Abstract
Indian hedgehog (Ihh) is essential for chondrocyte proliferation/differentiation and osteoblast differentiation during prenatal endochondral bone formation. Ihh expression in postnatal chondrocytes has a non-redundant role in maintaining a growth plate and sustaining trabecular bone after birth. Loss of Ihh in postnatal chondrocytes results in fusion of the growth plate and a decrease in trabecular bone. In order to normalize this abnormal chondrocyte phenotype and to investigate whether a putative rescue of the growth plate anomalies is sufficient to correct the severe alterations in the bone, we expressed a constitutively active PTH/PTHrP receptor, (an Ihh downstream target) in the chondrocytes of Col2α1-Cre ER*; Ihhdld mice by mating Col2α1-Cre ER*; Ihhfl/fl mice with Col2α1-constitutively active PTH/PTHrP receptor transgenic mice (Jansen, J). Col2α1-Cre ER*; Ihhf/f; J mice were then injected with tamoxifen at P0 to generate Col2α1-Cre ER*; Ihhd/d; J mice. In contrast with the previously reported growth plate phenotype of Col2α1-Cre ER*; Ihhd/d mice that displayed ectopic chondrocyte hypertrophy at P7, growth plates of Col2α1-Cre ER*; Ihhd/d; J double mutants were well organized, and exhibited a gene expression pattern similar to the one of control mice. However, expression of osteoblast markers and Dkk1, a Wnt signaling target, remain decreased in the bone collar of Col2α1-Cre ER*; Ihhd/d; J mice when compared to control mice despite the rescue of abnormal chondrocyte differentiation. Moreover, proliferation of chondrocytes was still significantly impaired in Col2α1-Cre ER*; Ihhd/d; J mice, and this eventually led to the fusion of the growth plate at P14. In summary, we have demonstrated that expression of a Jansen receptor in chondrocytes was able to rescue abnormal chondrocyte differentiation but not impaired chondrocyte proliferation and the bone anomalies in mice lacking the Ihh gene in chondrocytes after birth. Taken together, our findings suggest that Ihh has both PTHrP -dependent and –independent functions during postnatal endochondral bone development.
Keywords: Ihh, Bone, Growth plate, Jansen, PTHrP, Chondrocytes, Rescue
Introduction
The chondrocyte and osteoblast differentiation pathways are interrelated during endochondral bone formation where signaling molecules such as Indian hedgehog (Ihh), parathyroid hormone-related peptide (PTHrP), and Wnt play essential roles in regulating endochondral bone formation [1–4] Indian hedgehog signaling, in particular, plays essential roles in chondrocyte proliferation and differentiation, and in osteoblast differentiation during prenatal endochondral bone formation [1–3, 5, 6]. The Ihh signal is transduced through smoothened (Smo), a putative G protein-coupled seven-transmembrane domain protein [7]. In the absence of Ihh protein, Smo is repressed by the Ihh target gene patched (Ptch), another cell surface receptor for Ihh. Ihh is mainly expressed in prehypertrophic chondrocytes of the growth plate and suppresses their differentiation into hypertrophy by inducing PTHrP production in the periarticular region [1–3]. Moreover, Ihh has been shown to be essential for chondrocyte proliferation [8, 9] and osteoblast differentiation in the bone collar [3] during embryonic skeletogenesis. Finally, Ihh signaling is required for differentiation of osteoblast precursors from their progenitors. On the other hand, Wnt signaling promotes differentiation of osteoblast from their precursors [4].
The in vivo physiological role of Ihh has been determined mostly by mouse knockout studies [1, 2, 8]. The majority of Ihh-null embryos die during early development when Ihh expression is detected in the visceral endoderm. Ihh-null mice display abnormal chondrocyte proliferation and maturation, and lack of osteoblasts in long bones. Furthermore, overexpression of either Ihh or a constitutively active Smo allele specifically in cartilage results in increased activity of the Ihh signaling pathway to promote chondrocyte proliferation [9]. We have previously generated conditional Ihh-null mice in which Ihh was selectively ablated from chondrocytes. The phenotype of these mice resembled that of conventional Ihh-null mice at birth and provided evidence of the essential role of chondrocyte-derived Ihh in prenatal endochondral bone formation [6]. In order to study the postnatal role of Ihh in the growth plate, tamoxifen-inducible conditional Col2-Cre ER*; floxed Ihh-knockout mice were generated. Our results demonstrated that lack of Ihh expression in postnatal chondrocytes results in complete loss of the growth plate and the articular surface, leads to loss of the primary spongiosa, and to impaired bone growth after birth [10]. However, it is not clear from these results whether loss of trabecular bone is secondary to the abnormalities of the growth plate, which serves as a template for trabecular bone, or to loss of Ihh signaling per se from chondrocytes to osteoblast precursors. Interestingly, the use of a hedgehog inhibitor to treat tumors in young mice resulted in a similar bone phenotype with loss of the growth plate [11]. Therefore, maintenance of a growth plate is critical to study the role of chondrocyte derived Ihh during bone growth after birth. We attempted to maintain the growth plate by rescuing growth plate abnormalities in Col2-Cre ER*; Ihh-knockout mice with a constitutively active PTH/PTHrP receptor in chondrocytes.
