Abstract
Survival of all animals depends on effective protection against infection. In Drosophila, opportunistic infection kills larvae if they lack the Rel/NF-κB proteins Dorsal and Dif. We have used tissue-specific expression of Dif and Dorsal to reveal that these Rel proteins act in three different tissues to defend larvae from infection. Dif and Dorsal act in circulating blood cells, where they are required autonomously to promote blood-cell survival and phagocytosis of microorganisms. We show that a major transcriptional target of Dorsal and Dif in blood cells is Drosophila IAP1, a gene protecting these cells from death. We find that in addition to their autonomous role in blood-cell survival, Dif and Dorsal also act in the fat body to produce factors that promote blood-cell viability. These Rel proteins act in the epidermis to prevent infection by maintaining a barrier to microbial entry. Dorsal or Dif in any one of the three tissues is sufficient to defend the animal from opportunistic infection. Thus Drosophila has a multi-pronged system of defense and each branch of this network requires Rel proteins. Based on similarities between Drosophila and mammals, we propose that a Rel-dependent network is an ancient and robust framework of animal immune systems.
Keywords: Drosophila larva, Rel protein, Hemocyte, Innate immunity
Introduction
Mammals, insects and plants all use multiple, parallel strategies to avoid infection by the thousands of potential pathogens they encounter daily. In mammals, the mechanisms that defend against infection include anatomical and physiological barriers, as well as specialized immune tissues and immune cells. This complex group of organs and cells constitutes the body's natural defense and it is termed the immune system. Within the immune system, specialized immune cells such as circulating hematopoietic cells recognize and kill pathogens. Hematopoietic, epithelial and hepatic cells secrete humoral components that inhibit microbial growth and attract immune effector cells to the sites of infection. The components of the mammalian immune system define an interactive, mutually supportive and tightly regulated network that provides robust host defense. Although multiple tissues participate in the execution of the immune response, they all depend on signaling through the Rel/NF-κB family of transcription factors (Pasparakis et al., 2006; Schmidt-Ullrich et al., 2001). Thus, Rel proteins represent the molecular lynchpin in the mammalian host defense (Caamano and Hunter, 2002; Hayden et al., 2006).
In Drosophila, as in mammals, several tissues protect the animal from infection. In the larva, hemocytes (also called blood cells) have a central role in the defense against microbial infection (Braun et al., 1998; Defaye et al., 2009; Matova and Anderson, 2006). The vast majority of the hemocytes are phagocytic cells that rapidly and effectively remove microbes from circulation. The systemic humoral response in Drosophila is mediated primarily by the fat body (the liver analogue), which secretes antimicrobial peptides into the hemolymph in response to infection in both the larva and the adult (Lemaitre and Hoffmann, 2007). In addition, epithelial cells can express antimicrobial peptide genes as a local response to infection (Ferrandon et al., 1998; Onfelt Tingvall et al., 2001; Tzou et al., 2000).
Three Rel proteins, Dorsal-related immunity factor (Dif), Dorsal (Dl) and Relish, play central roles in the regulation of the Drosophila immune response. Relish is required for the production of most of the antimicrobial peptides in the larvae, but the cellular immune response appears to be normal in Relish mutants (Choe, 2002; Hedengren et al., 1999; Lu et al., 2001). Dif and dorsal (dl) single mutant larvae do not show defects in either the humoral or the cellular immune response. However, Dif dl double mutant hemocytes have a high rate of cell death and are unable to clear ingested microbes (Matova and Anderson, 2006). As a consequence of hemocyte death and defective cellular immune response, Dif dl double mutant animals die as larvae as a result of opportunistic infection.
Although multiple tissues in Drosophila participate in the defense against pathogens, it is not clear whether immune responses are coordinated among different tissues and whether these tissues form an interdependent network that could be called an immune system. Research in this area has been focused primarily on the possible instructive role of the blood cells in regard to the immune responses in the fat body. Blood cells appear to perform this role by producing cytokine-like molecules that in turn promote an immune response in the fat body. For example, blood cells secrete a JAK/STAT pathway ligand (Unpaired3) after septic injury, and this cytokine induces the production of stress proteins in the fat body (Agaisse et al., 2003). Analysis of mutants has shown that blood cells are required for the normal induction of the antimicrobial peptide gene Defensin in the fat body after septic injury (Brennan et al., 2007) and for the induction of several antimicrobial-peptide genes after natural infection (Basset et al., 2000; Dijkers and O'Farrell, 2007). However, other possible interactions between immune-responsive tissues have not been investigated.
In this study, we used tissue-specific expression to test whether there are coordinated immune interactions among three different immune tissues in the Drosophila larva. Previously we showed Dif and Dl act in circulating blood cells to promote their survival and to prevent opportunistic infection (Matova and Anderson, 2006). Here, we establish that a key target of these Rel proteins in hemocytes is the Drosophila IAP1 (diap1; also known as thread, th), a gene with a crucial role for cell survival in Drosophila (reviewed by Steller, 2008). We also demonstrate that Dl and Dif play a role in host defense in two other larval tissues. We show that expression of Dif and Dl in the larval fat body is important for host defense through regulation of blood-cell number. We also find that Dl acts in the epidermis, where it prevents infection by maintaining the proper barrier to the environment. Thus, Rel proteins are active in these three distinct immune tissues and control a coordinated network that mediates host defense and maintains immune homeostasis in Drosophila.
