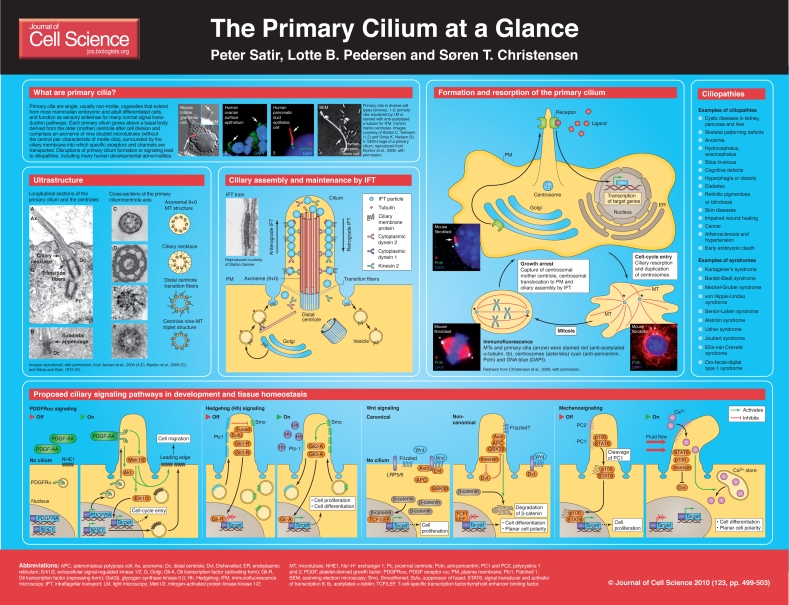
The primary cilium, which was first so named by Sergei Sorokin (Sorokin, 1968), is a solitary organelle that emanates from the cell surface of most mammalian cell types during growth arrest. Increasing evidence suggests that primary cilia are key coordinators of signaling pathways during development and in tissue homeostasis and, when defective, are a major cause of human diseases and developmental disorders (now commonly referred to as ciliopathies).
Primary cilia consist of an axoneme of nine doublet microtubules that extends from a basal body [which is derived from the older (mother) centriole of the centrosome, surrounded by the ciliary membrane (a specialized domain extension of the cell membrane)]. The microtubule pattern of the ciliary axoneme is traditionally abbreviated by referring to the numbers of peripheral doublets and single central microtubules as 9+2, 9+0 etc. In contrast to those of motile 9+2 cilia, axonemes of non-motile primary cilia lack key elements involved in ciliary motility, including the central pair of microtubules and the proteins that surround them, most if not all radial spokes and, importantly, outer and inner dynein arms that power microtubule sliding to produce motility (Satir and Christensen, 2007). Single 9+0 primary cilia are found on a large number of cells in the mammalian body, including stem, epithelial, endothelial, connective-tissue and muscle cells as well as neurons [for a more detailed list, please see Wheatley (Wheatley, 1982) and the website http://www.bowserlab.org/primarycilia/cilia3.htm]. The 9+0 pattern of the primary cilium is often lost towards the cilium tip, where doublet microtubules end or change position.
Until recently, three hypotheses existed regarding the functional significance of primary cilia: first, that the cilium was vestigial; second, that it provided a means of sequestering the centriole, so as to inhibit cell division; and third, that it was a cellular sensory structure. The first
hypothesis has been falsified by experiment. In one sense, the second hypothesis is correct — that is, the majority of cells that have primary cilia are non-cycling differentiated cells or stem cells in G0. The primary cilium is resorbed in cells that re-enter the cell cycle and divide, only to grow again on each daughter cell as the cells once again become quiescent (summarized in the poster). In addition, it is now clear that the third hypothesis is accurate: a major function of primary cilia is in cell signaling, because a variety of receptors, ion channels and transporter proteins, as well as some of their downstream effector molecules, localize to the cilium or basal body. Signaling in the cilium coordinates key processes during development and in tissue homeostasis, including cell migration, differentiation and/or re-entry into the cell cycle, specification of the plane of cell division, and apoptosis. Sensory modalities to which the primary cilium responds include mechanical stimulation (bending of the cilium) and chemosensation (detection of a specific ligand, growth factor, hormone or morphogen). In some specialized cases, primary cilia can also respond to light (Insinna and Besharse, 2008), temperature (Kuhara et al., 2008), osmolality (Christensen et al., 2005) or gravity (Moorman and Schorr, 2008) (note, however, that the ‘stereocilia’ of hair cells of the ear, which respond to mechanical displacement, are microvilli, not primary cilia). In invertebrates, including C. elegans and Drosophila, primary cilia form the basis of several types of sense organs or sensilla and are effectively dendritic extensions of specific neurons; in vertebrates, the outer segments of photoreceptors are modified primary cilia.
