Abstract
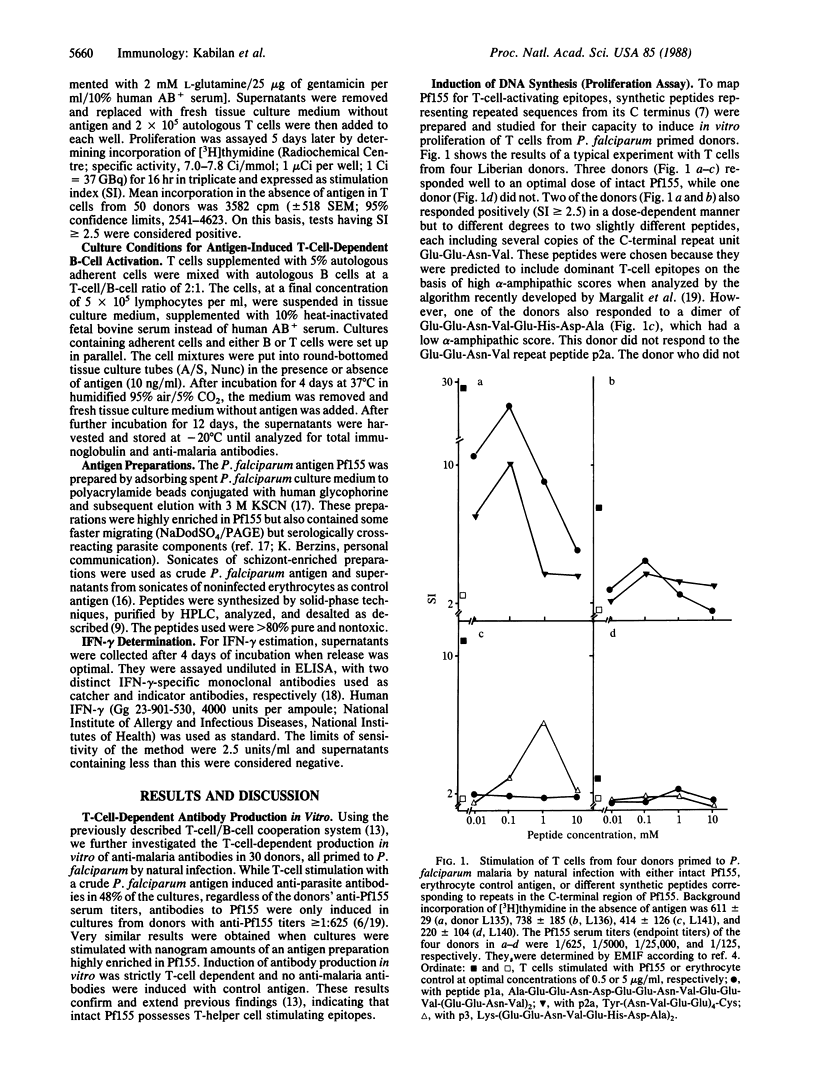
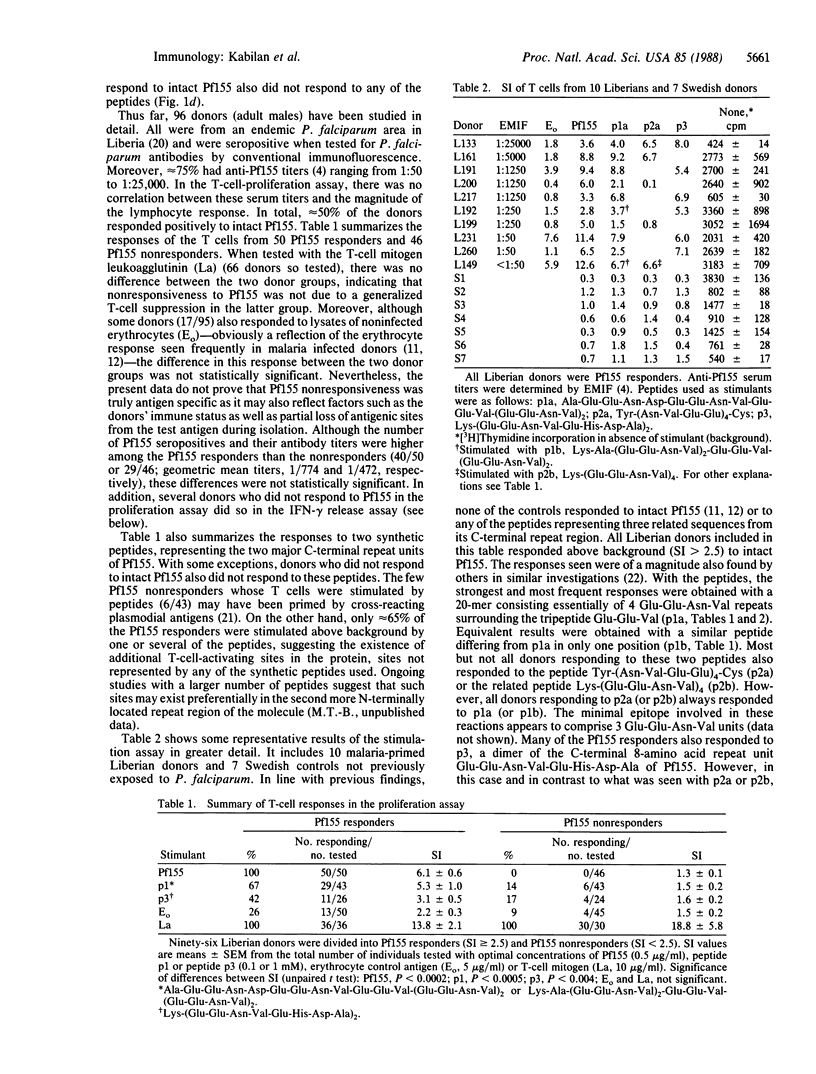
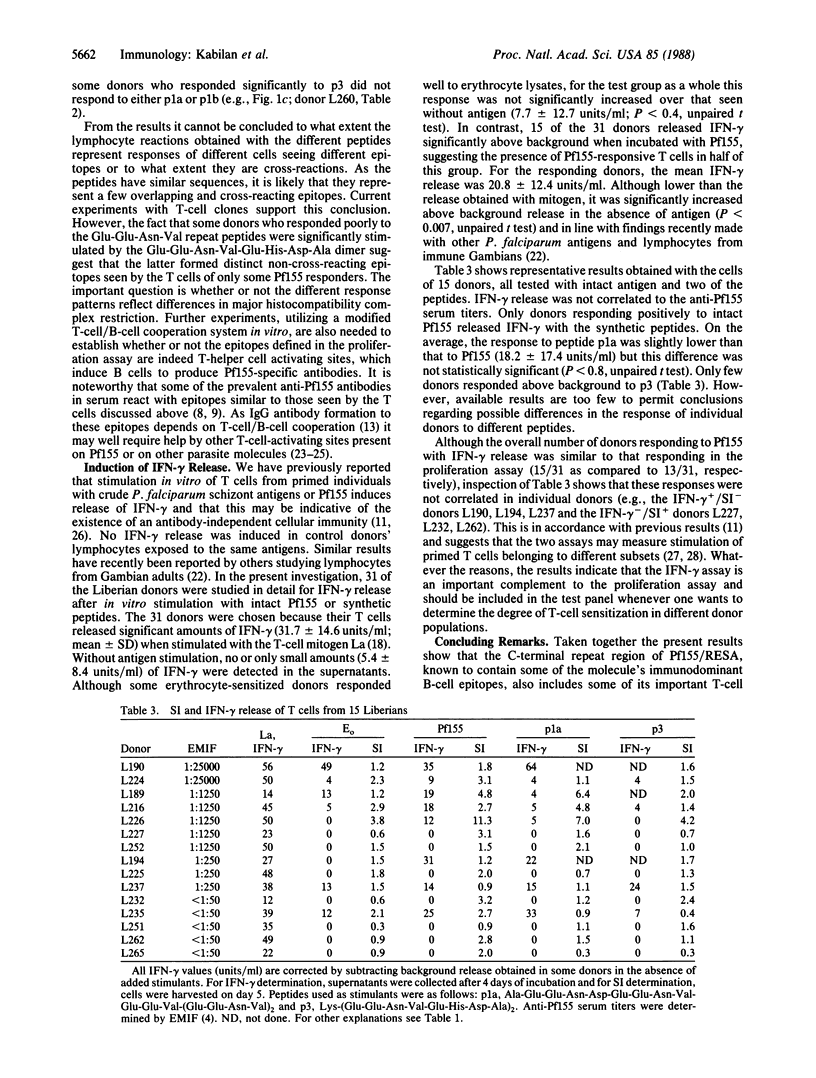
Immunogens included in a subunit vaccine should contain both B- and T-cell-activating sites to ensure anamnestic responses following reinfection after vaccination as well as antibody-independent cellular immunity. The Plasmodium falciparum antigen Pf155/RESA, a major candidate for a vaccine against the asexual blood stages of this malaria parasite, was investigated for T-cell epitopes in its C-terminal amino acid repeat region, a region known to be conserved in different P. falciparum strains. It was found to contain several related sequences that activated T cells from humans primed to P. falciparum by natural exposure, to proliferation, and/or interferon-gamma release in vitro. T cells from approximately half of the donor group investigated responded to the intact protein, and 65% of these responders also responded to short synthetic peptides, probably representing a small number of partly overlapping T-cell epitopes. Thus, sequences from the C terminus of Pf155 may be suitable constituents of a P. falciparum subunit vaccine and also provide a basis for epitope-specific epidemiological studies relating cellular immune responses in vitro to clinical immunity and P. falciparum endemicity.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berzins K., Perlmann H., Wåhlin B., Carlsson J., Wahlgren M., Udomsangpetch R., Björkman A., Patarroyo M. E., Perlmann P. Rabbit and human antibodies to a repeated amino acid sequence of a Plasmodium falciparum antigen, Pf 155, react with the native protein and inhibit merozoite invasion. Proc Natl Acad Sci U S A. 1986 Feb;83(4):1065–1069. doi: 10.1073/pnas.83.4.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björkman A., Hedman P., Brohult J., Willcox M., Diamant I., Pehrsson P. O., Rombo L., Bengtsson E. Different malaria control activities in an area of Liberia--effects on malariometric parameters. Ann Trop Med Parasitol. 1985 Jun;79(3):239–246. doi: 10.1080/00034983.1985.11811914. [DOI] [PubMed] [Google Scholar]