Results
Autonomous Dif or Dl activity promotes blood-cell survival through positive regulation of diap1
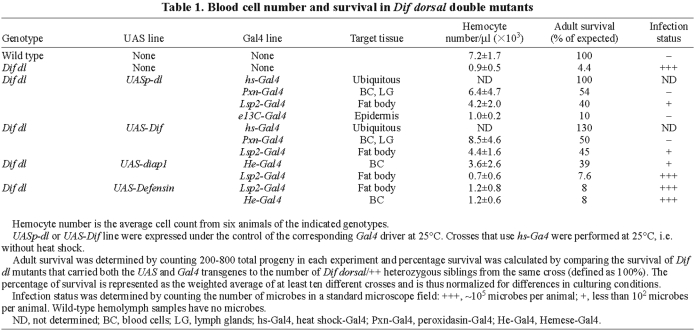
Previously we showed that more than 95% of Dif dl double mutant animals die during larval stages due to opportunistic infection by bacteria and fungi from the environment (Matova and Anderson, 2006) (Table 1). Defects in blood cells are a primary cause of infection and death in these animals: blood-cell number is reduced due to a high rate of cell death, and the surviving blood cells are abnormal in morphology and fail to phagocytose microorganisms (Matova and Anderson, 2006) (Fig. 1B,C). We used the UAS/GAL4 system to perform tissue-specific rescue experiments and showed that expression of either Dif or dl in Dif dl hemocytes rescues hemocyte number, morphology and function, prevents infection and allows survival to adulthood (Matova and Anderson, 2006). For example, when Peroxidasin-Gal4, a line that directs expression in the majority of the hemocytes but not in any other tissues (Stramer et al., 2005) (Materials and Methods) was used to express either Dif or dl in double mutant larvae, approximately 50% of Dif dl animals that carried UAS-Dif or UAS-dl survived to adulthood (Table 1). As observed with other blood-cell drivers (Matova and Anderson, 2006), hemolymph samples from these larvae were microbe-free, the morphology of the blood cells was normal (Fig. 1D) and hemocyte numbers approached the number in wild-type larvae (Table 1).
Table 1.
Blood cell number and survival in Dif dorsal double mutants
Fig. 1.
Tissue-specific rescue of Dif dl hemolymph and hemocyte phenotypes. Hemolymph samples were stained with Filamin antibody (Sokol and Cooley, 1999) (red; upper panels) to reveal the F-actin, and with DAPI (lower panels) in cyan (upper panels) to show nuclear and microbial DNA. (A) Wild-type hemocytes are either small round cells with pronounced subcortical F-actin or larger cells with numerous microspikes (arrow). Wild-type hemolymph samples do not contain microbes. (B,C) Dif dl mutant hemocytes appear ruptured (B) or have an irregular shape and a meshwork of cytoplasmic actin filaments (C). Dif dl hemolymph contains microbes in the absence of experimentally introduced infection (B′, arrow). (D) Dif dl larvae that express UASp-dl in blood cells under the control of Pxn-Gal4 line do not have microbes in the hemolymph. Hemocytes have the same morphology as wild-type hemocytes. (E) Dif dl larvae that express UAS-diap1 in hemocytes under the control of He-Gal4 line have only occasional microbes in the hemolymph. Hemocyte morphology is similar to the morphology of wild-type hemocytes. (F,G) Dif dl larvae that express UASp-dl in the fat body under the control of Lsp2-Gal4 have a low level of microbial infection in the hemolymph (F′, arrow). The cell shape and subcellular distribution of F-actin in 40-60% of the hemocytes is very abnormal (F). The remaining hemocytes have morphology that is more similar to wild type (G). (H) Dif dl larvae that express UASp-dl in the epidermis under the control of e13C-Gal4 line do not have microbes in the hemolymph. However, their hemocytes have an irregular shape and abnormal nuclei. Microbes are seen only occasionally inside the hemocytes (arrow in H′). Scale bar: 10 μm.
There are no known transcriptional targets of Dl or Dif in blood cells. Previously we have shown that Dl and Dif are required for blood-cell survival (Matova and Anderson, 2006): 15% of the hemocytes in third instar Dif dl larvae are TUNEL-positive, compared to 2.3% of hemocytes in wild-type larvae. Because of the potent anti-apoptotic activities of Dl and Dif and because diap1 is the principal negative regulator of apoptosis in somatic cells (Steller, 2008), we investigated whether Dif and Dl regulate diap1. We first tested whether constitutive expression of diap1 in Dif dl mutants could modify the Dif dl mutant phenotype. To that end, we expressed UAS-diap1 in the hemocyte lineage of Dif dl mutants using a Hemese (He)-Gal4 driver. 38.5% of these animals survived to adulthood compared with 6.7% of Dif dl animals that carried only one transgene (He-Gal4 without UAS-diap1). Hemolymph samples from Dif dl animals that expressed diap1 under the control of He-Gal4 showed that the hemocyte morphology was similar to that of wild-type hemocytes (Fig. 1E). The rescued larvae had only occasional microbes and their hemocyte numbers were four times higher than unrescued Dif dl animals (Table 1). Thus constitutive expression of diap1 in Dif dl mutants could rescue all phenotypes of Dif dl animals.
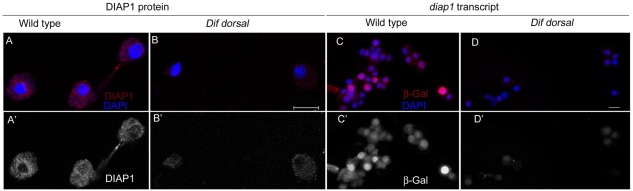
To test whether Dl and Dif are required for normal expression of DIAP1, we assayed DIAP1 protein levels in wild-type and Dif dl hemocytes. Staining with DIAP1 antibody (Ryoo et al., 2002) showed predominantly cytoplasmic distribution of DIAP1 in wild-type hemocytes (Fig. 2A). DIAP1 protein was severely reduced or undetectable in Dif dl hemocytes (Fig. 2B). By contrast, there was a robust DIAP1 expression in the fat body of Dif dl larvae (not shown). Thus, DIAP1 is specifically downregulated in Dif dl blood cells.
Fig. 2.
