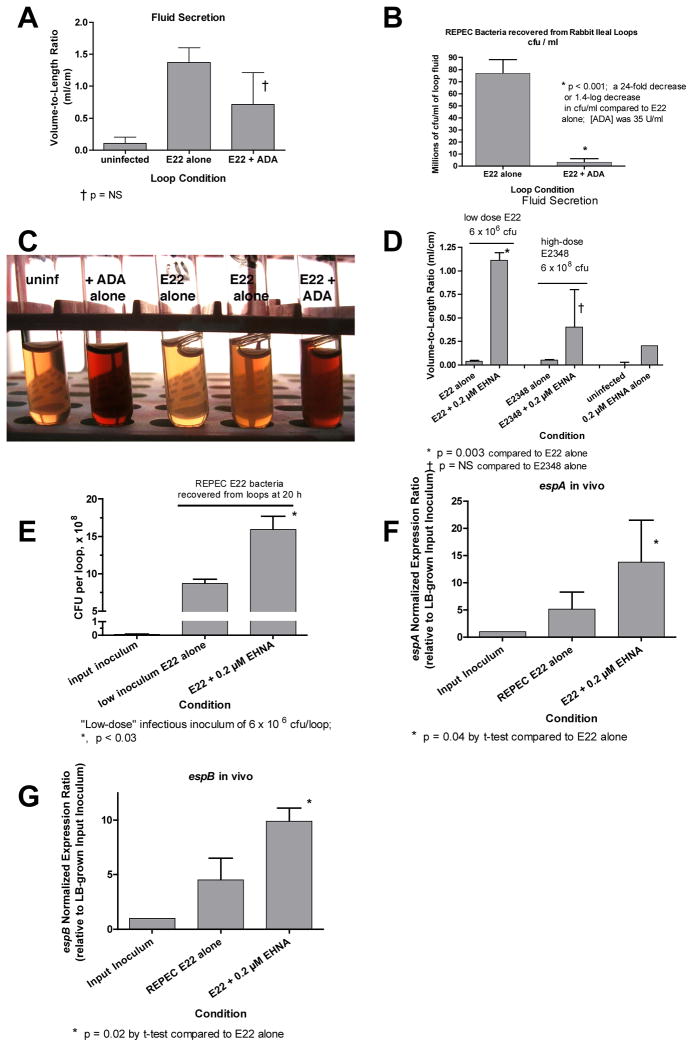
Next we tried to determine if the adenosine effects observed on EPEC in vitro are important in vivo as well. We used the ligated rabbit intestinal loop model and tested various manipulations to increase or decrease the availability of adenosine over the 20-hour course of a typical experiment. It should be noted that adenosine deaminase (ADA) is present in intestinal cells not just in the cytosol, but also on the lumenal surface of the cell, so-called ecto-ADA (Hashikawa, et al., 2004). Fig. 6, Panels A–C show the effect of adding exogenous ADA to rabbit ileal loops infected with 108 cfu of rabbit EPEC E22. Addition of ADA decreased the amount of fluid secretion in the E22-infected loops to about half of control loops (Fig. 6A), although this did not reach statistical significance. More strikingly, ADA decreased the number of E22 bacteria recovered from loop fluid by 24-fold, a 1.4-log decrease, a finding that was confirmed in two additional repetitions of the experiment. In loops injected with E22 + ADA, the number of E22 bacteria recovered at the end of 20 hours was very close to the initial inoculum; i.e., it appeared that ADA did not kill E22 but prevented it from multiplying in the intestinal environment. Histological examination of loops treated with E22 + ADA showed decreased adherence of REPEC to the epithelium, but there was a greater lumenal cellular exudate and more villi containing red blood cells than in those infected with E22 alone (Supporting Figure 3). Indeed, the loop fluids themselves were visibly bloodier in the loops receiving ADA than control loops or loops receiving E22 alone (Fig. 6C). These findings are consistent with the emerging role of adenosine as an anti-inflammatory mediator in the intestine and other tissues (Haskó & Cronstein, 2004, Cavalcante, et al., 2006).
Fig. 6.
Evidence for effects on adenosine on EPEC in vivo in ligated rabbit intestinal loops. Rabbits were subjected to laparatomy and 10 cm loops of ileum were ligated as described in Materials and Methods, then the loops were infected with human EPEC E2348/69 or rabbit EPEC strain E22. 19 to 20 h after infection, the rabbit was re-anesthetized, loop fluid was collected and the volume measured and bacterial cell counts were performed by dilution and plate counts on indicator agar. Because strain E22 is more virulent in the rabbit, the inoculum was reduced to 107 cfu/loop for most experiments, or even lower on occasion (Fig. 6D). Human EPEC strain was inoculated at 108 cfu/loop or occasionally even higher (Fig. 6D). Gene expression in vivo was also analyzed by quantitative RT-PCR. Panels A and B, effect of exogenous adenosine deaminase (ADA, 35 U/ml) on outcome of infection with REPEC E22 in vivo. Panel A, effect of ADA on fluid secretion, measured as the volume-to-length ratio; †, p not significant. Panel B, effect of ADA on the number of REPEC bacteria recovered from loop fluid; *, p < 0.001. Panel C, effect of ADA on the gross appearance of the intestinal fluids after low-speed centrifugation, showing bloodier fluids in the presence of ADA. Panels D–H, effect of the adenosine deaminase inhibitor erythro-9-(2-hydroxy-3-nonyl)adenine (EHNA) on infection with E2348 and E22. Panel D, effect of EHNA on fluid secretion into the ligated rabbit ileal loops. Panel E, effect of EHNA on E22 bacterial CFUs recovered from loop fluid, * p < 0.03. Panels F and G, effect of EHNA on expression of espA and espB in loop fluid, normalized to that in the input inoculum (E22 grown in LB broth medium). *, significantly increased compared to in vivo without EHNA, by Student’s t-test.