This poster article presents an outline of the mechanisms involved in the assembly and function of mammalian primary cilia, with a focus on some of the main signaling pathways that are associated with the cilium. In virtually every tissue, a set of specific receptors becomes localized to the ciliary membrane of the primary cilium to detect particular environmental signals. As indicated in the poster, the subsequent cascade within the cell influences the pattern of gene expression and potential physiological responses.
IFT builds primary cilia
To build a primary cilium, the centrosome migrates towards the cell surface as a cell enters G0. The mother centriole attaches to a Golgi-derived vesicle that then expands, with the axoneme of the primary cilium growing above the centriole within the ciliary membrane, which projects into the vesicle lumen (Sorokin, 1962). The axonemal microtubules polymerize at the growing tip of the projection, to which cargo is delivered by intraflagellar transport (IFT; see below). The vesicle is eventually exocytosed to expose the primary cilium at the cell surface, where ciliary growth continues for up to several micrometers to form the mature cilium. The docking of the mother centriole to the Golgi-derived membrane is thought to be mediated in part by the distal appendages of the centriole, also known as transition fibers, and the original attachment point to the membrane probably corresponds to the region known as the ciliary necklace (Gilula and Satir, 1972), the proposed barrier between the ciliary membrane and the general cell membrane. The transition fibers and necklace are thought to be part of a ‘ciliary pore complex’ through which only selected proteins are allowed passage into the ciliary compartment (Rosenbaum and Witman, 2002).
IFT is an evolutionarily conserved motility process that is required for growth and maintenance of both motile and primary cilia (Rosenbaum and Witman, 2002). IFT relies on the association of ciliary building blocks (e.g. tubulin, radial-spoke proteins and peripheral membrane proteins such as guanine-nucleotide exchange factors) with a scaffold of IFT-particle protein complexes, the components of which are orthologous from Chlamydomonas reinhardtii to humans. The IFT particles and their associated cargo proteins are transported along axonemal microtubules by kinesin 2 motor proteins in the anterograde (base-to-tip) direction, after which cargo is delivered to the growing tip, and by cytoplasmic dynein 2 in the retrograde (tip-to-base) direction. The process is schematized in the accompanying poster. Other reviews on IFT have been published recently (Cole and Snell, 2009; Pedersen and Rosenbaum, 2008).
Primary-cilium defects lead to kidney disease
The significance of primary cilia in signaling became clear on examination of a hypomorphic mouse mutant (Tg737orpk RpW) (Lehman et al., 2008) that was deficient in the homolog of IFT88, a Chlamydomonas IFT protein (Pazour et al., 2000). The mouse mutant had been developed as a model for human autosomal recessive polycystic kidney disease (ARPKD), because the mice developed polycystic kidneys and many other pleiomorphic phenotypes before dying a few days after birth. Just as in a Chlamydomonas Ift88 mutant, primary cilia did not grow normally in kidney cells (and elsewhere) in the mutant mouse. It was proposed that polycystic kidneys developed because primary-cilium signaling was defective. This was confirmed when the polycystins 1 and 2 (PC1 and PC2, respectively) — which form a TRP Ca2+ channel protein complex and are defective in models for autosomal dominant polycystic kidney disease (ADPKD) — were found to be normally localized to the primary cilium (Pazour et al., 2002; Yoder et al., 2002), but were mislocalized or absent when ADPKD developed. Meanwhile, Praetorius and Spring demonstrated that the kidney primary cilium acts as a flow sensor; when the cilium is bent, Ca2+ enters the cell as a messenger molecule (Praetorius and Spring, 2001). This mechanoregulation of intracellular Ca2+ is impaired in the mutant mouse kidney (Liu et al., 2005). Ciliary mechanoregulation appears not to be limited to kidney epithelial cells, but might operate in a variety of cell types and tissues, either in response to fluid flow, such as on the embryonic node (McGrath et al., 2003), endothelial cells (Iomini et al., 2004; Nauli et al., 2008; AbouAlaiwi et al., 2009; Hierck et al., 2008) and cholangiocytes (Masyuk et al., 2006), or through direct interaction between the primary cilium and the extracellular matrix (ECM), such as in chondrocytes (McGlashan et al., 2006) and smooth muscle cells (Lu et al., 2008).