Dif and Dl are positive regulators of diap1 expression. (A,B). Hemolymph samples were stained with DIAP1 antibody (red) and DAPI (blue). A′ and B′ show DIAP1 antibody staining only. Images of the wild-type and Dif dl hemocytes were acquired at identical settings. (A,A′) Wild-type hemocytes show predominantly cytoplasmic distribution of DIAP1. (B,B′) Dif dl hemocytes show a significant reduction in DIAP1 protein. Scale bar: 10 μm. (C,D) Hemolymph samples were stained with β-Gal antibody (red) and DAPI (blue). C′ and D′ show β-Gal staining only. Images of the wild-type and Dif dl hemocytes were acquired at identical settings. (C,C′) Wild-type hemocytes carrying a diap1 enhancer trap, thj5c8 P{lacZ}, show a predominantly nuclear localization of β-Gal. The nuclear staining reflects the presence of a nuclear localization signal in the P{lacZ} vector. (D,D′) Dif dl hemocytes carrying a diap1 enhancer trap, thj5c8 P{lacZ}, show a significant reduction in β-Gal staining. The intensity of β-Gal fluorescence in wild-type and Dif dl hemocytes is quantified in supplementary material Fig. S1. Scale bar: 10 μm.
To test whether diap1 is a transcriptional target of the Rel proteins in blood cells we employed a diap1 enhancer trap, thj5c8 P{lacZ}, that has been used previously to monitor diap1 transcription in the wing imaginal disc (Ryoo et al., 2002). Anti-β-galactosidase (β-Gal) labeling revealed a nuclear staining in wild-type hemocytes carrying the enhancer trap (Fig. 2C). The nuclear staining reflects the presence of a nuclear localization signal in the P{lacZ} vector. By contrast, in Dif dl hemocytes that carried the reporter enhancer trap, the nuclear staining was greatly diminished (Fig. 2D). We quantified diap1 transcription by measuring pixel intensity of nuclear β-Gal fluorescence in wild-type and Dif dl hemocytes. The measurements showed that expression levels of diap1 were 5.8-fold lower (P=4.4×10−8, Student's t-test) in Dif dl blood cells than in wild-type hemocytes (supplementary material Fig. S1). Thus Dif and Dl are positive regulators of diap1 expression in blood cells and diap1 is a key target of Dif and Dl in the regulation of blood-cell survival.
Dif and Dl activity in the fat body can regulate hemocyte number
To test whether the roles of Dif and Dl in immunity could be explained exclusively by their functions in blood cells, we examined the effect of expressing Dl or Dif in other immune-responsive tissues. We assayed survival to adulthood, presence of infection in hemolymph samples, hemocyte numbers and hemocyte morphology. We first focused on the fat body, a major immune organ in the Drosophila larva (Lemaitre and Hoffmann, 2007). To test the contribution of Dif and Dl in the larval fat body, we expressed UAS-Dif or UASp-dl in Dif dl animals using the Lsp2-Gal4 driver, a line that drives expression only in the fat body (Cherbas et al., 2003) (and our data). Forty to forty-five percent of the Lsp2-Gal4-rescued Dif dl double mutants survived to adulthood and their hemolymph samples had only few microbes, compared with the rampant infection in Dif dl siblings (Fig. 1F,G, Table 1). Thus, expression of either Dif or dl in the fat body protected the double mutant larvae from infection.
Because blood cells play a central role in the ability of larvae to fight infection, we examined the hemocyte number and morphology in the fat body-rescued Dif dl animals. Expression of either Dif or dl in the fat body increased the number of circulating hemocytes in Dif dl mutants nearly fivefold, to about 60% of wild-type numbers (Table 1).
As described above, 15% of the hemocytes in third instar Dif dl larva are TUNEL-positive (Matova and Anderson, 2006). By contrast, only 5.8% of the hemocytes in Dif dl larvae that expressed dl in the fat body were TUNEL-positive (supplementary material Table S1). When normalized to the total number of hemocytes (supplementary material Table S1), the percentage of TUNEL-positive cells in Dif dl larvae that expressed dl in the fat body is nearly threefold lower than in Dif dl mutants alone. The rescued hemocytes did not express Dl protein (supplementary material Fig. S2), indicating that Rel proteins in the fat body acted in a non-autonomous manner to promote survival of blood cells. The morphological abnormalities of Dif dl mutant hemocytes were not fully rescued by the expression of the Rel genes in the fat body: most hemocytes were irregular in shape and had a meshwork of cytoplasmic filamentous actin, like unrescued Dif dl blood cells (Fig. 1C,F,G). These observations indicate that a fat body-derived factor promotes survival of blood cells but cannot rescue a cell-autonomous requirement for Rel proteins in maintenance of blood-cell morphology.
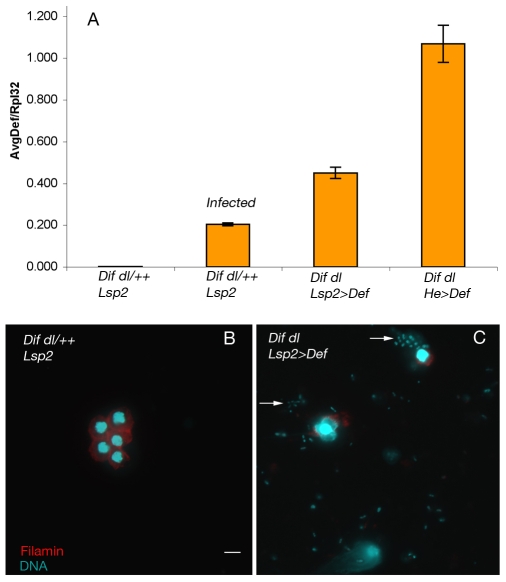
There are no obvious candidate genes that would encode the factor(s) made by the fat body and protecting blood cells. Expression of diap1 exclusively in the fat body with the Lsp2-Gal4 driver did not rescue either blood-cell number or survival to adulthood (Table 1). Because antibiotic treatment can protect Dif dl mutant larvae from infection (Matova and Anderson, 2006), it was possible that the antimicrobial peptides secreted by the fat body (Lemaitre and Hoffmann, 2007) might increase the likelihood of blood-cell survival. Although we previously demonstrated that Dif dl mutant larvae induce all of the major antimicrobial peptide genes (Matova and Anderson, 2006), Defensin (Def) was the only antimicrobial peptide whose induction after septic injury was significantly reduced in the double mutants, and was expressed at about 30% of the levels present in the wild type. Among the antimicrobial peptides, Def appears to have the most potent activity: constitutive expression of Def confers resistance to Gram-positive bacteria in immunocompromised adults (Tzou et al., 2002) and to Pseudomonas, a Gram-negative bacterium in wild-type adult Drosophila (Apidianakis et al., 2005). To test whether Def could protect Dif dl larvae from infection, we used the fat body-specific Lsp2-Gal4 driver and the hemocyte-specific He-Gal4 driver to express UAS-Def. Real-time PCR experiments showed that there was twice the level of Def in Dif dl larvae that expressed Def with the fat-body driver and five-fold more Def in double mutants that expressed Def with the blood-cell driver, compared with infected wild-type animals (Fig. 3A). High levels of Def expression did not significantly increase the number of blood cells (Table 1), indicating that Def is not the blood-cell survival factor. Expression of Def increased the likelihood of survival to adult stages only slightly (8% vs 4.4% survival) (Table 1) and did not prevent infection in most animals (five out of seven animals examined were infected; Fig. 3B). Even in those animals in which the infection load was reduced, the morphology of the blood cells was as abnormal as in the Dif dl mutant siblings. Thus, despite the potent activity of Def in adult animals, overexpression of Def was not sufficient to protect Dif dl double mutant larvae from opportunistic infection and Def is not the Rel-dependent fat-body factor that promotes hemocyte survival.
