Abstract
This report provides a comparison of the oral decorporation efficacy of L-glutathione (GSH), L-cysteine (Cys), and a liposomal GSH formulation (ReadiSorb) toward systemic cobalt-60 (60Co) to that observed following intravenous administration of GSH and Cys in F344 rats. Aminoacid L-histidine (His) containing no thiol functionality was tested intravenously to compare in vivo efficacy of the aminothiol (GSH, Cys) chelators with that of aminoimidazole (His) chelator. In these studies, 60Co was administered to animals by intravenous injection, followed by intravenous or oral gavage doses of a chelator repeated at 24 hour intervals for a total of 5 doses. The results suggest that GSH and Cys are potent decorporation agents for 60Co in the rat model, although the efficacy of treatment depends largely on systemic availability of the chelator. The intravenous route of administration of GSH or Cys was most effective in reducing tissue 60Co levels and in increasing excretion of radioactivity compared to control animals. Liposomal encapsulation was found to markedly enhance the oral bioavailability of GSH compared to non-formulated GSH. The oral administration of liposomal GSH reduced 60Co levels in nearly all tissues by 12-43% compared to that observed for non-formulated GSH. Efficacy of oral Cys was only slightly reduced in comparison with intravenous Cys. Further studies to optimize the dosing regimen in order to maximize decorporation efficiency are warranted.
Keywords: decorporation, 60Cobalt, aminothiol receptors, cysteine, glutathione
Introduction
Over the past decade, and especially since 11 September 2001, the growing threat of terrorism has become a national security priority. These threats consist of an increased awareness that tactics might involve unconventional weapons, including improvised weapons of mass destruction. Several threat scenarios (IAEA 2004; Levett 2006) predict that segments of the population could be exposed to external radiation and/or internal radioactive contamination. Risk assessments and surveys of commercial radioactive sources have identified cobalt-60 (Co-60) and several other radioisotopes as Category 1 hazard from a radiation safety perspective (Ferguson et al. 2003).
In the event of internal contamination of the body, treatment strategies will focus on preventing the occurrence of acute health effects attributable to a large dose radiation exposure, and/or to restrict the likelihood of late stage health effects such as cancers in a prolonged chronic exposure to relatively low levels of radiation (Valentin 2005). In medical triage protocols, early treatment is recommended in cases where a significant intake of radioactive material is suspected but may take time to confirm (Wood et al. 2000). However, this approach depends on the availability of non-toxic decorporation agents that can be safely and easily administered to the general population. Natural receptors, including polypeptides and proteins with known specificity for metals, are promising candidates to consider for such decorporation agents.
In biological systems, metal ions are strongly coordinated to aminothiol receptors. The tripeptide, glutathione (γGlu-Cys-Gly), is a ubiquitous small biological molecule, found in almost every cell of the living organism (Zhang et al. 2008). Glutathione contains an unusual peptide linkage between the amine group of cysteine (Cys) and the carboxyl group of the glutamate (Glu) side chain (Figure 1). In vivo, L-glutathione exists in reduced (GSH) and oxidized (GSSG) forms and exhibits a multitude of biological functions (Krezel et al. 2003). Literature reviews have reported high affinity of GSH to various metals including transition, lanthanide, certain actinide metal ions and metalloids (Krezel and Bal 1999; Singh 2005). This is due, in part, to a cysteine thiol functionality group, which exhibits a unique ability to bind various metal ions (Smith and Martell 1998). Both GSH and GSSH can form multiple coordination complexes with Co(II)/Co(III) at the physiological pH range of 5 – 9 (Krezel and Bal 1999). The interaction of Co(II) with GSH was first studied by Martin and Edsall (1959) who suggested multiple pH-dependent coordination modes for GSH with Co(II) via both the amino acid and thiol functional moieties. For example, at pH 5-7, Co(II) is predominantly complexed by the Glu residue; increasing pH results in both Glu and Cys coordination, and in strongly basic solutions Co(II) is also bound to the deprotonated amide donor group (Martin et al. 1981; Harman and Sovago 1983).
Figure 1.
Chemical structures of the studied compounds.
In addition to metal complexation, GSH exhibits a number of well-established biological activities, including redox-buffering of the cell environment, antioxidant activity, and detoxification of xenobiotics (Krezel and Bal 2003). Supplementation with GSH has been suggested as an effective way to mitigate metal-induced oxidative stress (Maines and Kappas 1977). GSH is essentially non-toxic at reasonable doses, with an oral LD50 of 5 g/kg in the mouse. Intravenous administration of 1200 mg GSH on a repeated basis for nine months showed no side effects and demonstrated benefit in preventing red blood cell hemolysis in patients undergoing hemodialysis (Usberti et al. 1997). These attributes make GSH an attractive candidate for decorporation therapy. To this end, GSH may uniquely play a double role as a decorporation agent accelerating the removal of internalized 60Co as well as reducing the associated health effects of whole-body exposure due to gamma radiation.