Analysis of mutants such as the Tg737orpk RpW mouse has indicated that signaling through the primary cilium is essential for normal development and function, not only of the kidney, but also of many other tissues and organs. Consequently, ciliary dysfunction might lead to an array of developmental abnormalities and diseases (ciliopathies), including randomization of the left-right body axis, abnormalities in neural-tube closure and patterning, skeletal defects, cystic diseases, blindness, behavioral and cognitive defects, and obesity (Lehman et al., 2008; Quinlan et al., 2008; Veland et al., 2009). An overview of ciliopathies and syndromes caused by defects in assembly or function of primary cilia is presented in the poster.
Orientation of primary cilia
At first glance, the primary cilium appears to be radially symmetrical, with each microtubule doublet equivalent to every other doublet; however, this is probably not the case because, in motile cilia, every doublet can be specifically numbered with respect to the effective stroke, whose direction (arbitrarily defined as cellular left) is identified by a basal foot. Similarly to motile cilia, primary cilia often have a basal foot (Wheatley, 1982) but, with or without this structure, basal bodies and primary cilia seemingly ‘know’ left from right (Bell et al., 2008). In conjunction with other factors, orientation of the basal body, and therefore orientation of cilia, translates into left-right cell orientation and nodal flow and then to body-axis determination at the embryonic node (Hirokawa et al., 2006). In a flow gradient (as in the kidney tubule) or in a chemotactic gradient (as in fibroblast migration during wound healing) (Christensen et al., 2008), all primary cilia align in a single direction, and cell orientation seems to be determined by this. In the kidney, the direction in which the cilia bend with flow depends on their orientation. The flow, and therefore the bend, is always in the anterior-posterior direction so the gradient of Ca2+ concentration or other signaling molecules along the cellular axes should be identically displayed in the anterior-posterior direction in every cell. Because the gradient is uniform from cell to cell, we can probably infer that this determines a consistent anterior-posterior orientation of the mitotic spindle for cell division. In the migrating fibroblasts at a wound edge, orientation of primary cilia ensures that the direction of cell migration is uniform. However, when the primary cilium is missing or defective, orientation is random, which translates into randomization of left-right asymmetry in body form, randomization of migration direction (Schneider et al., 2010) or randomization of mitotic-spindle orientation (Fischer et al., 2006).
Diversity and dynamics of signaling pathways in primary cilia
The signaling pathways coordinated by primary cilia are quite diverse, and depend on the cell type. As indicated in the accompanying poster, the pathways include signaling through Ca2+, receptor tyrosine kinases (RTKs), Hedgehog (Hh), Wingless (Wnt), neuronal and purinergic receptors, as well as through communication with the ECM (Christensen et al., 2007; Christensen et al., 2008; Eggenschwiler and Anderson, 2007; Kiprilov et al., 2008; Gerdes et al., 2009; Knight et al., 2009; Masyuk et al., 2008; Praetorius and Leipziger, 2009; Wong and Reiter, 2008; Jensen et al., 2004). A single primary cilium can be set up for several different kinds of signaling and can respond, for example, to mechanical strain as well as to several morphogens, hormones or growth factors. Different receptors or channels can be present in the same cilium at the same time or at different times. The diversity and dynamics of important ciliary membrane proteins and their effector molecules in the cilium-centrosome axis has only begun to be catalogued, and the list of which pathways are important for specific tissues, especially during development, is likely to be expanded in the future.
Primary cilia in adult tissues
Signaling via the primary cilium is of paramount importance during development, and probably remains so in stem-cell populations in various tissues. In the adult, primary cilia might still function in fibroblast cell-cycle control and/or cell migration during tissue regeneration and wound healing (Schneider et al., 2005; Schneider et al., 2009; Schneider et al., 2010). Most other differentiated, non-dividing cells of the adult body, including neurons and kidney cells, possess primary cilia. The primary cilia in these tissues might be necessary for maintenance of the differentiated state and suppression of cyst formation or oncogenesis. Davenport and colleagues used an inducible system to disrupt IFT and remove primary cilia from all adult mouse tissues or from specific tissues (Davenport et al., 2007). Even when all tissues were affected, the devastating abnormalities and lethality seen after embryonic loss of IFT did not occur, although PKD eventually developed after about a year, presumably because cell division was greatly reduced and cystogenesis in the adult is slow.