Loss of PTHrP or the PTH/PTHrP receptor has been shown to result in premature chondrocyte differentiation in the growth plate [1, 12]. In contrast, mice expressing a constitutively active PTH/PTHrP receptor (Jansen transgene) under the collagen (α1) type 2 promoter have been shown to exhibit a delay in chondrocyte differentiation, rescuing the massive hypertrophy in chondrocytes lacking PTHrP [13–15]. To test whether the Jansen transgene could rescue the loss of the growth plate in Col2-cre ER*; Ihhd/d mice after birth, we generated Col2a1-Cre ER*; Ihhfl/fl, J double mutants and injected them at P0 with tamoxifen to generate mice lacking Ihh, but expressing the Jansen receptor in postnatal chondrocytes. Using this system, we were able to transiently maintain a growth plate even in the absence of Ihh expression. However, the Jansen transgene did not rescue the dramatic impairment of proliferation of mutant growth plates lacking Ihh. This likely caused, at least in part, the premature fusion of the double mutant growth plates. Despite the successful maintenance of the growth plate for at least one week, osteoblast differentiation and expression of Wnt target genes were still decreased in the surrounding bone indicating that Ihh per se could be required to directly signal from chondrocytes to osteoblast precursors in order to maintain proper bone in postnatal life.
Materials and Methods
Generation of Col2-cre ER*; Ihhfl/fl; Col2 Jansen mice
The generation and characterization of the Col2-Cre ER* transgenic mouse line was described earlier [16]. The phenotype of Col2-Cre ER*; Ihhd/d animals (Ihh mutants) was also previously described [10]. In brief, these Ihh mutant mice show premature closure of the growth plate leading to dwarfism. Furthermore, lack of Ihh signaling from chondrocytes leads to significant decrease in Wnt signaling in osteoblasts resulting in loss of trabecular bone. Ihh mutants had a shorter lifespan and died around 10 months of age when compared to Ihhfl/fl litter mates (controls; about 24 months). The col-2; Jansen transgenic mouse line (Tg-A) was established by Dr. Schipani [13]. These mice misexpress a constitutively active PTH/PTHrP receptor in chondrocytes which causes a delay in their differentiation process. No further abnormalities were detected when compared to wild type.
Col2-Cre ER* transgenic mice were interbred with Ihhfl/fl animals [6] to obtain Col2-Cre ER*; Ihhfl/fl offspring. Subsequently, Col2-Cre ER*; Ihhfl/fl mice were interbred with Col2 Jansen transgenic mice (J) [13] to obtain Col2-cre ER*; Ihhfl/fl; J mice. These mice and their littermates were then injected at birth (P0) with tamoxifen (0.2 mg) to generate Col2-cre ER*; Ihhd/d; J (double mutants). All analyses were performed using at least three to five mice of each genotype. Animals were maintained in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and were studied according to Institutional Animal Care and Use Committee approved protocols.
PCR Analysis for Genotyping
Genotyping of mice was performed by PCR using the following specific primers: fl-Ihh forward 5′-AGC ACC TTT TTT CTC GAC TGC CTG-3′, fl-Ihh reverse 5′-TGT TAG GCC GAG AGG GAT TTC GTG-3′; Cre 275 5′ CGC GGT CTG GCA GTA AAA ACT ATC-3′, Cre 603 5′-CCC ACC GTC AGT ACG TGA ATA TC-3′. After an initial denaturation step for 8 min at 94°C, amplification cycles consisted of denaturation at 94°C for 30 sec, annealing at 68°C for 30 sec, and 45 sec extension at 72°C for 35 cycles, followed by a final extension for 10 min at 72°C. The expected amplicons for the wild-type Ihh allele were 320 bp, for the floxed Ihh allele 400 bp, for the Cre allele 328 bp. For Jansen transgene genotyping was performed using the following specific primers: Jansen forward 5′-TAG TTG GCC CAC GTC CTG T -3′, Jansen reverse 5′-TAA CCA TGT TCA TGC CTT CTT C -3′. After an initial denaturation step for 5 min at 95°C, amplification cycles consisted of denaturation at 95°C for 1 min, annealing at 58°C for 45 sec, and 1 min extension at 72°C for 35 cycles, followed by a final extension for 10 min at 72°C. The expected amplicon for the transgene was 560 bp.
Histology and Tissue Preparation
For histological analyses, paraffin sections of tibiae were generated from P7 and P14 mice. Tissues were fixed in 10% buffered formalin, decalcified in 20% EDTA, dehydrated at room temperature through an ethanol series, cleared in xylene, embedded in paraffin, and sectioned at 5 micrometer thickness. For routine morphological analyses sections were stained with hematoxylin/eosin.
