Abstract
We tested members of two non-cytotoxic classes of drugs, synthetic oleanane triterpenoids and rexinoids, both as individual agents and in combination, for the prevention and treatment of carcinogenesis in a highly relevant animal model of lung cancer. Lung adenocarcinomas were induced in A/J mice by injection of the carcinogen vinyl carbamate. Mice were fed drugs in diet, beginning 1 week after the carcinogen challenge for prevention or 8 weeks later for treatment. The number, size and severity of tumors in the lungs were then evaluated. In the prevention studies, the triterpenoids CDDO-ethyl amide (CDDO-EA) and CDDO-methyl ester (CDDO-Me) reduced the average tumor burden (ATB) in the lungs 86–92%, respectively, compared to the controls, and the rexinoid LG100268 (268) reduced ATB by 50%. The combination of CDDO-EA and 268 reduced ATB by 93%. We show for the first time that these drugs also were highly effective for treatment of experimental lung cancer, and all triterpenoid and rexinoid combinations reduced ATB 85–87% compared to the control group. The triterpenoids also potently inhibited proliferation of VC1 mouse lung carcinoma cells and directly interacted with key regulatory proteins in these cells. In contrast, the rexinoids had little anti-proliferative activity in VC1 cells but were potent inhibitors of the toll-like receptor pathway in macrophage-like cells. Triterpenoids and rexinoids are multifunctional, well-tolerated drugs that target different signaling pathways and are thus highly effective for prevention and treatment of experimental lung cancer.
Keywords: Triterpenoid, CDDO-ME, CDDO-EA, rexinoid, LG100268, NRX194204, combination therapy, lung cancer, prevention, treatment, A/J mice, inflammation
Introduction
The use of combinations of drugs for treatment of invasive and metastatic malignancy has become an accepted and widely used standard approach to clinical chemotherapy of cancer. Recent studies, which show that hundreds of critical genes, in multiple pathways, may be mutated in common forms of cancer, now provide mechanistic validation for combination chemotherapy directed at multiple targets (1,2). Unfortunately, similar clinical progress in the use of combinations of drugs for prevention of cancer has not yet occurred, in spite of abundant evidence from animal experiments that this is a highly effective approach. Thus, clinical validation of combination chemoprevention of cancer, an idea first proposed many years ago, is still in its infancy (3). The recent clinical demonstration that the combination of difluoromethylornithine (DFMO) and sulindac is significantly more effective for prevention of progression of colonic adenomas than the use of either of these drugs as single agents (4) now provides further impetus to pursue new studies in combination chemoprevention, with the practical goal of translating studies in experimental animals into clinical trials.
However, the choice of drugs for clinical chemoprevention, as compared to chemotherapy, is limited by the practical requirement that chemopreventive agents must have a much greater degree of safety of administration than chemotherapeutic agents, especially since chemoprevention is given to asymptomatic apparently “healthy” men and women over prolonged periods of time. Therefore, most conventional cytotoxic drugs, which have provided highly successful therapy for childhood leukemia, testicular cancer, Hodgkins lymphoma and other indications, are not suitable for clinical chemoprevention, and newer and safer agents must be developed (3,5). For this purpose, we report here the successful combined use of members of two classes of non-cytotoxic drugs, (6). Moreover, we also show for the first time that these same drugs may be used successfully for treatment of disease in this same lung cancer model.
The rationale for the use of synthetic oleanane triterpenoids and rexinoids as chemopreventive agents has been reviewed recently (7) and will not be repeated here at length. Suffice it to note that both classes of drugs represent multifunctional agents that emphatically do not have a single molecular target. The synthetic oleanane triterpenoids have multiple anti-inflammatory, anti-angiogenic, and anti-proliferative effects, mediated by interactions with targets as diverse as the transcription factors Nrf2, STAT3, and NF-κB, while the rexinoids control the transcription of hundreds of genes whose expression is controlled by their 3 nuclear receptors, the transcription factors RXR-α, -β, and –γ. The two triterpenoids we have studied in the present report, namely CDDO-methyl ester (CDDO-Me) and CDDO-ethyl amide (CDDO-EA), have already been shown to be effective for prevention of lung cancer in an experimental model (6), but they have not been previously used for this purpose in combination with a rexinoid. The converse is also true for the rexinoids, LG 100268 and NRX 194204, used in the present report; they are known to be effective as single agents for chemoprevention of lung cancer induced by vinyl carbamate (8,9), but have not been used for combination chemoprevention in this model. We also show new data indicating that the triterpenoids and rexinoids have different molecular targets; these findings provide further mechanistic basis for their combined use. The selection of drugs used in the present studies was based on multiple considerations, including information we already had in hand relating to their pharmacokinetic and pharmacodynamic activity, as well as the possibility that they might eventually be used clinically for chemoprevention. Thus, CDDO-Me and NRX 194204 already have entered phase 2 clinical trials for the treatment of cancer. CDDO-EA has been developed to have a better safety and pharmacodynamic profile in rodents. Although it has not yet been used in clinical trials, LG 100268 has been widely used in many experimental studies and has produced excellent preclinical results in rodents for more than 10 years (7).
Materials and Methods
Reagents
CDDO-Me and CDDO-EA were synthesized as previously described (10–12) by Reata Pharmaceuticals. LG 100268 (13) was generously provided by William Lamph, Ligand Pharmaceuticals and NRX 194204 (14) by Roshantha Chandraratna, NuRx Pharmaceuticals.
Prevention and treatment of lung cancer in vivo
Seven week-old female A/J mice (Jackson Laboratory) were injected i.p. with 0.32 mg vinyl carbamate (Toronto Research Chemicals) per mouse in a volume of 0.2 ml isotonic saline adjusted to pH 5. For the prevention studies, mice were randomized and fed triterpenoids or rexinoids in semisynthetic AIN-93G diet (Harlan Teklad) for 15 wks, beginning one week after initiation with the carcinogen. For treatment studies, mice were started on diet eight weeks after injection of vinyl carbamate and maintained on treatment diet for 8–16 wks. At the end of the experiments, lungs were removed and inflated with formalin. The number and size of grossly visible lesions on the inflated lungs, and the number, size and histopathology of tumors on lung sections were evaluated as described previously (6,8). Extensive description as well as color illustrations of the criteria we have used in the present study for the classification of tumors as adenocarcinomas (including their categorization as high grade and low grade) have been published previously (6). In this article, we have noted that under the conditions we have performed our experiments (including feeding of the semi-synthetic diet AIN-93G), and at the late time periods after carcinogen administration at which we have examined the mice, we have found essentially no benign tumors. The diagnosis of tumors as adenocarcinomas has been based on their invasion of stroma with accompanying obliteration of lung architecture, often accompanied by invasion into bronchi. In essentially all tumors, carcinoma cells can be seen infiltrating along cells of adjacent alveoli. Our consulting pathologist for these studies has been Dr. Thomas Sporn, Associate Professor of Pathology, Duke University Medical Center. Four mice per group in both treatment studies were also injected i.p. with bromodeoxyuridine (1 mg per mouse) to measure proliferation in the tumors. Two hrs after injection, lungs were harvested and lung sections (3–5 tumors per group) were stained using a BrdU in situ detection kit (BD PharMingen).