In many tissues, aberrant activation or absence of ciliary signaling is correlated with uncontrolled cell division and cancer (Christensen et al., 2008; Kuehn et al., 2007; Mans et al., 2008; Michaud and Yoder, 2006; Nielsen et al., 2008; Plotnikova et al., 2008; Wong et al., 2009; Han et al., 2009). However, an immediate effect of ciliary removal, either of all adult primary cilia or specifically of cilia on neurons of the hypothalamus in adult mice, is hyperphagia (compulsive eating), which leads to obesity. Secondarily, obesity leads to defects that resemble type II diabetes. These effects do not occur if the feeding of the knockout mice is restricted (Davenport et al., 2007). Specific hormone receptors associated with feeding behavior localize to cilia of the hypothalamus, including somatostatin sst 3 receptor (Sst3R) (Handel et al., 1999) and melanin-concentrating hormone receptor 1 (Mchr1) (Berbari et al., 2008) in neuronal primary cilia, as well as leptin receptor (LepR) in olfactory cilia (Baly et al., 2007). The Sst3R and Mchr1 receptors are mislocalized in neurons of mice that have mutations in proteins that correspond to those observed in individuals with the syndromic obesity condition Bardet-Biedl syndrome (Berbari et al., 2008; Seo et al., 2009). Furthermore, type 3 adenylyl cyclase, which is also associated with obesity in mice and humans, is also specifically localized to hypothalamic neurons (Wang et al., 2009). A ‘yin-yang’ relationship, in which activation between the ciliary receptors in hypothalamic neurons alternates to stop and start feeding behavior, might be involved in the satiety response (Satir, 2007).
Obesity might also be linked to primary cilia in adipose tissue. Adipogenic differentiation and fat accumulation is associated with transient formation of the primary cilium, containing Wnt and Hh signaling components, such that adipocytes in culture derived from dermal fibroblasts of individuals with Bardet-Biedl syndrome exhibit a higher predisposition for fat accumulation and a higher secreted leptin level than control cells (Marion et al., 2009). Zhu and colleagues demonstrated that the primary cilium and its basal body form an organized signaling pathway for the IGF-1 receptor to induce adipocyte differentiation in confluent 3T3-L1 preadipocytes (Zhu et al., 2009). In addition, childhood obesity and type II diabetes in Alström syndrome patients is caused by mutations in Alström syndrome 1 protein (ALMS1), which localizes to the base of primary cilia (Hearn et al., 2005) and is regulated during adipogenesis (Romano et al., 2008). Evidently, multiple ciliary signaling pathways, involving Wnt, Hh and RTK signaling take part in the regulation of adipogenic differentiation.
Perspective
Because of its near-ubiquity on cells of the human body, and because of the multiple signaling pathways that require it both during development and in the adult, the primary cilium has moved from being nearly forgotten to a position of considerable importance in biomedicine. This is amply demonstrated for primary cilia in the kidney and there are important data that indicate how signaling pathways involving the cilium might affect other tissues.
One persistent unanswered question about ciliary function is why certain receptors and channels are concentrated more or less exclusively in the membrane of the primary cilium. Clearly, signaling molecules or second messengers that leave the cilium are initially spatially localized at the basal body or centrosome, which would not be true of signals arising from receptors or channels at the leading edge of the cell or dispersed in the cell membrane. Signals from the cilium might therefore interact with, activate or inactivate specific centrosomal proteins to control trafficking to the Golgi, to the leading edge of a migrating cell, to cell junctions or, in the case of transcription factors, to the nucleus. Ciliary orientation might impose a gradient of second messengers or effector molecules within the cytoplasm to help determine positioning of organelles and the mitotic spindle.
In certain cases, the amplitude of the signal or the concentration of signaling molecules arising from the cilium might be compared at the centrosome to signals arising from elsewhere in the cell to determine a specific physiological outcome, such as entry into the cell cycle and resorption of the cilium. At present, there are only hints of how this computation might be performed. As we learn more about IFT-complex assembly and IFT cargo, the role of activation of vesicular trafficking and exocytosis in building the cilium, and targeting processes in the cell in general, we might come to understand reasons for sequestration within the primary cilium more completely. In turn, we might be able to understand why different receptors are sequestered in different cilia, why there are ‘yin-yang’ pairs of ciliary receptors and why sequestration of ciliary receptors and effectors is so dynamic.