Unfortunately, GSH suffers from low oral bioavailability, and treatment with non-formulated GSH requires intravenous (IV) administration, thus preventing simple dispersal to the general public in the event of a radiological/nuclear emergency (Witschi et al. 1992). A recently developed novel liposomal formulation of GSH (ReadiSorb) is specifically designed to maximize gastrointestinal absorption of GSH (ReadiSorb 2008). The study reported here compared 60Co decorporation efficacy of ReadiSorb with oral and IV administration of non-formulated GSH using a rat model.
It was also of interest to evaluate in vivo 60Co affinity of common amino acid L-cysteine (Cys) possessing aminothiol binding moiety similar to that of GSH and of the aminoimidazole receptor L-histidine (His). Several literature studies have demonstrated the formation of stable Co(II)/Co(III) complexes with Cys in vitro (Harman and Sovago 1983; Bresson et al. 2006, 2007). Coordination of Co(II) to imidazole nitrogen was previously demonstrated (Harman and Sovago 1983) and the resulting complexes have been shown to possess antibacterial and antifungal properties (Chohan et al. 2006). A comparison of in vivo efficacy of GSH, Cys, and His can provide important information on structure-function relationship of Co-amino acid complexes and their metabolism and provide valuable information for design of more effective detoxification therapies for cobalt.
Materials and Methods
General
The 60Co tracer solution in 1 M HCl was purchased from Eckert and Ziegler Isotope Products (Valencia, CA). Aqueous solutions were prepared using distilled water deionized to 18 MΩ-cm with a Barnstead Nanopure water purification system (Thermo Fisher Scientific, Dubuque, IA). The 60Co dosing solutions were individually prepared for each animal and counted for radioactivity before and after dosing to accurately determine the administered dose per animal. Animals were administered 60Co as CoCl2 at an approximate activity of 14.5 kBq per animal, which on a body weight (BW) basis, is equivalent to 4×10-5 mmol kg-1.
L-cysteine (Cys), L-histidine (His), and reduced L-glutathione (GSH) were purchased from Sigma-Aldrich (St. Louis, MO) as reagent grade. Liquid liposomal GSH (ReadiSorb, Your Energy Solution, Inc.) was provided in a liquid suspension consisting of purified water (72.4%), L-glutathione (8.5%), glycerin (15%), hydroxylated lecithin (1.5%), and potassium sorbate (0.1%) by weight, and administered to animals by oral gavage without modification.
Experimental Animals
Male F344 (130-170 g BW) were obtained from Charles River Breeding Laboratory (Raleigh, NC). All animals were purchased with indwelling jugular-vein cannula. During acclimation, animals were housed in solid-bottom cages with hardwood chips and provided certified PMI 5002 Rodent Diet (Animal Specialties, Inc., Hubbard, OR) and water ad libitum. Animals were acclimated in a humidity- and temperature-controlled room with a 12-h light/dark cycle for at least 3 days prior to use. Cannula patency was ensured by establishing that blood could be drawn into the cannula tubing, followed by flushing with heparinized saline using a disposable 1-mL syringe with a 23-gauge blunt-tip needle. All animal protocols were approved by the Institutional Animal Care and Use Committee at Pacific Northwest National laboratory and studies were performed according to the “Guide for the Care and Use of Laboratory Animals” (National Research Council 1996).
Study Design
Animals were randomly assigned to one of 7 groups of n = 3 – 12 animals per group. All animals received a single IV injection (0.2 mL) of 60Co in a sterile saline solution at a dose of 14.5 ± 0.5 kBq via the indwelling jugular vein cannula. The administration of the radionuclide by the IV route was purposely selected to model systemic circulation of radioactivity, without delays in absorption as might be observed with dermal, oral or inhalation exposures. Immediately following IV injection, groups of animals received an oral gavage dose of 0.5 mL ReadiSorb (containing GSH of 1.0 mmol kg BW-1), GSH (1.0 mmol kg BW-1), or Cys (1.0 mmol kg BW-1); additional sets of animals received an IV dose (0.3 mL) of GSH (0.28 mmol kg BW-1), Cys (1.4 mmol kg BW-1) or His (1.3 mmol kg BW-1). For IV administration, 60Co and chelating agents were dissolved in buffered 0.9% saline and administered at near-neural pH (pH 5 – 6); sodium bicarbonate or HEPES buffer was used to adjust pH as needed. For oral administration, chelators were dissolved in deionized water. Animals in the control group received only the IV dose of 60Co without subsequent administration of any chelation material. Animals receiving oral administration of the chelator were fasted overnight prior to 60Co exposure. Following dosing, all animals were housed in Nalgene® metabolism cages and provided food and water ad libitum. Repeat doses of chelation materials were administered to animals at 24-hour intervals until sacrifice. Urine and feces were collected per metabolism cage daily and analyzed for radioactivity. Animals were sacrificed 5 days following the 60Co exposure, and selected tissues (liver, kidney, skin, muscle, femur, heart, blood, lung, spleen and brain) were collected, weighed, and analyzed for radioactivity.