Tissue culture and in vitro assays
RAW264.7 macrophage-like cells (ATCC) were grown in DMEM and 10% FBS. RAW cells were treated with the triterpenoid CDDO-Me or the rexinoid 268 and stimulated with LPS (Sigma) for 16 hrs. Total RNA was isolated, reverse transcribed, and hybridized to a mouse TLR signaling pathway Oligo GEArray (SuperArray). RAW cells were also treated with triterpenoids or rexinoids and stimulated with LPS or IFNγ (R & D Systems) for 24 hrs, and IL-1β, TNFα and GM-CSF released into the medium were measured with specific Quantikine ELISA kits (R&D Systems). To generate a new cell line from an A/J mouse, a lung tumor generated as described above was minced with a scalpel and digested in 0.25% trypsin/0.02% EDTA (Invitrogen) for 15 min at 37°C with gentle agitation from a stir bar. The cell suspension was filtered through a 40 µm Cell Strainer (BD Bioscience), centrifuged at 220 g for 10 min and plated in RPMI 1640 + 10% FBS. After 10 passages in this medium, a stable cell line (VC1) was obtained and then used for mechanistic studies. To measure effects on proliferation, VC1 cells were treated with drugs for 48 hrs using a [3H]-thymidine incorporation assay. To determine targets of the triterpenoids, VC1 cells were treated with 3 µmol/L of a biotinylated triterpenoid (15) for 1 hr. Cell lysates were incubated with 50 µl DynaBeads MyOne Strepavidin (Invitrogen) as described (16), and then analyzed by Western blotting using antibodies against cyclin D1, p21, IKK, IKBα, cEBPβ (Santa Cruz), tubulin (Calbiochem), STAT3, CREB and EGFR (Cell Signaling).
Statistical analysis
Results are described as mean ± SE and were analyzed by one-way ANOVA and a Tukey test or by one-way ANOVA on ranks (Kruskal-Wallis) and Dunn’s method, if the data did not fit a normal distribution (SigmaStat3.5). All p values are two-sided.
Results
The combination of a triterpenoid and rexinoid prevent lung cancer in A/J mice injected with vinyl carbamate
We have previously reported that triterpenoids and rexinoids, as single agents, can prevent lung carcinogenesis in A/J mice (6,9). Because these unique classes of drugs target different cellular proteins and pathways (7), we tested the combination in the A/J mouse model of lung cancer. At an advanced age, these mice develop spontaneous lung tumors, but carcinogens such as vinyl carbamate rapidly and reproducibly accelerate tumorigenesis, both in terms of tumor incidence and severity. In our laboratory, lung tumors induced by vinyl carbamate in A/J mice fed AIN-93G diet are almost entirely adenocarcinomas (6) as described in the methods section. For our prevention studies, A/J mice were challenged with 2 doses of vinyl carbamate, one week apart. CDDO-Me (40 mg/kg diet or ~ 10 mg/kg body weight), CDDO-EA (400 mg/kg diet or ~ 100 mg/kg body weight) and LG100268 (45 mg/kg diet or ~ 11 mg/kg body weight) were then given in the diet, beginning two weeks after the initial carcinogenic insult, and thus did not interfere with tumor initiation by vinyl carbamate, which is rapidly metabolized. As shown in Table 1, the combination of the triterpenoids CDDO-Me and CDDO-EA with the rexinoid 268 significantly (P < 0.05) decreased the number, size, and severity of the histopathology of lung tumors compared to the controls. The average number of tumors on the surface of the inflated lungs in the groups with the combination of a triterpenoid and 268 (7.8 and 5.1 for CDDO-EA and CDDO-Me with 268, respectively) was 47–65% lower than in the control group (14.7), and the percentage of tumors < 0.5 mm in diameter was only 2% in control lungs vs. 51–58% in the combination groups. Moreover, 19% of the tumors in control mice were larger than 1 mm in diameter, while not a single tumor larger than 1 mm was found in either of the groups given two drugs. Similar reductions in the number and size of tumors were observed in lung sections, as the average tumor burden (ATB) on the slides in mice fed the combination of a triterpenoid and rexinoid was only 0.5–0.6 mm3, which was 92–93% lower than the ATB of 7.7 mm3 in the controls. The percentage of low grade adenocarcinomas on the lung slides was 58–77% in the combined treatment groups vs. 19% in the control lungs, and the percentage of high grade adenocarcinomas decreased from 61% in the control group to only 6–7% of tumors in the combined treatment groups.
Table 1.
The combination of a triterpenoid and the rexinoid LG268 (268) prevents lung carcinogenesis in A/J mice injected with vinyl carbamate
| Control - |
LG268 45 mg/kg diet |
CDDO-Me 40 mg/kg diet |
CDDO-Me + 268 |
CDDO-EA 400 mg/kg diet |
CDDO-EA + 268 |
|
|---|---|---|---|---|---|---|
| Inflated lungs: | ||||||
| # of mice/group | 22 | 12 | 12 | 12 | 12 | 12 |
| Ave # of tumors/mouse (% control) |
14.7 ± 0.8 (100) |
10.0 ± 1.3* (68) |
7.5 ± 1.0† (51) |
5.1 ± 0.9† (35) |
8.8 ± 0.7 † (59) |
7.8 ± 1.1 † (53) |
| # of tumors ≤ 0.5 mm (% of total tumors) | 6 (2) | 11(9) | 52 (58)* | 35 (57)* | 42 (40)* | 48 (51)* |
| # of tumors > 1 mm (% of total tumors) | 63 (19) | 6 (5)* | 0 (0)* | 0 (0)* | 0 (0)* | 0 (0)* |
| Slides: | ||||||
| # of slides/group | 44 | 24 | 24 | 24 | 24 | 24 |
| Ave # tumors/slide (% control) |
3.0 ± 0.2 (100) |
2.3 ± 0.3 (77) |
1.4 ± 0.2* (46) |
1.2 ± 0.2* (38) |
1.8 ± 0.3* (58) |
1.3 ± 0.3 * (42) |
| Ave tumor size, mm3 (% control) |
2.5 ± 0.3 (100) |
1.7 ± 0.3* (66) |
0.4 ± 0.1* (17) |
0.3 ± 0.1* (11) |
0.6 ± 0.1 * (24) |
0.4 ± 0.1* (16) |
| Ave tumor burden, mm3 (% control) |
7.7 ± 1.2 (100) |
3.9 ± 0.8* (50) |
0.6 ± 0.1* (8) |
0.3 ± 0.1* (4) |
1.1 ± 0.2 * (14) |
0.5 ± 0.1 *§ (7) |
| Histopathology: | ||||||
| # low grade tumors (% of total tumors) | 25 (19) | 24 (43)† | 19 (58)† | 20 (72)† | 24 (57)† | 24 (77)†§ |
| # medium grade tumors (% of total) | 27 (20) | 8 (14) | 12 (36)* | 4 (14) | 8 (19) | 5 (16) |
| # high grade tumors (% of total) | 82 (61) | 24 (43)† | 2 (6)† | 4 (14)† | 10 (24)† | 2 (7)†§ |
Female A/J mice were injected i.p. with 2 doses of vinyl carbamate (0.32 mg/mouse), one week apart. One week after the final injection with the carcinogen, mice were fed compounds in diet for 15 weeks. Values are mean ± SEM.
