Abstract
Objective:
Dysfunction within corticostriatal dopaminergic neurocircuitry has been implicated in neuropsychiatric symptoms associated with Alzheimer disease (AD). This study aimed to test the hypothesis that the symptom domains delusions and apathy would be associated with striatal dopamine (D2) receptor function in AD.
Methods:
In vivo dopamine (D2/D3) receptor availability was determined with [11C]raclopride (RAC) PET in 23 patients with mild and moderate probable AD. Behavioral symptoms were measured using the Neuropsychiatric Inventory and the Apathy Inventory. Imaging data were analyzed using a region-of-interest approach. The potential confounding effects of age, sex, and disease stage were explored using a linear mixed model. Correlational and independent samples comparisons were used to examine the relationship between behavioral and binding potential (BPND) measures.
Results:
Mean [11C]RAC BPND was higher in patients with delusions (n = 7; 5 men) than in patients without delusions (n = 16; 6 men) (p = 0.006). When women were excluded from the analysis, [11C]RAC BPND was higher in men with delusions than in men without delusions (p = 0.05). Apathy measures showed no association with [11C]RAC BPND.
Conclusions:
Striatal dopamine (D2/D3) receptor availability is increased in Alzheimer disease patients with delusions, to an extent comparable to that observed in drug-naive patients with schizophrenia. Whether this represents up-regulation of dopamine (D2) or possibly dopamine (D3) receptors and how this relates to responsivity of the striatal dopaminergic system merit further exploration.
GLOSSARY
AD = Alzheimer disease; AI = Apathy Inventory; AST = associative striatum; BPND = binding potential; CAMCOG = Cambridge Cognitive Examination; DA = dopamine; LS = limbic striatum; MMSE = Mini-Mental State Examination; MRC = Medical Research Council; non-AC = non–attenuated corrected; NPI = Neuropsychiatric Inventory; RAC = raclopride; ROI = region of interest; SMST = sensorimotor striatum.
The neurochemical changes that underpin neuropsychiatric symptoms in Alzheimer disease (AD) have been poorly elucidated. In vivo studies that avoid the confounding influences of illness duration and exposure to psychotropics that are encountered postmortem1 could identify potential treatment targets. Perturbation of cholinergic–dopaminergic influences within corticostriatal neurocircuitry have been implicated in the development of psychosis2 and apathy.3 Excessive striatal dopamine (DA) activity is viewed as the final common pathway in the development of psychotic symptoms; and in AD progressive cholinergic loss, within the context of a functionally intact dopaminergic system, may increase the propensity of patients with AD to develop psychosis because of a relative striatal hyperdopaminergia.2 Apathy is believed to represent a deficit in corticostriatal (ventral striatum, prefrontal cortex) processing,4 characterized by a reduced ability to process emotionally significant stimuli.5 In vivo studies in small numbers of patients with moderately severe AD (n = 10) have reported higher DA (D2) receptor availability in association with wandering behavior6 and lower striatal DA (D2) receptor availability in more behaviorally disturbed patients.7 More recently, higher striatal DA transporter availability has been correlated with apathy (“loss of initiative”) in a larger sample (n = 24) that included 8 patients with Lewy body dementia.8 We aimed to test the hypothesis that striatal DA (D2) receptor availability—using the DA (D2/D3) receptor ligand [11C]raclopride (RAC)—would be associated with delusions and apathy in psychotropic-naive patients with mild to moderate AD.
METHODS
Sample.
Twenty-three patients with probable late-onset Alzheimer dementia were recruited as part of wider study aiming to investigate the influence of donepezil on striatal DA release. Twenty-one patients were recruited from a dedicated memory clinic (the Croydon Memory Service), and two were recruited from community mental health teams. All patients fulfilled National Institute of Neurological and Communicative Disorders and Stroke–Alzheimer’s Disease and Related Disorders Association criteria for AD9 and were eligible to receive treatment with a cholinesterase inhibitor but had not yet commenced medication. Clinical assessment included screening with the Mini-Mental State Examination (MMSE)10 (all patients) and, in patients assessed by the Croydon Memory Service (n = 21), the Cambridge Cognitive Examination (CAMCOG).11 Where scores were above the suggested cutoff for questionable dementia (>24/30 on the MMSE or >79/105 on the CAMCOG), neuropsychological testing was conducted to establish the diagnosis. All subjects underwent standard laboratory investigation and structural (MRI) imaging to rule out other cause of memory loss and to quantify the extent of vascular pathology: Seven patients (30.4%) showed radiologic signs of vascular pathology—small vessel disease (n = 4) or silent lacunar infarcts (n = 3). Patients were excluded on the basis of past or current psychiatric illness or the prescription of medication that might influence DA function. The study was approved by the Charing Cross and Joint South London and Maudsley and The Institute of Psychiatry Research Ethics Committees. The Administration of Radioactive Substances Advisory Committee granted permission to administer [11C]RAC. Written and verbal consent was obtained at the time of recruitment and repeated before the scanning procedure.