- Collins W. E., Anders R. F., Pappaioanou M., Campbell G. H., Brown G. V., Kemp D. J., Coppel R. L., Skinner J. C., Andrysiak P. M., Favaloro J. M. Immunization of Aotus monkeys with recombinant proteins of an erythrocyte surface antigen of Plasmodium falciparum. Nature. 1986 Sep 18;323(6085):259–262. doi: 10.1038/323259a0. [DOI] [PubMed] [Google Scholar]
- Coppel R. L., Cowman A. F., Anders R. F., Bianco A. E., Saint R. B., Lingelbach K. R., Kemp D. J., Brown G. V. Immune sera recognize on erythrocytes Plasmodium falciparum antigen composed of repeated amino acid sequences. 1984 Aug 30-Sep 5Nature. 310(5980):789–792. doi: 10.1038/310789a0. [DOI] [PubMed] [Google Scholar]
- Favaloro J. M., Coppel R. L., Corcoran L. M., Foote S. J., Brown G. V., Anders R. F., Kemp D. J. Structure of the RESA gene of Plasmodium falciparum. Nucleic Acids Res. 1986 Nov 11;14(21):8265–8277. doi: 10.1093/nar/14.21.8265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good M. F., Pombo D., Quakyi I. A., Riley E. M., Houghten R. A., Menon A., Alling D. W., Berzofsky J. A., Miller L. H. Human T-cell recognition of the circumsporozoite protein of Plasmodium falciparum: immunodominant T-cell domains map to the polymorphic regions of the molecule. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1199–1203. doi: 10.1073/pnas.85.4.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabstein K., Dower S., Gillis S., Urdal D., Larsen A. Expression of interleukin 2, interferon-gamma, and the IL 2 receptor by human peripheral blood lymphocytes. J Immunol. 1986 Jun 15;136(12):4503–4508. [PubMed] [Google Scholar]
- Jónsdóttir I., Dillner-Centerlind M. L., Perlmann H., Perlmann P. Antibody dependent cellular cytotoxicity and mitogen responsiveness of human peripheral blood lymphocytes differing in avidity for sheep erythrocytes. Scand J Immunol. 1979;10(6):525–533. doi: 10.1111/j.1365-3083.1979.tb01386.x. [DOI] [PubMed] [Google Scholar]
- Kabilan L., Troye-Blomberg M., Patarroyo M. E., Björkman A., Perlmann P. Regulation of the immune response in Plasmodium falciparum malaria: IV. T cell dependent production of immunoglobulin and anti-P. falciparum antibodies in vitro. Clin Exp Immunol. 1987 May;68(2):288–297. [PMC free article] [PubMed] [Google Scholar]