Tissue counting
Collected urine, feces, and whole tissues were counted for radioactivity using an automated Wallac 1480 (Perkin Elmer, Waltham, MA) gamma counter equipped with 3″ NaI(Tl) crystal shielded detector. Gamma count data were normalized to percent administered dose after adjusting the grams of tissue collected for total organ mass. Total organ mass calculations for blood, skeleton, muscle and skin/hair assume that these organs are approximately 6, 7, 40 or 19 %, respectively, of the body weight of the animal (Brown et al. 1997). Further, it was assumed that radioactivity in femur was representative of the skeleton as a whole.
Data Statistical Evaluation
Each data group was subjected to Dixon's Q-test to evaluate for potential outliers. In this test, Q parameter of 0.625 (n=6, 95% confidence level, α = 0.05) was used (Rorabacher 1991); the final n values are shown in Tables 1 and 2. For other 60Co and 210Po data groups, no outliers were found and n=6 was used in all cases. For each tissue, a preliminary F-test (95% confidence level, α = 0.05) for the equality of variances of the controls and each treatment group was performed using Excel software. If the calculated probability p-value was less than 0.05 (p < 0.05), the variances were assumed to be not equal. Based on this information, a T-test: two-sample assuming unequal variances or T-test: two-sample assuming equal variances (95% confidence level, α = 0.05) was performed using Excel software. The calculated p-value less than 0.05 provide evidence to reject the null hypothesis of equal means.
Table 1.
Urinary and fecal elimination of 60Co following IV administration in F344 rats: Effect of oral versus IV administered chelators (expressed as average % administered radioactivity ± SD).
| Control (n=12) |
Oral ReadiSorb (n=5) |
Oral GSH (n=6) |
IV GSH (n=4) |
Oral Cys (n=6) |
IV Cys (n=4) |
IV His (n=3) |
|
|---|---|---|---|---|---|---|---|
| Urine | |||||||
| Day 1 | 62.5 ± 5.4 | 64.8 ± 5.7 | 57.4 ± 11.7 | 80.9 ± 2.7a | 67.7 ± 7.7 | 77.2 ± 8.1a | 66.2b |
| Day 2 | 7.4 ± 1.3 | 5.6 ± 2.2 | 6.7 ± 2.5 | 7.3 ± 1.2 | 7.79 ± 0.69 | 6.8 ± 1.7 | 8.13 ± 0.26 |
| Day 3 | 1.92 ± 0.23 | 1.49 ± 0.21 | 2.3 ± 1.5 | 1.66 ± 0.39 | 1.79 ± 0.23 | 1.50 ± 0.08 | 1.95 ± 013 |
| Day 4 | 0.98 ± 0.12 | 1.03 ± 0.75 | 1.34 ± 0.61 | 0.44 ± 0.41 | 0.79 ± 0.07 | 0.89 ± 0.43 | 1.36 ± 0.08 |
| Day 5 | 0.56 ± 0.18 | 0.77 ± 0.53 | 0.87 ± 0.58 | 0.24 ± 0.20 | 0.58 ± 0.15 | 0.30 ± 0.10 | 0.95 ± 0.25 |
| Feces | |||||||
| Day 1 | 3.32 ± 0.79 | 4.1 ± 1.7 | 3.9 ± 1.1 | 1.54 ± 0.32 | 3.71 ± 0.72 | 1.8 ± 1.4 | 5.1 ± 1.1 |
| Day 2 | 2.9 ± 1.2 | 1.90 ± 0.21 | 2.6 ± 1.7 | 1.59 ± 0.25 | 1.86 ± 0.49 | 1.78 ± 0.69 | 2.90 ± 0.03 |
| Day 3 | 1.47 ± 0.58 | 0.94 ± 0.21 | 1.41 ± 0.66 | 0.89 ± 0.08 | 0.97 ± 0.35 | 0.95 ± 0.22 | 1.23 ± 0.17 |
| Day 4 | 0.77 ± 0.21 | 0.50 ± 0.19 | 0.67 ± 0.11 | 0.32 ± 0.15 | 0.39 ± 0.10 | 0.42 ± 0.24 | 0.76 ± 0.03 |
| Day 5 | 0.49 ± 0.16 | 0.32 ± 0.12 | 0.45 ± 0.20 | 0.12 ± 0.03 | 0.28 ± 0.07 | 0.25 ± 0.11 | 0.33 ± 0.19 |
Statistically different from corresponding control group by two tailed t-test (P < 0.05).