P < 0.05 vs. control,
P < 0.001 vs. control,
P < 0.05 vs. CDDO-EA and 268 alone.
Although the drug concentrations used in the present studies were lower than those used in previously effective studies, the individual drugs were still all effective as single agents for preventing lung cancer in this model. However, the combination of a triterpenoid and rexinoid was consistently more effective than either drug used singly, and both the ATB (0.5 mm3) and the percentage of low grade tumors (77%) were significantly (P < 0.05) improved with the combination of CDDO-EA and 268 vs. each individual drug (3.9 and 1.1 mm3 for ATB and 43% and 57% for low grade tumors, respectively, as individual drugs). All of the drugs were well-tolerated at these doses as all groups continued to gain weight throughout the study; the average weight of the mice at the end of the study was 21.8 ± 3.1 g for the control group, 22.9 ± 2.2 g for the 268 group, 19.8 ± 1.0 for the CDDO-EA group, and 22.1 ± 1.5 g for the combination of CDDO-EA and 268.
Triterpenoids and rexinoids, either alone or in combination, also effectively treat established lung tumors
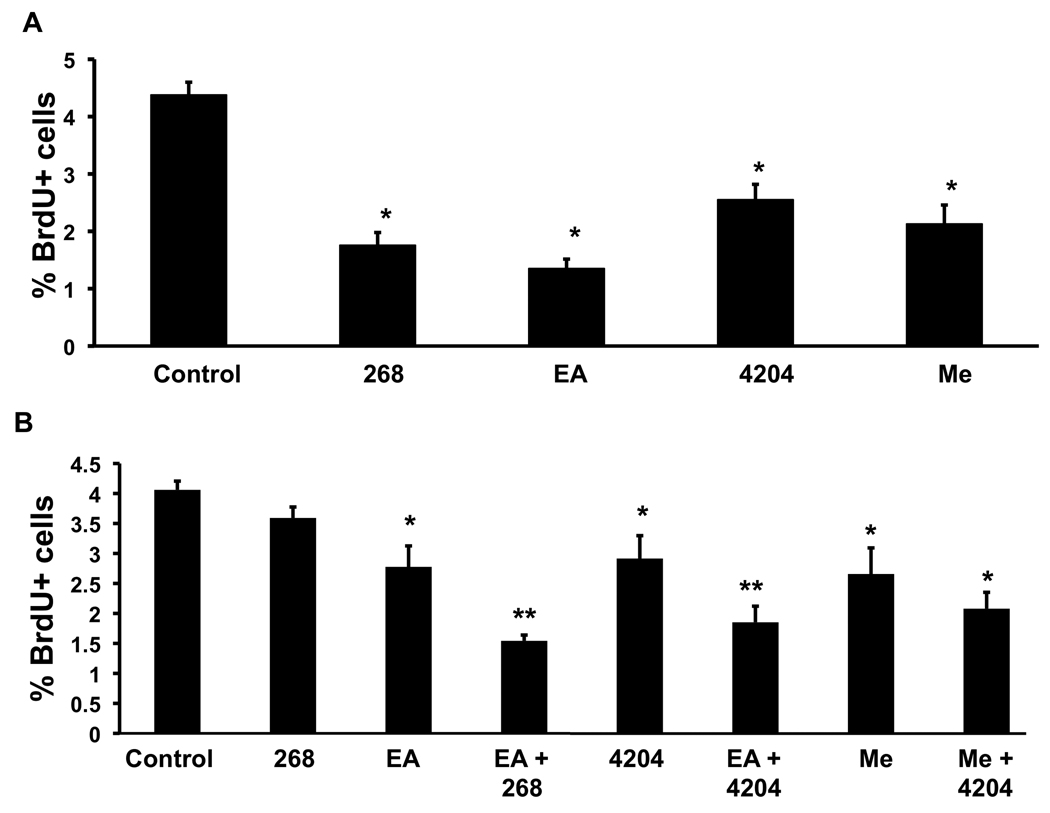
Because the triterpenoids and rexinoids reduced the lung cancer burden in A/J mice by more than 90% in our prevention studies, we next tested whether these drugs could be used for treating lung cancer in this model. For these studies, treatment was not started until 8 weeks after the final injection of vinyl carbamate; by this time, an average of 8 lesions are grossly visible on the surface of the lungs (9). Even at this early time point, these lesions are adenocarcinomas, as shown in our previous studies and in histological sections from these tumors (6). Higher doses of drugs, at least twice the concentrations used in the prevention studies, were given for a total of 8 weeks in order to block proliferation and/or induce apoptosis in these established tumors (7). These higher concentrations were still below the maximum tolerated dose, as all groups continued to gain weight throughout the study. As shown in Table 2, the triterpenoids CDDO-Me and CDDO-EA significantly (P < 0.001) reduced the size but not the number of tumors visible on the surface of the lungs, while the rexinoids significantly (P < 0.001) decreased both the size and number of tumors on the inflated lungs. Similar results were observed on lung sections, as the ATB was 2.0–3.4 mm3 in the lungs of mice fed CDDO-EA and CDDO-Me, respectively, a reduction of 58–75% compared to the ATB of 7.9 mm3 in the lungs of control mice. The rexinoids 268 and 4204 also decreased ATB on slides by ~ 65% (2.7–2.8 mm3) compared to the controls (7.9 mm3). All four drugs also significantly decreased the percentage of high grade adenocarcinomas from 59% in the control group to 31–43% in the treated groups. The bigger differences in average tumor sizes per group than in the number of tumors per group suggested that these drugs may have stopped the growth and histological progression of the established tumors rather than cause them to regress. At the end of the treatment study, mice were injected with BrdU 2 hours before the lungs were harvested, and immunohistochemical analysis of the lungs revealed a significant (P < 0.05) decrease of 40–70% in the percentage of BrdU-positive cells in all four treatment groups (Fig. 1A).