Fig. 6, Panels D–G show the effect of adding an adenosine deaminase inhibitor, erythro-9-(2-hydroxy-3-nonyl)adenine (EHNA) on the outcome of EPEC infection in ligated rabbit intestine. Fig. 6D shows the effect of EHNA on fluid secretion into the loops. Since E22 infection alone often produces a maximal distention of the loops, it was necessary to reduce the inoculum of E22 used in these experiments in order to observe any potentiation of fluid secretion by EHNA. Fig. 6D shows that EHNA did increase fluid secretion triggered by infection with low-inoculum REPEC E22 (p = 0.003). EHNA also seemed to increase the fluid accumulation triggered by infection with human EPEC strain E2348/69, but this was not statistically significant.
Fig. 6E shows the effect of EHNA on EPEC bacteria recovered from intestinal loops. 0.2 μM EHNA significantly increased the numbers of E22 bacteria recovered from loop fluid after a 20-hour infection (Fig. 6E), while the numbers of E2348/69 recovered were about the same in control loops and those receiving the inhibitor (data not shown). In interpreting the results of Figs. 6E, it is notable that in the rabbit intestinal environment the rabbit pathogen E22 proliferates rapidly and adheres avidly, while the human-derived strain E2348 grows only slowly, adheres less well (Crane, et al., 2007), and probably triggers less adenosine release. However, E2348/69 adheres avidly to human intestinal tissues, so in the human gut this pathogen may behave similarly to strain E22 in the rabbit.
Last, we also analyzed gene expression in vivo by qRT-PCR to see if ADA or EHNA changed the pattern of expression of the esp genes. When we attempted to analyze in vivo gene expression in loops treated with ADA (Fig. 6B) there were insufficient numbers of EPEC bacteria in the ADA-treated loops and therefore insufficient quantity of extracted bacterial RNA to detect amplification by PCR, although espA and espB were readily detected in control loops. In contrast, in the experiments with EHNA we were able to extract and purify sufficient RNA to complete the analysis in both control and EHNA-treated loops. In vivo, both espA and espB expression were increased ~5-fold compared to the input inoculum grown in LB broth (Figs. 6G and H). Addition of EHNA increased the abundance of espA transcripts by an additional 2.7-fold and that of espB by an additional 2.2-fold (p < 0.05 for both). The results of Fig. 6 provide evidence that adenosine plays an important role in vivo in EPEC growth, EPEC-induced fluid secretion, and regulation of virulence genes. In addition, Fig. 6C and the intestinal histology findings (Supporting Figure 3) suggests that adenosine plays an anti-inflammatory role in the host innate immune response to EPEC as well.
Since the effect of EHNA in vivo in Fig. 6 was large, we returned to in vitro growth experiments to see if EHNA affected EPEC growth. Addition of EHNA did not stimulate the growth of strains E2348/69 or E22 in the absence or presence of added adenosine in any medium tested (data not shown). The contrast between the lack of effect of EHNA on growth in vitro and strong effects of EHNA on EPEC growth in vivo is consistent with EHNA inhibiting ADA of host origin, which is the vast majority of ADA in the intestine.
