Abstract
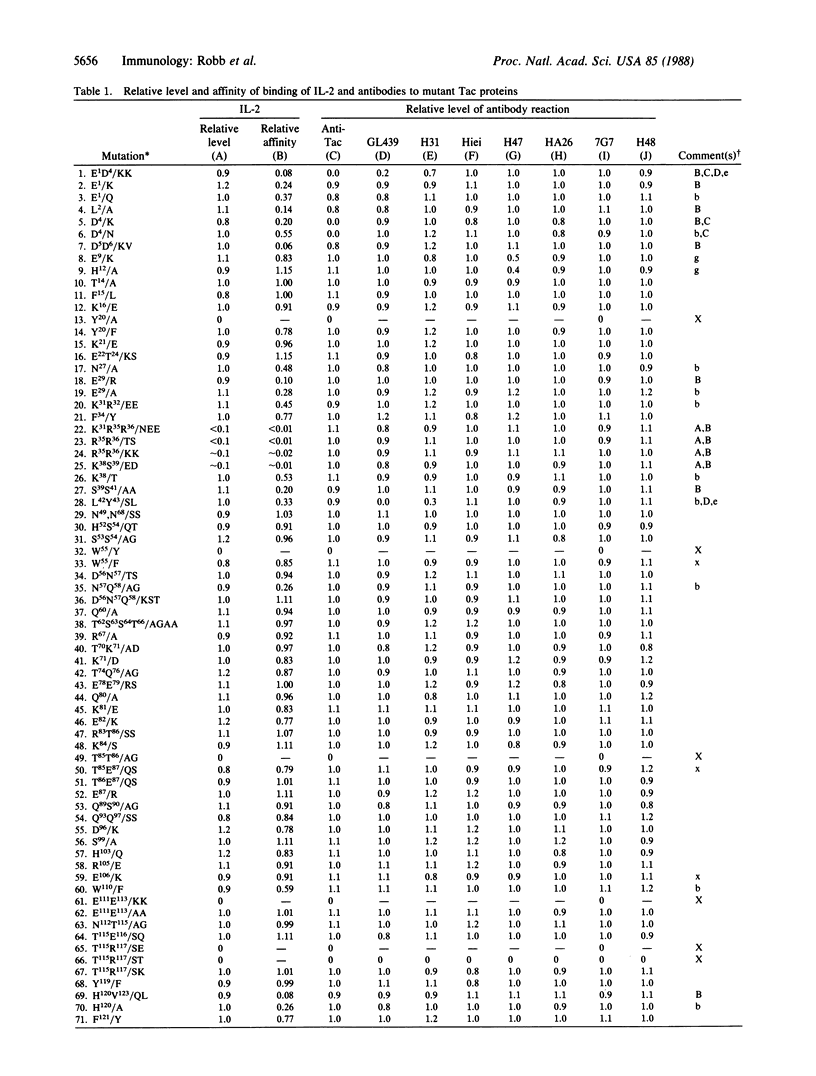
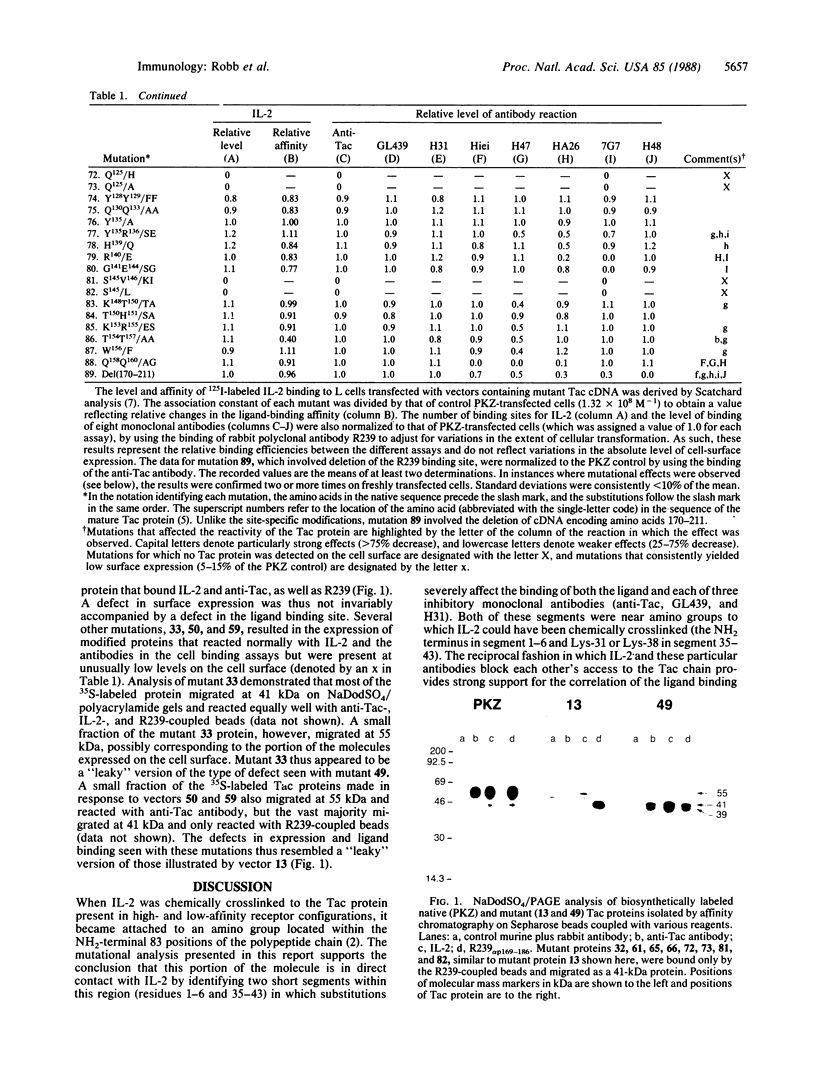
The Tac protein plays a role in high- and low-affinity interleukin 2 (IL-2) receptors. A mutational survey of this molecule identified several small segments in which the binding of IL-2 was particularly sensitive to amino acid substitutions. Two of the segments (residues 1-6 and 35-43) located in the exon 2-encoded region of the molecule overlapped the apparent binding sites of three monoclonal antibodies (anti-Tac, GL439, and H31) that block high- and low-affinity Tac-IL-2 interactions, thus supporting the hypothesis that these segments of the protein are at or near sites of receptor-ligand contact. In contrast, the apparent binding sites of antibodies (Hiei and H47) that selectively inhibit high-affinity IL-2 binding were mapped to a distinct location (residues 158-160) within the region encoded by exon 4 of the Tac gene. Since high-affinity receptors consist of a heterodimer of Tac and a second ligand-binding protein (p70), this portion of the Tac molecule may be involved in the interaction between the two receptor subunits. As expected, the binding sites of noninhibitory antibodies (7G7/B6, residues 140-144; H48, residues 170-211) did not overlap those segments in which IL-2-binding mutants were observed. These results provide a preliminary correlation of structure and function for the Tac protein that should prove useful in evaluating detailed models of the IL-2-receptor complex.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Fujii M., Sugamura K., Nakai S., Tanaka Y., Tozawa H., Hinuma Y. High- and low-affinity interleukin 2 receptors: distinctive effects of monoclonal antibodies. J Immunol. 1986 Sep 1;137(5):1552–1556. [PubMed] [Google Scholar]
- Gething M. J., McCammon K., Sambrook J. Expression of wild-type and mutant forms of influenza hemagglutinin: the role of folding in intracellular transport. Cell. 1986 Sep 12;46(6):939–950. doi: 10.1016/0092-8674(86)90076-0. [DOI] [PubMed] [Google Scholar]
