Summary
Objective
Osteoarthritis is a disease process of cellular degradation of articular cartilage caused by mechanical loads and inflammatory cytokines. We studied the cellular response in native cartilage subjected to a mechanical load administered simultaneously with an inflammatory cytokine (IL-1), hypothesizing that the combination of load and cytokine would result in accelerated extracellular matrix (ECM) degradation.
Methods
Mature bovine articular cartilage was loaded for three days (stimulation) with 0.2 and 0.5 MPa stresses, with and without interleukin-1 (IL-1α, 10 ng/ml), followed by three days of no stimulation (recovery). Aggrecan and collagen loss were measured as well as aggrecan cleavage using monoclonal antibodies AF-28 and BC-3 for cleavage by aggrecanases (ADAMTS) and matrix metalloproteinases (MMPs), respectively.
Results
Incubation with IL-1 caused aggrecan cleavage by aggrecanases and MMPs during the three days of stimulation. A load of 0.5 MPa inhibited the IL-1-induced aggrecan loss while no inhibition was found for the 0.2 MPa stress. There was no collagen loss during the treatments but upon load and IL-1 removal proteoglycan and collagen loss increased. Load itself under these conditions was found to have no effect when compared to the unloaded controls.
Conclusions
A mechanical load of sufficient magnitude can inhibit ECM degradation by chondrocytes when stimulated by IL-1. The molecular mechanisms involved in this process are not clear but probably involve altered mechanochemical signal transduction between the ECM and chondrocyte.
Keywords: Cartilage, Mechanobiology, Load, IL-1, MMPs, ADAMTS
Introduction
Osteoarthritis (OA) is a pathological condition of articular cartilage associated with extracellular matrix (ECM) degradation related to mechanical (wear-and-tear, high stress, trauma) and inflammatory (physicochemical) mechanisms, both of which have been shown to increase enzyme synthesis by chondfrocytes1–4. Matrix metalloproteinases-1, 3 & 13 (MMPs) and aggrecanases-1 & 2 (i.e., ADAMTS 4 & 5 - A Disintegrin And Metalloproteinase with ThromboSpondin-like motifs) are important enzymes responsible for the degradation of articular cartilage. Both enzymes cleave aggrecan within the interglobular domain (IGD) between the G1 and G2 regions, MMPs at the Asn341-Phe342 site and ADAMTS-4 & 5 at the Glu373-Ala374 site2,3,5–7. Each cleavage site produces a unique neoepitope which can be recognized by specific antibodies. Several studies have hypothesized that aggrecanase is the primary enzyme responsible for aggrecan proteolysis in the early stages of cartilage degeneration, well before aggrecan and collagen cleavage by MMPs2,3,7. These studies used physiochemically induced models of OA in which IL-1 was used to stimulate a catabolic response by the chondrocytes. While the inflammatory-induced injury model of OA has been well characterized, the mechanically-induced injury model of OA is not.
Several mechanically-induced in-vitro cell and tissue injury models have reported increased synthesis of MMPs and ECM degradation while one study found increased aggrecanase but only at the messenger level4,8–15. Recent evidence indicates that the mechanical and inflammatory mechanisms may be synergistic, resulting in a mechanically-induced down-regulation of the cellular catabolic response caused by IL-1 stimulation16–21. Thus, a mechanical load appears to inhibit the cleavage of aggrecan induced by IL-1 stimulation. Based on previous investigations4,12,22, we hypothesized that different magnitudes of load applied in the presence of an inflammatory cytokine (IL-1) might result in different mechanisms of cellular catabolism of the ECM. To test this hypothesis we measured aggrecan loss and cleavage before, during and after subjecting mature bovine cartilage to inflammatory (10 ng/ml of IL-1α) and mechanical (0.2 and 0.5 MPa) stimulations. We found that when stimulated with IL-1 both aggrecanases and MMPs degraded the ECM (aggrecan and collagen) in unloaded and 0.2 MPa loaded cartilage while the 0.5 MPa load was found to completely inhibited the cellular catabolism of the ECM produced by the IL-1 stimulation. Although several studies have reported preventative affects of load on IL-1-induced matrix degradation in isolated chondrocyte systems, to our knowledge this is the first report documenting that mechanical loading of native articular cartilage can inhibit chondrocyte catabolic degradation of the ECM when stimulated by IL-1.
Materials and Methods
CHEMICALS
Unless otherwise stated, all chemicals were purchased from Sigma (St. Louis, MO) or Gibco BRL (Grand Island, NY). Culture media (CM) consisted of Dulbecco’s Modified Eagle’s Medium (DMEM) containing 1% antibiotic/antimycotic (ABAM), 10mM HEPES buffer and 50μg/mL ascorbic acid, used as is or supplemented with 10% fetal bovine serum (FBS) or 1% ITS+ (BD bioscience) as described below. Interleukin-1 (IL-1α and IL-1β) was obtained from R&D Systems (Minneapolis, MN), aggrecanase-1 and MMPs-1,3 and 13 from Chemicon International (Temecula, CA), and chondroitinase ABC, keratanase and keratanase II from Seikagaku (Tokyo, Japan). Monoclonal antibodies (Mab) AF-28 and BC-3 for aggrecan cleavage sites within the IGD were obtained from Chemicon and Abcam (Cambridge, MA), respectively, and Mab G1, which recognizes the G1 domain within aggrecan, was kindly provided by Dr. Peter J. Roughley (Shriners Hospital, McGill University, Montreal, Canada).