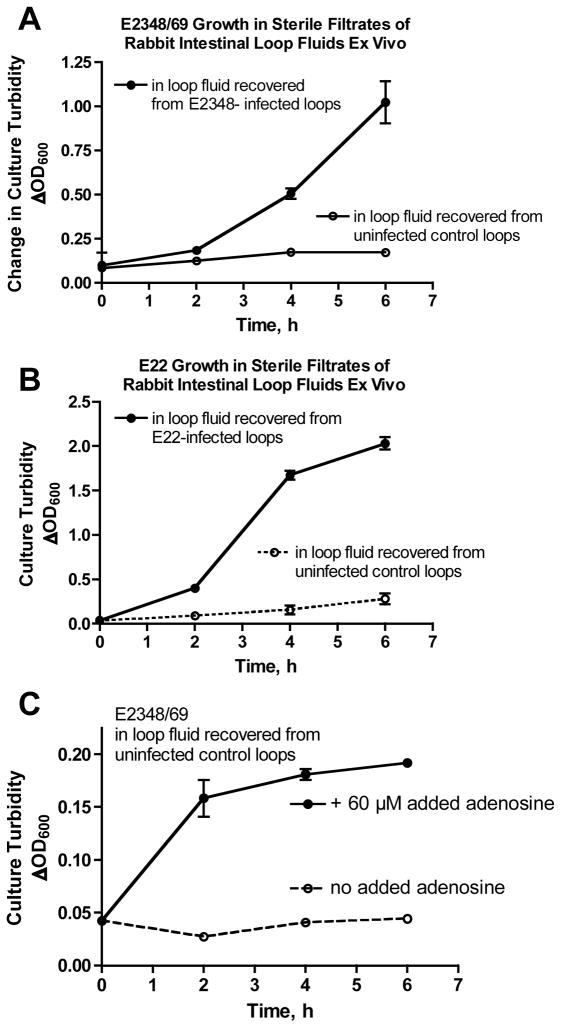
To try to provide additional evidence that secreted mediators from the host can regulate growth of EPEC, we did growth experiments ex vivo using loop fluids recovered from ligated intestinal loop experiments. Loop fluids were subjected to sterile filtration, then inoculated with human or rabbit EPEC and growth curves were measured. Fig. 7, Panels A and B show that strains E2348/69 and E22 grew much faster in the sterile filtrates of fluid recovered from infected loops than in the fluid recovered from the uninfected (saline-treated) control loop in the same animal. In Fig. 7C, addition of 60 μM adenosine to the filtered, uninfected loop fluid significantly increased EPEC growth compared to the same loop fluid without adenosine supplementation. Addition of adenosine to loop fluid did not increase the EPEC growth rate to the same level observed in filtrates of infected loop fluid, however (compare OD600 in top curves in Fig. 7, Panels A and C). This hints that in addition to adenosine, there may be other, yet-undiscovered soluble mediators released in response to infection that can promote EPEC growth.
Fig. 7.
Panel A, growth of E2348/69 ex vivo in sterile filtrates of fluids collected from E2348-infected and uninfected control ileal loops. Panel B, growth of REPEC E22 ex vivo in sterile filtrates of fluids collected from E22-infected and uninfected (buffered saline control) ligated ileal loops. Panel C, growth of E2348/69 in sterile filtrates of uninfected loop fluids alone (bottom curve, dotted line) or supplemented with 60 μM exogenous adenosine (top curve, closed circles). In Panels A – C growth in the upper curves was significantly increased compared to the lower curves by ANOVA (p < 0.05).
Discussion
In earlier studies in our laboratory we discovered that EPEC infection of cultured cells triggers a large release of ATP from the host cell, and that the extracellular ATP is subsequently broken down by host ecto-enzymes to ADP, AMP, and then adenosine (Crane, et al., 2002, Crane, et al., 2007). Like others (Barrett, et al., 1989, Madara, et al., 1993, Strohmeier, et al., 1995), we noted that adenosine is a potent secretagogue in the small and large intestine and we felt that adenosine was likely an important mediator of the watery diarrhea that is the hallmark of EPEC infection. Our view of adenosine acting on host tissues as a secretory mediator was apparently too limiting, however, for as shown in this study adenosine has strong effects on EPEC bacteria as well.
Our studies of EPEC infection in a rabbit model showed that adenosine, or substances which can be quickly converted into adenosine, accumulate in vivo to concentrations of ~ 30 μM or more in cecal fluid (Fig. 1). In our in vitro studies of adenosine on EPEC (Figs. 2–5) we studied responses to adenosine at concentrations above 30 μM for several reasons. First, Okada et al. showed in respiratory epithelium that the concentration of adenine nucleotides measured at the plasma membrane surface was 10 times higher than that obtained by measuring bulk aliquots of airway surface liquid (Okada, et al., 2006). Second, when we analyzed expression of the EPEC secreted proteins (Esps), we observed interesting biphasic responses to adenosine which seemed important to take into account when interpreting the pathophysiologic significance of the adenosine effects.
Adenosine has strong growth-promoting effects on EPEC strains in several types of media, including minimal medium with glucose, minimal medium with succinate, and serum-free DMEM (mammalian tissue culture medium). The ability of E. coli strains, including some laboratory strains, to utilize adenosine for growth has been known for decades (Zalkin & Nygaard, 1996) but is of particular relevance in the case of the diarrhea-producing E. coli strains, because the intestinal lumen is a nutrient-limited and purine-limited environment (Chiang & Mekalanos, 1998, Heithoff, et al., 2000). Other pathogens whose growth is dependent on extracellular adenine nucleotides or adenosine include Hemophilus influenzae (dependent on extracellular nicotinamide adenine dinucleotide, NAD, the so-called V factor) and the parasite Toxoplasma gondii, which must obtain adenosine from the extracellular medium or the host since it lacks de novo purine biosynthesis pathways (el Kouni, 2007).