In Situ Hybridization
In situ hybridization was performed as described previously [6]. In brief, complementary 35S-UTP-labeled riboprobes were used to perform in situ hybridization on paraffin sections for collagen type 2 (Col 2), collagen type X (Col X), Ihh, Ptch, collagen type 1 (Col1), and osteocalcin (OCN). Since the Ihh gene was removed manually by injection of Tamoxifen, only the mutant samples in which deletion of Ihh was confirmed were used for the further analysis with other marker genes. Three to five samples from each genotype were examined, respectively.
Proliferation
To evaluate the cell cycle of the proliferating chondrocytes, sections were routinely processed for immunohistochemistry by using a PCNA kit purchased from Zymed (Invitrogen), according to the manufacturer’s protocols. Sections were counter stained with methyl green. Since positive signals were seen as brown nuclei the percentage of positive cells could be calculated.
Quantitative PCR
At P7, 60 μm frozen sections were prepared from the tibiae of tamoxifen-injected (P0) Ihh mutant, double mutant, control and Jansen control mice. Primary spongiosa and bone collar were microdissected from frozen sections and tissue was collected in lysis buffer (Agilent Technologies, Santa Clara, CA) and 2-mercaptoethanol (Sigma– Aldrich, St. Louis, MO). Total RNA was extracted (Agilent Technologies), and processed further for real-time PCR with the following specific primers: beta-actin forward 5′-AAG GCC AAC CGT GAA AAG AT-3′, beta-actin reverse 5′-GTG GTA CGA CCA GAG GCA TAC-3′, Dkk1 forward 5′-CTT GCG CTG AAG ATG AGG AGT-3′, Dkk1 reverse 5′-GAG GGC ATG CAT ATT CCA TTT-3′. The relative expression of mutants was normalized to that of controls by using the calculations described earlier [17].
Statistical Analysis
Statistically significant differences between groups were evaluated by Mann–Whitney’s U test. A P value of ≤0.05 was considered to be statistically significant. Analyses were performed by using Excel (Microsoft, Redmond, WA) and Graph Prism 4.0 (GraphPad, San Diego, CA).
Results
Rescue of growth plate of postnatal Ihh mutants by introducing a constitutively active PTH/PTHrP receptor into chondrocytes
To examine whether the Jansen transgene could rescue the abnormal growth plate of postnatal Ihh mutants, we crossed Col2 Jansen transgenic mice (J) with our Col2-cre ER*; Ihhfl/fl animals. The resulting Col2-cre ER*; Ihhfl/fl; J mice and their littermates (Ihhfl/fl, Ihhfl/fl; J and Col2-cre ER*; Ihhfl/fl) were injected with tamoxifen at P0 to generate double mutants (Col2-cre ER*; Ihhd/d; J), which are lacking Ihh but expressing the Jansen receptor in postnatal chondrocytes, and their controls (Ihhfl/fl), Jansen controls (Ihhfl/fl; J) and Ihh mutants (Col2-cre ER*; Ihhd/d). Seven days after tamoxifen injection, at P7, all mutant mice appeared macroscopically normal and were indistinguishable from their control littermates.
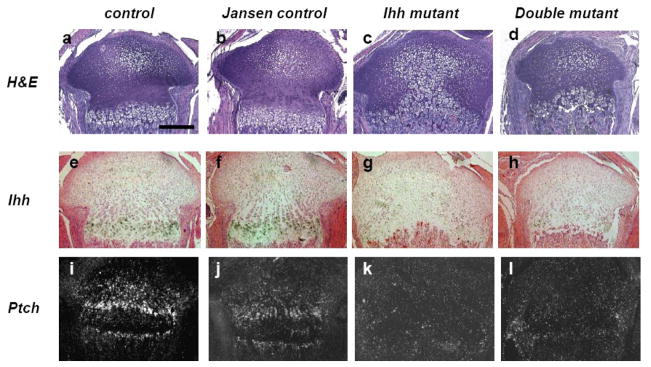
Efficiency of Ihh gene deletion was examined by in situ hybridization. Ihh is normally expressed in prehypertrophic chondrocytes of both control and Jansen control mice (Fig. 1e, f). Loss of Ihh expression was confirmed in Ihh mutants and in double mutants (Fig. 1g, h), respectively. Furthermore, expression of Patched (Ptch), a classical downstream target gene of Ihh signaling, was clearly present in chondrocytes and osteoblasts in the primary spongiosa and bone collar of both control and Jansen control mice (Fig. 1i, j). However, Patched was hardly detectable in Ihh mutants and double mutants (Fig. 1k, l), confirming efficient deletion of Ihh signaling in these mutant mice.
Fig. 1.