TISSUE PREPARATION
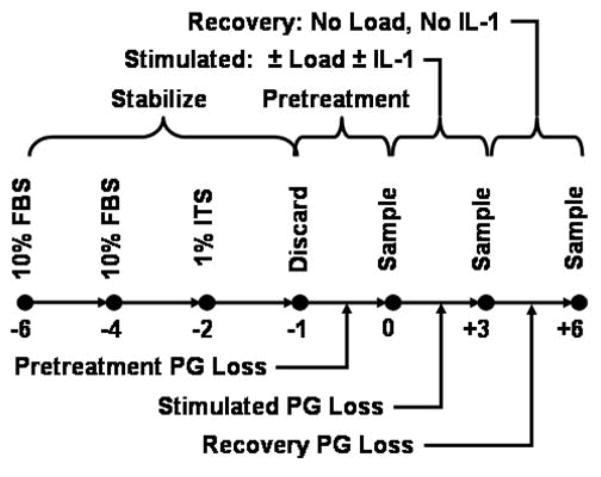
Mature bovine knees (young adult 18–24 months old) were obtained from a local abattoir within twenty-four hour’s postmortem. Seven-millimeter diameter full-thickness (~1.5 mm) cartilage discs (explants) were removed from the underlying bone in the load-bearing regions of the trochlear notch using a biopsy punch and surgical blade. The explants were separated into 24-well plates containing CM + 10% FBS and incubated at 100% humidity, 5% C02 and 95% air at 37°C for four days (denoted as days −6 to −2, Fig. 1). The media was replaced with CM + 1% ITS, the explants incubated for another two days (days −2 to 0), and the media from the last day (day 0) used to compare the initial 24-hour proteoglycan loss between the different treatment groups as described below. For specimens analyzed for aggrecan cleavage using Mab the ITS+ was not included in the media to avoid nonspecific cross-reaction with the antibodies. Separate tests found no effect of ITS+ on PG loss in any treatment group (data not shown).
Figure 1.
Cartilage specimens were removed from mature bovine knees (day −6) and incubated (Pretreatment) for 5 days (days −6 to −1) in culture media supplemented with 10% FBS (4 days) or 1% ITS (1 day); all media was discarded. The specimens were then separated into four treatment groups (Control, IL-1, Load, Load+IL-1), incubated in culture media for one additional day (days −1 to 0), and the media sampled (day 0), Treatments began on day 0 (Treatment) and continued for 3 days, with media sampled at 1, 3, 6, 9 and 24 hours for the first day and at 24 hr intervals thereafter. On day +3 the treatments were removed (Recovery) and the media sampled on each day. Analyses for PG and collagen content in the media and explant were performed.
EFFECT OF IL-1α AND IL-1β ON PROTEOGLYCAN LOSS
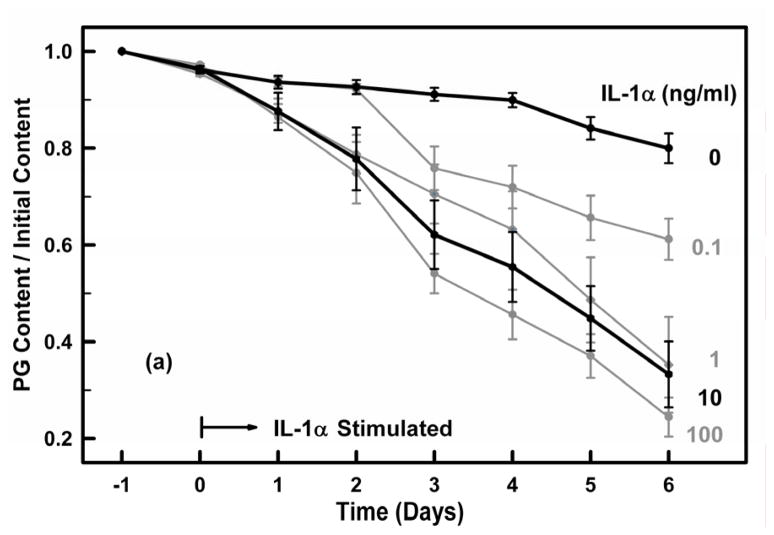
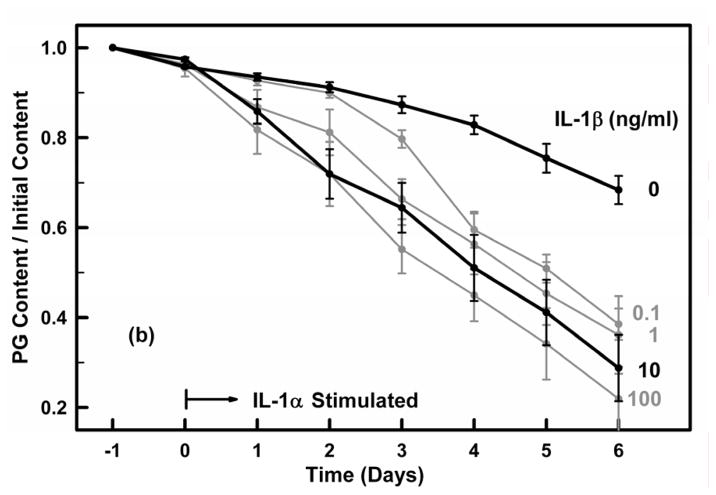
Preliminary studies were performed to measure the PG loss in cartilage explants exposed to different concentrations of IL-1 α and IL-1β over a six-day period. Explants (N=5 each treatment) were incubated with IL-1α or IL-1β at concentrations of 0, 0.1, 1, 10 or 100 ng/ml for six days. The media was collected at the end of each day with total PG content in the media (days 0 to 6) and explant (day 6) determined as described below.