Fig. 3.
Constitutive expression of Def does not rescue Dif dl hemocyte phenotypes. (A) Expression of Def as assayed by real-time PCR. Uninfected wild-type larvae (genotype: Dif dl/++; Lsp2) do not express Def. 2 hours after injection with E. coli DH5α, Def expression is induced 85-fold in wild-type larvae. Dif dl larvae expressing Def under the control of Lsp2-Gal4 (Dif dl; Lsp2>Def) or under the control of Hemese-Gal4 (Dif dl; He>Def) express very high levels of Def. Def expression is represented on the y-axis as the ratio of the average Def induction to the induction of Rpl32 (AvgDef/Rpl32). (B,C) Hemolymph samples from wild-type larvae (genotype: Dif dl/++; Lsp2) have intact blood cells and are microbe-free (B). By contrast, hemolymph samples of Dif dl larvae expressing Def under the control of Lsp2-Gal4 (Dif dl; Lsp2>Def) show abundant bacteria (arrows) and ruptured hemocytes (C). Similar results were obtained in hemolymph samples from Dif dl larvae expressing Def under the control of Hemese-Gal4 (Dif dl; He>Def). Hemolymph samples were stained with Filamin antibody (red) to reveal the F-actin, and DAPI (cyan) to show nuclei and microbial DNA. Scale bar: 10 μm.
Rel protein activity in the epidermis protects from infection
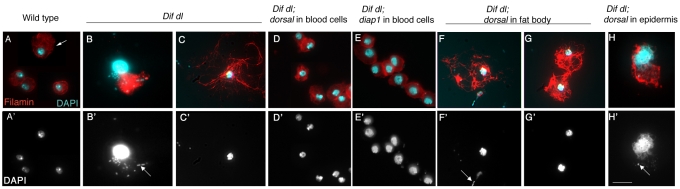
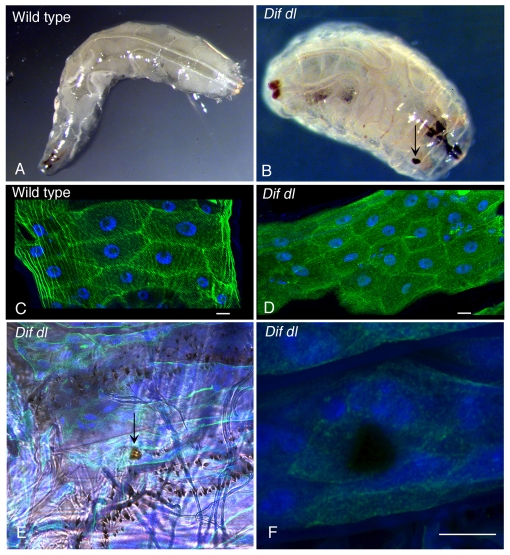
In addition to hemocytes and the fat body, the larval epidermis is another immune organ that provides a mechanical barrier to infection and also expresses antimicrobial peptides that could defend the animal from invading microbes (Onfelt Tingvall et al., 2001). We observed that Dif dl larvae had obvious defects in the epidermis: 70% of the double mutants exhibited melanized spots of variable size and location throughout the epidermis (Fig. 4B; supplementary material Table S2), whereas only 5.5% of the wild-type larvae had detectable epidermal defects (supplementary material Table S2). These spots did not clear and persisted throughout the life of Dif dl larvae. To look more closely at the structure of the larval epidermis in wild-type and in Dif dl animals, we marked the epidermal cells with GFP::actin and DAPI. This examination revealed that the global organization of the epidermis was similar in wild-type and Dif dl animals (Fig. 4C,D). Confocal scanning microscopy through the melanized lesions in Dif dl larvae showed that the lesions spanned the entire epidermal layer and did not have detectable GFP::actin or DAPI fluorescence (Fig. 4F).
Fig. 4.
Epidermal lesions in Dif dl larvae. (A,B) Images of wild-type larva (A) and Dif dl larva (B). Dif dl larvae have melanized epidermal spots of variable size (arrow in B) that are not present in wild-type animals. (C,D) Confocal images of the epidermal cells of wild-type larvae (C) resemble the epidermal cells of Dif dl larvae (D). Epidermal cells express GFP::actin (green) under the control of e13-Gal4 line and are stained with DAPI (blue) to show their nuclei. Scale bars: 10 μm. (E) Dif dl larva: a DIC image showing a portion of the ventral epidermis with rows of denticles and a melanized epidermal lesion (arrow). (F) High magnification confocal image of the lesion shown in E. Epidermal cells expressing GFP::actin (green) under the control of e13-Gal4 line were additionally stained with DAPI (blue). Note that the lesion does not express GFP::actin and does not stain with DAPI. Scale bar: 10 μm.