Growth plate morphology and mRNA expression of Ihh signaling at P7. Hematoxylin/eosin staining (a–d) and in situ hybridization (e–l) for Ihh (e–h), Ptch (i–l). At P7, the growth plate of double mutants (d) was restored when compared to the one of Ihh mutants (c) and appears similar to the one of control and Jansen control mice (a and b). Ihh expression is detected in prehypertrophic chondrocytes in control and Jansen control mice (e and f). Significant decrease of Ihh expression was confirmed in Ihh mutants and double mutants (g and h). The expression of Ptch was also found to be significantly decreased in Ihh mutants and double mutants (k and l). Magnification bar =0.5mm.
The growth plate of Ihh mutants was completely disorganized and was comprised of ectopic hypertrophic chondrocytes (Fig. 1c) as reported previously [10]. In contrast, the morphology of the growth plate of double mutants (Fig. 1d) appeared to be corrected through expression of the Jansen transgene and closely resembled the growth plate of control and Jansen control mice (Fig. 1a, b). The size of the bones was similar in double mutants and controls, but the distance between the primary and the predictive secondary ossification center was smaller in double mutants, indicating a reduction in length of chondrocyte columnar layers.
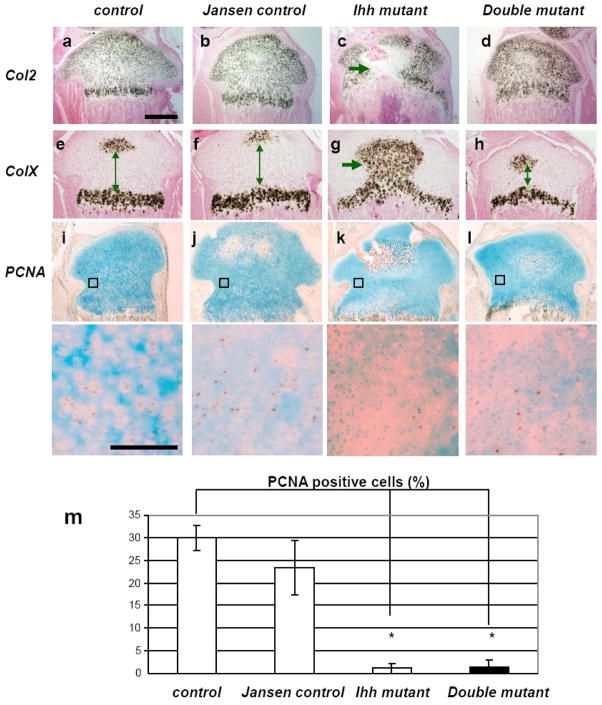
Expression of collagen type 2, which is present in all chondrocytes with exception of late hypertrophic cells, was found to be normal in double mutants (Fig. 2d) and resembled the expression pattern present in control (Fig. 2a) and Jansen control mice (Fig. 2b). In contrast, ectopic hypertrophic chondrocytes were detected in Ihh mutants and failed to express Col2 (Fig. 2c). Moreover, the expression pattern of collagen type X (ColX), a marker for hypertrophic chondrocytes, was similar in double mutants, control (Fig. 2h and 2e), and Jansen control mice (Fig. 2f), whereas the growth plate of Ihh mutants exhibited an abnormal pattern of ColX expression (Fig. 2g). Consistent with the histological findings, we found that the distance between the two expression domains of ColX in double mutants was reduced (Fig. 2h arrows).
Fig. 2.
mRNA expression and proliferation of chondrocytes. In situ hybridization for Col2 (a–d), and ColX (e–h). Expression of Col2 was found to be normal in double mutants (d). Ectopic hypertrophic chondrocytes in Ihh mutant completely lacked Col2 expression (c, arrow). Expression of ColX in double mutants (h), was similar to the one in control and Jansen control mice (e and f), despite the narrowing of the space between the two expression centers in double mutants (h, arrow). Ihh mutants showed ColX expression in abnormal ectopic hypertrophic chondrocytes (g, arrow). Proliferation was analyzed by PCNA staining (i–l) and was significantly decreased in Ihh mutants and double mutants when compared with control and Jansen control mice (m) (*, P<0.05; n =5 mice, one section each.) All chondrocytes were counted in the boxed area and the percentage of PCNA positive (brown nuclei) chondrocytes was calculated (lower panels represent magnification of the boxed areas. Magnification bar = 0.05mm, Magnification bar for a-I = 0.5mm).
Lastly, chondrocyte proliferation was examined by PCNA staining (Fig. 2i-m). Proliferation of chondrocytes was dramatically decreased in Ihh mutants (Fig. 2k). Proliferation was also reduced in double mutants (Fig. 2l) when compared to the controls and Jansen controls (Fig. 2i and j) despite the presence of the Jansen transgene. This result confirms and expands previous findings that demonstrate transgenic expression of a constitutively active PTH/PTHrP receptor can rescue the premature differentiation of chondrocytes but not the impaired proliferation of chondrocytes in Ihh mutants’ growth plates [18], even after birth.