Urine was collected from only two animals
Table 2.
Tissue distribution of IV administered 60Co in F344 rats at day 5 post radionuclide exposure: Effect of chelation treatment with decorporation agents (expressed as average % administered radioactivity ± SD).
| Tissue |
60Co Control (n=12) |
Oral ReadiSorb (n=6) |
GSH | Cys | His | ||
|---|---|---|---|---|---|---|---|
| Oral (n=6) | IV (n=6) | Oral (n=6) | IV (n=4) | IV (n=3) | |||
| Liver | 2.22 ± 0.61 | 1.17 ± 0.43 a | 2.04 ± 0.52 | 0.81 ± 0.01 a | 0.84 ± 0.12 a | 0.29 ± 0.06 a | 1.29 ± 0.11 a |
| Muscle b | 1.25 ± 0.32 | 0.82 ± 0.23 a | 0.98 ± 0.09 a | 0.34 ± 0.02 a | 0.66 ± 0.21 a | 0.26 ± 0.14 a | 0.72 ± 0.15 a |
| Kidney | 0.64 ± 0.10 | 0.51 ± 0.13 a | 0.62 ± 0.07 | 0.32 ± 0.02 a | 0.53 ± 0.07 a | 0.35 ± 0.09 a | 0.43 ± 0.03 a |
| Skeleton b | 0.62 ± 0.17 | 0.38 ± 0.10 a | 0.53 ± 0.07 | 0.27 ± 0.02 a | 0.30 ± 0.05 a | 0.17 ± 0.06 a | 0.50 ± 0.05 |
| Skin b | 0.56 ± 0.13 | 0.36 ± 0.06 a | 0.41 ± 0.04 a | 0.19 ± 0.06 a | 0.32 ± 0.10 a | 0.15 ± 0.06 a | 0.28 ± 0.05 a |
| Heart | 0.07 ± 0.02 | 0.04 ± 0.01 a | 0.045 ± 0.007 a | 0.03 ± 0.01 a | 0.030 ± 0.006 a | 0.010 ± 0.001 a | 0.036 ± 0.009 |
| Blood b | 0.06 ± 0.01 | 0.04 ± 0.01 a | 0.06 ± 0.02 | 0.022 ± 0.008 a | 0.033 ± 0.008 a | 0.013 ± 0.008 a | 0.04 ± 0.03 a |
| Spleen | 0.06 ± 0.02 | 0.039 ± 0.006 a | 0.051 ± 0.008 | 0.03 ± 0.01 a | 0.034 ± 0.006 a | 0.017 ± 0.002 a | 0.026 ± 0.001 a |
| Lung | 0.05 ± 0.01 | 0.032 ± 0.009 a | 0.041 ± 0.006 | 0.014 ± 0.008 a | 0.027 ± 0.003 a | 0.013 ± 0.005 a | 0.025 ± 0.008 a |
| Brain | 0.028 ± 0.006 | 0.022 ± 0.001 a | 0.024 ± 0.004 a | 0.014 ± 0.006 a | 0.018 ± 0.005 a | 0.009 ± 0.002 a | 0.017 ± 0.004 a |
Statistically different from corresponding control group by two tailed t-test (P < 0.05).
Calculation assumes that total skeleton, blood, muscle, or skin is approximately 7.3, 6, 40, or 19% of the body weight of the animal, respectively (Brown et al. 1997); radioactivity in skeleton is calculated based on the femur data.
Results
Following IV administration, the elimination of 60Co was predominantly through the kidney, with a cumulative 5-day urinary excretion of approximately 73% of the administered dose in control animals (Table 1). This result is in excellent agreement with data by Gregus and Klaasen (1986) who reported that following a single IV injection of 1 mg Co(II) kg-1, 72.6% of non-radioactive cobalt was excreted in urine by day 4. In comparison to urine, fecal elimination was much lower, with approximately 9% of the administered dose eliminated over the 5-day period from control animals. For both urine and fecal routes, elimination of radioactivity peaked on the first day post exposure and decreased sharply thereafter (Table 1).
Regardless of treatment group, total cumulative excretion of 60Co ranged from approximately 78 to 95% over the 5-day period. Of the chelators evaluated, IV administration of GSH, His and Cys appeared to enhance overall elimination of radioactivity compared to control animals, although this result was statistically significant only for GSH and Cys. For animals treated intravenously with either GSH or Cys, urinary elimination of 60Co was markedly increased at 24 hr post radionuclide exposure with 80.9 ± 2.7% and 77.2 ± 8.1% eliminated, respectively, compared to that observed for control animals (62.5 ± 5.4%); this difference was statistically significant (P<0.05). In contrast, urinary elimination of radioactivity from animals receiving oral chelators (ReadiSorb, GSH, or Cys) or His by the IV route did not differ from control animals.