Behavioral ratings.
The Neuropsychiatric Inventory (NPI)12 evaluates 12 symptom domains (shown in table 1) over the preceding month, and scores range from 0 (not present) to 12 if present very frequently4 and are very disruptive.3 The Apathy Inventory (AI)13 separates apathy into three components: emotional blunting, lack of interest, and lack of initiative. Scoring follows the same rules as the NPI (maximum score of 12 on each of the three domains). Ratings were made by a live-in carer on the day of the scan. No cutoff point was used to categorize any of the symptom domains.
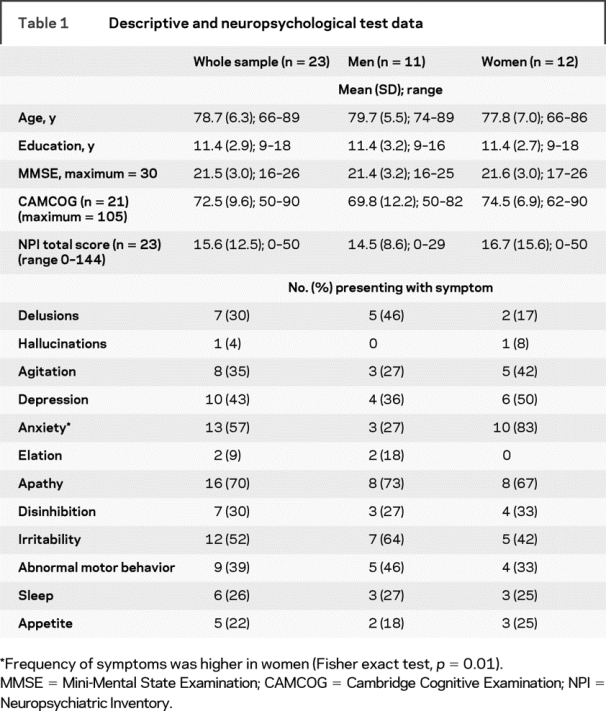
Table 1 Descriptive and neuropsychological test data
PET procedure.
[11C]RAC PET is an established in vivo method for estimating the availability of DA (D2) receptors in the brain. Participants were scanned at rest on a 962-Siemens/CTI PET camera in three-dimensional mode. Head movement was minimized using a molded headrest and straps. Each scan consisted of 1) a short (10-minute) transmission scan to enable correction for tissue attenuation of radioactivity and 2) dynamic scanning: [11C]RAC was administered as a bolus injection followed by a constant-rate infusion with a κbol of 60 minutes.14 The bolus infusion method enables a state of equilibrium to be reached which avoids potential artifacts introduced through blood flow change during the scan.15 Total administered activity was 370 MBq per scan. The sampling period for calculation of the binding potential (BPND), after RAC equilibrium had been established, was 35 to 60 minutes collected over 20 serial time frames. Head movement was corrected using a frame-by-frame realignment procedure, previously described.16 Non–attenuated corrected (non-AC) images were used for realignment, to provide additional information by reducing the influence of redistribution of radiotracer producing erroneous realignments. The non-AC image was denoised using a level 2, order 64 Battle Lemarie wavelet. A mutual information algorithm was applied for frame realignment to a single frame acquired 35 minutes after injection, in which there was a high signal-to-noise ratio. Finally, transformation parameters were applied to the corresponding attenuation-corrected dynamic images for frames 15 through 20 to generate a frame-by-frame corrected dynamic image.
Region-of-interest analysis.