Table 2.
Triterpenoids and rexinoids are effective as single agents for the treatment of lung cancer in A/J mice
| Control - |
CDDO-Me 80 mg/kg diet |
CDDO-EA 800 mg/kg diet |
LG268 100 mg/kg diet |
NRX4204 80 mg/kg diet |
|
|---|---|---|---|---|---|
| Inflated lungs: | |||||
| # of mice/group | 31 | 12 | 16 | 16 | 18 |
| Ave # of tumors/mouse (% control) | 14.6 ± 0.7 (100) | 12.2 ± 1.1 (84) | 12.6 ± 0.6 (86) | 9.7 ± 0.8 (67)† | 9.8 ± 0.7 (67)† |
| # of tumors ≤ 0.5 mm (% of total tumors) | 14 (3) | 18 (12)† | 47 (23)† | 15 (10)* | 29 (16)† |
| # of tumors > 1 mm (% of total tumors) | 66 (15) | 12 (8) | 5 (3)† | 4 (2)† | 4 (2)† |
| Slides: | |||||
| # of slides/group | 62 | 24 | 32 | 32 | 36 |
| Ave # tumors/slide (% control) | 4.0 ± 0.3 (100) | 2.3 ± 0.3* (58) | 2.3 ± 0.3* (59) | 2.6 ± 0.3* (66) | 3.0 ± 0.3* (75) |
| Ave tumor size, mm3 (% control) | 2.0 ± 0.1 (100) | 1.5 ± 0.2 (73) | 0.9 ± 0.1* (43) | 1.1 ± 0.1* (53) | 0.9 ± 0.1* (46) |
| Ave tumor burden, mm3 (% control) | 7.9 ± 0.7 (100) | 3.4 ± 0.7* (42) | 2.0 ± 0.3* (25) | 2.8 ± 0.5* (35) | 2.7 ± 0.5* (34) |
| Histopathology: | |||||
| # low grade tumors (% of total tumors) | 37 (15) | 15 (28) | 15 (20) | 26 (30)* | 39 (36)† |
| # medium grade tumors (% of total) | 64 (26) | 20 (36) | 28 (37) | 29 (35) | 35 (33) |
| # high grade tumors (% of total) | 144 (59) | 20 (36)* | 32 (43)* | 29 (35)† | 33 (31)† |
Female A/J mice were injected i.p. with 2 doses of vinyl carbamate (0.32 mg/mouse), one week apart. Eight weeks after the final injection of carcinogen, mice were fed compounds in diet for 8 weeks. Results were pooled from 2 independent experiments. Values are mean ± SEM.
P < 0.05 vs. control,
P < 0.001 vs. control.
Fig. 1. The triterpenoids CDDO-EA and CDDO-Me and the rexinoids 268 and 4204 decrease proliferation in vivo.
Eight wks after initiation of lung carcinogenesis with vinyl carbamate, A/J mice were fed powdered diet containing triterpenoids (80 and 800 mg triterpenoid per kg of diet for Me and EA, respectively) or rexinoids (80 and 100 mg 4204 or 268 per kg diet) for 8 wks (A) or 16 wks (B). At the end of the study, mice were injected with BrdU for 2 hrs, and lung sections were stained with a BrdU antibody. The number of BrdU+ cells in tumors and the total number of tumor cells per field were counted in two lung sections per mouse. Values shown are mean ± SEM of > 1,500 cells; n = 4 mice per group. *, P < 0.05 vs. control; **, P < 0.05 vs. both individual drugs.
Because the combination of a triterpenoid and rexinoid was more effective for preventing lung cancer than the individual drugs, we next tested several drug combinations for treating established lung cancer. As shown in Table 3, each individual drug significantly (P < 0.05) reduced the ATB per slide by 47–75% compared to the control group. The ATB after 16 weeks of treatment on the three combination groups was only 3.9–4.6 mm3 compared to an ATB of 26.7 mm3 in the control group, a reduction of 83–85%. The drugs also significantly (P < 0.05) reduced the number of high grade tumors but again were not as effective at reducing the average number of tumors per lung or per slide. The percentage of BrdU-positive cells was only 1.5–2.8% compared to 4.1% in the control tumors, and the combination of CDDO-EA with either 268 or 4204 was also significantly (P < 0.05) more effective than either the triterpenoid or rexinoid alone.
Table 3.
Combinations of triterpenoids and rexinoids are effective for the treatment of lung carcinogenesis in A/J mice
| Control - |
LG268 100 mg/kg diet |
CDDO-EA 800 mg/kg diet |
CDDO-EA + 268 |
NRX4204 80 mg/kg diet |
CDDO-EA + 4204 |
CDDO-Me 80 mg/kg diet |
CDDO-Me + 4204 |
|
|---|---|---|---|---|---|---|---|---|
| Inflated lungs: | ||||||||
| # of mice/group | 21 | 12 | 12 | 12 | 10 | 12 | 12 | 12 |
| Ave # of tumors/mouse (% control) |
15.9 ± 0.8 (100) |
13.5 ± 0.7* (85) |
14.2 ± 0.7 (89) |
12.8 ± 1.2* (80) |
13.8 ± 1.2 (87) |
10 ± 1.2*§ (63) |
13 ± 1.2* (82) |
11.8 ± 1.1* (74) |
| # of tumors ≤ 0.5 mm (% of total tumors) |
4 (1) |
14 (9)† |
28 (16)† |
28 (18)† |
8 (6)* |
29 (24)† |
11 (7)† |
22 (15)†§ |
| # of tumors > 1 mm (% of total tumors) |
105 (32) |
10 (6)† |
10 (6)† |
4 (3)† |
16 (12)† |
2 (2)† |
12 (8)† |
4 (3)† |
| Slides: | ||||||||
| # of slides/group | 42 | 24 | 24 | 24 | 20 | 24 | 24 | 24 |
| Ave # tumors/slide (% control) |
4.9 ± 0.3 (100) |
4.4 ± 0.5 (91) |
3.0 ± 0.4* (61) |
3.0 ± 0.4* (61) |
4.1 ± 0.4 (84) |
3.0 ± 0.4* (62) |
2.8 ± 0.3* (57) |
3.2 ± 0.3* (65) |
| Ave tumor size, mm3 (% control) |
5.5 ± 0.6 (100) |
3.2 ± 0.4* (58) |
2.2 ± 0.4* (41) |
1.3 ± 0.1* (24) |
2.8 ± 0.5* (51) |
1.5 ± 0.3* (28) |
2.7 ± 0.4* (49) |
1.4 ± 0.1*§ (25) |
| Ave tumor burden, mm3 (% control) |
26.7 ± 3.3 (100) |
14.1 ± 2.0* (53) |
6.6 ± 1.0* (25) |
3.9 ± 0.7* (15) |
11.6 ± 2.1* (43) |
4.6 ± 0.9* (17) |
7.6 ± 1.4* (28) |
4.3 ± 0.6*§ (16) |
| Histopathology: | ||||||||
| # low grade tumors (% of total tumors) |
10 (5) |
15 (14)* |
8 (11) |
13 (18)† |
10 (12) |
12 (16)* |
8 (12) |
2 (3) |
| # medium grade tumors (% of total) |
61 (30) |
37 (35) |
34 (48)* |
29 (40) |
34 (42) |
31 (43) |
29 (43) |
32 (42) |
| # high grade tumors (% of total) |
134 (65) |
54 (51)* |
29 (41)† |
30 (42)† |
38 (46)* |
30 (41)† |
30 (45)* |
42 (55) |
Female A/J mice were injected i.p. with 2 doses of vinyl carbamate (0.32 mg/mouse), one week apart. Eight weeks after the final injection of carcinogen, mice were fed compounds in diet for 16 weeks. For the combination groups, triterpenoids and rexinoids were fed on alternate weeks, instead of simultaneously. Values are mean ± SEM.