Supplementary Material
Acknowledgments
We apologize to those authors whose work has not been cited because of space limitations. P.S. is partially funded by the NIH (NIDDK). S.T.C. and L.B.P. are partially funded by grants from the Lundbeck Foundation (R9-A969), the Novo Nordisk Foundation, and the Danish Natural Science Research Council (272-070530). We thank Bradley K. Yoder for valuable comments on the manuscript and the poster. Deposited in PMC for release after 12 months.
Footnotes
This article is part of a Minifocus on cilia and flagella. For further reading, please see related articles: ‘Sensory reception is an attribute of both primary cilia and motile cilia’ by Robert A. Bloodgood (J. Cell Sci. 123, 505-509), ‘The perennial organelle: assembly and disassembly of the primary cilium’ by E. Scott Seeley and Maxence V. Nachury (J. Cell Sci. 123, 511-518), ‘Flagellar and ciliary beating: the proven and the possible’ by Charles B. Lindemann and Kathleen A. Lesich (J. Cell Sci. 123, 519-528) and ‘Molecular mechanisms of protein and lipid targeting to ciliary membranes’ by Brian T. Emmer et al. (J. Cell Sci. 123, 529-536).
References
- AbouAlaiwi W. A., Takahashi M., Mell B. R., Jones T. J., Ratnam S., Kolb R. J., Nauli S. M. (2009). Ciliary polycystin-2 is a mechanosensitive calcium channel involved in nitric oxide signaling cascades. Circ. Res. 104, 860-869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baly C., Aioun J., Badonnel K., Lacroix M. C., Durieux D., Schlegel C., Salesse R., Caillol M. (2007). Leptin and its receptors are present in the rat olfactory mucosa and modulated by the nutritional status. Brain Res. 1129, 130-141 [DOI] [PubMed] [Google Scholar]
- Bell A. J., Satir P., Grimes G. W. (2008). Mirror-imaged doublets of Tetmemena pustulata: implications for the development of left-right asymmetry. Dev. Biol. 314, 150-160 [DOI] [PubMed] [Google Scholar]
- Berbari N. F., Lewis J. S., Bishop G. A., Askwith C. C., Mykytyn K. (2008). Bardet-Biedl syndrome proteins are required for the localization of G protein-coupled receptors to primary cilia. Proc. Natl. Acad. Sci. USA 105, 4242-4246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen S. T., Voss J. W., Teilman S. C., Lambert I. H. (2005). High expression of the taurine receptor TauT in primary cilia of NIH3T3 fibroblasts. Cell Biol. Int. 29, 347-351 [DOI] [PubMed] [Google Scholar]
- Christensen S. T., Pedersen L. B., Schneider L., Satir P. (2007). Sensory cilia and integration of signal transduction in human health and disease. Traffic 8, 97-109 [DOI] [PubMed] [Google Scholar]
- Christensen S. T., Pedersen S. F., Satir P., Veland I. R., Schneider L. (2008). The primary cilium coordinates signaling pathways in cell cycle control and migration during development and tissue repair. Curr. Top. Dev. Biol. 85, 261-301 [DOI] [PubMed] [Google Scholar]
- Cole D. G., Snell W. J. (2009). SnapShot: Intraflagellar transport. Cell 137, 784 [DOI] [PubMed] [Google Scholar]
- Davenport J. R., Watts A. J., Roper V. C., Croyle M. J., van Groen T., Wyss J. M., Nagy T. R., Kesterson R. A., Yoder B. K. (2007). Disruption of intraflagellar transport in adult mice leads to obesity and slow-onset cystic kidney disease. Curr. Biol. 17, 1586-1594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggenschwiler J. T., Anderson K. V. (2007). Cilia and developmental signaling. Annu. Rev. Cell Dev. Biol. 23, 345-373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer E., Legue E., Doyen A., Nato F., Nicolas J. F., Torres V., Yaniv M., Pontoglio M. (2006). Defective planar cell polarity in polycystic kidney disease. Nat. Genet. 38, 21-23 [DOI] [PubMed] [Google Scholar]