Region-of-interest (ROI) templates for striatal and cerebellar areas were defined on a magnetic resonance scan positioned in standard Montreal Neurological Institute space. Three striatal subregions were defined bilaterally, based on previously described criteria17: the limbic striatum (LS) corresponds to the ventral striatum, and the caudate and putamen were subdivided along their rostrocaudal axis using the anterior commissure to derive the associative striatum (AST; precommissural and postcommissural dorsal caudate and precommissural dorsal putamen) and the sensorimotor striatum (SMST; postcommissural putamen). Delineation of ROI templates in the right striatum are shown in figure 1. Templates were applied to dynamic images to generate the time–activity curves for striatal (ROI) and cerebellar (reference) regions. Images were sampled between 35 and 60 minutes, and BPND18 was expressed as the ratio at equilibrium of specifically bound radioligand to that of nondisplaceable radioligand in tissue [(striatal/cerebellar binding) − 1].
Figure 1 Striatal regions of interest
Right-sided sensorimotor (SMS), associative (AST), and limbic (LS) striatal regions of interest, defined on a magnetic resonance scan, have been superimposed on a [11C]raclopride (dynamic) image positioned in standard Montreal Neurological Institute space. (A) Transverse view, SMS and AST. (B) Coronal view, AST and LS. (C) Sagittal view, AST and LS.
Statistical analysis.
Data were analyzed using SPSS 15.0. Previous imaging studies have shown that age,19 sex,20 and global impairment21 may potentially modulate the relationship between dopaminergic and behavioral indices. It was thus important to explore and control for their potentially confounding effects. A linear mixed model was used to determine the effect of sex on mean [11C]RAC BP and the interaction between age and sex. Correlation coefficients (Spearman ρ) were used to describe the associations between behavioral ratings and other variables, including age, MMSE, and [11C]RAC BPND. Sex differences were investigated using the independent-samples t test, Mann–Whitney U test, and Fisher exact test.
RESULTS
Descriptive and neuropsychological test performance.
The mean age of the sample was 78.7 years (range 66–89 years), and there were 11 men (48%). The majority of patients (n = 14; 61%) were in the mild stage of disease (MMSE >20 out of 30), 21 (91%) were right-handed, and 14 (61%) were prescribed medication for chronic physical health problems. There were no sex differences in mean age, educational level, or global cognitive or behavioral function (table 1).
Dopamine (D2) receptor measures.
The mean [11C]RAC BPND for the whole striatum was 2.17 (SD 0.29, range 1.74–2.97). There was an effect of sex [F(1,97) = 27.8, p < 0.0001]: mean values for the striatum were higher in men (mean 2.26, SD 0.34) than in women (mean 2.08, SD 0.21). The sex difference was explained by an interaction between age and sex [F(1,97) = 30.4, p < 0.0001]: mean BP decreased with age in women (r = −0.77, p = 0.003) but not in men (r = 0.41, p = 0.21) (figure e-1 on the Neurology® Web site at www.neurology.org). The presence or absence of vascular pathology had no effect on mean [11C]RAC BPND (p = 0.77).
Neuropsychiatric symptoms.
Frequencies of each of the symptom domains from the NPI are shown in table 1. Apathy was present in 16 (69%) of the sample. Delusions were present in 7 patients (30%; 5 men) and were characteristic of AD, including beliefs regarding theft or the presence of an intruder in the house. One woman exhibited hallucinations as well as delusions. Anxiety was more common in women (Fisher exact test, p = 0.01). Mean (SD) scores and ranges for the AI and NPI (delusions, apathy) are shown in table 2. Carer ratings of emotional blunting were higher in men (Mann–Whitney U, p = 0.05). Total AI scores were correlated with the NPI apathy domain (Spearman ρ = 0.72, p < 0.0001). Delusions showed no interrelationships with other symptom domains or with the presence of vascular pathology (Mann–Whitney U, p = 0.41), and mean age, educational level, and MMSE were not correlated with scores on any of the symptom domains.
Table 2 Correlation between neuropsychiatric symptoms and [11C]raclopride binding potential

Relationship between [11C]RAC BPND and neuropsychiatric symptoms.
A correlation was found between delusions and [11C]RAC BP in the whole striatum (ρ = 0.54, p = 0.008) and in each subregion (SMST: ρ = 0.61, p = 0.002; AST: ρ = 0.48, p = 0.02; LS: ρ = 0.44, p = 0.03) (table 2). When patients were analyzed by group (presence/absence of delusions), mean [11C]RAC BPND was higher in patients with delusions (mean 2.4, SD 0.3) than in patients without delusions (mean 2.1, SD 0.2) (independent-samples t test: t21 = −3.1, p = 0.006), and this was seen in all striatal subregions. Table 3 summarizes descriptive and BPND data in patients with and without delusions. There were no differences in mean age and global impairment, but the proportion of men in the group with delusions was higher (71.4%) than in patients without delusions (37.5%) (Fisher exact test, p = 0.19). It was possible that the higher mean [11C]RAC BPND in patients with delusions might be explained simply by the higher BPND values in men. When the analysis was restricted to men, however, differences between patients with delusions (mean 2.5, SD 0.3) and patients without delusions (mean 2.1, SD 0.3) were comparable to the total sample (p = 0.05). Mean BP values for the whole striatum in the presence or absence of delusions are shown in figure 2.