The increase in urinary elimination observed with IV administration of GSH and Cys was accompanied by a corresponding reduction in fecal elimination (Table 1). Administration of His by the IV route significantly increased (P<0.05) fecal elimination of 60Co at the initial 24 hr post exposure time interval, but not at subsequent times. None of the other administered chelators had any impact on fecal elimination of radioactivity over the 5-day period. The cumulative total 5 day urinary and fecal elimination of 60Co is shown in Figure 2. The increase in total excretion of 60Co was observed for animal groups receiving GSH, Cys, and His by the IV route. On the other hand, orally administered chelators had no significant impact on the elimination of 60Co.
Figure 2.
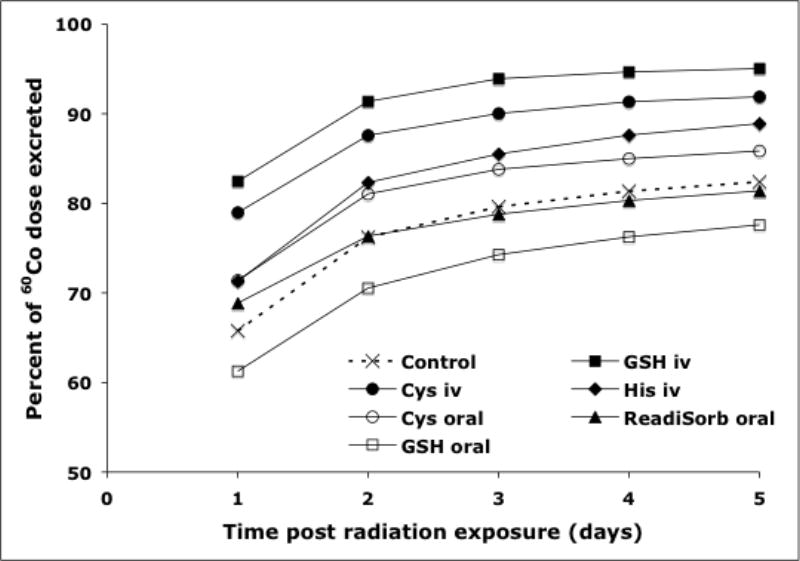
Comparison of combined 5 day excretion of 60Co in the treatment and control animal groups expressed as percent of the administered dose.
All animals were sacrificed at 5 days post 60Co exposure and tissues collected and analyzed for radioactivity. Of the tissues collected, the liver contained the highest amount of radioactivity, followed by muscle, kidney, skeleton and skin (Table 2). Low levels were observed in blood, heart, spleen, lung and brain tissues. In total, tissue radioactivity accounted for approximately 1.3 to 5.6% of the administered 60Co, with total cumulative recovery (excretion plus tissues) ranging from 82 to 97% of the administered radioactivity. Residual and radioactivity unaccounted for ranged from a low of 3% in animals treated with IV administration of GSH, to a high of approximately 18% in animals treated with GSH by the oral route.
Overall, tissue 60Co levels were consistent with excretion data, in that animals with the highest 60Co elimination had the lowest tissue concentrations. For example, animals treated with either GSH or Cys by the IV route excreted approximately 92 to 95% of the administered radioactivity with 1 to 2% of the administered dose recovered in tissues. In contrast, control animals excreted approximately 82% of the administered radioactivity over the 5-day period with over 5% remaining in tissues. In general, tissue 60Co levels in animals treated by oral administration of GSH were equivalent to those observed in control animals, with several exceptions; radioactivity in muscle, skin/hair, heart and brain tissues were significantly lower than corresponding levels in control animals. In comparison, radioactivity in all tissues analyzed from animals treated with oral ReadiSorb, oral Cys, or IV Cys were significantly lower (P<0.05) than observed in control animals (Table 2). Radioactivity in tissues from animals treated with IV His was significantly reduced compared to control animals for all tissues with the exception of skeleton and heart.
The impact of chelation on tissue 60Co levels can be discerned by comparison of the percent reduction in radioactivity in tissues from chelator-treated animals versus control animals (Table 3). Of the chelators evaluated, IV administration of Cys was most effective at reducing 60Co levels in tissues, with statistically significantly reductions of 45-87% compared to control animals. In contrast, oral administration of non-formulated GSH was the least effective, with tissue reductions of 0 to 26% compared to control animals. Oral administration of ReadiSorb reduced levels of 60Co in all tissues by 21-47% compared to control animals.
Table 3.