- Gerdes J. M., Davis E. E., Katsanis N. (2009). The vertebrate primary cilium in development, homeostasis, and disease. Cell 137, 32-45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilula N. B., Satir P. (1972). The ciliary necklace. A ciliary membrane specialization. J. Cell Biol. 53, 494-509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y.-G., Kim H. J., Dlugosz A. A., Ellison D. W., Gilbertson R. J., Alvarez-Buylla A. (2009). Dual and opposing roles of primary cilia in medulloblastoma development. Nat. Med. 15, 1062-1065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handel M., Schulz S., Stanarius A., Schreff M., Erdtmann-Vourliotis M., Schmidt H., Wolf G., Hollt V. (1999). Selective targeting of somatostatin receptor 3 to neuronal cilia. Neuroscience 89, 909-926 [DOI] [PubMed] [Google Scholar]
- Hearn T., Spalluto C., Phillips V. J., Renforth G. L., Copin N., Hanley N. A., Wilson D. I. (2005). Subcellular localization of ALMS1 supports involvement of centrosome and basal body dysfunction in the pathogenesis of obesity, insulin resistance, and type 2 diabetes. Diabetes 54, 1581-1587 [DOI] [PubMed] [Google Scholar]
- Hierck B. P., Van der Heiden K., Alkemade F. E., Van de Pas S., Van Thienen J. V., Groenendijk B. C., Bax W. H., Van der Laarse A., Deruiter M. C., Horrevoets A. J., Poelmann R. E. (2008). Primary cilia sensitize endothelial cells for fluid shear stress. Dev. Dyn. 237, 725-735 [DOI] [PubMed] [Google Scholar]
- Hirokawa N., Tanaka Y., Okada Y., Takeda S. (2006). Nodal flow and the generation of left-right asymmetry. Cell 125, 33-45 [DOI] [PubMed] [Google Scholar]
- Insinna C., Besharse J. C. (2008). Intraflagellar transport and the sensory outer segment of vertebrate photoreceptors Dev. Dyn. 237, 1982-1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iomini C., Tejada K., Mo W., Vaananen H., Piperno G. (2004). Primary cilia of human endothelial cells disassemble under laminar shear stress. J. Cell Biol. 164, 811-817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen C. G., Poole C. A., McGlashan S. R., Marko M., Issa Z. I., Vujcich K. V., Bowser S. S. (2004). Ultrastructural, tomographic and confocal imaging of the chondrocyte primary cilium in situ. Cell Biol. Int. 28, 101-110 [DOI] [PubMed] [Google Scholar]
- Kiprilov E. N., Awan A., Desprat R., Velho M., Clement C. A., Byskov A. G., Andersen C. Y., Satir P., Bouhassira E. E., Christensen S. T., Hirsch R. E. (2008). Human embryonic stem cells in culture possess primary cilia with hedgehog signaling machinery. J. Cell Biol. 180, 897-904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight M. M., McGlashan S. R., Garcia M., Jensen C. G., Poole C. A. (2009). Articular chondrocytes express connexin 43 hemichannels and P2 receptors-a putative mechanoreceptor complex involving the primary cilium? J. Anat. 214, 275-283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuehn E. W., Walz G., Benzing T. (2007). Von Hippel-Lindau: a tumor suppressor links microtubules to ciliogenesis and cancer development. Cancer Res. 67, 4537-4540 [DOI] [PubMed] [Google Scholar]
- Kuhara A., Okumura M., Kimata T., Tanzizawa Y., Takano R., et al. (2008). Temperature sensing by a olfactory neuron in a circuit contolling behavior of C. elegans. Science 320, 803-807 [DOI] [PubMed] [Google Scholar]
- Lehman J. M., Michaud E. J., Schoeb T. R., Aydin-Son Y., Miller M., Yoder B. K. (2008). The Oak Ridge Polycystic Kidney mouse: Modeling ciliopathies of mice and men. Dev. Dyn. 237, 1960-1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W., Murcia N. S., Duan Y., Weinbaum S., Yoder B. K., Schwiebert E., Satlin L. M. (2005). Mechanoregulation of intracellular Ca2+ concentration is attenuated in collecting duct of monocilium-impaired orpk mice. Am. J. Physiol. Renal Physiol. 289, F978-F988 [DOI] [PubMed] [Google Scholar]