Table 3 Mean [11C]raclopride binding potential in the presence (n = 7) or absence (n = 16) of delusions


Figure 2 [11C]raclopride binding potential in patients with and without delusions
Mean [11C]raclopride (RAC) binding potential (BPND) in the striatum, in the presence or absence of delusions.
None of the apathy measures was found to correlate with [11C]RAC BPND in the whole striatum or in any of the subregions (table 2). An exploratory analysis of the associations between [11C]RAC BPND and other symptom domains from the NPI revealed a relationship only with disinhibition (ρ = 0.42, p = 0.05; SMST: ρ = 0.31, p = 0.15; AST: ρ = 0.40, p = 0.06; LS: ρ = 0.41, p = 0.06). However, because this analysis was not hypothesis driven, it did not survive correction for multiple comparisons.
DISCUSSION
We found patients with delusions to have significantly (14%) higher striatal DA (D2/D3) receptor availability than their counterparts without delusions, a difference not explained by age, disease stage, or vascular pathology. After controlling for the significant effect of sex on BPND by excluding women from the analysis, [11C]RAC BPND was found to be 16% higher in men with delusions than in men without delusions.
Contrary to our hypothesis, there was no relationship between [11C]RAC BPND and apathy. This was not explained by a low frequency or narrow range of apathy scores, but the possibility of a type II error cannot be ruled out. Our findings are somewhat at odds with recently published data in a mixed sample of patients with AD and Lewy body dementia,8 and there are several possible explanations for this. It is possible that previous findings reflect primarily an association between apathy and striatal DA transporter function in patients with Lewy body dementia, or—assuming that the association is representative of the AD population—that presynaptic neuronal integrity is a more sensitive indicator of apathy than postsynaptic receptor sites. We cannot rule out the involvement of extrastriatal DA (D2/D3) receptors in apathy in AD, because this was beyond the scope of our study. The relative importance of noradrenergic innervation of the ventral striatum—implicated in apathy in PD22—must also be considered, because alterations in the cholinergic–noradrenergic balance might similarly contribute to symptoms in AD.
Our finding of a significant age × sex interaction warrants discussion, because it does not seem to have been explained by differences in the age distribution, disease stage, or presence of vascular pathology in our sample. It is unclear whether lower [11C]RAC BPND in women with AD reflects a reduction in receptor density or differences in endogenous DA levels, as has been reported in young adults.20 It is also possible that higher BPND in patients with delusions may have contributed to our findings (figure e-1), because this reduced our ability to detect an influence of age on BPND in men. This will need to be explored through further studies in patients without psychosis.
Contemporary models of psychosis suggest that a variety of pathologic processes—neonatal hypoxia, hippocampal damage, cortical lesions, and cholinergic denervation—may lead to the sensitization of striatal dopaminergic neurons and the expression of positive symptoms.23–25 In support of this model are data on young adults with schizophrenia, who exhibit increased presynaptic DA synthesis capacity, increased postsynaptic receptor DA (D2/D3) receptor availability, and an exaggerated response to amphetamine, in terms of both endogenous DA release and symptom exacerbation.26 Young psychotic patients with bipolar illness exhibit similar increases in postsynaptic striatal DA (D2/D3) receptor availability,27 without evidence of the alterations in presynaptic function observed in schizophrenia,28,29 and it is thus probable that the relative importance of presynaptic and postsynaptic mechanisms may depend on the primary pathologic process. Our findings provide in vivo evidence of a direct relationship between the expression of positive symptoms and altered postsynaptic striatal DA (D2/D3) receptor function in AD. Further, the extent of the increase in [11C]RAC BPND in patients with delusions is comparable to that observed in young adults with psychosis.27,30
The functional significance of an increase in DA (D2) receptor availability is difficult to interpret, because [11C]RAC binding does not simply reflect the density of DA (D2/D3) receptors, but competes with endogenous DA for receptor sites.31 It is thus not possible to determine the extent to which differences in endogenous DA between the two groups contributed to our findings. The potential contribution of higher DA (D3) receptor availability in the group with delusions must also be considered. In vitro, a 70% increase in DA (D3) receptor density has been found in the ventral caudate of psychotic (neuroleptic naive or intolerant) compared with nonpsychotic patients with AD.32 It is not possible to distinguish between DA (D2 and D3) receptors through the use of [11C]RAC, because it binds with equal affinity to both subtypes. However, DA (D3) receptor binding accounts for only 10% of the total signal because of the lower density of expression in regions other than the ventral striatum.33 Although it remains possible that our findings represent differences in DA (D3) receptor availability in patients with delusions, the fact that the greatest percentage differences between the two groups were found in the associative striatum would tend to argue against this. It is possible that partial volume effects—as a result of greater atrophy in the striatum of patients without delusions—might have contributed to the observed differences. Although data suggest that there may be structural differences between patients with and without psychosis in relation to cortical atrophy—right frontal34 and temporal35 regions have been specifically implicated—there are no such data relating to the striatum.