Expression of Ihh in chondrocytes is required for normal expression of osteoblast markers and Wnt activity in the surrounding bone tissue
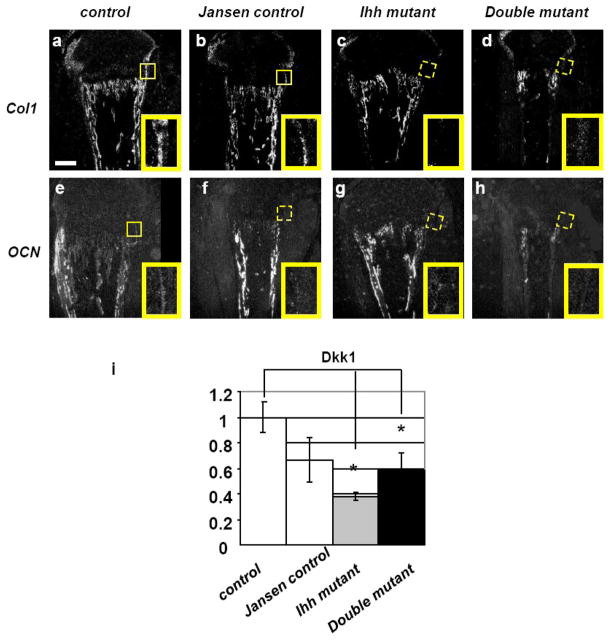
To test whether the correction of chondrocyte differentiation is also sufficient to rescue the previously described defect in osteoblast differentiation in the bone collar of postnatal Ihh mutants [10], expression of osteoblast markers such as collagen type I (Col1) and osteocalcin (OCN) were analyzed by in situ hybridization. Expression of both markers was reduced or even completely absent in Ihh mutants (Fig. 3c and g) as well as in double mutants (Fig. 3d and h) when compared to controls (Fig. 3a and e). Interestingly, expression of OCN was also absent in the bone collar of Jansen control, a new finding that has not been previously reported (Fig. 3f). Since Wnt signaling is known to play a critical role in stimulating osteoblast differentiation, we further analyzed the expression of Dickkopf 1 (Dkk1), a Wnt signaling target gene, in the bone collar and primary spongiosa of mutant mice and controls by qPCR. First, we confirmed our previous observation [10] that Dkk1 mRNA expression was significantly decreased in the bone collar and primary spongiosa of Ihh mutants when compared to controls. Secondly, we also observed a decrease in Dkk1 expression in double mutants despite the presence of a normal appearing growth plate (Fig. 3i). These findings suggest that chondrocyte derived Ihh is directly required for early osteoblast differentiation.
Fig. 3.
mRNA expression of osteoblast markers. Expression of Col1 and OCN was decreased in the bone collar of both Ihh mutants and double mutants (c, d, g, h, boxed area) when compared to controls (a and e, boxed area). Magnification bar = 0.1mm. Magnification of the boxed area =0.06 X 0.04mm. Bar graph is showing mRNA expression of Dkk1 in the bone collar and primary spongiosa of controls and mutants at P7 (i). Data are expressed as fold over control. The expression of Dkk1 was significantly reduced in both Ihh mutants and double mutants when compared to controls (*, P<0.05; n = 5 mice, measured in triplicates each). No other significant changes were found.
Premature closure of the growth plate cannot be prevented despite expression of constitutively active PTH/PTHrP receptor
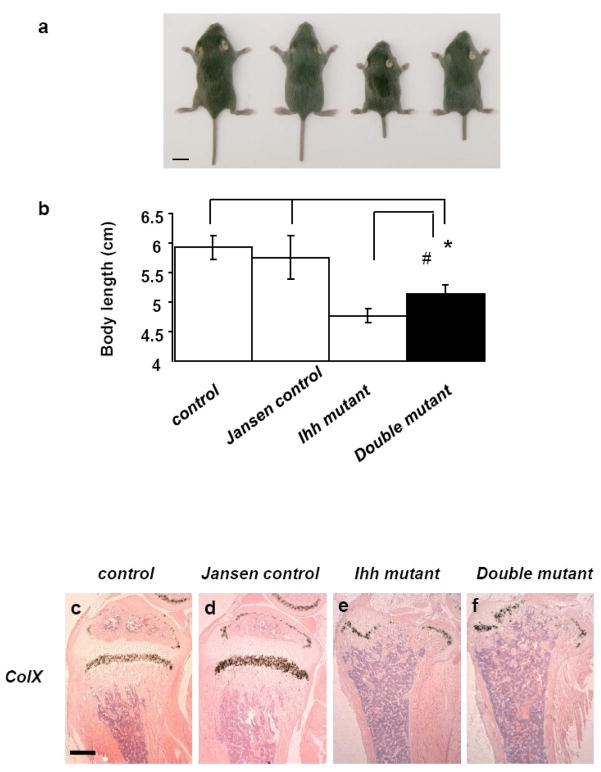
We further extended our studies and examined the phenotype of all mice at P14, two weeks after deletion of Ihh from chondrocytes by tamoxifen injection. At that age, double mutants were already smaller in size when compared to both control and Jansen control mice (Fig. 4a). However, the double mutants were 8 % larger by body length than Ihh mutant littermates (P<0.05) (Fig. 4b). Routine histology and in situ hybridization showed normal ColX expression pattern in the growth plate of control and Jansen control mice at P14 (Fig. 4c, d). In both Ihh mutant and double mutants, however, the growth plate had virtually disappeared with only a thin layer of ColX positive hypertrophic chondrocytes remaining at the distal end of the bones (Fig. 4e, f), suggesting that expression of a constitutively active PTH/PTHrP receptor is not sufficient to maintain a growth plate in the absence of Ihh.