- Lu C. J., Du H., Wu J., Jansen D. A., Jordan K. L., Xu N., Sieck G. C., Qian Q. (2008). Non-random distribution and sensory functions of primary cilia in vascular smooth muscle cells. Kidney Blood Press. Res. 31, 171-184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mans D. A., Voest E. E., Giles R. H. (2008). All along the watchtower: Is the cilium a tumor suppressor organelle? Biochim. Biophys. Acta 1786, 114-125 [DOI] [PubMed] [Google Scholar]
- Marion V., Stoetzel C., Schlicht D., Messaddeq N., Koch M., Flori E., Danse J. M., Mandel J. L., Dollfus H. (2009). Transient ciliogenesis involving Bardet-Biedl syndrome proteins is a fundamental characteristic of adipogenic differentiation. Proc. Natl. Acad. Sci. USA 106, 1820-1825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masyuk A. I., Masyuk T. V., Splinter P. L., Huang B. Q., Stroope A. J., LaRusso N. F. (2006). Cholangiocyte cilia detect changes in luminal fluid flow and transmit them into intracellular Ca2+ and cAMP signaling. Gastroenterology 131, 911-920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masyuk A. I., Masyuk T. V., LaRusso N. F. (2008). Cholangiocyte primary cilia in liver health and disease. Dev. Dyn. 237, 2007-2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGlashan S. R., Jensen C. G., Poole C. A. (2006). Localization of extracellular matrix receptors on the chondrocyte primary cilium. J. Histochem. Cytochem. 54, 1005-1014 [DOI] [PubMed] [Google Scholar]
- McGrath J., Somlo S., Makova S., Tian X., Brueckner M. (2003). Two populations of node monocilia initiate left-right asymmetry in the mouse. Cell 114, 61-73 [DOI] [PubMed] [Google Scholar]
- Michaud E. J., Yoder B. K. (2006). The primary cilium in cell signaling and cancer. Cancer Res. 66, 6463-6467 [DOI] [PubMed] [Google Scholar]
- Moorman S. J., Schorr A. Z. (2008). The primary cilium as a gravitational force transducer and a regulator of transcriptional noise. Dev. Dyn. 237, 1955-1959 [DOI] [PubMed] [Google Scholar]
- Nauli S. M., Kawanabe Y., Kaminski J. J., Pearce W. J., Ingber D. E., Zhou J. (2008). Endothelial cilia are fluid shear sensors that regulate calcium signaling and nitric oxide production through polycystin-1. Circulation 117, 1161-1171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen S. K., Mollgard K., Clement C. A., Veland I. R., Awan A., Yoder B. K., Novak I., Christensen S. T. (2008). Characterization of primary cilia and Hedgehog signaling during development of the human pancreas and in human pancreatic duct cancer cell lines. Dev. Dyn. 237, 2039-2052 [DOI] [PubMed] [Google Scholar]
- Pazour G. J., Dickert B. L., Vucica Y., Seeley E. S., Rosenbaum J. L., Witman G. B., Cole D. G. (2000). Chlamydomonas IFT88 and its mouse homologue, polycystic kidney disease gene tg737, are required for assembly of cilia and flagella. J. Cell Biol. 151, 709-718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazour G. J., San Agustin J. T., Follit J. A., Rosenbaum J. L., Witman G. B. (2002). Polycystin-2 localizes to kidney cilia and the ciliary level is elevated in orpk mice with polycystic kidney disease. Curr. Biol. 12, R378-R380 [DOI] [PubMed] [Google Scholar]
- Pedersen L. B., Rosenbaum J. L. (2008). Intraflagellar transport (IFT): role in ciliary assembly, resorption and signalling. Curr. Top. Dev. Biol. 85, 23-61 [DOI] [PubMed] [Google Scholar]
- Plotnikova O. V., Golemis E. A., Pugacheva E. N. (2008). Cell cycle-dependent ciliogenesis and cancer. Cancer Res. 68, 2058-2061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praetorius H. A., Spring K. R. (2001). Bending the MDCK cell primary cilium increases intracellular calcium. J. Membr. Biol. 184, 71-79 [DOI] [PubMed] [Google Scholar]
- Praetorius H., Leipziger J. (2009). Released nucleotides amplify the cilium-dependent, flow-induced [Ca2+]i response in MDCK cells. Acta Physiol. 197, 241-251 [DOI] [PubMed] [Google Scholar]
- Quinlan R. J., Tobin J. L., Beales P. L. (2008). Modeling ciliopathies: primary cilia in development and disease. Curr. Top. Dev. Biol. 84, 249-310 [DOI] [PubMed] [Google Scholar]