P < 0.05 vs. control,
P < 0.001 vs. control,
P < 0.05 vs. triterpenoid or rexinoid alone.
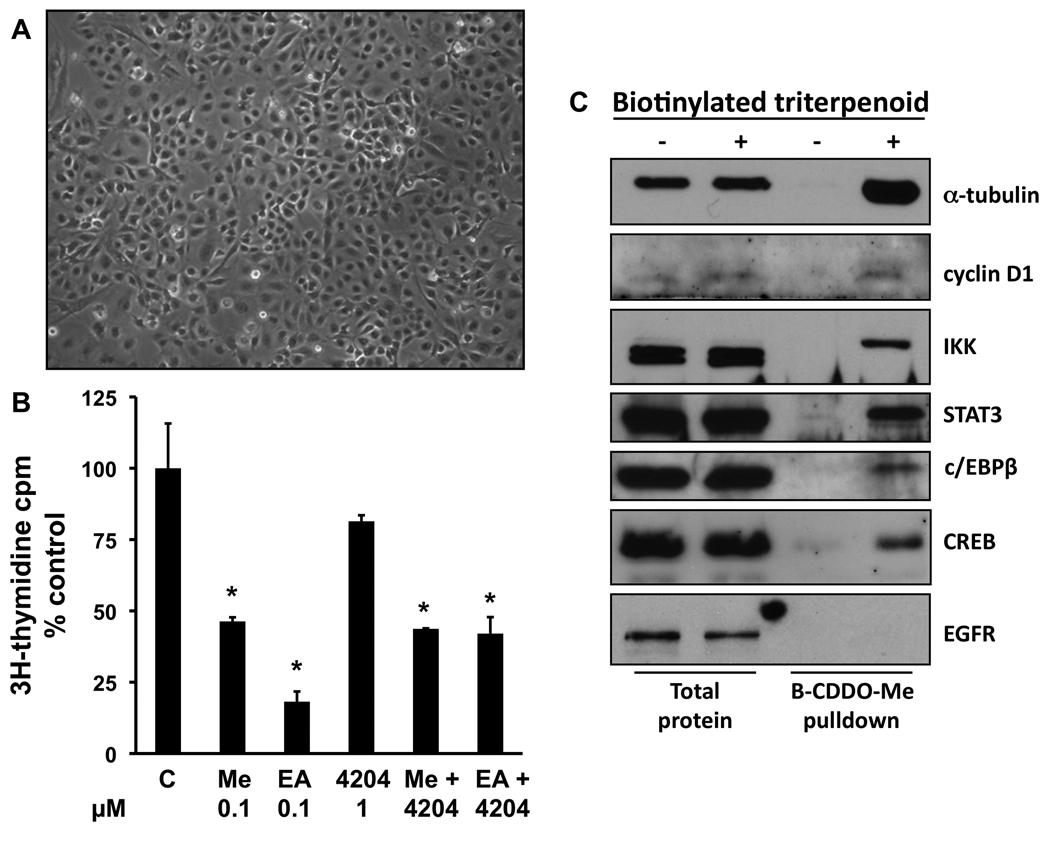
The triterpenoids inhibit proliferation in primary lung cancer cells from A/J mice
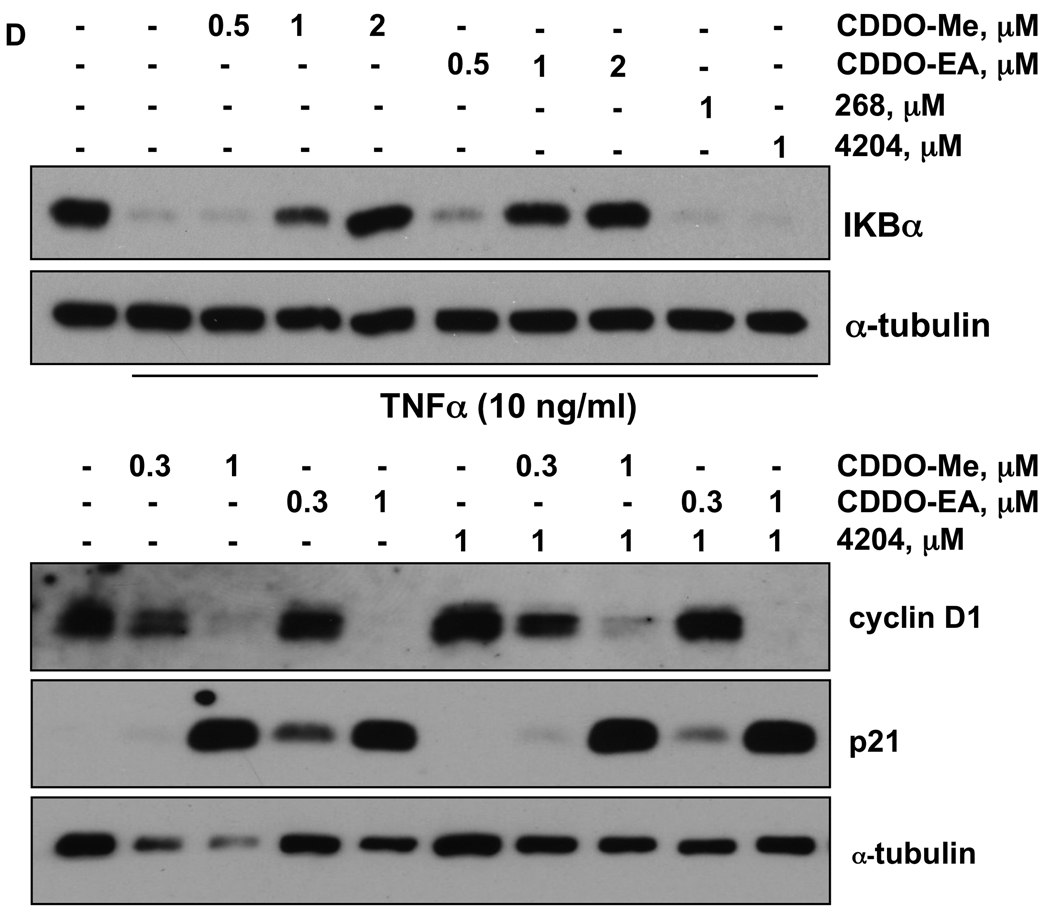
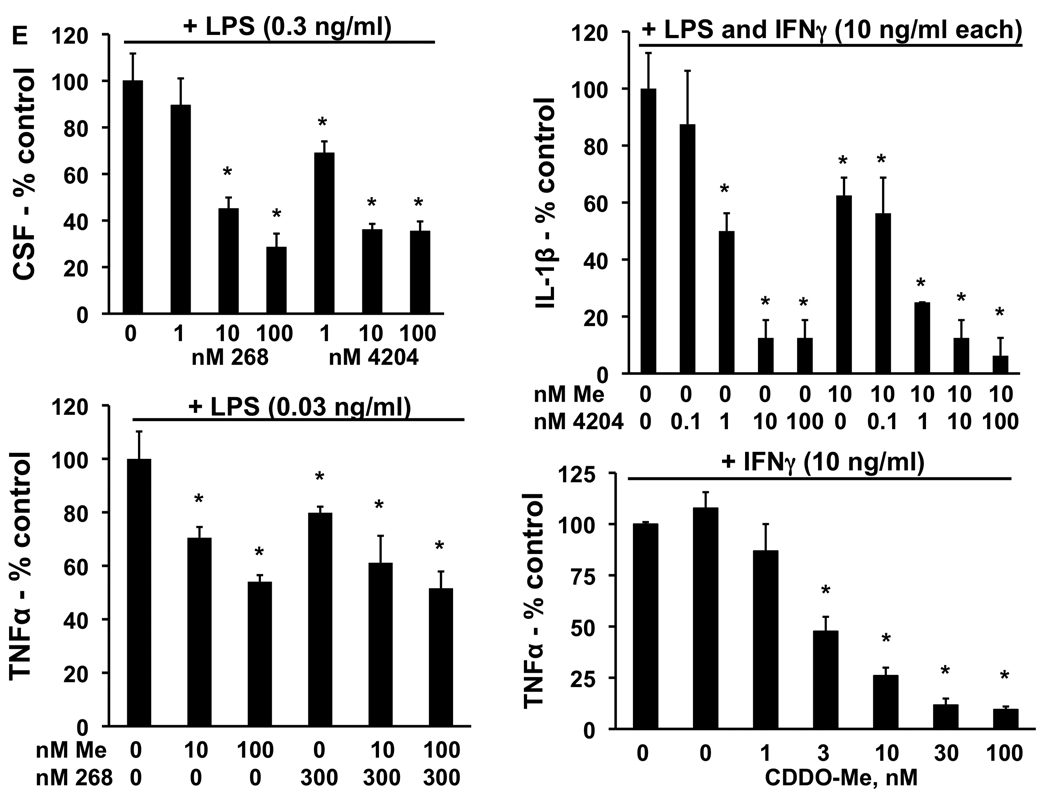
In order to study the mechanisms of action for the effects of these drugs, we have developed a new cell line (VC1), derived from an adenocarcinoma of the lung in an A/J mouse treated with vinyl carbamate. VC1 cells are easily grown in monolayer culture in standard media (Fig. 2A). Both the triterpenoids CDDO-Me and CDDO-EA significantly (P < 0.05) inhibited the proliferation of these VC1 cells, while the rexinoids had no effect (Fig. 2B). As shown by treating VC1 cells with a biotinylated triterpenoid (compound 6 in ref. 15) followed by strepavidin precipitation (17), the triterpenoids directly interact with a number of important regulatory targets in lung cancer, including α-tubulin, cyclin D1, IKK, STAT3, C/EBPβ, and CREB (Fig. 2C). Notably, the triterpenoids do not interact randomly with all proteins in a cell, as there was no interaction with the receptor for EGF (EGFR). Furthermore, it has been shown that biotinylated compound is highly specific for binding to particular cysteine residues in target proteins, such as cysteine 179 in IKK; when this cysteine was mutated to alanine in IKK, the biotinylated triterpenoid did not bind to this target (17). These protein interactions are biologically relevant as the triterpenoids CDDO-Me and CDDO-EA, but not the rexinoid 4204, induced the degradation of cyclin D1, and pretreatment with triterpenoids also prevented