The ability of EPEC to utilize adenosine as a nutrient for growth is not unique, but the ability of EPEC to release ATP from epithelial cells is a trait shared by only a few enteric pathogens. Of the strains examined, only EPEC, EHEC (serotype O157:H7), and Salmonella enterica triggered ATP release from cultured cells, whereas enterotoxigenic E. coli (ETEC), enteroaggregative E. coli (EAEC), diffuse-adherent E. coli (DAEC), laboratory E. coli strains, and Shigella sonnei did not (Crane, et al., 2002, Crane, et al., 2006). However, even bacteria unable to trigger adenine nucleotide or nucleoside release from host cells might still be able to respond to extracellular adenosine if its release was triggered by another stimulus. For example, intestinal ischemia stimulated the release of adenosine into the gut lumen and this adenosine was able to increase the expression of a Pseudomonas aeruginosa lectin/adhesin, called PA–I, by 3 to 7-fold (Patel, et al., 2007).
In addition to effects on growth, adenosine had profound effects on the pattern of EPEC adherence to cultured cells, abolishing the localized adherence (LA) pattern, a pattern so distinctive that it was once commonly used as a test for diagnosing EPEC infection in diarrheal fecal samples (Fig. 3, and Ref. Cravioto, et al., 1991). Localized adherence is dependent on expression of BFP, and BFP is known to be dynamically regulated. For example, even after bundle-forming pili are produced, they are capable of being retracted and of changing their morphology from thin to thick filaments (Puente, et al., 1996, Knutton, et al., 1999). In the present work, we found that adenosine strongly inhibits expression of BFP (BFP protein and bfpA RNA transcripts). Even 20 μM adenosine inhibited bfpA expression by 50 %, so this effect is within the adenosine concentration range we have measured in the intestine during infection. Other investigators have noted that BFP expression is regulated by environmental signals (Vuopio-Varkila & Schoolnik, 1991, Puente, et al., 1996), and the results of the present work suggest that adenosine, derived from EPEC-induced damage to the host, is one such inhibitory signal.
Adenosine’s inhibitory effect on bfpA is clearly mediated via Per, the plasmid-encoded regulator, but how adenosine inhibits Per is not yet known. For example, it is not known if adenosine is acting via a receptor at the EPEC cell surface or if adenosine must enter the cell, bind to a signaling target, and then alter transcription of Per.
The effect of adenosine on expression of the esps is a bit more complicated than its effect on BFP because it is biphasic, with an approximate 2-fold stimulation of esps A, B, and C at lower adenosine concentrations followed by an inhibition at higher concentrations. While the 2-fold stimulation of esp expression (normalized to rrsB) may seem modest, these changes in gene expression are accompanied by strong stimulation of EPEC growth, with the result that 20–40 μM adenosine increases the amount of EspA protein secreted into the medium by 6 to 10-fold, depending on the strain.
Concentrations of adenosine of 60– 80 μM, if they occur in vivo (for example in areas of epithelium heavily damaged by EPEC infection, in the unstirred layer of the brush border, or other protected environments), would result in partial inhibition of espA as well as strong inhibition of bfpA. EspA filaments and BFP are two of the most important EPEC adhesins (Cleary, et al., 2004). The inhibitory effect of adenosine on BFP, together with its strong stimulatory effects on growth, makes it tempting to speculate that adenosine serves as a detachment-and-dispersal signal to EPEC. In this scenario, high local adenosine concentrations would trigger EPEC to disperse to other segments of the intestinal tract, and possibly eventually even to leave the host and disperse into the environment. Precedent for this hypothesis is found in other enteric pathogens, such as enteroaggregative E. coli, where the protein dispersin causes disaggregation of EAEC bacterial clumps, allowing individual bacterial cells to penetrate through intestinal mucus (Sheikh, et al., 2002, Huang, et al., 2008). Likewise, signals in the intestinal environment trigger major phenotypic and genotypic changes in Vibrio cholerae which help prepare it to survive in the aquatic environment after leaving the host (Tischler & Camilli, 2005, Schild, et al., 2007) while maintaining readiness to cause disease in a new host (Merrell, et al., 2002).
The ability of Gram-positive and Gram-negative bacterial pathogens to sense and respond to host-derived signals such as hormones, neurotransmitters, and autacoids has been noted by others investigators; indeed, Lyte and colleagues have coined the term “microbial endocrinology” to describe this ability. Most of the work in this area has dealt with catecholamines such as epinephrine and norepinephrine, with other families of hormones not well studied (Freestone, et al., 2000, Freestone, et al., 2007, Hughes & Sperandio, 2008). An example of a pathogen sensing a mammalian hormone other than a catecholamine includes the ability of the fungus Paracoccidioides brasiliensis to bind and respond to estrogen (Loose, et al., 1983); estrogen inhibits transition to a yeast form, an explanation invoked for the strongly skewed sex ratio in cases of paracoccidiosis ( > 20: 1 male: female). Examples of microbial pathogens responding to mammalian peptide hormones are hard to find in the literature but Pseudomonas aeruginosa responds to κ-opiate agonists such as dynorphin (Zaborina, et al., 2007). The results of the present study show that adenosine should be added to the list of host-derived signaling molecules which are sensed by bacteria.
