Abstract
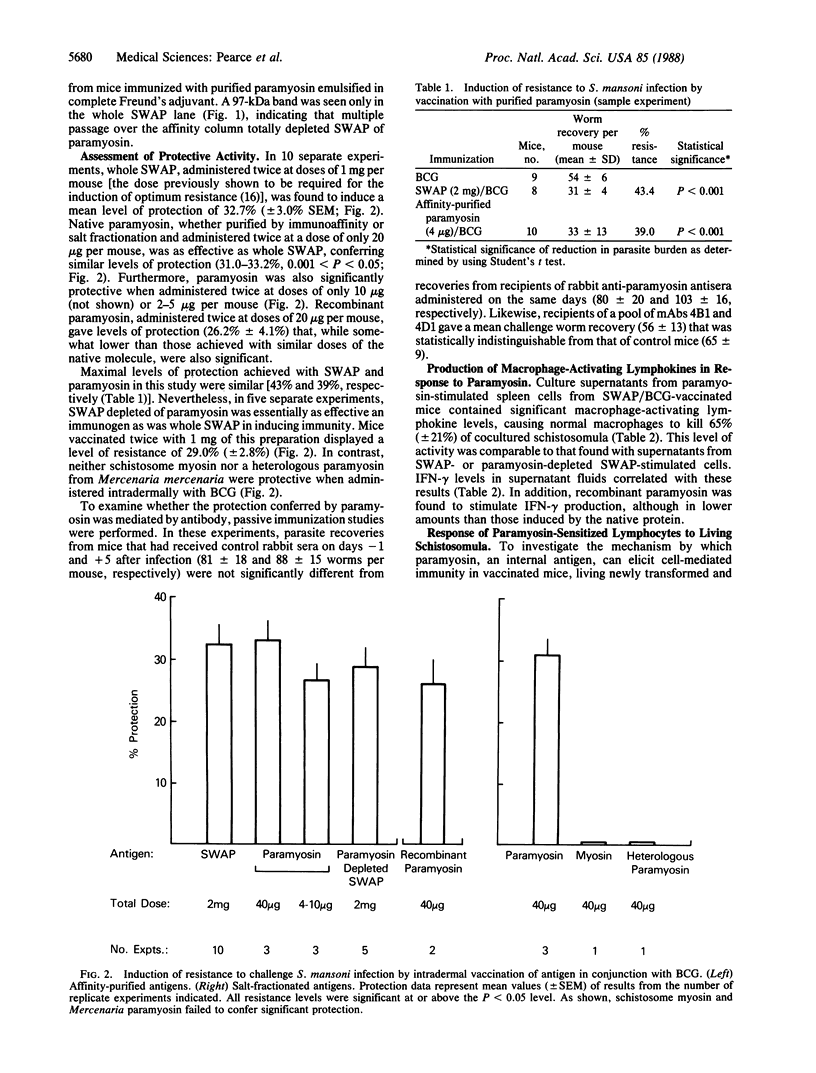
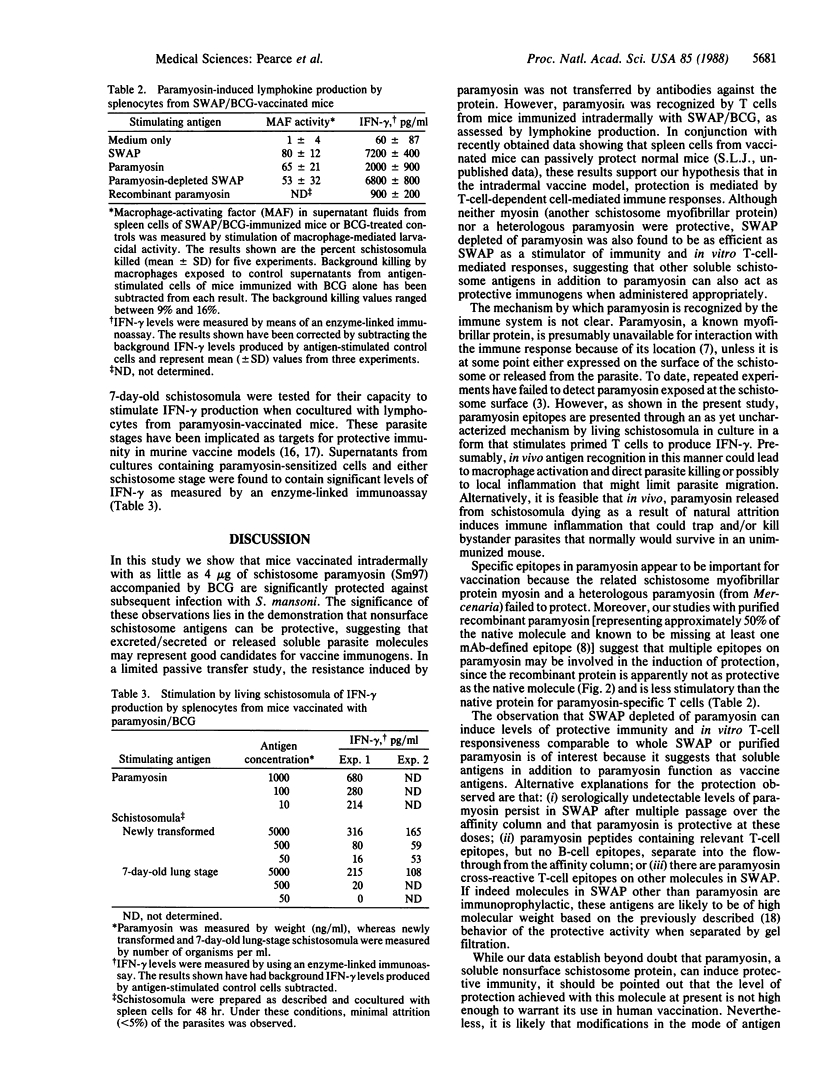
Paramyosin (Sm97), a 97-kDa myofibrillar protein identified by the unusually monospecific antibody response induced by intradermal vaccination of mice with a complex soluble worm antigen preparation (SWAP) of adult Schistosoma mansoni administered with bacillus Calmette-Guérin (BCG), was purified and tested for its capacity to protect mice against challenge infection. When administered intradermally with BCG at total doses of only 4-40 micrograms per mouse, both the native molecule and a recombinant expression product containing approximately 50% of the whole protein were found to confer significant resistance (26-33%) against challenge infection, while 2 mg of unfractionated SWAP was required to induce similar levels of protection. In addition, paramyosin was shown to stimulate T lymphocytes from vaccinated mice to produce lymphokines [e.g., gamma interferon (IFN-gamma)] that activate macrophages to kill schistosomula. Neither schistosome myosin nor a heterologous paramyosin from a different invertebrate genus were protective, indicating a requirement for specific epitopes in the immunization. That the protection induced by paramyosin involves a T-cell-mediated mechanism was supported by the failure of anti-paramyosin antibodies to passively transfer significant resistance to infection to recipient mice. Lymphocytes from mice vaccinated with paramyosin were found to produce IFN-gamma in response to living schistosomula, suggesting that during challenge infection of vaccinated hosts, paramyosin (a nonsurface antigen) may elicit a protective T-cell response as a consequence of its release from migrating parasite larvae. Paramyosin-depleted SWAP was also found to be protective as well as stimulatory for T lymphocytes from SWAP-vaccinated mice, indicating that other antigens in this preparation may have immunoprophylactic potential. In summary, these results (i) suggest that the induction of T-cell-dependent cell-mediated immunity against soluble nonsurface antigens may be an effective strategy for immunization against multicellular parasites and (ii) in the case of schistosomes, identify paramyosin as a candidate vaccine immunogen in this category.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balloul J. M., Grzych J. M., Pierce R. J., Capron A. A purified 28,000 dalton protein from Schistosoma mansoni adult worms protects rats and mice against experimental schistosomiasis. J Immunol. 1987 May 15;138(10):3448–3453. [PubMed] [Google Scholar]