The presence of psychosis has considerable clinical and economic implications, generally predicting a greater speed of cognitive decline and early institutionalization.36 It has been suggested that psychosis in AD may represent not one but several different phenotypes, each with genetically, biologically, and neurochemically distinct markers.37,38 Attempts to further categorize psychotic symptoms through the use of factor analytic approaches have identified two broad subtypes: a paranoid subtype (defined by the presence of persecutory delusions) and a “misidentification” subtype (defined by misidentification phenomena and hallucinations).39 Whether increased striatal DA (D2) receptor availability primarily reflects the paranoid subtype is a question that will need to be answered through future studies.
Perhaps most relevant clinically is the fact that we found measurable differences between patients with and without delusions who were at an early stage in the disease process and in whom the presence of delusional beliefs was not associated with other behavioral symptoms, such as agitation or aggression. This would indicate that increased DA (D2/D3) receptor availability—and possibly other indices of striatal dopaminergic function—might act as markers of psychosis proneness in AD. Future studies should similarly aim to better characterize psychosis in AD in terms of presynaptic and postsynaptic dopaminergic indices, to identify those most vulnerable to its development.
AUTHOR CONTRIBUTIONS
All statistical analyses were performed by S.R.
ACKNOWLEDGMENT
The authors thank the staff at the Croydon Memory Service, the team of radiographers at the MRC Cyclotron Unit, Steve Nobes for his help in transporting participants to scanning sessions, and all those who kindly gave up their time to take part in the study.
Supplementary Material
Address correspondence and reprint requests to Dr. Suzanne Reeves, Section of Old Age Psychiatry, MRC Centre for Neurodegeneration Research, Institute of Psychiatry, De Crespigny Park, Camberwell, London SE5 8AF, UK s.reeves@iop.kcl.ac.uk
Supplemental data at www.neurology.org
Supported by the Wellcome Research Trust, as part of a Research Training Fellowship, and by the MRC.
Disclosure: The authors report no competing interests. R.H. discloses that he has received honoraria as speaker’s fee from Lundbeck and Pfizer-Eisai. Pfizer-Eisai and Lundbeck have provided medication and placebo for independently funded randomized controlled trials on which R.H. is Chief Investigator. P.G. has served as an occasional consultant to GlaxoSmithKline, Merck, and Pfizer.
Received July 28, 2008. Accepted in final form November 10, 2008.