- Kingsley D. M., Kozarsky K. F., Hobbie L., Krieger M. Reversible defects in O-linked glycosylation and LDL receptor expression in a UDP-Gal/UDP-GalNAc 4-epimerase deficient mutant. Cell. 1986 Mar 14;44(5):749–759. doi: 10.1016/0092-8674(86)90841-x. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo L. M., Rusk C. M., Robb R. J. Structure-function relationships for the IL 2-receptor system. II. Localization of an IL 2 binding site on high and low affinity receptors. J Immunol. 1986 Sep 1;137(5):1544–1551. [PubMed] [Google Scholar]
- Leonard W. J., Depper J. M., Kanehisa M., Krönke M., Peffer N. J., Svetlik P. B., Sullivan M., Greene W. C. Structure of the human interleukin-2 receptor gene. Science. 1985 Nov 8;230(4726):633–639. doi: 10.1126/science.2996141. [DOI] [PubMed] [Google Scholar]
- Neeper M. P., Kuo L. M., Kiefer M. C., Robb R. J. Structure function relationships for the IL 2 receptor system. III. Tac protein missing amino acids 102 to 173 (exon 4) is unable to bind IL 2: detection of spliced protein after L cell transfection. J Immunol. 1987 May 15;138(10):3532–3538. [PubMed] [Google Scholar]
- Robb R. J., Greene W. C., Rusk C. M. Low and high affinity cellular receptors for interleukin 2. Implications for the level of Tac antigen. J Exp Med. 1984 Oct 1;160(4):1126–1146. doi: 10.1084/jem.160.4.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin L. A., Kurman C. C., Biddison W. E., Goldman N. D., Nelson D. L. A monoclonal antibody 7G7/B6, binds to an epitope on the human interleukin-2 (IL-2) receptor that is distinct from that recognized by IL-2 or anti-Tac. Hybridoma. 1985 Summer;4(2):91–102. doi: 10.1089/hyb.1985.4.91. [DOI] [PubMed] [Google Scholar]
- Rusk C. M., Neeper M. P., Kuo L. M., Kutny R. M., Robb R. J. Structure-function relationships for the IL-2 receptor system. V. Structure-activity analysis of modified and truncated forms of the Tac receptor protein: site-specific mutagenesis of cysteine residues. J Immunol. 1988 Apr 1;140(7):2249–2259. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf H., Modrow S., Motz M., Jameson B. A., Hermann G., Förtsch B. An integrated family of amino acid sequence analysis programs. Comput Appl Biosci. 1988 Mar;4(1):187–191. doi: 10.1093/bioinformatics/4.1.187. [DOI] [PubMed] [Google Scholar]