- Capron A., Dessaint J. P., Capron M., Ouma J. H., Butterworth A. E. Immunity to schistosomes: progress toward vaccine. Science. 1987 Nov 20;238(4830):1065–1072. doi: 10.1126/science.3317823. [DOI] [PubMed] [Google Scholar]
- Curry R. C., Kiener P. A., Spitalny G. L. A sensitive immunochemical assay for biologically active MuIFN-gamma. J Immunol Methods. 1987 Nov 23;104(1-2):137–142. doi: 10.1016/0022-1759(87)90497-2. [DOI] [PubMed] [Google Scholar]
- Dean D. A., Mangold B. L., Georgi J. R., Jacobson R. H. Comparison of Schistosoma mansoni migration patterns in normal and irradiated cercaria-immunized mice by means of autoradiographic analysis. Evidence that worm elimination occurs after the skin phase in immunized mice. Am J Trop Med Hyg. 1984 Jan;33(1):89–96. doi: 10.4269/ajtmh.1984.33.89. [DOI] [PubMed] [Google Scholar]
- Harris H. E., Epstein H. F. Myosin and paramyosin of Caenorhabditis elegans: biochemical and structural properties of wild-type and mutant proteins. Cell. 1977 Apr;10(4):709–719. doi: 10.1016/0092-8674(77)90105-2. [DOI] [PubMed] [Google Scholar]
- James S. L., Correa-Oliveira R., Leonard E. J. Defective vaccine-induced immunity to Schistosoma mansoni in P strain mice. II. Analysis of cellular responses. J Immunol. 1984 Sep;133(3):1587–1593. [PubMed] [Google Scholar]
- James S. L., DeBlois L. A. Induction of protective immunity against Schistosoma mansoni by a nonliving vaccine. II. Response of mouse strains with selective immune defects. J Immunol. 1986 May 15;136(10):3864–3871. [PubMed] [Google Scholar]
- James S. L. In vitro proliferative response to living schistosomula by T lymphocytes from mice infected with Schistosoma mansoni. Parasitology. 1981 Aug;83(Pt 1):147–162. doi: 10.1017/s0031182000050125. [DOI] [PubMed] [Google Scholar]
- James S. L. Induction of protective immunity against Schistosoma mansoni by a non-living vaccine. V. Effects of varying the immunization and infection schedule and site. Parasite Immunol. 1987 Sep;9(5):531–541. doi: 10.1111/j.1365-3024.1987.tb00528.x. [DOI] [PubMed] [Google Scholar]
- James S. L. Induction of protective immunity against Schistosoma mansoni by a nonliving vaccine is dependent on the method of antigen presentation. J Immunol. 1985 Mar;134(3):1956–1960. [PubMed] [Google Scholar]
- James S. L. Induction of protective immunity against Schistosoma mansoni by a nonliving vaccine. III. Correlation of resistance with induction of activated larvacidal macrophages. J Immunol. 1986 May 15;136(10):3872–3877. [PubMed] [Google Scholar]