the degradation of IKBα in the lung cancer cells when stimulated with TNFα (Fig. 2D). As we have reported previously, the rexinoids have almost no effects on epithelial cells but can inhibit the release of the inflammatory mediators IL-6 and nitric oxide in RAW264.7 macrophage-like cells (16). Both triterpenoids and rexinoids are also potent inhibitors of inflammation (7,8) and the rexinoids are particularly effective against LPS challenge. To explore the effects of these drugs on the toll-like receptor (TLR) signaling pathway, RAW cells were treated with 0.3 µM CDDO-Me or 1 µM 268 for 16 h, and the expression of 113 genes was analyzed using a TLR microarray. As shown in Table 4, 268 regulates a number of TLR-pathway genes, including effectors such as Caspase-8 and Map3k7ip1 (TAB1) and downstream pathways/target genes such as Chuk (IKKα), Clecsf9, Csf3, Cxcl10, IL-1β, Irf-1, and Ptgs2 (COX-2). The changes in gene expression for all of the 9 genes mentioned above have been confirmed by RT-PCR (data not shown). Moreover, when LPS concentrations were optimized for different cytokines released into conditioned media, rexinoids were potent inhibitors of CSF-3 and IL-1β, while CDDO-Me inhibited IL-1β and TNF-α as shown by ELISAs (Fig. 2E). Although low nanomolar concentrations of the triterpenoids inhibit the induction of inflammatory cytokines, including IL-1β and TNFα, by IFNγ, they had little effect on many of the genes in the TLR pathway induced by LPS. Interestingly, the triterpenoid CDDO-Me induced the opposite response of 268 for Cxcl10, IL-6, and COX-2 expression, and these results also have been confirmed by RT-PCR or ELISA (data not shown).