The melanized-spot phenotype of Dif dl larvae was rescued by expression of dl in the epidermis with e13C-Gal4, a line that drives strong expression in the epidermis but not in hemocytes or in the fat body (supplementary material Table S2). Thus, Dl is required autonomously in larval epidermal cells to prevent epidermal lesions. Because expression of UAS-Dif with the e13C-Gal4 caused lethality in both the wild-type and Dif dl mutant background, the effect of Dif expression in the epidermis of Dif dl mutants could not be assayed.
To test whether dl in the epidermis protects the larva from infection, we examined hemolymph samples of Dif dl mutants that expressed dl with the e13C-Gal4 line. Although a few bacteria were still detectable inside hemocytes (Fig. 1H′, arrow), no microbes were seen in the hemolymph of these animals. Nevertheless, the number of circulating blood cells in these larvae remained as low as in unrescued Dif dl double mutants (Table 1) and these blood cells retained the abnormal morphology seen in Dif dl double mutant larvae (Fig. 1H). Epidermal expression of dl improved the rate of survival of Dif dl mutants to the third instar larvae, but these animals arrested at the onset of metamorphosis and did not survive to adult stages. We conclude that Rel protein activity in the epidermis protects larvae from microbes in the environment without affecting the number or the morphology of blood cells.
Discussion
In this study we show that Rel proteins in three different tissues — the epidermis, the circulating blood cells and the fat body — protect Drosophila larvae from microbial infection. These functions of the Drosophila Rel proteins have close parallels to the functions of the mammalian Rel proteins in immune-responsive tissues that form the backbone of the vertebrate host defense.
Rel activity in blood cells and in the fat body controls hemocyte numbers
Because circulating phagocytic cells play an essential role in the clearance of microbial infection in the Drosophila larva, maintenance of correct number of healthy hemocytes is crucial for the survival of the animal. We find that two Rel proteins, Dl and Dif, function both in hemocytes and in the fat body to promote hemocyte survival. Even though the mechanisms of blood-cell protection are different, these results revealed that the immune-responsive organs in Drosophila interact and form a mutually supportive network. This conclusion is further strengthened by earlier findings indicating that signals from blood cells can stimulate production of antimicrobial and stress peptides in the fat body (Agaisse et al., 2003; Basset et al., 2000; Brennan et al., 2007; Dijkers and O'Farrell, 2007). Thus there appear to be bidirectional signals between the fat body and the blood cells that coordinate host defense responses in Drosophila larvae.
Our experiments identified diap1 as the first target of Dl and Dif in Drosophila blood cells. Dif and Dl autonomously promote hemocyte survival and diap1 is likely to be their principal target, as hemocyte number, hemocyte morphology and survival to adulthood of Dif dl double mutants can be rescued to nearly the same extent by expression of diap1 in the hemocyte lineage as when Dif or dl is expressed in those cells.
Mammalian blood cells also rely on Rel proteins to prevent apoptosis: c-Rel RelA double mutant hematopoietic progenitors cannot rescue lethally irradiated wild-type mouse hosts, and the double-mutant macrophages have a high rate of apoptosis (Grossmann et al., 1999). The parallels with mammals extend further: just as Drosophila Rel proteins regulate the expression of the inhibitor of apoptosis DIAP1 in blood cells, mammalian Rel proteins control the expression of homologous molecules such as c-IAP and XIAP (Karin and Lin, 2002).
Our analysis revealed a previously unsuspected role of the fat body in controlling the number of blood cells. The results suggest that Dif and Dl in the fat body protect the animal from infection because they regulate blood-cell number and not because they are required for the production of antimicrobial peptides. Although it is conceivable that a particular cocktail of Rel-controlled antimicrobial peptides made in the fat body prevents blood-cell death, it is unlikely, given that the peptides are expressed in Dif dl larvae (Matova and Anderson, 2006) and that overexpression of Def has no effect on blood-cell survival (Fig. 3). Instead, we propose that Dl and Dif in the fat body control the production of survival factors that are released into the hemolymph. It is known that the fat body secretes a family of proteins that promote survival or proliferation of imaginal disc cells in a concentration-dependent manner (Kawamura et al., 1999) and these growth factors, or other unidentified factors, might also be active on blood cells.
Just as Rel protein activity in the Drosophila fat body provides factors that promote survival or proliferation of blood cells, the mammalian liver (the analogue of the fat body) secretes growth factors such as the hepatic growth factor (HGF) and interleukin-7 that act as survival and proliferation molecules for blood cells (Sawa et al., 2009; Sugiura et al., 2007; Yu et al., 1998), although it is not known whether Rel proteins directly regulate their production.
Distinct functions of Rel proteins in blood cells and the epidermis protect the animal from infection
The opportunistic microbial infection in Dif dl hemolymph can be rescued by expression of Dif or Dl in either the blood cells or in the epidermis. Expression of dl in the epidermis of Dif dl animals does not affect hemocyte number, morphology or ability to phagocytose bacteria; nevertheless, dl expression in the epidermis is sufficient to protect the animal from microbial infection. Hence, these two tissues have different roles in host defense — the blood cells remove microbes from circulation whereas the epidermis prevents entry of microbes into the animal — but both rely on Rel proteins for their immune functions.
The function of the Rel proteins in the epidermis is not clear. It is possible that the lesions in the epidermis of Dif dl larvae are the result of a developmental defect. Alternatively, because each animal has only one or a few epidermal lesions and their location varies, we prefer the hypothesis that epidermal gaps arise because of defective healing of wounds that occur normally in the life of the larva (Brock et al., 2008). In addition, Rel signaling in epidermal cells might help to prevent infection by local expression of products with antimicrobial activity (Onfelt Tingvall et al., 2001).
