Abstract
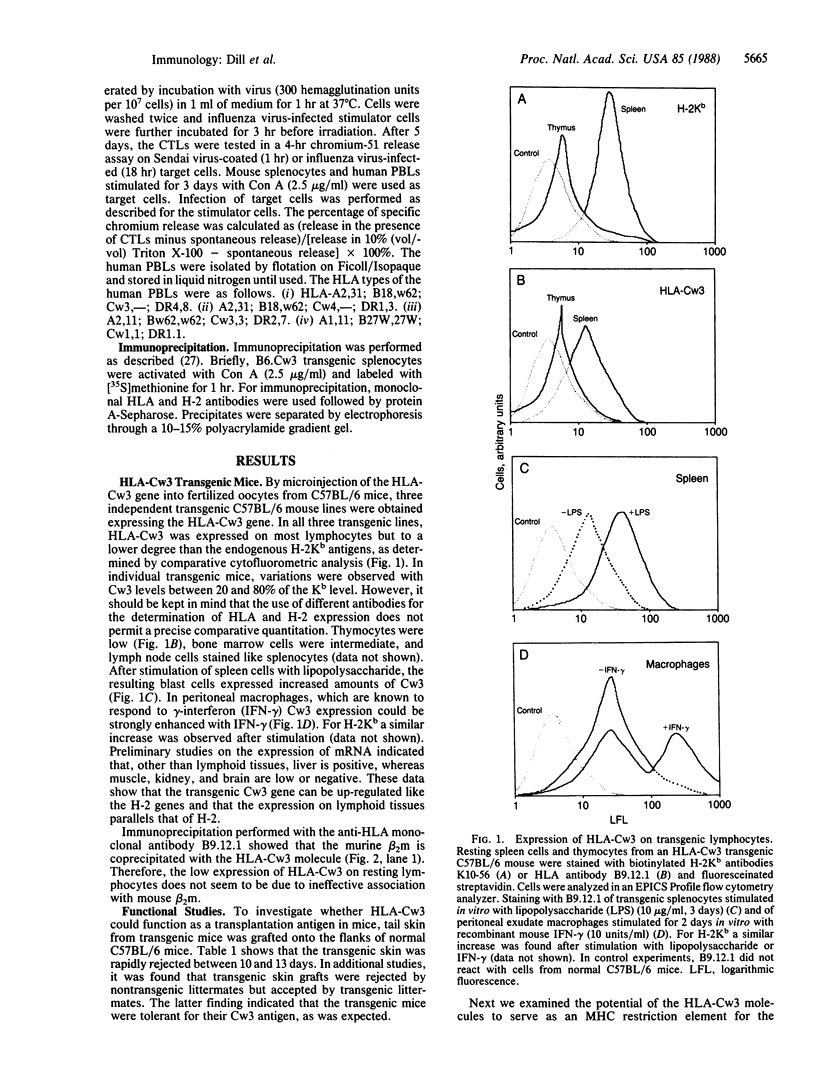
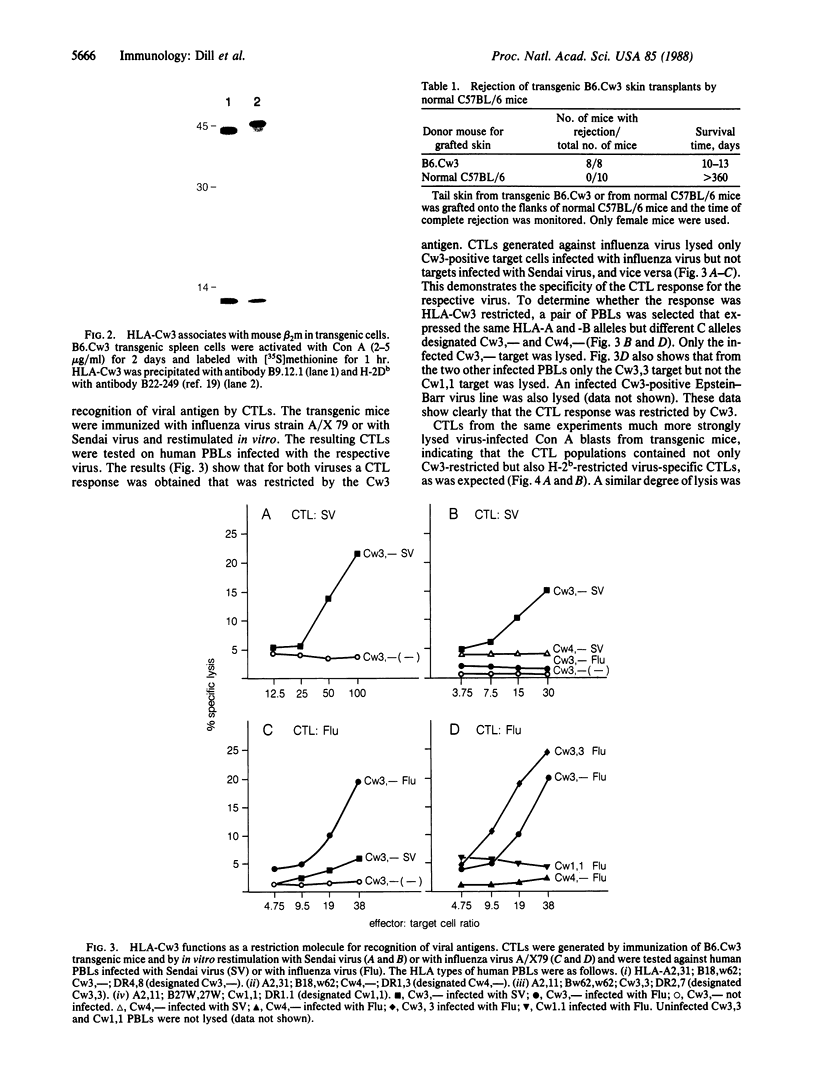
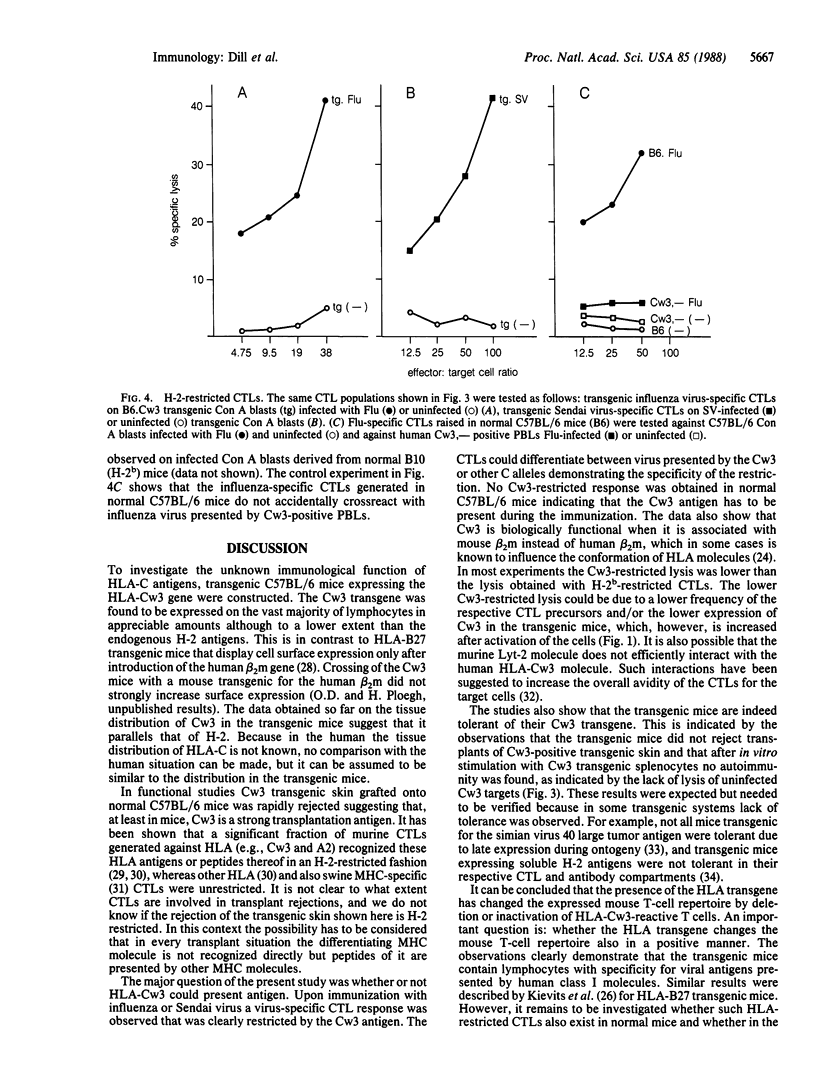
The human major histocompatibility complex encodes three classical class I antigens, HLA-A, -B, and -C. Of these HLA-A and -B act as strong transplantation antigens and as restriction molecules for recognition of foreign antigen by cytotoxic T lymphocytes. In contrast, little is known about HLA-C and it is not clear whether HLA-C has the same functional properties as HLA-A and -B. Transgenic C57BL/6 mice expressing the HLA-Cw3 gene were established. Functional studies demonstrated that transgenic skin was rapidly rejected by normal C57BL/6 mice and that cytotoxic T lymphocytes generated by immunization of the Cw3 transgenic mice with influenza and Sendai virus were restricted by the Cw3 molecule. These data suggest that HLA-Cw3 has immunological functions comparable to those of HLA-A and -B.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams T. E., Alpert S., Hanahan D. Non-tolerance and autoantibodies to a transgenic self antigen expressed in pancreatic beta cells. Nature. 1987 Jan 15;325(6101):223–228. doi: 10.1038/325223a0. [DOI] [PubMed] [Google Scholar]
- Albrechtsen D., Moen T., Flatmark A., Halvorsen S., Jakobsen A., Jervell J., Solheim B. G., Thorsby E. Influence of HLA-A, B, C, D, and DR matching in renal transplantation. Transplant Proc. 1981 Mar;13(1 Pt 2):924–929. [PubMed] [Google Scholar]
- Arnold B., Dill O., Küblbeck G., Jatsch L., Simon M. M., Tucker J., Hämmerling G. J. Alloreactive immune responses of transgenic mice expressing a foreign transplantation antigen in a soluble form. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2269–2273. doi: 10.1073/pnas.85.7.2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhard E. J., Le A. X., Yannelli J. R., Holterman M. J., Hogan K. T., Parham P., Engelhard V. H. The ability of cytotoxic T cells to recognize HLA-A2.1 or HLA-B7 antigens expressed on murine cells correlates with their epitope specificity. J Immunol. 1987 Dec 1;139(11):3614–3621. [PubMed] [Google Scholar]