REFERENCES
- 1.Perry E, Court J, Goodchild R, et al. Clinical neurochemistry: developments in dementia research based on brain bank material. J Neural Transm 1998;105:915–933. [DOI] [PubMed] [Google Scholar]
- 2.Cummings JL, Back C. The cholinergic hypothesis of neuropsychiatric symptoms in Alzheimer’s disease. Am J Geriatr Psychiatry 1998;6:S64–S78. [DOI] [PubMed] [Google Scholar]
- 3.White KE, Cummings JL. Schizophrenia and Alzheimer’s disease: clinical and pathophysiologic analogies. Compr Psychiatry 1996;37:188–195. [DOI] [PubMed] [Google Scholar]
- 4.Levy R, Dubois B. Apathy and the functional anatomy of the prefrontal cortex-basal ganglia circuits. Cereb Cortex 2006;16:916–928. [DOI] [PubMed] [Google Scholar]
- 5.Cummings JL, Kaufer D. Neuropsychiatric aspects of Alzheimer’s disease: the cholinergic hypothesis revisited. Neurology 1996;47:876–883. [DOI] [PubMed] [Google Scholar]
- 6.Meguro K, Yamaguchi S, Itoh M, Fujiwara T, Yamadori A. Striatal dopamine metabolism correlated with frontotemporal glucose utilization in Alzheimer’s disease: a double-tracer PET study. Neurology 1997;49:941–945. [DOI] [PubMed] [Google Scholar]
- 7.Tanaka Y, Meguro K, Yamaguchi S, et al. Decreased striatal D2 receptor density associated with severe behavioral abnormality in Alzheimer’s disease. Ann Nucl Med 2003;17:567–573. [DOI] [PubMed] [Google Scholar]
- 8.David R, Koulibaly M, Benoit M, et al. Striatal dopamine transporter levels correlate with apathy in neurodegenerative diseases: a SPECT study with partial volume effect correction. Clin Neurol Neurosurg 2008;110:19–24. [DOI] [PubMed] [Google Scholar]
- 9.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 1984;34:939–944. [DOI] [PubMed] [Google Scholar]
- 10.Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–198. [DOI] [PubMed] [Google Scholar]
- 11.Huppert FA, Brayne C, Gill C, Paykel ES, Beardsall L. CAMCOG—a concise neuropsychological test to assist dementia diagnosis: socio-demographic determinants in an elderly population sample. Br J Clin Psychol 1995;34(pt 4):529–541. [DOI] [PubMed] [Google Scholar]
- 12.Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology 1994;44:2308–2314. [DOI] [PubMed] [Google Scholar]
- 13.Robert PH, Clairet S, Benoit M, et al. The apathy inventory: assessment of apathy and awareness in Alzheimer’s disease, Parkinson’s disease and mild cognitive impairment. Int J Geriatr Psychiatry 2002;17:1099–1105. [DOI] [PubMed] [Google Scholar]
- 14.Watabe H, Endres CJ, Breier A, Schmall B, Eckelman WC, Carson RE. Measurement of dopamine release with continuous infusion of [11C]raclopride: optimization and signal-to-noise considerations. J Nucl Med 2000;41:522–530. [PubMed] [Google Scholar]
- 15.Carson RE, Breier A, de Bartolomeis A, et al. Quantification of amphetamine-induced changes in [11C]raclopride binding with continuous infusion. J Cereb Blood Flow Metab 1997;17:437–447. [DOI] [PubMed] [Google Scholar]
- 16.Montgomery AJ, Thielemans K, Mehta MA, Turkheimer F, Mustafovic S, Grasby PM. Correction of head movement on PET studies: comparison of methods. J Nucl Med 2006;47:1936–1944. [PubMed] [Google Scholar]
- 17.Martinez D, Slifstein M, Broft A, et al. Imaging human mesolimbic dopamine transmission with positron emission tomography, part II: amphetamine-induced dopamine release in the functional subdivisions of the striatum. J Cereb Blood Flow Metab 2003;23:285–300. [DOI] [PubMed] [Google Scholar]
- 18.Innis RB, Cunningham VJ, Delforge J, et al. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab 2007;27:1533–1539. [DOI] [PubMed] [Google Scholar]
- 19.Backman L, Nyberg L, Lindenberger U, Li SC, Farde L. The correlative triad among aging, dopamine, and cognition: current status and future prospects. Neurosci Biobehav Rev 2006;30:791–807. [DOI] [PubMed] [Google Scholar]
- 20.Pohjalainen T, Rinne JO, Nagren K, Syvalahti E, Hietala J. Sex differences in the striatal dopamine D2 receptor binding characteristics in vivo. Am J Psychiatry 1998;155:768–773. [DOI] [PubMed] [Google Scholar]