Percent reduction of 60Co in tissues of treated animals groups compared to the control group.
| Tissue | Oral ReadiSorb (n=5) |
GSH | Cys | His | ||
|---|---|---|---|---|---|---|
| Oral (n=6) | IV (n=4) | Oral (n=6) | IV (n=4) | IV (n=3) | ||
| Liver | 47 | 0 | 64 | 62 | 87 | 42 |
| Muscle | 34 | 21 | 73 | 47 | 80 | 42 |
| Kidney | 21 | 0 | 50 | 16 | 45 | 33 |
| Skeleton | 38 | 14 a | 57 | 51 | 73 | 19 a |
| Skin/Hair | 36 | 26 | 66 | 42 | 74 | 49 |
| Heart | 41 | 31 | 59 | 54 | 84 | 44 a |
| Blood | 33 | 0 | 65 | 46 | 79 | 37 |
| Spleen | 30 | 0 | 53 | 39 | 70 | 53 |
| Lung | 35 | 16 a | 71 | 46 | 74 | 50 |
| Brain | 22 | 14 | 49 | 33 | 67 | 38 |
Statistically non-significant by t-test (P > 0.05)
Discussion
Reduced glutathione (GSH) possesses as many as eight coordination sites, including two carboxyl, one thiol, one amino, and two pairs of carbonyl and amide donors within two peptide bonds, making it an important endogenous complexing agent for transporting metals between tissues and body fluids. L- Cysteine (Figure 1) is a non-essential amino acid synthesized by the body under normal physiological conditions, and one of the peptides of GSH. With a thiol side chain, Cys is an important structural and functional component of many proteins and enzymes. Oxidation of Cys produces the disulfide cystine, which is more stable in the gastrointestinal tract.
L-Cystine travels through the gastrointestinal tract and in blood plasma, and is promptly reduced upon cell entry. Griffith et al. (1942) first demonstrated the utility of Cys added to the diet for the detoxification of dietary cobalt in the rat model. Further, supplementation of a cobalt-containing diet with Cys or His was shown to inhibit the development of cobalt-induced polycythemia (Orten and Bucciero 1948). In later studies, both GSH and Cys were evaluated for effectiveness of chelating cobalt following intraperitoneal administration in mice, with both showing good antidotal action (Llobet et al. 1985, 1986).
The studies reported here were designed to compare the oral decorporation efficacy of non-formulated GSH, Cys or a liposomal GSH formulation toward systemic 60Co to that observed following IV administration of GSH, Cys or His. The administered chelator doses ranged from 0.28 to 1.4 mmol kg BW-1 and significantly exceeded the amount of administered 60Co (4×10-5 mmol kg BW-1). Oral doses of ReadiSorb, GSH, and Cys contained equal amounts of the chelator (1.0 mmol kg BW-1) for direct comparison. The systemic dose of GSH was reduced to 0.28 mmol kg BW-1; IV doses of Cys and His were 1.3 and 1.4 mmol kg BW-1, respectively.
Overall, a significant increase in the elimination of 60Co was observed for animals receiving either GSH or Cys by the IV route compared to control animals, which is in agreement with previous literature (Llobet et al. 1985, 1986). Radioactivity in tissues collected from animals treated with GSH or Cys by the IV route was reduced 45-87% compared to control animals. Cumulative 60Co excretion from animals receiving oral chelators or His by the IV route was generally similar to the control animals. However, despite roughly equivalent elimination levels, tissue 60Co, as a percent of administered radioactivity, was found to be significantly lower in animals that received chelation materials for most tissues collected.
Of the chelators evaluated, oral administration of non-formulated GSH was least effective in reducing tissue levels of 60Co. In comparison of route of administration, non-formulated GSH by the IV route reduced tissue 60Co levels by 40 to 75% compared to non-formulated GSH by the oral route, thus supporting prior reports of low oral bioavailability of GSH (Witschi et al. 1992). Oral administration of ReadiSorb reduced 60Co levels in all tissues except brain by approximately 12 to 43% compared to that observed for oral administration of non-formulated GSH.
This study suggests that reduced GSH is a potent decorporation agent for 60Co in the rat model. The obtained data support the prerequisite that oral delivery of liposomal glutathione markedly enhances its bioavailability compared to oral administration of non-formulated glutathione. Unmodified GSH has limited systemic availability and exhibits little functional effect in terms of decorporation; liposomal GSH appears to have the functional capacity to remove 60Co. The IV administration of non-formulated GSH, however, was still more effective at decorporation, with tissue 60Co reductions of 25 to 67% compared to that observed with ReadiSorb treatment. This difference might be attributed to lower systemic absorption of ReadiSorb compared to IV delivery, or might be dependent on the kinetics of absorption from the GI tract. For example, the IV administration of GSH may provide immediate chelation opportunities within the blood, and decrease 60Co binding to metallo-enzymes within tissues. This supports the suggestion that chelation treatment for 60Co be initiated with an hour of exposure, while greater than 20% of the radionuclide is still in circulation (Taylor et al. 2000). Tissue radioactivity levels following oral administration of Cys were generally similar to those observed for ReadiSorb treatment, but 30-70% higher than levels following IV administration of Cys. The IV administration of His was found to be as effective, or slightly more so, as oral administration of ReadiSorb at reducing tissue levels of 60Co compared to control animals. Tissue 60Co levels were mostly comparable following treatment of either Cys or GSH by the IV route of administration, with the exception of liver, which was significantly lower for Cys-treated animals.
