Abstract
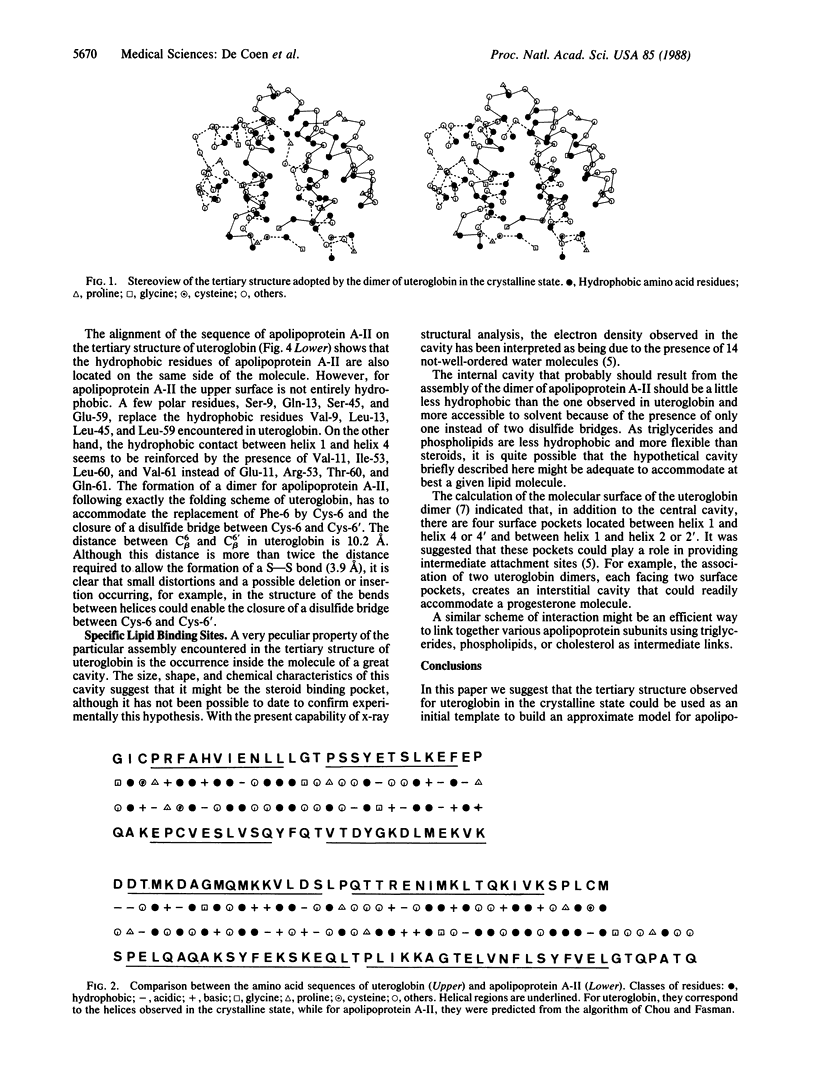
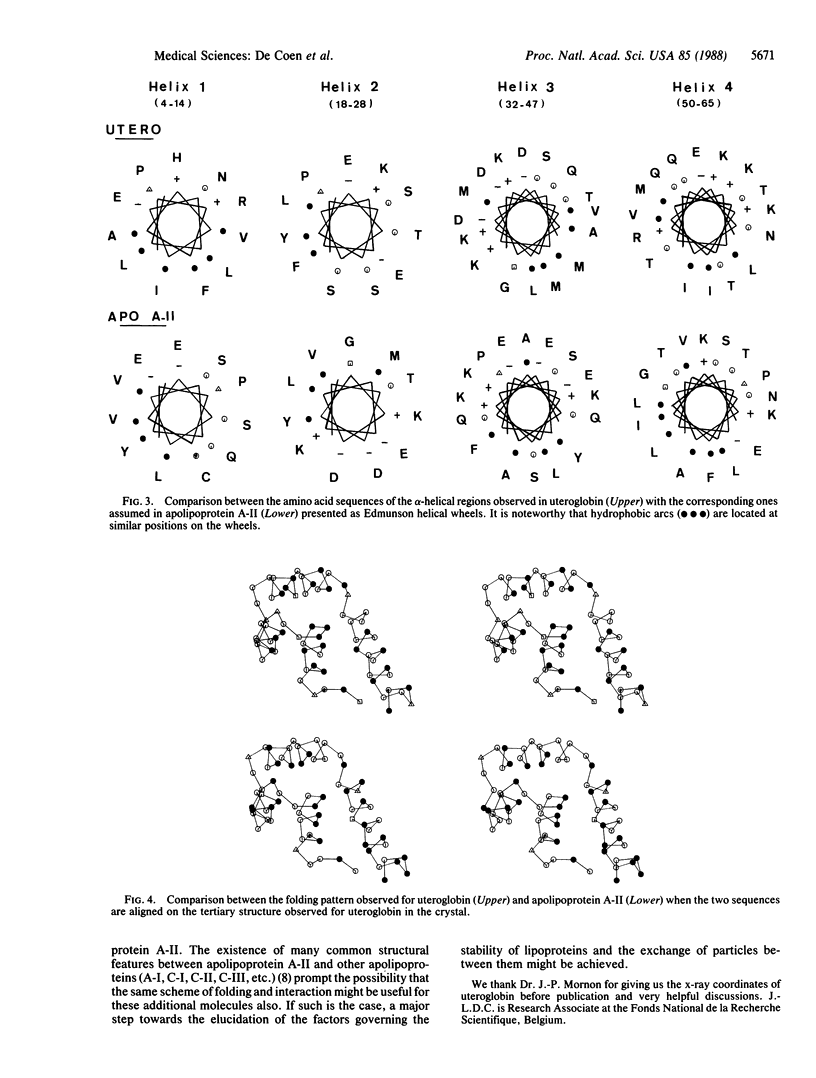
The tertiary structure observed in the crystalline state for uteroglobin, a small steroid binding protein, is used as a template to build an approximated model for apolipoprotein A-II. The presence of four proline residues and four hydrophobic clusters located at similar positions in apolipoprotein A-II and uteroglobin is taken as the major source of stability in such tertiary structures. A brief description of plausible specific binding sites appearing on the model of apolipoprotein A-II is given. It is suggested that the internal cavity and the four surface pockets observed for uteroglobin and postulated for apolipoprotein A-II might be used to insure specific binding of triglycerides, phospholipids, or cholesterol.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Gaboriaud C., Bissery V., Benchetrit T., Mornon J. P. Hydrophobic cluster analysis: an efficient new way to compare and analyse amino acid sequences. FEBS Lett. 1987 Nov 16;224(1):149–155. doi: 10.1016/0014-5793(87)80439-8. [DOI] [PubMed] [Google Scholar]
