The mechanisms by which neural signals are encoded to produce conscious sensations remain a central question in neuroscience. Invasive recordings from human brain structures in vivo give us the opportunity to study neural correlates of these sensations. Pain is a sensation fundamental to survival and its subjective nature in the clinical setting makes it difficult to quantify. It remains without direct objective neuronal correlates. Here we describe an 8–14 Hz, spindle-shaped neural signal present in both the sensory thalamus and periaqueductal gray area (PAG) in humans that directly correlates to the subjective reporting of pain intensity.
Local field potentials (LFPs) recorded by deep brain macroelectrodes reveal the ensemble activity of neuronal groups in particular brain regions.1 The oscillatory amplitude of such ensembles is proportionate to the degree of synchrony with which they oscillate.2 Properties of oscillations including their synchrony, frequency, and corresponding power spectra vary both between brain structures and dynamically, depending upon the activity performed.3
Methods.
Twelve patients (11 male, 1 female) underwent deep brain stimulation for treatment of chronic neuropathic pain. Etiology was as follows; poststroke pain (4), phantom limb pain (3), facial pain of various causes (4), and brachial plexus injury (1). Nuclei targeted were periaqueductal gray (PAG) alone in 3 patients, ventroposterolateral/medial nucleus of the thalamus (VPL/VPM) in 6 patients, and both in 3 patients. This was decided upon based on clinical grounds (in general, sensory thalamus was avoided if the patient had had a thalamic stroke). Three patients had both nuclei implanted as stimulation of the first did not show convincing pain relief intraoperatively. Mean age was 51.6 years (range 35–74 years).
LFPs were recorded postoperatively. The experiment was started with 10 minutes of rest before recording. Recording of LFPs then lasted 10 minutes, during which time Visual Analogue Score (VAS) of pain was recorded every 60 seconds. This protocol was repeated three times for each patient, on 3 separate days.
Results.
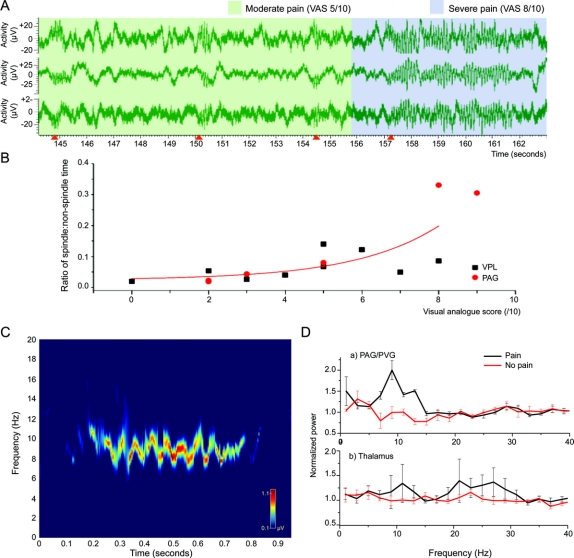
The most prominent finding was characteristic spindle-shaped bursts of increased amplitude at approximately 10 Hz (slightly different in different subjects, range 8–14 Hz, mean 10 Hz) in both the sensory thalamus and PAG concomitant with subjective awareness of pain (figure, A). Statistical analysis revealed a significant increase in the number of bursts and increasing VAS for each individual subject as well as an increased ratio of burst-time to non-burst-time activity in the pain state (figure, B). Plotting burst activity against the background power spectrum of LFP activity over a finite time course reveals an individual neural signature of pain for each subject (figure, C). In general, power spectra reveal that there is a significant rise in the 8–12 Hz activity in the PAG and a rise in the 17–30 Hz activity, i.e., beta frequency, in the sensory thalamus (figure, D).