- Bluestone J. A., Pescovitz M. D., Frels W. I., Singer D. S., Hodes R. J. Cytotoxic T lymphocyte recognition of a xenogeneic major histocompatibility complex antigen expressed in transgenic mice. Eur J Immunol. 1987 Jul;17(7):1035–1041. doi: 10.1002/eji.1830170721. [DOI] [PubMed] [Google Scholar]
- Daar A. S., Fuggle S. V., Fabre J. W., Ting A., Morris P. J. The detailed distribution of HLA-A, B, C antigens in normal human organs. Transplantation. 1984 Sep;38(3):287–292. doi: 10.1097/00007890-198409000-00018. [DOI] [PubMed] [Google Scholar]
- Dausset J., Colombani J., Legrand L., Feingold N. Les sub-loci du système HL-A. Le système principal d'histocompatibilité de l'homme. Presse Med. 1969 May 10;77(23):849–852. [PubMed] [Google Scholar]
- Dickmeiss E., Soeberg B., Svejgaard A. Human cell-mediated cytotoxicity against modified target cells is restricted by HLA. Nature. 1977 Dec 8;270(5637):526–528. doi: 10.1038/270526a0. [DOI] [PubMed] [Google Scholar]
- Ferrier P., Fontecilla-Camps J. C., Bucchini D., Caillol D. H., Jordan B. R., Lemonnier F. A. Altered structure of HLA class I heavy chains associated with mouse beta-2 microglobulin. Immunogenetics. 1985;21(4):321–331. doi: 10.1007/BF00430798. [DOI] [PubMed] [Google Scholar]
- Gordon J. W., Ruddle F. H. Gene transfer into mouse embryos: production of transgenic mice by pronuclear injection. Methods Enzymol. 1983;101:411–433. doi: 10.1016/0076-6879(83)01031-9. [DOI] [PubMed] [Google Scholar]
- Goulmy E., Termijtelen A., Bradley B. A., van Rood J. J. Y-antigen killing by T cells of women is restricted by HLA. Nature. 1977 Apr 7;266(5602):544–545. doi: 10.1038/266544a0. [DOI] [PubMed] [Google Scholar]
- Grunnet N., Kristensen T., Kissmeyer-Nielsen F. Cell mediated lympholysis in man. The impact of HLA-C antigens. Tissue Antigens. 1976 May;7(5):301–309. doi: 10.1111/j.1399-0039.1976.tb01071.x. [DOI] [PubMed] [Google Scholar]
- Güssow D., Rein R. S., Meijer I., de Hoog W., Seemann G. H., Hochstenbach F. M., Ploegh H. L. Isolation, expression, and the primary structure of HLA-Cw1 and HLA-Cw2 genes: evolutionary aspects. Immunogenetics. 1987;25(5):313–322. doi: 10.1007/BF00404424. [DOI] [PubMed] [Google Scholar]