- Romano S., Milan G., Veronese C., Collin G. B., Marshall J. D., Centobene C., Favaretto F., Dal Pra C., Scarda A., Leandri S., Naggert J. K., Maffei P., Vettor R. (2008). Regulation of Alstrom syndrome gene expression during adipogenesis and its relationship with fat cell insulin sensitivity. Int. J. Mol. Med. 21, 731-736 [PubMed] [Google Scholar]
- Rosenbaum J. L., Witman G. B. (2002). Intraflagellar transport. Nat. Rev. Mol. Cell. Biol. 3, 813-825 [DOI] [PubMed] [Google Scholar]
- Satir P. (2007). Cilia biology: stop overeating now! Curr. Biol. 17, R963-R965 [DOI] [PubMed] [Google Scholar]
- Satir P., Christensen S. T. (2007). Overview of structure and function of mammalian cilia. Annu. Rev. Physiol. 69, 377-400 [DOI] [PubMed] [Google Scholar]
- Schneider L., Clement C. A., Teilmann S. C., Pazour G. P., Hoffmann E. K., Satir P., Christensen S. T. (2005). PDGFRαα signaling is regulated through the primary cilium in fibroblasts. Curr. Biol. 15, 1861-1866 [DOI] [PubMed] [Google Scholar]
- Schneider L., Stock C., Dieterich P., Satir P., Schwab A., Christensen S. T., Pedersen S. F. (2009). The Na+/H+ exchanger NHE1 plays a central role in directional migration stimulated via PDGFRα in the primary cilium. J. Cell Biol. 185, 163-176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider L., Cammer M., Lehman J., Nielsen S. K., Guerra C. F., Veland I. R., Stock C., Hoffmann E. K., Yoder B. K., Schwab A., Satir P., Christensen S. T. (2010). Directional cell migration and chemotaxis in wound healing response to PDGF-AA are coordinated by the primary cilium in fibroblasts. Cell. Physiol. Biochem. 25, 279-292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo S., Guo D. F., Bugge K., Morgan D. A., Rahmouni K., Sheffield V. C. (2009). Requirement of Bardet-Biedl syndrome proteins for leptin receptor signaling. Hum. Mol. Genet. 18, 1323-1331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorokin S. (1962). Centrioles and the formation of rudimentary cilia by fibroblasts and smooth muscle cells. J. Cell Biol. 15, 363-377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorokin S. P. (1968). Reconstructions of centriole formation and ciliogenesis in mammalian lungs. J. Cell Sci. 3, 207-230 [DOI] [PubMed] [Google Scholar]
- Veland I. R., Awan A., Pedersen L. B., Yoder B. K., Christensen S. T. (2009). Primary cilia and signaling pathways in mammalian development, health and disease. Nephron. Physiol. 111, 39-53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Li V., Chan G. C. K., Phan T., Nudelman A., SW., et al. (2009). Adult type 3 adenylyl cyclase-deficient mice are obese. PLoS One 4, e6979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheatley D. N. (1982). The Centriole: A Central Enigma of Cell Biology New York: Elsevier Science; [Google Scholar]
- Wong S. Y., Reiter J. F. (2008). The primary cilium at the crossroads of mammalian hedgehog signaling. Curr. Top. Dev. Biol. 85, 225-260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong S. Y., Seol A. D., So P.-L., Ermilov A. N., Bichakjian C. K., Epstein E. H., Dlugosz A. A., Reiter J. F. (2009). Primary cilia can both mediate and suppress Hedgehog pathway-dependent tumorigenesis. Nat. Med. 15, 1055-1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder B. K., Hou X., Guay-Woodford L. M. (2002). The polycystic kidney disease proteins, polycystin-1, polycystin-2, polaris, and cystin, are co-localized in renal cilia. J. Am. Soc. Nephrol. 13, 2508-2516 [DOI] [PubMed] [Google Scholar]
- Zhu D., Shi S., Wang H., Liao K. (2009). Growth arrest induces primary cilium formation and sensitizes IGF-1-receptor signaling during differentiation induction of 3T3-L1 preadipocytes. J. Cell Sci. 122, 2760-2768 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.