Our work with adenosine deaminase (ADA) and ADA inhibitors (Fig. 6) provides evidence that adenosine is important in EPEC growth, pathogenesis, and regulation of gene expression in the intestinal lumen. In addition, these experiments demonstrate the role of adenosine as an anti-inflammatory mediator in the GI tract, supporting other recent reports (Cavalcante, et al., 2006). Moreover, our work suggests that ADA should itself be considered a host defense molecule, because its enzymatic activity limits the accumulation of adenosine in the intestinal lumen and thereby checks the proliferation of intra-lumenal pathogens. A great deal of older literature on ADA in infectious diseases focused narrowly on the use of ADA measurements in body fluids as a diagnostic test for infection, especially mycobacterial infection (Kataria & Khurshid, 2001), with no consideration of whether the increased ADA levels might actually play a role in the outcome of infection. The results presented here and recent reports (Boldrick, et al., 2002) suggest that ADA should not just be viewed as a marker of infection but also a mediator of host response. Our results also lead us to wonder if the anti-inflammatory effects of adenosine blunt or slow the onset of an effective host immune response to EPEC. EPEC-induced disease in humans and animals is known to be especially long-lasting (Ulshen & Rollo, 1980, Rothbaum, et al., 1982). More research on adenosine and ADA using longer duration experimental models may answer these questions.
Supplementary Material
1. Supporting Fig. 1. Effect of Adenosine on Expression of BFP Protein by Western Immunoblot.
2. Supporting Fig. 2. Effect of Adenosine on the Abundance of EPEC Secreted Proteins
3. Supporting Fig. 3. Effect of Adenosine Deaminase (ADA) on Histology of REPEC E22 in Rabbit Ileal Loops.
Acknowledgments
We thank Dr. James B. Kaper, University of Maryland at Baltimore, for supplying the Per and Ler mutants of EPEC used in this report. We thank Dr. Michael S. Donnenberg, also of the University of Maryland at Baltimore, for the gift of antibody against the bundlin subunit of BFP, and Dr. Joel Linden, Univ of Virginia, for MRS1754. The expert technical assistance of Ms. Tonniele M. Naeher and of Ms. Ruth Olson is also thankfully acknowledged.
This work was supported by NIH Grants RO1 AI 50652 and R21 AI 066055 (to J.K.C.).
List of abbreviations used
- EPEC
enteropathogenic Escherichia coli
- EHNA
erythro-9-(2-hydroxy-3-nonyl)adenine
- ADA
adenosine deaminase
- BFP
bundle-forming pilus
Footnotes
Authors’ contributions: I.S. performed most of the experiments and refined the techniques for qRT-PCR analysis of gene expression. J.K.C. conceived of the experimental plan, analyzed data, and wrote the manuscript.
References
- 1.Barrett K, Huott P, Shah S, Dharmsathophorn K, Wasserman S. Differing effects of apical and basolateral adenosine on colonic epithelial cell line T84. Am J Physiol. 1989;256 :C197–C203. doi: 10.1152/ajpcell.1989.256.1.C197. Cell Physiol. 25. [DOI] [PubMed] [Google Scholar]
- 2.Bieber D, Ramer S, Wu C-Y, Murray W, Tobe T, Fernandez R, Schoolnik G. Type IV pili, transient bacterial aggregates, and virulence of enteropathogenic Escherichia coli. Science. 1998;280:2114–2118. doi: 10.1126/science.280.5372.2114. [DOI] [PubMed] [Google Scholar]
- 3.Boldrick JC, Alizadeh AA, Diehn M, et al. Stereotyped and specific gene expression programs in human innate immune responses to bacteria. Proceedings of the National Academy of Sciences of the United States of America; 2002. pp. 972–977. %R 910.1073/pnas.231625398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cantey JR, Inman LR. Diarrhea due to Escherichia coli strain RDEC-1 in the rabbit: the peyer’s patch as the initial site of attachment and colonization. Journal of Infectious Diseases. 1981;143:440–446. doi: 10.1093/infdis/143.3.440. [DOI] [PubMed] [Google Scholar]
- 5.Cavalcante I, Castro M, Barreto A, et al. Effect of a novel A2a adenosine receptor agonist ATL 313 on Clostridium difficile toxin A-induced murine ileal enteritis. Infect Immun. 2006;74:2606–2612. doi: 10.1128/IAI.74.5.2606-2612.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chiang S, Mekalanos J. Use of signature-tagged mutagenesis to identify Vibrio cholerae genes critical for colonization. Mol Microbiol. 1998;27:797–805. doi: 10.1046/j.1365-2958.1998.00726.x. [DOI] [PubMed] [Google Scholar]
- 7.Cleary J, Lai L-C, Shaw R, Strattman-Iwanowska A, Donnenberg M, Frankel G, Knutton S. Enteropathogenic Escherichia coli (EPEC) adhesion to intestinal epithelial cells: role of bundle-forming pili (BFP), EspA filaments, and intimin. Microbiology. 2004;150:527–538. doi: 10.1099/mic.0.26740-0. [DOI] [PubMed] [Google Scholar]