Fig. 4.
Body size and histology of bones at P14. Macroscopic image of the double mutant (a). Magnification bar =1 cm. Double mutants appear smaller in size when compared to the control and Jansen control mice, however are 8 % larger by body length than Ihh mutant littermates (*, #, P<0.05) (n = 6, 15, 6, and 3 mice in each group, respectively from left to right) (a and b). In situ hybridization showed normal ColX expression pattern in the growth plate of control and Jansen control mice at P14 (c and d). In both Ihh mutants and the double mutants, however, the growth plate has disappeared (e and f). Magnification bar =0.1mm.
Discussion
Maintenance of a growth plate in postnatal life is critical for bone growth. Hedgehog signaling has been involved in the pathogenesis of numerous malignancies in children and its inhibition is currently exploited as a potentially promising therapeutical approach. However, in light of the critical role of Ihh in endochondral bone development, this treatment could severely impair bone growth in young patients. A study of whether and how Ihh affects growth plate development postnatally is thus an important and highly significant question.
In this study, we attempted to prevent the closure of the growth plate due to loss of Ihh signaling from chondrocytes after birth [10]. We demonstrate that the activation of the PTHrP signaling pathway in Ihh deleted postnatal chondrocytes could only partially correct the growth plate abnormalities caused by lack of Ihh signaling. Introduction of the constitutively active PTH/PTHrP receptor (Jansen receptor) into chondrocytes was able to prevent premature chondrocyte differentiation of Ihh mutant growth plates. However, it was not sufficient to restore the impairment of chondrocyte proliferation. Moreover, the incomplete rescue of osteoblast differentiation in these mutants suggests a role of Ihh per se in regulating osteoblast differentiation.
To target the Ihh gene deletion to chondrocytes and to express the constitutively active PTH/PTHrP receptor in chondrocytes the collagen (α1) type 2 promoter was used in this study. A recent report showed that collagen type 2 is also expressed in the kidney [19]. Notably, the Ihh transcript has been detected in the proximal convoluted tubule and proximal straight tubule of the adult kidney [20]. Since the kidney is the critical organ for maintaining balanced calcium-phosphate homeostasis we wanted to assure that none of the bone abnormalities we observed in Ihh mutants and/or in mice expressing the Jansen transgene were due to altered mineral ion homeostasis in these mice. We therefore performed serum calcium and phosphate measurements and were able to exclude this possibility. No changes in serum calcium and serum phosphate levels could be detected in our mutant mice when compared to controls (data not shown).
Ihh signaling is known to directly promote chondrocyte proliferation through a PTHrP independent pathway before birth. Here we show that even after birth expression of a constitutively active PTH/PTHrP receptor was unable to correct the decreased chondrocyte proliferation observed in the Ihh mutant growth plate, suggesting that Ihh also acts postnatally as a positive modulator of chondrocyte proliferation in a PTHrP-independent manner. Although Ihh is known to induce Cyclin D1, a classical cell cycle gene, the mechanism by which Ihh promotes chondrocyte proliferation is not fully understood [9]. In any event, it is likely that the impaired chondrocyte proliferation contributes to the premature closure of the epiphysis observed in double mutant mice at P14.
One of the key functions of Ihh is to suppress hypertrophic chondrocyte differentiation in a PTHrP-dependent manner. In our investigations the constitutively active PTH/PTHrP receptor was able to rescue the abnormal differentiation of cells to hypertrophic chondrocytes temporally. Interestingly, a recent report showed, that differentiation from slowly proliferating periarticular chondrocytes to fast proliferating columnar chondrocytes is promoted by Ihh independently of PTHrP signaling [21]. Consistent with these findings, we describe a reduction of the height of the columnar layer in the growth plate of double mutants (Fig. 2h). The failure of constitutively active PTH/PTHrP receptors to prevent the growth plate closure at P14 in double mutants could also be due to reduced columnar chondrocyte differentiation and an age-dependent reduction in the number of cells that express the transgene. Likewise, the loss of growth plate of PTHrP−/−; Col2 Jansen mice was rescued only temporally [13]. Interestingly, it has been reported that Ihh also promotes hypertrophic chondrocyte differentiation in a PTHrP independent manner [22]. Although we did not observe this effect of Ihh in our study, it was suggested that the outcome of chondrocyte hypertrophy when Ihh signaling is perturbed depends on the relative strength of the pro- (by Bmps and Wnts) and anti- (i.e. by PTHrP) hypertrophy signaling in the surrounding area. Since PTHrP signaling was selectively upregulated in our experimental design, we probably observed only the anti-hypertrophy actions of Ihh signaling.