- Luo C. C., Li W. H., Moore M. N., Chan L. Structure and evolution of the apolipoprotein multigene family. J Mol Biol. 1986 Feb 5;187(3):325–340. doi: 10.1016/0022-2836(86)90436-5. [DOI] [PubMed] [Google Scholar]
- Morize I., Surcouf E., Vaney M. C., Epelboin Y., Buehner M., Fridlansky F., Milgrom E., Mornon J. P. Refinement of the C222(1) crystal form of oxidized uteroglobin at 1.34 A resolution. J Mol Biol. 1987 Apr 20;194(4):725–739. doi: 10.1016/0022-2836(87)90250-6. [DOI] [PubMed] [Google Scholar]
- Osborne J. C., Jr, Brewer H. B., Jr The plasma lipoproteins. Adv Protein Chem. 1977;31:253–337. doi: 10.1016/s0065-3233(08)60220-x. [DOI] [PubMed] [Google Scholar]
- Pownall H. J., Pao Q., Hickson D., Sparrow J., Gotto A. M. Thermodynamics of lipid-protein association in human plasma lipoproteins. Biophys J. 1982 Jan;37(1):175–177. doi: 10.1016/s0006-3495(82)84658-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segrest J. P., Jackson R. L., Morrisett J. D., Gotto A. M., Jr A molecular theory of lipid-protein interactions in the plasma lipoproteins. FEBS Lett. 1974 Jan 15;38(3):247–258. doi: 10.1016/0014-5793(74)80064-5. [DOI] [PubMed] [Google Scholar]