- 21.Rodriguez G, Morbelli S, Brugnolo A, et al. Global cognitive impairment should be taken into account in SPECT-neuropsychology correlations: the example of verbal memory in very mild Alzheimer’s disease. Eur J Nucl Med Mol Imaging 2005;32:1186–1192. [DOI] [PubMed] [Google Scholar]
- 22.Remy P, Doder M, Lees A, Turjanski N, Brooks D. Depression in Parkinson’s disease: loss of dopamine and noradrenaline innervation in the limbic system. Brain 2005;128:1314–1322. [DOI] [PubMed] [Google Scholar]
- 23.Laruelle M, Abi-Dargham A, Gil R, Kegeles L, Innis R. Increased dopamine transmission in schizophrenia: relationship to illness phases. Biol Psychiatry 1999;46:56–72. [DOI] [PubMed] [Google Scholar]
- 24.Seeman P, Weinshenker D, Quirion R, et al. Dopamine supersensitivity correlates with D2High states, implying many paths to psychosis. Proc Natl Acad Sci USA 2005;102:3513–3518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mattsson A, Olson L, Svensson TH, Schilstrom B. Cortical cholinergic deficiency enhances amphetamine-induced dopamine release in the accumbens but not striatum. Exp Neurol 2007;208:73–79. [DOI] [PubMed] [Google Scholar]
- 26.Breier A, Su TP, Saunders R, et al. Schizophrenia is associated with elevated amphetamine-induced synaptic dopamine concentrations: evidence from a novel positron emission tomography method. Proc Natl Acad Sci USA 1997;94:2569–2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pearlson GD, Wong DF, Tune LE, et al. In vivo D2 dopamine receptor density in psychotic and nonpsychotic patients with bipolar disorder. Arch Gen Psychiatry 1995;52:471–477. [DOI] [PubMed] [Google Scholar]
- 28.Anand A, Verhoeff P, Seneca N, et al. Brain SPECT imaging of amphetamine-induced dopamine release in euthymic bipolar disorder patients. Am J Psychiatry 2000;157:1108–1114. [DOI] [PubMed] [Google Scholar]
- 29.Yatham LN, Liddle PF, Shiah IS, et al. PET study of [(18)F]6-fluoro-L-dopa uptake in neuroleptic- and mood-stabilizer-naive first-episode nonpsychotic mania: effects of treatment with divalproex sodium. Am J Psychiatry 2002;159:768–774. [DOI] [PubMed] [Google Scholar]
- 30.Laruelle M. Imaging dopamine transmission in schizophrenia: a review and meta-analysis. Q J Nucl Med 1998;42:211–221. [PubMed] [Google Scholar]
- 31.Laruelle M. Imaging synaptic neurotransmission with in vivo binding competition techniques: a critical review. J Cereb Blood Flow Metab 2000;20:423–451. [DOI] [PubMed] [Google Scholar]
- 32.Sweet RA, Hamilton RL, Healy MT, et al. Alterations of striatal dopamine receptor binding in Alzheimer disease are associated with Lewy body pathology and antemortem psychosis. Arch Neurol 2001;58:466–472. [DOI] [PubMed] [Google Scholar]
- 33.Gurevich EV, Joyce JN. Distribution of dopamine D3 receptor expressing neurons in the human forebrain: comparison with D2 receptor expressing neurons. Neuropsychopharmacology 1999;20:60–80. [DOI] [PubMed] [Google Scholar]
- 34.Forstl H, Besthorn C, Burns A, Geiger-Kabisch C, Levy R, Sattel A. Delusional misidentification in Alzheimer’s disease: a summary of clinical and biological aspects. Psychopathology 1994;27:194–199. [DOI] [PubMed] [Google Scholar]
- 35.Geroldi C, Akkawi NM, Galluzzi S, et al. Temporal lobe asymmetry in patients with Alzheimer’s disease with delusions. J Neurol Neurosurg Psychiatry 2000;69:187–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ropacki SA, Jeste DV. Epidemiology of and risk factors for psychosis of Alzheimer’s disease: a review of 55 studies published from 1990 to 2003. Am J Psychiatry 2005;162:2022–2030. [DOI] [PubMed] [Google Scholar]
- 37.Sweet RA, Nimgaonkar VL, Devlin B, Jeste DV. Psychotic symptoms in Alzheimer disease: evidence for a distinct phenotype. Mol Psychiatry 2003;8:383–392. [DOI] [PubMed] [Google Scholar]
- 38.Borroni B, Grassi M, Agosti C, et al. Cumulative effect of COMT and 5-HTTLPR polymorphisms and their interaction with disease severity and comorbidities on the risk of psychosis in Alzheimer disease. Am J Geriatr Psychiatry 2006;14:343–351. [DOI] [PubMed] [Google Scholar]
- 39.Cook SE, Miyahara S, Bacanu SA, et al. Psychotic symptoms in Alzheimer disease: evidence for subtypes. Am J Geriatr Psychiatry 2003;11:406–413. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