The epidermal function of Drosophila Rel proteins might also parallel Rel functions in mammals, as mice that lack both RelA and c-rel have defects in epidermal development and immune homeostasis (Gugasyan et al., 2004). In agreement with these findings, expression of a dominant negative IkBa transgene in the epidermis of the mouse, which should block activity of several Rel proteins, causes progressive skin disease and a strong local inflammatory response (van Hogerlinden et al., 2004). However, future experiments that involve conditional inactivation of essential Rel family genes might provide a definitive assay for the function of Rel proteins in the mammalian epidermis.
An evolutionarily conserved network of Rel-dependent responses prevents infection
It is striking that, as in mammals, host defense in Drosophila depends on Rel proteins in multiple tissues, although it is likely that the Rel transcriptional targets are different in each tissue. These parallels with the mammalian immune response suggest that the action of Rel proteins in multiple tissues was selected early in metazoan evolution as a mechanism to provide a robust immune network that effectively prevents microbial infection. Future studies on the tissue-specific targets of Rel proteins should define how much of this immune network was present in the common ancestor of vertebrates and invertebrates. Because of the remarkable conservation in the framework of the immune system between mammals and insects, characterization of Rel targets in Drosophila, which has only three Rel genes and can be easily manipulated genetically, could guide future experiments in mammals.
Materials and Methods
Drosophila stocks
Dif dl double mutants were transheterozygotes for two overlapping deficiencies Df(2L)J4 and Df(2L)TW119, as described previously (Matova and Anderson, 2006). Lsp2-Gal4 was obtained from the Bloomington Stock Center and the e13-Gal4 line came from Norbert Perrimon (Harvard Medical School, Boston, MA). The UASp-GFP::actin and UAS-Def transgenic flies were obtained from Andrew Hudson and Lynn Cooley (Yale University, New Haven, CT) and Bruno Lemaitre (École Polytechnique Fédérale de Lausanne, Lausanne, Switzerland), respectively. The UAS-diap1 line (Betz et al., 2008) and thj5c8 allele were provided by Hermann Steller (Rockefeller University, New York, NY). UASp-dl and UAS-Dif have been described previously (Matova and Anderson, 2006).
We determined the expression pattern of the Gal4 lines by crossing to stocks carrying UAS-GFP. Pxn-Gal4 drove expression in approximately 70% of the blood cells, lymph glands (first pair), in a part of the proventriculus, and in a subset of brain cells. Lsp2-Gal4 drove expression exclusively in fat body cells (Cherbas et al., 2003) (and our data). He-Gal4 drove expression in blood cells, lymph glands (a few cells), midgut, salivary glands (Zettervall et al., 2004) (and our data). e13C-Gal4 drove expression in the epidermis, pharynx, salivary glands, ventral nerve cord, brain and Malpighian tubules.
Tissue-specific expression of Dif and dl
Dif dl double mutants were obtained from the cross between Df(2L)J4/T(2;3)CyO;TM6B and Df(2L)TW119/T(2;3)CyO;TM6B. Double mutants were identified by the absence of the Tubby marker carried by the CyO;TM6B translocation. The CyO;TM6B balancer translocation was also used to identify Dif dl larvae that expressed Dif or dl in a tissue-specific manner. In these rescue experiments, the UASp-dl transgene was recombined onto the Df(2L)J4 chromosome or a third chromosome insertion of UAS-Dif line was used. All Gal4 lines used in the experiments mapped to the third chromosome. Dif dl larvae that carried both the UAS and Gal4 transgenes were the non-Tubby larvae from crosses from two doubly balanced lines: e.g. Df(2L)J4 UASp-dl/T(2;3)CyO;TM6B × Df(2L)TW119; e13C-Gal4/T(2;3)CyO;TM6B.
For each genotype, hemolymph from three to eight non-Tubby larvae was analyzed. Expression of UAS-Dif with the e13C-Gal4 caused lethality in both the wild-type and Dif dl mutant backgrounds. Because of that, the effect of Dif expression in the epidermis of Dif dl mutants could not be assayed.
Immunocytochemistry and microscopy
Blood cells samples were collected, stained and imaged as described previously (Matova and Anderson, 2006). Hemocyte counts, infection and TUNEL were performed as described previously (Matova and Anderson, 2006). Staining for β-Gal was performed with a rabbit antibody from Cappel, which was used at a dilution of 1:2200. DIAP1 stainings were performed either with the rabbit anti-DIAP1 serum (diluted at 1:2000) or with the affinity-purified DIAP1 antibody (diluted at 1:300). Both preparations produced similar results. The stainings with β-Gal and DIAP1 antibody were performed in animals reared on apple-juice agar plates, which had a lower level of hemolymph infection than the animals grown on standard cornmeal-molasses-yeast medium.
The epidermis of Dif dl mutants and Dif dl/++ siblings expressing GFP::actin under the control of e13C-Gal4 was dissected and fixed for 20 minutes in 4% paraformaldehyde. The epidermal samples were subsequently washed in PBT and stained with DAPI (4 mg/ml) for 2 hours at room temperature. Samples were washed again and mounted in Vectashield (Vector laboratories) for examination. Images of the epidermis were acquired with an inverted Leica TCS SP2 confocal microscope using a 20× 0.7 NA lens.
Analysis of Def expression
Infected wild-type animals were obtained by injecting third-instar larvae with E. coli DH5α. These larvae were homogenized 2 hours later and RNA was prepared using RNA STAT-60 (TEL-TEST). For all other genotypes, uninfected third instar larvae were homogenized and RNA was prepared using RNA STAT-60. Q-PCR was performed with pre-designed TaqMan Gene expression assays for Def (Dm01818074_s1) and Ribosomal protein L32 (Dm02151827_s1) as described previously (Matova and Anderson, 2006). Fold changes reported are the average value of triplicate experiments.