Fig. 2. Triterpenoids and rexinoids target different pathways and cell types.
A. A new lung cancer cell line (VC1) was derived from a lung tumor from an A/J mouse injected with vinyl carbamate. B. VC1 cells were treated with drugs for 48 hrs, and proliferation was measured using a [3H]-thymidine incorporation assay. C. VC1 cells were treated with a biotinylated triterpenoid (B-CDDO-Me, 3 µM) for 1 hr and triterpenoid-protein complexes were precipitated from cell lysates with strepavidin DynaBeads and proteins were detected by Western blot. D. Cells were pretreated with triterpenoids or rexinoids for 2 hrs and then stimulated with TNFα for 15 min (upper panel) or were treated with compounds alone for 24 hrs (lower panel). E. RAW264.7 mouse macrophage-like cells were treated with rexinoids and/or triterpenoids and stimulated with LPS (0.03–10 ng/ml) or IFNγ (10 ng/ml) for 24 hrs, and the amount of GM-CSF, IL-1β or TNFα released into the medium was detected using specific ELISAs. *, P < 0.05 vs. control.
Table 4.
CDDO-Me and LG268 regulate different genes after treatment with LPS
| Gene | CDDO-Me Fold change |
LG268 Fold change |
|
|---|---|---|---|
| Casp8 | 1.7 | 2.1 | |
| Ccl2 | 0.8 | 1.1 | |
| CEBPβ | 1.1 | 1.3 | |
| Chuk | 1.2 | 2.1 | |
| Clecsf9 | 1.7 | 1.7 | |
| Csf3 | 1.0 | 0.7 | |
| Cxcl10 | 0.6 | 1.7 | |
| IL12a | 0.9 | 1.6 | |
| IL1b | 0.7 | 0.5 | |
| IL-6 | 2.7 | 0.9 | |
| Irf1 | 1.4 | 2.0 | |
| Ly86 | 1.0 | 1.6 | |
| Ly96 | 1.6 | 1.7 | |
| Map3k1 | 1.0 | 1.7 | |
| Map3k14 | 1.0 | 1.6 | |
| Map3k7 | 1.0 | 1.5 | |
| Map3k7ip1 | 1.0 | 2.1 | |
| Mapk14 | 1.2 | 1.5 | |
| Nfkb2 | 1.3 | 1.5 | |
| Prkra | 1.0 | 1.6 | |
| Ptgs2 | 1.6 | 0.7 | |
| Tbk1 | 1.1 | 1.8 | |
| Tlr4 | 1.0 | 2.2 | |
| Tlr7 | 0.8 | 1.5 | |
| TNF | 0.9 | 1.1 | |
| Tollip | 1.4 | 2.0 | |
| Ube2v1 | 1.0 | 1.6 | |
RAW264.7 cells were treated with 5 ng/ml LPS and either 300 nmol/L CDDO-Methyl ester (CDDO-Me) or 1000 nmol/L LG100268 (268) for 16 hours. Total RNA from these cells was analyzed using a mouse TLR signaling pathway DNA microarray. The listed fold changes are relative to LPS stimulated control samples.
Discussion
The most important conclusion to be drawn from the present data is that combinations of synthetic oleanane triterpenoids and rexinoids are more effective than individual members of these two classes of agents, with respect to either preventing or treating lung cancer in a highly relevant mouse model of the human disease. Using this model, we have previously shown that either triterpenoids or rexinoids are quite effective as single agents for suppressing the progression of premalignant lesions of the lung toward full-blown invasive adenocarcinoma (6,8,9). Now, we are reporting for the first time that combining these two classes of drugs can result in a marked increase in chemopreventive activity in the lung. Thus, we have shown here that the combination of either CDDO-Me or CDDO-EA, together with 268 can suppress tumor burden by greater than 90% when these drugs are used together in a preventive regimen. These reductions in tumor burden are significantly greater (P < 0.05) for the combination of CDDO-EA and LG 268, compared to the effects of either of the two agents when used singly. Moreover, we have also shown that this same combination has a marked ability to suppress the emergence of high grade tumors, again statistically significant when compared to the effects of either of the two agents when used singly. The present study also shows for the first time that both synthetic oleanane triterpenoids and rexinoids are effective drugs, both as single agents and in combination, for chemotherapy of adenocarcinoma of the lung induced in mice by vinyl carbamate. Reductions in tumor burden of greater than 80% were achieved with combination therapy.
In support of the beneficial actions of the use of combinations of triterpenoids and rexinoids, our mechanistic studies suggest that the striking results obtained here are the result of the multifunctional nature of the two classes of drugs. Although our studies with bromodeoxyuridine (Figure 1) have shown that both triterpenoids and rexinoids exert a marked anti-proliferative effect on adenocarcinomas of the lung in vivo, similar results were not obtained with cultures of lung cancer cells derived from these tumors. Thus, in contrast to triterpenoids which target regulatory proteins in addition to the classic cell cycle proteins cyclin D1 and α-tubulin (Fig. 2C), rexinoids do not have any marked anti-proliferative effect (Fig. 2B) in the new VC1 lung cancer cell line, suggesting that some effects of rexinoids are not mediated by their direct actions on epithelial cell components of the carcinoma, but rather are the results of effects mediated by actions on cells of the tumor microenvironment. This conclusion is supported by recent studies which have shown that 268 has potent anti-angiogenic activity, both in cell culture and in vivo (16). Thus the concerted action of the combination of a triterpenoid and a rexinoid, acting on both the epithelial and stromal components of a tumor, could exert a synergistic effect on the growth of that neoplasm.