- James S. L., Pearce E. J., Sher A. Induction of protective immunity against Schistosoma mansoni by a non living vaccine. I. Partial characterization of antigens recognized by antibodies from mice immunized with soluble schistosome extracts. J Immunol. 1985 May;134(5):3432–3438. [PubMed] [Google Scholar]
- James S. L., Salzman C., Pearce E. J. Induction of protective immunity against Schistosoma mansoni by a non-living vaccine. VI. Antigen recognition by non-responder mouse strains. Parasite Immunol. 1988 Jan;10(1):71–83. doi: 10.1111/j.1365-3024.1988.tb00204.x. [DOI] [PubMed] [Google Scholar]
- Lanar D. E., Pearce E. J., James S. L., Sher A. Identification of paramyosin as schistosome antigen recognized by intradermally vaccinated mice. Science. 1986 Oct 31;234(4776):593–596. doi: 10.1126/science.3094144. [DOI] [PubMed] [Google Scholar]
- Lazdins J. K., Stein M. J., David J. R., Sher A. Schistosoma mansoni: rapid isolation and purification of schistosomula of different developmental stages by centrifugation on discontinuous density gradients of Percoll. Exp Parasitol. 1982 Feb;53(1):39–44. doi: 10.1016/0014-4894(82)90090-x. [DOI] [PubMed] [Google Scholar]
- McLaren D. J., James S. L. Ultrastructural studies of the killing of schistosomula of Schistosoma mansoni by activated macrophages in vitro. Parasite Immunol. 1985 May;7(3):315–331. doi: 10.1111/j.1365-3024.1985.tb00079.x. [DOI] [PubMed] [Google Scholar]
- Pearce E. J., James S. L., Dalton J., Barrall A., Ramos C., Strand M., Sher A. Immunochemical characterization and purification of Sm-97, a Schistosoma mansoni antigen monospecifically recognized by antibodies from mice protectively immunized with a nonliving vaccine. J Immunol. 1986 Dec 1;137(11):3593–3600. [PubMed] [Google Scholar]
- Pearce E. J., James S. L. Post lung-stage schistosomula of Schistosoma mansoni exhibit transient susceptibility to macrophage-mediated cytotoxicity in vitro that may relate to late phase killing in vivo. Parasite Immunol. 1986 Sep;8(5):513–527. doi: 10.1111/j.1365-3024.1986.tb00866.x. [DOI] [PubMed] [Google Scholar]
- Sher A., Pearce E., Hieny S., James S. Induction of protective immunity against Schistosoma mansoni by a nonliving vaccine. IV. Fractionation and antigenic properties of a soluble adult worm immunoprophylactic activity. J Immunol. 1986 May 15;136(10):3878–3883. [PubMed] [Google Scholar]
- Taylor J. B., Vidal A., Torpier G., Meyer D. J., Roitsch C., Balloul J. M., Southan C., Sondermeyer P., Pemble S., Lecocq J. P. The glutathione transferase activity and tissue distribution of a cloned Mr28K protective antigen of Schistosoma mansoni. EMBO J. 1988 Feb;7(2):465–472. doi: 10.1002/j.1460-2075.1988.tb02834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]