- 8.Crane J, Shulgina I, Naeher T. Ecto-5′-nucleotidase and Intestinal Ion Secretion by Enteropathogenic Escherichia coli. Purinergic Signalling. 2007;3:233–246. doi: 10.1007/s11302-007-9056-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crane J, Olson R, Jones H, Duffey M. Release of ATP during host cell killing by enteropathogenic E. coli and its role as a secretory mediator. Am J Physiol (Gastrointest Liver Physiol) 2002;282:G74–G86. doi: 10.1152/ajpgi.00484.2001. [DOI] [PubMed] [Google Scholar]
- 10.Crane J, Choudhari S, Naeher T, Duffey M. Mutual enhancement of virulence by enterotoxigenic and enteropathogenic Escherichia coli. Infect Immun. 2006;74:1505–1515. doi: 10.1128/IAI.74.3.1505-1515.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crane J, Naeher T, Shulgina I, Zhu C, Boedeker E. Effect of zinc in enteropathogenic Escherichia coli infection. Infect Immun. 2007;75:5974–5984. doi: 10.1128/IAI.00750-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crane JK, Majumdar S, Pickhardt DP. Host cell death due to enteropathogenic Escherichia coli has features of apoptosis. Infect Immun. 1999;67:2575–2584. doi: 10.1128/iai.67.5.2575-2584.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cravioto A, Tello A, Navarro A, Ruiz J, Villafán H, Uribe F, Eslava C. Association of Escherichia coli HEp-2 adherence patterns with type and duration of diarrhoea. Lancet. 1991;337:262–264. doi: 10.1016/0140-6736(91)90868-p. [DOI] [PubMed] [Google Scholar]
- 14.Donnenberg M, Nataro J. Methods for studying adhesion of diarrheagenic Escherichia coli. Methods in Enzymol. 1995;253:324–336. doi: 10.1016/s0076-6879(95)53028-2. [DOI] [PubMed] [Google Scholar]
- 15.el Kouni M. Adenosine metabolism in Toxoplasma gondii: potential targets for chemotherapy. Current Pharmaceut Design. 2007;13:581–597. doi: 10.2174/138161207780162836. [DOI] [PubMed] [Google Scholar]
- 16.Elliott S, Sperandio V, Giron J, et al. The locus of enterocyte effacement (LEE)-encoded regulator controls expression of both LEE- and non-LEE encoded virulence factors in enteropathogenic and enterohemorrhagic Escherichia coli. Infect Immun. 2000;68:6115–6126. doi: 10.1128/iai.68.11.6115-6126.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Freestone P, Haigh R, Lyte M. Specificity of catecholamine-induced growth in Escherichia coli O157:H7, Salmonella enterica and Yersinia enterocolitica. FEMS Microbiology Letters. 2007;269:221–228. doi: 10.1111/j.1574-6968.2006.00619.x. [DOI] [PubMed] [Google Scholar]
- 18.Freestone PPE, Lyte M, Neal CP, Maggs AF, Haigh RD, Williams PH. The Mammalian Neuroendocrine Hormone Norepinephrine Supplies Iron for Bacterial Growth in the Presence of Transferrin or Lactoferrin. J Bacteriol %R 10.1128/JB.182.21.6091-6098.2000. 2000;182:6091–6098. doi: 10.1128/jb.182.21.6091-6098.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giron J, Jones T, Millan-Velasco F, et al. Diffuse-adhering Escherichia coli (DAEC) as a putative cause of diarrhea in Mayan children in Mexico. J Infect Dis. 1991;163:507–513. doi: 10.1093/infdis/163.3.507. [DOI] [PubMed] [Google Scholar]
- 20.Gomez-Duarte O, Kaper JB. A plasmid-encoded regulatory region activates chromosomal eaeA expression in enteropathogenic Escherichia coli. Infect Immun. 1995;63:1767–1776. doi: 10.1128/iai.63.5.1767-1776.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hashikawa T, Hooker S, Maj J, Knott-Craig C, Takedachi M, Murakami S, Thompson L. Regulation of adenosine receptor engagement by ecto-adenosine deaminase. FASEB J. 2004;18:131–133. doi: 10.1096/fj.03-0011fje. [DOI] [PubMed] [Google Scholar]
- 22.Haskó G, Cronstein BN. Adenosine: an endogenous regulator of innate immunity. Trends in Immunology. 2004;25:33. doi: 10.1016/j.it.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 23.Heithoff D, Sinsheimer R, Low D, Maham M. In vivo gene expression and the adaptive response: from pathogenesis to vaccines and antimicrobials. Phil Trans Royal Soc London. 2000;355:633–642. doi: 10.1098/rstb.2000.0604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang DB, Brown EL, DuPont HL, et al. Seroprevalence of the enteroaggregative Escherichia coli virulence factor dispersin among USA travellers to Cuernavaca, Mexico: a pilot study. J Med Microbiol %R 10.1099/jmm.0.47495-0. 2008;57:476–479. doi: 10.1099/jmm.0.47495-0. [DOI] [PubMed] [Google Scholar]