- Hämmerling G. J., Rüsch E., Tada N., Kimura S., Hämmerling U. Localization of allodeterminants on H-2Kb antigens determined with monoclonal antibodies and H-2 mutant mice. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4737–4741. doi: 10.1073/pnas.79.15.4737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kievits F., Ivanyi P., Krimpenfort P., Berns A., Ploegh H. L. HLA-restricted recognition of viral antigens in HLA transgenic mice. Nature. 1987 Oct 1;329(6138):447–449. doi: 10.1038/329447a0. [DOI] [PubMed] [Google Scholar]
- Koch S., Schultz A., Koch N. The production of recombinant HLA-DR beta and invariant chain polypeptides by cDNA expression in E. coli. J Immunol Methods. 1987 Nov 5;103(2):211–220. doi: 10.1016/0022-1759(87)90292-4. [DOI] [PubMed] [Google Scholar]
- Krimpenfort P., Rudenko G., Hochstenbach F., Guessow D., Berns A., Ploegh H. Crosses of two independently derived transgenic mice demonstrate functional complementation of the genes encoding heavy (HLA-B27) and light (beta 2-microglobulin) chains of HLA class I antigens. EMBO J. 1987 Jun;6(6):1673–1676. doi: 10.1002/j.1460-2075.1987.tb02416.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemke H., Hämmerling G. J., Hämmerling U. Fine specificity analysis with monoclonal antibodies of antigens controlled by the major histocompatibility complex and by the Qa/TL region in mice. Immunol Rev. 1979;47:175–206. doi: 10.1111/j.1600-065x.1979.tb00293.x. [DOI] [PubMed] [Google Scholar]
- MacDonald H. R., Glasebrook A. L., Cerottini J. C. Clonal heterogeneity in the functional requirement for Lyt-2/3 molecules on cytolytic T lymphocytes: analysis by antibody blocking and selective trypsinization. J Exp Med. 1982 Dec 1;156(6):1711–1722. doi: 10.1084/jem.156.6.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malissen B., Kristensen T., Goridis C., Madsen M., Mawas C. Clones of human cytotoxic T lymphocytes derived from an allosensitized individual: HLA specificity and cell surface markers. Scand J Immunol. 1981 Sep;14(3):213–224. doi: 10.1111/j.1365-3083.1981.tb00558.x. [DOI] [PubMed] [Google Scholar]