Figure Local field potential changes in different pain states
(A) 10-Hz spindle-shaped activity in the ventroposterolateral nucleus of the thalamus in a patient with chronic neuropathic poststroke pain. The three graphs represent three bipolar channels of the same electrode (four contacts). Note the onset of each period of activity as indicated by the orange arrows on the x-axis as well as the relative pain that the patient was experiencing, as indicated by the colored overlay (green = moderate pain [VAS = 5/10], blue = severe pain [VAS = 9/10]). (B) Comparison of spindle–nonspindle activity in each nucleus showed a significant exponential correlation between VAS and spindle activity. Each point represents a different patient (15 electrodes in 12 patients, as 3 had PAG and VPL). R2 = 0.67, χ2 = 0.00373. (C) Time-frequency analysis of local field potentials during pain. This shows a 1-second segment of the power spectrum of the local field potentials recorded from the sensory thalamus in a patient with intense pain (VAS = 8/10). Based on time-variant autoregressive modeling, this time-frequency distribution demonstrates the changes in dominant frequencies during a 1-second burst of spindle activity over time. It demonstrates the maximum power of approximately 8–10 Hz with entry from high frequency to low frequency during the 1-second burst of spindle activity. This provides an individual signature of pain for this patient. Frequencies above 20 Hz have been omitted for clarity. (D) Mean power spectral changes for each nucleus. Power spectra showing the dominant frequencies for each nucleus in two conditions. In the nonpain condition, patients were at rest. In the painful condition, pain was evoked using an ice-cold stimulus on the skin of the affected part. Pain increases power in the 8–12 Hz frequency in the PAG and 17–30 Hz in the sensory thalamus. Light-colored lines show 1 SD of the mean. VAS = Visual Analogue Score; PVG = periventricular gray area; PAG = periaqueductal gray area; VPL = ventroposterolateral.
Discussion.
Noninvasive electrophysiologic and functional neuroimaging technologies have, until now, provided the most convincing evidence of changes in human brain activity associated with pain.4 They have also enhanced understanding of whole brain responses to deep brain stimulation.5 Although previous studies have looked at electrical coupling between brain nuclei in pain,6 they have not revealed convincing characteristic waveforms that may represent pain perception. These previous studies have suggested 0.2–0.4 Hz frequency changes in the sensory thalamus in the pain state, but these frequencies were reduced during analgesia induced by periventricular gray area stimulation and this frequency is rather close to that of respiration. The correlation of the pain spindles in the thalamus may be explained by the fact that they represent direct activity in a relay nucleus. In the PAG, they are harder to understand as this is an antinociceptive area but they may be due to the “pain inhibits pain” mechanism of diffuse noxious inhibitory controls on which the PAG has been shown to have an influence.7 Our findings are significant in that not only do they provide a direct neural signature of a subjective conscious state but they may also allow for objective measurements and predictions of the relative level of subjective pain. We intend to investigate these preliminary findings in a more methodical and robust manner.
AUTHOR CONTRIBUTIONS
The statistical analysis was performed by A.L.G.
Shouyan Wang is supported by the Norman Collison Foundation, and Tipu Aziz by the Medical Research Council, Wellcome Trust, and Templeton Foundation.
Disclosure: The authors report no disclosures.
Received July 16, 2008. Accepted in final form September 16, 2008.
Address correspondence and reprint requests to Dr. Alexander L. Green, Department of Neurosurgery, West Wing Level 3, John Radcliffe Hospital, Oxford, OX3 9DU, UK; alex.green@nds.ox.ac.uk
&NA;
- 1.Buzsaki G. Large-scale recording of neuronal ensembles. Nat Neurosci 2004;7:446–451. [DOI] [PubMed] [Google Scholar]
- 2.Pfurtscheller G, Lopes da Silva FH. Event-related EEG/MEG synchronization and desynchronization: basic principles. Clin Neurophysiol 1999;110:1842–1857. [DOI] [PubMed] [Google Scholar]
- 3.Engel AK, Fries P, Singer W. Dynamic predictions: oscillations and synchrony in top-down processing. Nat Rev Neurosci 2001;2:704–716. [DOI] [PubMed] [Google Scholar]
- 4.Peyron R, Laurent B, Garcia-Larrea L. Functional imaging of brain responses to pain. A review and meta-analysis. Neurophysiol Clin 2000;30:263–288. [DOI] [PubMed] [Google Scholar]
- 5.Kringelbach ML, Jenkinson N, Green AL, et al. Deep brain stimulation for chronic pain investigated with magnetoencephalography. Neuroreport 2007;18:223–228. [DOI] [PubMed] [Google Scholar]
- 6.Nandi D, Aziz T, Carter H, et al. Thalamic field potentials in chronic central pain treated by periventricular gray stimulation: a series of eight cases. Pain 2003;101:97–107. [DOI] [PubMed] [Google Scholar]
- 7.Bouhassira D, Villanueva L, Le Bars D. Effects of systemic morphine on diffuse noxious inhibitory controls: role of the periaqueductal grey. Eur J Pharmacol 1992;216:149–156. [DOI] [PubMed] [Google Scholar]