- 25.Hughes DT, Sperandio V. Inter-kingdom signalling: communication between bacteria and their hosts. Nat Rev Micro. 2008;6:111–120. doi: 10.1038/nrmicro1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jerse A, Yu J, Tall B, Kaper J. A genetic locus of enteropathogenic Escherichia coli necessary for the production of attaching and effacing lesions on tissue culture cells. Proc Natl Acad Sci USA. 1990;87:7839–7843. doi: 10.1073/pnas.87.20.7839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kataria YP, Khurshid I. Adenosine Deaminase in the Diagnosis of Tuberculous Pleural Effusion. Chest %R 10.1378/chest.120.2.334. 2001;120:334–336. doi: 10.1378/chest.120.2.334. [DOI] [PubMed] [Google Scholar]
- 28.Knutton S, Shaw R, Anantha R, Donnenberg M, Zorgani A. The type IV bundle-forming pilus of enteropathogenic Escherichia coli undergoes dramatic alterations in structure associated with bacterial adherence, aggregation, and dispersal. Mol Microbiol. 1999;33:499–509. doi: 10.1046/j.1365-2958.1999.01495.x. [DOI] [PubMed] [Google Scholar]
- 29.Koszalka P, Ozuyaman B, Huo Y, et al. Targeted disruption of cd73/ecto-5′-nucleotidase alters thromboregulation and augments vascular inflammatory response. [see comment] Circulation Research. 2004;95:814–821. doi: 10.1161/01.RES.0000144796.82787.6f. [DOI] [PubMed] [Google Scholar]
- 30.Leverton L, kaper J. Temporal expression of enteropathogenic Escherichia coli virulence genes in an in vitro model of infection. Infect Immun. 2005;73:1034–1043. doi: 10.1128/IAI.73.2.1034-1043.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Levine M, Nalin D, Hornick R, et al. Escherichia coli strains that cause diarrhea but do not produce heat-labile or heat-stable enterotoxins and are not invasive. Lancet. 1978;1:1119–1122. doi: 10.1016/s0140-6736(78)90299-4. [DOI] [PubMed] [Google Scholar]
- 32.Linden J. Molecular approach to adenosine receptors: receptor-mediated mechanisms of tissue protection. Annu Rev Pharmacol Toxicol. 2001;41:775–787. doi: 10.1146/annurev.pharmtox.41.1.775. [DOI] [PubMed] [Google Scholar]
- 33.Linden J. New insights into the regulation of inflammation by adenosine. J Clin Invest. 2006;116:1835–1837. doi: 10.1172/JCI29125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Linden J. Purinergic chemotaxis. Science. 2006;314:1689–1690. doi: 10.1126/science.1137190. [DOI] [PubMed] [Google Scholar]
- 35.Loose D, Stover E, Restrepo A, Stevens D, Feldman D. Estradiol binds to a receptor-like cytosol binding protein and initiates a biological response in Paracoccidioides brasiliensis. Proc Natl Acad Sci USA. 1983;80:7659–7663. doi: 10.1073/pnas.80.24.7659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Madara J, Patapoff T, Gillece-Castro B, Colgan S, Parkos C, Delp C, Mrsny R. 5′-adenosine monophosphate is the neutrophil-derived paracrine factor that elicits chloride secretion from T84 intestinal epithelial monolayers. J Clin Invest. 1993;91:2320–2325. doi: 10.1172/JCI116462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mellies JL, Elliott SJ, Sperandio V, Donnenberg MS, Kaper JB. The Per regulon of enteropathogenic Escherichia coli: identification of a regulatory cascade and a novel transcriptional activator, the locus of enterocyte effacement (LEE)-encoded regulator (Ler) Molecular Microbiology. 1999;33:296–306. doi: 10.1046/j.1365-2958.1999.01473.x. [DOI] [PubMed] [Google Scholar]
- 38.Merrell D, Butler S, Qadri F, et al. Host-induced epidemic spread of the cholera bacterium. Nature. 2002;417:642–645. doi: 10.1038/nature00778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Milon A, Oswald E, De Rycke J. Rabbit EPEC: a model for the study of enteropathogenic Escherichia coli. Veterinary Res. 1999;30:203–219. [PubMed] [Google Scholar]
- 40.Nataro J, Steiner T, Guerrant R. Enteroaggregative Escherichia coli. Emerging Infect Dis. 1998;4:251–261. doi: 10.3201/eid0402.980212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Okada SF, Nicholas RA, Kreda SM, Lazarowski ER, Boucher RC. Physiological Regulation of ATP Release at the Apical Surface of Human Airway Epithelia. J Biol Chem. 2006;281:22992–23002. doi: 10.1074/jbc.M603019200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Olsson RA. Cardiovascular ecto-5′-nucleotidase: an end to 40 years in the wilderness? [comment] Circulation Research. 2004;95:752–753. doi: 10.1161/01.RES.0000146278.94064.4b. [DOI] [PubMed] [Google Scholar]
- 43.Patel NJ, Zaborina O, Wu L, et al. Recognition of intestinal epithelial HIF-1{alpha} activation by Pseudomonas aeruginosa. Am J Physiol Gastrointest Liver Physiol. 2007;292:G134–142. doi: 10.1152/ajpgi.00276.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Puente JL, Bieber D, Ramer SW, Murray W, Schoolnik GK. The bundle-forming pili of enteropathogenic Escherichia coli: transcriptional regulation by environmental signals. Molecular Microbiology. 1996;20:87–100. doi: 10.1111/j.1365-2958.1996.tb02491.x. [DOI] [PubMed] [Google Scholar]