Quantification of diap1 expression levels
The levels of diap1 transcription were determined by crossing the diap1 enhancer trap, thj5c8 P{lacZ}, into wild-type and Dif dl animals. Hemocytes were stained with β-Gal antibody and DAPI. Fluorescence images were captured with a Zeiss Axiovert 200M microscope equipped with a CoolSNAP HQ CCD (Roper Scientific Photometrics) and the MetaMorph software (Universal Imaging Corporation). All hemocyte nuclei were identified by DAPI staining. Fluorescence intensity of nuclear β-Gal was measured in arbitrary units in 50 individual nuclei using the ImageJ 1.410 software package (Particle analysis plug-in).
Statistical analysis
Hemocyte numbers in Table 1 were compared using unpaired two-tailed Student's t-test. When compared with Dif dl larvae, hemocyte numbers were significantly higher in Dif dl mutants that expressed UASp-dl with Pxn-Gal4, UAS-Dif with Pxn-Gal4, UASp-dl with Lsp2-Gal4, UAS-Dif with Lsp2-Gal4 or UAS-diap1 with He-Gal4 (Student's t-test, P<0.05).
When compared with Dif dl larvae, hemocyte numbers were not significantly higher in Dif dl mutants that expressed UASp-dl with e13C-Gal4, UAS-Def with Lsp2-Gal4, UAS-Def with He-Gal4 or UAS-diap1 with Lsp2-Gal4 (Student's t-test, P>0.05).
Percentages of TUNEL-positive hemocytes in different genetic backgrounds were compared using a Chi-square test in supplementary material Table S1. Chi-square test was also used to compare animals with epidermal lesions versus animals without lesions in supplementary material Table S2.
Supplementary Material
Acknowledgments
We are grateful to Katia Manova and the Molecular Cytology Facility and to Agnes Viale and the Genomics Core Facility for experimental support. We thank Andrew Hudson and Lynn Cooley for the UASp-GFP::actin flies, Bruno Lemaitre for the UAS-Def line, Hermann Steller and Joe Rodriguez for DIAP1 reagents, Hyung Don Ryoo for the affinity-purified polyclonal DIAP1 antibody, and Céline Carret, Polloneal Ocbina and José Rino for the help with statistics and the quantification of diap1. We thank J. Bloomekatz, J. Lee, I. Migeotte and S. Weatherbee for comments on the manuscript. This work was supported by NIH grant AI45149 to K.V.A. and the Lita Annenberg Hazen Foundation. N.M. was a recipient of a fellowship from the Irvington Institute for Immunological Research and is currently supported by Fundação para a Ciência e Tecnologia (FCT) of the Portuguese Ministry of Science. Deposited in PMC for release after 12 months.
Footnotes
Supplementary material available online at http://jcs.biologists.org/cgi/content/full/123/4/627/DC1
References
- Agaisse H., Petersen U. M., Boutros M., Mathey-Prevot B., Perrimon N. (2003). Signaling role of hemocytes in Drosophila JAK/STAT-dependent response to septic injury. Dev. Cell 5, 441-450 [DOI] [PubMed] [Google Scholar]
- Apidianakis Y., Mindrinos M. N., Xiao W., Lau G. W., Baldini R. L., Davis R. W., Rahme L. G. (2005). Profiling early infection responses: Pseudomonas aeruginosa eludes host defenses by suppressing antimicrobial peptide gene expression. Proc. Natl. Acad. Sci. USA 102, 2573-2578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basset A., Khush R. S., Braun A., Gardan L., Boccard F., Hoffmann J. A., Lemaitre B. (2000). The phytopathogenic bacteria Erwinia carotovora infects Drosophila and activates an immune response. Proc. Natl. Acad. Sci. USA 97, 3376-3381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betz A., Ryoo H. D., Steller H., Darnell J. E., Jr (2008). STAT92E is a positive regulator of Drosophila inhibitor of apoptosis 1 (DIAP/1) and protects against radiation-induced apoptosis. Proc. Natl. Acad. Sci. USA 105, 13805-13810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun A., Hoffmann J. A., Meister M. (1998). Analysis of the Drosophila host defense in domino mutant larvae, which are devoid of hemocytes. Proc. Natl. Acad. Sci. USA 95, 14337-14342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan C. A., Delaney J. R., Schneider D. S., Anderson K. V. (2007). Psidin is required in Drosophila blood cells for both phagocytic degradation and immune activation of the fat body. Curr. Biol. 17, 67-72 [DOI] [PubMed] [Google Scholar]
- Brock A. R., Babcock D. T., Galko M. J. (2008). Active cop, passive cop: developmental stage-specific modes of wound-induced blood cell recruitment in Drosophila. Fly (Austin) 2, 303-305 [DOI] [PubMed] [Google Scholar]
- Caamano J., Hunter C. A. (2002). NF-κB family of transcription factors: central regulators of innate and adaptive immune functions. Clin. Microbiol. Rev. 15, 414-429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherbas L., Hu X., Zhimulev I., Belyaeva E., Cherbas P. (2003). EcR isoforms in Drosophila: testing tissue-specific requirements by targeted blockade and rescue. Development 130, 271-284 [DOI] [PubMed] [Google Scholar]
- Choe K.-M. (2002). Molecular Characterization of Drosophila Immunity Mutants New York: Cornell University; [Google Scholar]
- Defaye A., Evans I., Crozatier M., Wood W., Lemaitre B., Leulier F. (2009). Genetic ablation of Drosophila phagocytes reveals their contribution to both development and resistance to bacterial infection. J. Innate Immunity 1, 322-334 [DOI] [PubMed] [Google Scholar]