- Karttunen R., Andersson G., Ekre H. P., Juutinen K., Surcel H. M., Syrjälä H., Herva E. Interleukin 2 and gamma interferon production, interleukin 2 receptor expression, and DNA synthesis induced by tularemia antigen in vitro after natural infection or vaccination. J Clin Microbiol. 1987 Jun;25(6):1074–1078. doi: 10.1128/jcm.25.6.1074-1078.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp D. J., Coppel R. L., Anders R. F. Repetitive proteins and genes of malaria. Annu Rev Microbiol. 1987;41:181–208. doi: 10.1146/annurev.mi.41.100187.001145. [DOI] [PubMed] [Google Scholar]
- Lamb J. R., Ivanyi J., Rees A. D., Rothbard J. B., Howland K., Young R. A., Young D. B. Mapping of T cell epitopes using recombinant antigens and synthetic peptides. EMBO J. 1987 May;6(5):1245–1249. doi: 10.1002/j.1460-2075.1987.tb02360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margalit H., Spouge J. L., Cornette J. L., Cease K. B., Delisi C., Berzofsky J. A. Prediction of immunodominant helper T cell antigenic sites from the primary sequence. J Immunol. 1987 Apr 1;138(7):2213–2229. [PubMed] [Google Scholar]
- Milich D. R., McLachlan A., Thornton G. B., Hughes J. L. Antibody production to the nucleocapsid and envelope of the hepatitis B virus primed by a single synthetic T cell site. Nature. 1987 Oct 8;329(6139):547–549. doi: 10.1038/329547a0. [DOI] [PubMed] [Google Scholar]
- Miller L. H., Howard R. J., Carter R., Good M. F., Nussenzweig V., Nussenzweig R. S. Research toward malaria vaccines. Science. 1986 Dec 12;234(4782):1349–1356. doi: 10.1126/science.2431481. [DOI] [PubMed] [Google Scholar]
- Ozaki S., Berzofsky J. A. Antibody conjugates mimic specific B cell presentation of antigen: relationship between T and B cell specificity. J Immunol. 1987 Jun 15;138(12):4133–4142. [PubMed] [Google Scholar]