- 45.Roman R, Fitz J. Emerging roles of purinergic signaling in gastrointestinal epithelial secretion and hepatobiliary function. Gastroenterol. 1999;116:964–979. doi: 10.1016/s0016-5085(99)70081-8. [DOI] [PubMed] [Google Scholar]
- 46.Rothbaum R, McAdams A, Giannella R, Partin J. A clinicopathologic study of enterocyte-adherent Escherichia coli: a cause of protracted diarrhea in infants. Gastroenterology. 1982;83:441–454. [PubMed] [Google Scholar]
- 47.Rothbaum R, Partin J, Saalfield K, McAdams A. An ultrastructural study of enteropathogenic Escherichia coli infection in human infants. Ultrastruct Pathol. 1983;4:291–304. doi: 10.3109/01913128309140582. [DOI] [PubMed] [Google Scholar]
- 48.Schild S, Tamayo R, Nelson EJ, Qadri F, Calderwood SB, Camilli A. Genes Induced Late in Infection Increase Fitness of Vibrio cholerae after Release into the Environment. Cell Host & Microbe. 2007;2:264. doi: 10.1016/j.chom.2007.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sheikh J, Czeczulin J, Harrington S, et al. A novel dispersin protein in enteroaggregative Escherichia coli. J Clin Invest. 2002;110:1329–1337. doi: 10.1172/JCI16172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sircili M, Walters M, Trabulsi L, Sperandio V. Modulation of enteropathogenic Escherichia coli virulence by quorum sensing. Infect Immun. 2004;72:2329–2337. doi: 10.1128/IAI.72.4.2329-2337.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Spears K, Roe A, Gally D. A comparison of enteropathogenic and enterohaemorraghic Escherichia coli pathogenesis. FEMS Microbiol Lett. 2006;255:187–202. doi: 10.1111/j.1574-6968.2006.00119.x. [DOI] [PubMed] [Google Scholar]
- 52.Strohmeier GR, Reppert SM, Lencer WI, Madara JL. The A2b adenosine receptor mediates cAMP responses to adenosine receptor agonists in human intestinal epithelia. Journal of Biological Chemistry. 1995;270:2387–2394. doi: 10.1074/jbc.270.5.2387. [DOI] [PubMed] [Google Scholar]
- 53.Synnestvedt K, Furuta GT, Comerford KM, et al. Ecto-5′-nucleotidase (CD73) regulation by hypoxia-inducible factor-1 mediates permeability changes in intestinal epithelia. Journal of Clinical Investigation. 2002;110:993–1002. doi: 10.1172/JCI15337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tischler AD, Camilli A. Cyclic Diguanylate Regulates Vibrio cholerae Virulence Gene Expression. Infect Immun %R 10.1128/IAI.73.9.5873-5882.2005. 2005;73:5873–5882. doi: 10.1128/IAI.73.9.5873-5882.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ulshen M, Rollo J. Pathogenesis of Escherichia coli gastroenteritis in man-another mechanism. N Engl J Med. 1980;302:99–101. doi: 10.1056/NEJM198001103020207. [DOI] [PubMed] [Google Scholar]
- 56.Vuopio-Varkila J, Schoolnik G. Localized adherence by enteropathogenic Escherichia coli is an inducible phenotype associated with the expression of new outer membrane proteins. J Exp Med. 1991;174:1167–1177. doi: 10.1084/jem.174.5.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Walters M, Sperandio V. Autoinducer 3 and Epinephrine Signaling in the Kinetics of Locus of Enterocyte Effacement Gene Expression in Enterohemorrhagic Escherichia coli. Infect Immun %R 10.1128/IAI.00099-06. 2006;74:5445–5455. doi: 10.1128/IAI.00099-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ye J, van den Berg B. Crystal structure of the bacterial nucleoside transporter Tsx. EMBO J. 2004;23:3187–3195. doi: 10.1038/sj.emboj.7600330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zaborina O, Lepine F, Xiao G, et al. Dynorphin Activates Quorum Sensing Quinolone Signaling in Pseudomonas aeruginosa. PLoS Pathogens. 2007;3:e35. doi: 10.1371/journal.ppat.0030035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zalkin H, Nygaard P. Biosynthesis of purine nucleotides. In: Neidhardt F, editor. Escherichia coli and Salmonella. Vol. 1. ASM Press; Washington, D.C: 1996. pp. 575–576. [Google Scholar]
- 61.Zhu C, Agin TS, Elliott SJ, Johnson LA, Thate TE, Kaper JB, Boedeker EC. Complete nucleotide sequence and analysis of the locus of enterocyte effacement from rabbit diarrheagenic Escherichia coli RDEC-1. Infection & Immunity. 2001;69:2107–2115. doi: 10.1128/IAI.69.4.2107-2115.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
1. Supporting Fig. 1. Effect of Adenosine on Expression of BFP Protein by Western Immunoblot.
2. Supporting Fig. 2. Effect of Adenosine on the Abundance of EPEC Secreted Proteins
3. Supporting Fig. 3. Effect of Adenosine Deaminase (ADA) on Histology of REPEC E22 in Rabbit Ileal Loops.