It is known that Ihh is directly required for osteoblast differentiation prenatally [3]. Moreover, a recent study reported that activation of Ihh signal in OCN positive osteoblasts increased bone formation after birth [23]. These results support our findings. Since osteoblast differentiation could not be recovered in the double mutants despite recovery of a relatively normal growth plate in these mice at P7, it is possible to assume that chondrocytes might be the crucial source of Ihh production required for osteoblast differentiation after birth. The unexpected new finding that osteoblast maturation was also partially disturbed in Jansen control mice per se, as shown by decreased OCN expression in the bone collar of the tibia, despite the presence of a normal growth plate, (Fig. 3f and i) raises the question whether abnormal osteoblast differentiation in double mutants could be also due to the effect of the Jansen transgene in addition to the lack of direct Ihh signaling from chondrocytes in our new animal model. Jansen control mice might show a slight delay in endochondral bone formation after birth, a findings that has been made also prenatally [13]. Additional studies are required to answer these questions.
Ihh is a key factor required for normal endochondral bone formation. To target postnatal skeletal disease it is important to understand how Ihh regulates postnatal bone formation and maintenance. In this study, we clarified the PTHrP signaling-dependent role of Ihh in chondrocyte differentiation, and the PTHrP signaling-independent role of Ihh in chondrocyte proliferation and osteoblast differentiation in postnatal bone. Due to the loss of the growth plate in double mutants at P14, we were unable to further investigate the role of Ihh on bone remodeling at later stages. Further studies using alternate animal models are required to address the questions how chondrocyte–derived Ihh is involved in this process.
Acknowledgments
We thank Drs T Sato and T Kobayashi for helpful suggestions. This work was supported by NIH/NIAMS AR050560 funding (BL).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lanske B, Karaplis AC, Lee K, Luz A, Vortkamp A, Pirro A, Karperien M, Defize LH, Ho C, Mulligan RC, Abou-Samra AB, Juppner H, Segre GV, Kronenberg HM. PTH/PTHrP receptor in early development and Indian hedgehog-regulated bone growth. Science. 1996;273:663–6. doi: 10.1126/science.273.5275.663. [DOI] [PubMed] [Google Scholar]
- 2.Vortkamp A, Lee K, Lanske B, Segre GV, Kronenberg HM, Tabin CJ. Regulation of rate of cartilage differentiation by Indian hedgehog and PTH-related protein. Science. 1996;273:613–22. doi: 10.1126/science.273.5275.613. [DOI] [PubMed] [Google Scholar]
- 3.Long F, Chung UI, Ohba S, McMahon J, Kronenberg HM, McMahon AP. Ihh signaling is directly required for the osteoblast lineage in the endochondral skeleton. Development. 2004;131:1309–18. doi: 10.1242/dev.01006. [DOI] [PubMed] [Google Scholar]
- 4.Rodda SJ, McMahon AP. Distinct roles for Hedgehog and canonical Wnt signaling in specification, differentiation and maintenance of osteoblast progenitors. Development. 2006;133:3231–44. doi: 10.1242/dev.02480. [DOI] [PubMed] [Google Scholar]
- 5.Chung UI, Schipani E, McMahon AP, Kronenberg HM. Indian hedgehog couples chondrogenesis to osteogenesis in endochondral bone development. J Clin Invest. 2001;107:295–304. doi: 10.1172/JCI11706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Razzaque MS, Soegiarto DW, Chang D, Long F, Lanske B. Conditional deletion of Indian hedgehog from collagen type 2alpha1-expressing cells results in abnormal endochondral bone formation. J Pathol. 2005;207:453–61. doi: 10.1002/path.1870. [DOI] [PubMed] [Google Scholar]
- 7.Alcedo J, Noll M. Hedgehog and its patched-smoothened receptor complex: a novel signalling mechanism at the cell surface. Biol Chem. 1997;378:583–90. doi: 10.1515/bchm.1997.378.7.583. [DOI] [PubMed] [Google Scholar]
- 8.St-Jacques B, Hammerschmidt M, McMahon AP. Indian hedgehog signaling regulates proliferation and differentiation of chondrocytes and is essential for bone formation. Genes Dev. 1999;13:2072–86. doi: 10.1101/gad.13.16.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Long F, Schipani E, Asahara H, Kronenberg H, Montminy M. The CREB family of activators is required for endochondral bone development. Development. 2001;128:541–50. doi: 10.1242/dev.128.4.541. [DOI] [PubMed] [Google Scholar]