Moreover, the anti-inflammatory effects of both the triterpenoids and the rexinoids add to this preventive and therapeutic synergy. As shown in Figure 2E and Table 4, both sets of agents can suppress the ability of LPS (or the combination of LPS and IFN-γ) to induce the expression of inflammatory cytokines such as TNF-α or IL-1β. However, there is a markedly different pattern of gene expression regulated by either the triterpenoid or the rexinoid, if one evaluates effects on a set of genes involved in the TLR signaling pathway. Although its importance for carcinogenesis has been greatly neglected in the past, the role of inflammation in enhancing tumor progression has become a major theme in recent carcinogenesis studies (18,19), and significant advances have been made in the use of anti-inflammatory drugs to control cancer. Because the process of inflammation involves the concerted contextual actions of many different types of cells, including neutrophils, macrophages, lymphocytes, and mast cells, as well as endothelial cells and fibroblasts, all interacting in functional networks (20), it is not surprising that a pharmacological approach that uses combinations of multifunctional anti-inflammatory drugs (such as the triterpenoids and rexinoids we have used here) can produce desirable anti-carcinogenic results. Thus, it will be increasingly important to study the process of inflammation from the perspective of network pharmacology, which has been proposed as the next paradigm in drug discovery (21). Advances in this new area of polypharmacology offer immense opportunities for control of cancer and should be vigorously pursued. In summary, we have found that these two classes of drugs, triterpenoids and rexinoids, are useful for prevention and treatment of lung cancer in an important animal model. It remains to be seen if these promising results can be translated into a practical clinical application.
Acknowledgements
We thank William W. Lamph and Roshantha A. Chandraratna for supplying the rexinoids and Thomas Sporn for his assistance with the lung pathology.
Grant support: National Foundation for Cancer Research, NIH R01 CA78814, and Reata Pharmaceuticals, Inc.
References
- 1.Jones S, Zhang X, Parsons DW, et al. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science. 2008;321:1801–1806. doi: 10.1126/science.1164368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parsons DW, Jones S, Zhang X, et al. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321:1807–1812. doi: 10.1126/science.1164382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sporn MB, Liby KT. Cancer chemoprevention: scientific promise, clinical uncertainty. Nat Clin Pract Oncol. 2005;2:518–525. doi: 10.1038/ncponc0319. [DOI] [PubMed] [Google Scholar]
- 4.Meyskens FL, Jr, McLaren CE, Pelot D, et al. Difluoromethylornithine plus sulindac for the prevention of sporadic colorectal adenomas: a randomized placebo-controlled, double-blind trial. Cancer Prev Res. 2008;1:32–38. doi: 10.1158/1940-6207.CAPR-08-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sporn MB. Dichotomies in cancer research: some suggestions for a new synthesis. Nat Clin Pract Oncol. 2006;3:364–373. doi: 10.1038/ncponc0536. [DOI] [PubMed] [Google Scholar]
- 6.Liby K, Royce DB, Williams CR, et al. The synthetic triterpenoids, CDDO-methyl ester and CDDO-ethyl amide, prevent lung cancer induced by vinyl carbamate in A/J mice. Cancer Res. 2007;67:2414–2419. doi: 10.1158/0008-5472.CAN-06-4534. [DOI] [PubMed] [Google Scholar]
- 7.Liby KT, Yore MM, Sporn MB. Triterpenoids and rexinoids as multifunctional agents for the prevention and treatment of cancer. Nat Rev Cancer. 2007;7:357–369. doi: 10.1038/nrc2129. [DOI] [PubMed] [Google Scholar]
- 8.Liby K, Royce DB, Risingsong R, et al. A new rexinoid, NRX194204, prevents carcinogenesis in both the lung and mammary gland. Clin Cancer Res. 2007;13:6237–6243. doi: 10.1158/1078-0432.CCR-07-1342. [DOI] [PubMed] [Google Scholar]
- 9.Liby K, Black CC, Royce DB, et al. The rexinoid LG100268 and the synthetic triterpenoid CDDO-methyl amide are more potent than erlotinib for prevention of mouse lung carcinogenesis. Mol Cancer Ther. 2008;7:1251–1257. doi: 10.1158/1535-7163.MCT-08-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Honda T, Rounds BV, Bore L, et al. Novel synthetic oleanane triterpenoids: a series of highly active inhibitors of nitric oxide production in mouse macrophages. Bioorg Med Chem Lett. 1999;9:3429–3434. doi: 10.1016/s0960-894x(99)00623-x. [DOI] [PubMed] [Google Scholar]
- 11.Honda T, Honda Y, Favaloro FG, Jr, et al. A novel dicyanotriterpenoid, 2-cyano-3,12-dioxooleana-1,9(11)-dien-28-onitrile, active at picomolar concentrations for inhibition of nitric oxide production. Bioorg Med Chem Lett. 2002;12:1027–1030. doi: 10.1016/s0960-894x(02)00105-1. [DOI] [PubMed] [Google Scholar]
- 12.Yates MS, Tauchi M, Katsuoka F, et al. Pharmacodynamic characterization of chemopreventive triterpenoids as exceptionally potent inducers of Nrf2-regulated genes. Mol Cancer Ther. 2007;6:154–162. doi: 10.1158/1535-7163.MCT-06-0516. [DOI] [PubMed] [Google Scholar]
- 13.Boehm MF, Zhang L, Zhi L, et al. Design and synthesis of potent retinoid X receptor selective ligands that induce apoptosis in leukemia cells. J Med Chem. 1995;38:3146–3155. doi: 10.1021/jm00016a018. [DOI] [PubMed] [Google Scholar]
- 14.Vuligonda V, Thacher SM, Chandraratna RA. Enantioselective syntheses of potent retinoid X receptor ligands: differential biological activities of individual antipodes. J Med Chem. 2001;44:2298–2303. doi: 10.1021/jm0100584. [DOI] [PubMed] [Google Scholar]
- 15.Honda T, Janosik T, Honda Y, et al. Design, synthesis, and biological evaluation of biotin conjugates of CDDO for the isolation of the protein targets. J Med Chem. 2004;47:4923–4932. doi: 10.1021/jm049727e. [DOI] [PubMed] [Google Scholar]
- 16.Liby K, Risingsong R, Royce DB, et al. Prevention and treatment of experimental estrogen receptor-negative mammary carcinogenesis by the synthetic triterpenoid CDDO-methyl ester and the rexinoid LG100268. Clin Cancer Res. 2008;14:4556–4563. doi: 10.1158/1078-0432.CCR-08-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yore MM, Liby KT, Honda T, Gribble GW, Sporn MB. The synthetic triterpenoid CDDO-imidazole blocks NF-κB activation through direct inhibition of IKK. Mol Cancer Ther. 2006;5:3232–3239. doi: 10.1158/1535-7163.MCT-06-0444. [DOI] [PubMed] [Google Scholar]
- 18.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 19.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Albini A, Sporn MB. The tumour microenvironment as a target for chemoprevention. Nat Rev Cancer. 2007;7:139–147. doi: 10.1038/nrc2067. [DOI] [PubMed] [Google Scholar]
- 21.Hopkins AL. Network pharmacology: the next paradigm in drug discovery. Nat Chem Biol. 2008;4:682–690. doi: 10.1038/nchembio.118. [DOI] [PubMed] [Google Scholar]