- Perlmann H. K., Berzins K., Wåhlin B., Udomsangpetch R., Ruangjirachuporn W., Wahlgren M., Perlmann P. H. Absence of antigenic diversity in Pf155, a major parasite antigen in membranes of erythrocytes infected with Plasmodium falciparum. J Clin Microbiol. 1987 Dec;25(12):2347–2354. doi: 10.1128/jcm.25.12.2347-2354.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlmann H., Berzins K., Wahlgren M., Carlsson J., Björkman A., Patarroyo M. E., Perlmann P. Antibodies in malarial sera to parasite antigens in the membrane of erythrocytes infected with early asexual stages of Plasmodium falciparum. J Exp Med. 1984 Jun 1;159(6):1686–1704. doi: 10.1084/jem.159.6.1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlmann H., Perlmann P., Pape G. R., Halldén G. Purification, fractionation and assay of antibody-dependent lymphocytic effector cells (K cells) in human blood. Scand J Immunol. 1976 Jun;Suppl 5:57–68. doi: 10.1111/j.1365-3083.1976.tb03856.x. [DOI] [PubMed] [Google Scholar]
- Riley E. M., Jepsen S., Andersson G., Otoo L. N., Greenwood B. M. Cell-mediated immune responses to Plasmodium falciparum antigens in adult Gambians. Clin Exp Immunol. 1988 Mar;71(3):377–382. [PMC free article] [PubMed] [Google Scholar]
- Troye-Blomberg M., Andersson G., Stoczkowska M., Shabo R., Romero P., Patarroyo M. E., Wigzell H., Perlmann P. Production of IL 2 and IFN-gamma by T cells from malaria patients in response to Plasmodium falciparum or erythrocyte antigens in vitro. J Immunol. 1985 Nov;135(5):3498–3504. [PubMed] [Google Scholar]
- Troye-Blomberg M., Perlmann H., Patarroyo M. E., Perlmann P. Regulation of the immune response in Plasmodium falciparum malaria. II. Antigen specific proliferative responses in vitro. Clin Exp Immunol. 1983 Aug;53(2):345–353. [PMC free article] [PubMed] [Google Scholar]
- Troye-Blomberg M., Perlmann P. T cell functions in Plasmodium falciparum and other malarias. Prog Allergy. 1988;41:253–287. doi: 10.1159/000415226. [DOI] [PubMed] [Google Scholar]
- Udomsangpetch R., Lundgren K., Berzins K., Wåhlin B., Perlmann H., Troye-Blomberg M., Carlsson J., Wahlgren M., Perlmann P., Björkman A. Human monoclonal antibodies to Pf 155, a major antigen of malaria parasite Plasmodium falciparum. Science. 1986 Jan 3;231(4733):57–59. doi: 10.1126/science.3510452. [DOI] [PubMed] [Google Scholar]
- Weidanz W. P., Long C. A. The role of T cells in immunity to malaria. Prog Allergy. 1988;41:215–252. [PubMed] [Google Scholar]