Although IV administration of GSH or Cys was most effective in both reducing tissue 60Co levels and increasing excretion of radioactivity, effective delivery of a decorporation agent could be severely limited if necessitated by this route. The rapid triage treatment of large numbers of the population following a radiological or nuclear emergency will be problematic if decorporation agents must be administered and monitored using an invasive procedure, such as the IV route. The accessibility of an oral, non-toxic chelator for 60Co connotes that the chelation therapy can be continued for a prolonged period of time to continue the removal of 60Co, which may lessen the long term effects of this exposure.
Conclusions
The studies reported here evaluated the decorporation efficacy of GSH and Cys while comparing the impact of the route of administration on their ability to remove 60Co from the body. A novel liposomal formulation of GSH designed to maximize oral bioavailability was additionally examined. Further, the role of the cysteine residue of GSH was comparatively evaluated for both IV and oral routes of administration.
The obtained results suggest that both GSH and Cys are potent decorporation agents for 60Co in the rat model. The efficacy of the treatment largely depends on systemic availability, with administration by the IV route more effective than that observed by the oral route. The liposomal formulation of GSH was more effective by the oral route of administration at reducing tissue radioactivity concentrations compared to non-formulated oral GSH. Further studies to optimize the dosing regimen in order to maximize decorporation efficiency are warranted.
Acknowledgments
This research was supported by the Laboratory Directed Research and Development Program at the Pacific Northwest National Laboratory operated by Battelle for the U.S. Department of Energy under Contract DE-AC05-76RL01830 and by National Institute of Allergy and Infectious Diseases, Project Bioshield No 1R01AI074067-01.
References
- Bresson C, Esnouf S, Lamouroux C, Solari PL, Auwer CD. XAS investigation of biorelevant cobalt complexes in aqueous media. New J Chem. 2006;30:416–424. [Google Scholar]
- Bresson C, Spezia R, Esnouf S, Solari PL, Coantice S, Auwer CD. A combined spectroscopic and theoretical approach to investigate structural properties of Co(II)/Co(III) tri-cysteinato complexes in aqueous medium. New J Chem. 2007;31:1789–1797. [Google Scholar]
- Brown RP, Delp MD, Lindstedt SL, Rhomberg LR, Beliles RP. Physiological parameter values for physiologically based pharmacokinetic models. Toxicol Ind Health. 1997;13:407–484. doi: 10.1177/074823379701300401. [DOI] [PubMed] [Google Scholar]
- Chohan ZC, Akhtar MA, Cupuran CT. Metal-based antibacterial and antifungal agents: Synthesis, characterization, and in vitro biological evaluation of Co(II), Cu(II), Ni(II) and Zn(II) complexes with amino acid-derived compounds. Bioinorg Chem Appl. 2006;1-13 doi: 10.1155/BCA/2006/83131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson CD, Kazi T, Perera J. Commercial Radioactive Sources: Surveying the Security Risks. Center for Nonproliferation Studies. Monterey Institute of International Studies; 2003. Available at: http://cns.miis.edu/pubs/opapers/op11/index.htm.