- Maryanski J. L., Pala P., Corradin G., Jordan B. R., Cerottini J. C. H-2-restricted cytolytic T cells specific for HLA can recognize a synthetic HLA peptide. Nature. 1986 Dec 11;324(6097):578–579. doi: 10.1038/324578a0. [DOI] [PubMed] [Google Scholar]
- Mayr W. R., Bernoco D., De Marchi M., Ceppellini R. Genetic analysis and biological properties of products of the third SD (AJ) locus of the HL-A region. Transplant Proc. 1973 Dec;5(4):1581–1593. [PubMed] [Google Scholar]
- McMichael A. J., Ting A., Zweerink H. J., Askonas B. A. HLA restriction of cell-mediated lysis of influenza virus-infected human cells. Nature. 1977 Dec 8;270(5637):524–526. doi: 10.1038/270524a0. [DOI] [PubMed] [Google Scholar]
- Natali P. G., Bigotti A., Nicotra M. R., Viora M., Manfredi D., Ferrone S. Distribution of human Class I (HLA-A,B,C) histocompatibility antigens in normal and malignant tissues of nonlymphoid origin. Cancer Res. 1984 Oct;44(10):4679–4687. [PubMed] [Google Scholar]
- Rebaï N., Malissen B. Structural and genetic analyses of HLA class I molecules using monoclonal xenoantibodies. Tissue Antigens. 1983 Aug;22(2):107–117. doi: 10.1111/j.1399-0039.1983.tb01176.x. [DOI] [PubMed] [Google Scholar]
- Snary D., Barnstable C. J., Bodmer W. F., Crumpton M. J. Molecular structure of human histocompatibility antigens: the HLA-C series. Eur J Immunol. 1977 Aug;7(8):580–585. doi: 10.1002/eji.1830070816. [DOI] [PubMed] [Google Scholar]
- Sodoyer R., Damotte M., Delovitch T. L., Trucy J., Jordan B. R., Strachan T. Complete nucleotide sequence of a gene encoding a functional human class I histocompatibility antigen (HLA-CW3). EMBO J. 1984 Apr;3(4):879–885. doi: 10.1002/j.1460-2075.1984.tb01900.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinkernagel R. M., Doherty P. C. MHC-restricted cytotoxic T cells: studies on the biological role of polymorphic major transplantation antigens determining T-cell restriction-specificity, function, and responsiveness. Adv Immunol. 1979;27:51–177. doi: 10.1016/s0065-2776(08)60262-x. [DOI] [PubMed] [Google Scholar]