- Dijkers P. F., O'Farrell P. H. (2007). Drosophila calcineurin promotes induction of innate immune responses. Curr. Biol. 17, 2087-2093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrandon D., Jung A. C., Criqui M., Lemaitre B., Uttenweiler-Joseph S., Michaut L., Reichhart J., Hoffmann J. A. (1998). A drosomycin-GFP reporter transgene reveals a local immune response in Drosophila that is not dependent on the Toll pathway. EMBO J. 17, 1217-1227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossmann M., Metcalf D., Merryfull J., Beg A., Baltimore D., Gerondakis S. (1999). The combined absence of the transcription factors Rel and RelA leads to multiple hemopoietic cell defects. Proc. Natl. Acad. Sci. USA 96, 11848-11853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gugasyan R., Voss A., Varigos G., Thomas T., Grumont R. J., Kaur P., Grigoriadis G., Gerondakis S. (2004). The transcription factors c-rel and RelA control epidermal development and homeostasis in embryonic and adult skin via distinct mechanisms. Mol. Cell. Biol. 24, 5733-5745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden M. S., West A. P., Ghosh S. (2006). NF-κB and the immune response. Oncogene 25, 6758-6780 [DOI] [PubMed] [Google Scholar]
- Hedengren M., Asling B., Dushay M. S., Ando I., Ekengren S., Wihlborg M., Hultmark D. (1999). Relish, a central factor in the control of humoral but not cellular immunity in Drosophila. Mol. Cell 4, 827-837 [DOI] [PubMed] [Google Scholar]
- Karin M., Lin A. (2002). NF-κB at the crossroads of life and death. Nat. Immunol. 3, 221-227 [DOI] [PubMed] [Google Scholar]
- Kawamura K., Shibata T., Saget O., Peel D., Bryant P. J. (1999). A new family of growth factors produced by the fat body and active on Drosophila imaginal disc cells. Development 126, 211-219 [DOI] [PubMed] [Google Scholar]
- Lemaitre B., Hoffmann J. (2007). The host defense of Drosophila melanogaster. Annu. Rev. Immunol. 25, 697-743 [DOI] [PubMed] [Google Scholar]
- Lu Y., Wu L. P., Anderson K. V. (2001). The antibacterial arm of the Drosophila innate immune response requires an IκB kinase. Genes Dev. 15, 104-110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matova N., Anderson K. V. (2006). Rel/NF-κB double mutants reveal that cellular immunity is central to Drosophila host defense. Proc. Natl. Acad. Sci. USA 103, 16424-16429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onfelt Tingvall T., Roos E., Engstrom Y. (2001). The imd gene is required for local Cecropin expression in Drosophila barrier epithelia. EMBO Rep. 2, 239-243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasparakis M., Luedde T., Schmidt-Supprian M. (2006). Dissection of the NF-κB signalling cascade in transgenic and knockout mice. Cell Death Differ. 13, 861-872 [DOI] [PubMed] [Google Scholar]
- Ryoo H. D., Bergmann A., Gonen H., Ciechanover A., Steller H. (2002). Regulation of Drosophila IAP1 degradation and apoptosis by reaper and ubcD1. Nat. Cell Biol. 4, 432-438 [DOI] [PubMed] [Google Scholar]
- Sawa Y., Arima Y., Ogura H., Kitabayashi C., Jiang J. J., Fukushima T., Kamimura D., Hirano T., Murakami M. (2009). Hepatic interleukin-7 expression regulates T cell responses. Immunity 30, 447-457 [DOI] [PubMed] [Google Scholar]
- Schmidt-Ullrich R., Aebischer T., Hulsken J., Birchmeier W., Klemm U., Scheidereit C. (2001). Requirement of NF-κB/Rel for the development of hair follicles and other epidermal appendices. Development 128, 3843-3853 [DOI] [PubMed] [Google Scholar]
- Sokol N. S., Cooley L. (1999). Drosophila filamin encoded by the cheerio locus is a component of ovarian ring canals. Curr. Biol. 9, 1221-1230 [DOI] [PubMed] [Google Scholar]
- Steller H. (2008). Regulation of apoptosis in Drosophila. Cell Death Differ. 15, 1132-1138 [DOI] [PubMed] [Google Scholar]
- Stramer B., Wood W., Galko M. J., Redd M. J., Jacinto A., Parkhurst S. M., Martin P. (2005). Live imaging of wound inflammation in Drosophila embryos reveals key roles for small GTPases during in vivo cell migration. J. Cell Biol. 168, 567-573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura K., Taketani S., Yoshimura T., Nishino T., Nishino N., Fujisawa J., Hisha H., Inaba T., Ikehara S. (2007). Effect of hepatocyte growth factor on long term hematopoiesis of human progenitor cells in transgenic-severe combined immunodeficiency mice. Cytokine 37, 218-226 [DOI] [PubMed] [Google Scholar]
- Tzou P., Ohresser S., Ferrandon D., Capovilla M., Reichhart J. M., Lemaitre B., Hoffmann J. A., Imler J. L. (2000). Tissue-specific inducible expression of antimicrobial peptide genes in Drosophila surface epithelia. Immunity 13, 737-748 [DOI] [PubMed] [Google Scholar]
- Tzou P., Reichhart J. M., Lemaitre B. (2002). Constitutive expression of a single antimicrobial peptide can restore wild-type resistance to infection in immunodeficient Drosophila mutants. Proc. Natl. Acad. Sci. USA 99, 2152-2157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hogerlinden M., Rozell B. L., Toftgard R., Sundberg J. P. (2004). Characterization of the progressive skin disease and inflammatory cell infiltrate in mice with inhibited NF-κB signaling. J. Invest. Dermatol. 123, 101-108 [DOI] [PubMed] [Google Scholar]
- Whalen A. M., Steward R. (1993). Dissociation of the dorsal-cactus complex and phosphorylation of the dorsal protein correlate with the nuclear localization of dorsal. J. Cell Biol. 123, 523-534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C. Z., Hisha H., Li Y., Lian Z., Nishino T., Toki J., Adachi Y., Inaba M., Fan T. X., Jin T., et al. (1998). Stimulatory effects of hepatocyte growth factor on hemopoiesis of SCF/c-kit system-deficient mice. Stem Cells 16, 66-77 [DOI] [PubMed] [Google Scholar]
- Zettervall C. J., Anderl I., Williams M. J., Palmer R., Kurucz E., Ando I., Hultmark D. (2004). A directed screen for genes involved in Drosophila blood cell activation. Proc. Natl. Acad. Sci. USA 101, 14192-14197 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.