- Gregus Z, Klassen CD. Disposition of metals in rats: A comprehensive study of fecal, urinary, and biliary excretion and tissue distribution of eighteen metals. Toxicol Appl Pharm. 1986;85:24–38. doi: 10.1016/0041-008x(86)90384-4. [DOI] [PubMed] [Google Scholar]
- Griffith WH, Pauvcek PL, Mulford DJ. The relationship of the sulfur amino acids to the toxicity of cobalt and nickel in the rat. J Nutrition. 1942;23:603–612. [Google Scholar]
- Harman B, Sovago I. Metal complexes of sulphur-containing ligands. V. Interactions of cobalt(II) ion with L-cysteine and its derivatives. Inorg Chim Acta. 1983;80:75–83. [Google Scholar]
- IAEA. International Atomic Energy Agency; Vienna, Austria: [17 October 2008]. Promoting Nuclear Security: Possible Terrorist Scenarios. Available at: http://www.iaea.org/NewsCenter/Features/NuclearSecurity/scenarios20040601.html. [Google Scholar]
- Krezel A, Bal W. Coordination chemistry of glutathione. Acta Biochim Polonica. 1999;46:567–580. [PubMed] [Google Scholar]
- Krezel A, Bal W. Structure-function relationships in glutathione and its analogues. Organic and Biomolec Chem. 2003;22:3885–3890. doi: 10.1039/b309306a. [DOI] [PubMed] [Google Scholar]
- Krezel A, Szczepanik W, Sokolowka M, Jezowska-Bojczuk M, Bal W. Correlations between complexation modes and redox activities of Ni(II)-GSH complexes. Chem Res Toxicol. 2003;16:855–864. doi: 10.1021/tx034012k. [DOI] [PubMed] [Google Scholar]
- Levett J. Radiological terrorism scenarios. Prehospital and Disaster Medicine. 2006;21 [PubMed] [Google Scholar]
- Llobet JM, Domingo JL, Corbella J. Comparison of antidotal efficacy of chelating agents upon acute toxicity of cobalt in mice. Res Commun Chem Pathol Pharmacol. 1985;50:305–308. [PubMed] [Google Scholar]
- Llobet JM, Domingo JL, Corbella J. Comparison of effectiveness of several chelators after single administration on the toxicity, excretion and distribution of cobalt. Arch Toxicol. 1986;58:278–281. doi: 10.1007/BF00297121. [DOI] [PubMed] [Google Scholar]
- Maines MD, Kappas A. Regulation of heme pathway enzymes and cellular glutathione content by metals that do not chelate with tetrapyrroles: Blockade of metal effects by thiols. Proc National Acad Sci. 1977;74:1875–1878. doi: 10.1073/pnas.74.5.1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin R, Edsall J. The association of divalent cations with glutathione. J Am Chem Soc. 1959;81:4044–4047. [Google Scholar]
- Martin X, Abello L, Ensuque A, Toniti R, Lapluye G. Contribution to the study of the chelated complexes of glutathione. 3. Study of the complexation of cobalt (ii) with reduced glutathione, oxidized glutathione and glycyl-methionine. J de Chimie Physique et de Physico-Chemie Biologique. 1981;78:615–619. [Google Scholar]
- National Research Council. Guide for the Care and Use of Laboratory Animals. National Academy Press; Washington, DC: 1996. [Google Scholar]
- Orten JM, Bucciero MC. The effect of cysteine, histidine, and methionine on the production of polycythemia by cobalt. J Biol Chem. 1948;176:961–968. [PubMed] [Google Scholar]
- Readisorb. Your Energy Systems, LLC. [2 April 2008]; Available at http://www.readisorb.com.
- Rorabacher DB. Statistical treatment for rejection of deviant values: Critical values of Dixon's “Q” parameter and related subrange ratios at the 95% confidence level. Anal Chem. 1991;63:139–146. [Google Scholar]
- Singh B. Complexation behavior of glutathione with metal ions. Asian J Chem. 2005;17:1–32. [Google Scholar]
- Smith RM, Martell AE. Critical stability constants. In: Martell AE, Smith RM, Motekaitis RJ, editors. Critically Selected Stability Constants of Metal Complexes Database Version 5.0. New York, NY: Plenum Press; 1998. [Google Scholar]
- Taylor DM, Stradling GN, Henge-Napoli MH. The scientific background to decorporation. Radiat Prot Dosim. 2000;87:11–17. [Google Scholar]
- Usberti M, Lima G, Arisi M, Bufano G, D'Avanzo L, Gazzotti RM. Effects of exogenous reduced glutathione on the survival of red blood cells in hemodialyzed patients. J Nephrol. 1997;10:261–265. [PubMed] [Google Scholar]
- Valentin J. Protecting people against radiation exposure in the event of a radiological attack. A Report of the International Commission on Radiological Protection. Annals of ICRP. 2005;35:1–110. doi: 10.1016/j.icrp.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Witschi A, Reddy S, Stofer B, Lauterburg BH. The systemic availability of oral glutathione. Eur J Clin Pharmacol. 1992;43:667–669. doi: 10.1007/BF02284971. [DOI] [PubMed] [Google Scholar]
- Wood R, Sharp C, Gourmelon P, Le Guen B, Stradling GN, Taylor DM, Henge-Napoli MH. Decorporation treatment – Medical overview. Radiat Prot Dosim. 2000;87:51–56. [Google Scholar]
- Zhang F, Bartels MJ, Geter DR, Jeong YC, Schisler MR, Wood AJ, Kan L, Gollapudi BB. Quantitation of glutathione by liquid chromatography/positive electrospray ionization tandem mass spectrometry. Rapid Commun Mass Spectrom. 2008;22:3608–3614. doi: 10.1002/rcm.3776. [DOI] [PubMed] [Google Scholar]