- 10.Maeda Y, Nakamura E, Nguyen MT, Suva LJ, Swain FL, Razzaque MS, Mackem S, Lanske B. Indian Hedgehog produced by postnatal chondrocytes is essential for maintaining a growth plate and trabecular bone. Proc Natl Acad Sci U S A. 2007;104:6382–7. doi: 10.1073/pnas.0608449104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kimura H, Ng JM, Curran T. Transient inhibition of the Hedgehog pathway in young mice causes permanent defects in bone structure. Cancer Cell. 2008;13:249–60. doi: 10.1016/j.ccr.2008.01.027. [DOI] [PubMed] [Google Scholar]
- 12.Karaplis AC, Luz A, Glowacki J, Bronson RT, Tybulewicz VL, Kronenberg HM, Mulligan RC. Lethal skeletal dysplasia from targeted disruption of the parathyroid hormone-related peptide gene. Genes Dev. 1994;8:277–89. doi: 10.1101/gad.8.3.277. [DOI] [PubMed] [Google Scholar]
- 13.Schipani E, Lanske B, Hunzelman J, Luz A, Kovacs CS, Lee K, Pirro A, Kronenberg HM, Juppner H. Targeted expression of constitutively active receptors for parathyroid hormone and parathyroid hormone-related peptide delays endochondral bone formation and rescues mice that lack parathyroid hormone-related peptide. Proc Natl Acad Sci U S A. 1997;94:13689–94. doi: 10.1073/pnas.94.25.13689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schipani E, Kruse K, Juppner H. A constitutively active mutant PTH-PTHrP receptor in Jansen-type metaphyseal chondrodysplasia. Science. 1995;268:98–100. doi: 10.1126/science.7701349. [DOI] [PubMed] [Google Scholar]
- 15.Soegiarto DW, Kiachopoulos S, Schipani E, Juppner H, Erben RG, Lanske B. Partial rescue of PTH/PTHrP receptor knockout mice by targeted expression of the Jansen transgene. Endocrinology. 2001;142:5303–10. doi: 10.1210/endo.142.12.8553. [DOI] [PubMed] [Google Scholar]
- 16.Nakamura E, Nguyen MT, Mackem S. Kinetics of tamoxifen-regulated Cre activity in mice using a cartilage-specific CreER(T) to assay temporal activity windows along the proximodistal limb skeleton. Dev Dyn. 2006;235:2603–12. doi: 10.1002/dvdy.20892. [DOI] [PubMed] [Google Scholar]
- 17.Razzaque MS, Sitara D, Taguchi T, St-Arnaud R, Lanske B. Premature aging-like phenotype in fibroblast growth factor 23 null mice is a vitamin D-mediated process. Faseb J. 2006;20:720–2. doi: 10.1096/fj.05-5432fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karp SJ, Schipani E, St-Jacques B, Hunzelman J, Kronenberg H, McMahon AP. Indian hedgehog coordinates endochondral bone growth and morphogenesis via parathyroid hormone related-protein-dependent and -independent pathways. Development. 2000;127:543–8. doi: 10.1242/dev.127.3.543. [DOI] [PubMed] [Google Scholar]
- 19.Kolpakova-Hart E, Nicolae C, Zhou J, Olsen BR. Col2-Cre recombinase is co-expressed with endogenous type II collagen in embryonic renal epithelium and drives development of polycystic kidney disease following inactivation of ciliary genes. Matrix Biol. 2008;27:505–12. doi: 10.1016/j.matbio.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Valentini RP, Brookhiser WT, Park J, Yang T, Briggs J, Dressler G, Holzman LB. Post-translational processing and renal expression of mouse Indian hedgehog. J Biol Chem. 1997;272:8466–73. doi: 10.1074/jbc.272.13.8466. [DOI] [PubMed] [Google Scholar]
- 21.Kobayashi T, Soegiarto DW, Yang Y, Lanske B, Schipani E, McMahon AP, Kronenberg HM. Indian hedgehog stimulates periarticular chondrocyte differentiation to regulate growth plate length independently of PTHrP. J Clin Invest. 2005;115:1734–42. doi: 10.1172/JCI24397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mak KK, Kronenberg HM, Chuang PT, Mackem S, Yang Y. Indian hedgehog signals independently of PTHrP to promote chondrocyte hypertrophy. Development. 2008;135:1947–56. doi: 10.1242/dev.018044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mundy GR, Yang X. Hedgehog coordination of postnatal osteoclast and osteoblast activities. Dev Cell. 2008;14:637–8. doi: 10.1016/j.devcel.2008.04.010. [DOI] [PubMed] [Google Scholar]