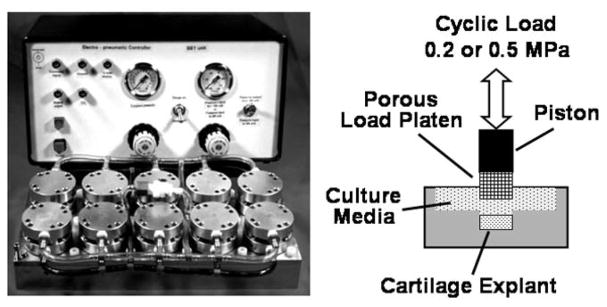
MECHANICAL LOADING PROTOCOL
Mechanical loading of the explant was performed using a Mechanical Explant Test System (METS, Fig. 2), as previously described12,23,24. The explants were cyclically loaded with a peak stress of 0.2 or 0.5 MPa (0-to-peak, sinusoidal waveform, 0.5 Hz). Four treatment groups (N=10 explants each) were used: (1) unloaded explants (Control), (2) unloaded explants stimulated with 10 ng/ml of IL-1α (IL-1), (3) loaded explants (Load), and (4) loaded explants stimulated with 10 ng/ml of IL-1α (Load+IL-1). Stimulation by mechanical load and IL-1α, individually and in combination, was applied for three days (stimulation phase) followed by six or three days of no stimulation (recovery phase) for the 0.2 and 0.5 MPa tests, respectively. Media was exchanged every 24 hrs for the 0.2 MPa groups and for the 0.5 MPa groups at 1, 3, 6, 9 and 24 hrs, then every 24 hrs thereafter.
Figure 2.
The Mechanical Explant Test System (METS) consists of dual 10-well loading chambers (bottom left) controlled by a personal computer interfaced with an electronic pneumatic controller (top left). The full-depth cartilage explant is confined laterally with its articular surface in contact with the culture media (right). A uniaxial compressive load is applied to the articular surface by a porous load platen attached to the piston of the air cylinder. Modified from Lucchinetti et al.24
ANALYSIS OF CELL DEATH
Cell viability was assessed at harvest and each time interval. A ~0.5 mm specimen was removed perpendicular to the articular surface, incubated in 60 μM propidium iodide (indicator of cell death) and 10μM fluorescein diacetate (indicator of cell viability), washed, and imaged using a fluorescent microscope (excitation 488 nm; wavelength’s 535 and 630 nm, respectively; Nikon Optiphot-2, Melville, NY) and CCD camera (Optronics, Goleta, CA).
PROTEOGLYCAN AND COLLAGEN LOSS
Each explant was digested at the end of the experiment with papain (1 mg/ml at 65°C for 4 hours) and the PG content (sulfated glycosaminoglycans or GAGs) in the explant digest and media determined using the dimethylmethylene blue (DMMB) assay25. Likewise collagen content (0.5 MPa only) was determined using the hydroxyproline assay26.
ANALYSIS OF ENZYME-GENERATED AGGRECAN FRAGMENTS
In brief, proteoglycans were extracted from the explant and media using 4 M GuHCl in sodium acetate buffer solution, deglycosylated with keratanase (0.001 U/ug GAG), keratanase II (0.001 U/ug GAG) and chondroitinase ABC (0.001 U/ug GAG), and analyzed using Western blots. Western blot analysis was performed using Mab BC-3 for detection of aggrecanase generated aggrecan cleavage at the N-terminal ARGS fragment at the Asn373-Ala374 bond27, and Mabs AF 28 and BC14 were used for detection of MMP mediated cleavage, both of which recognize the N-terminal FFGV fragment at the Asn341-Phe342 bond28. As a positive control, purified bovine aggrecan (10 mg/ml) was digested with human recombinant aggrecanase-1 and MMPs-1, 3 and 13, all activated with 1mM APMA. To ensure that the aggrecan and its catabolites were deglycosylated, Western blot analysis was performed using Mab G1.
STATISTICAL ANALYSIS
For each explant, PG and collagen released to the media were normalized to their total (initial) contents at the start of the experiment (day −1) prior to mechanical and IL-1 stimulation on day 0 (total = digest + media summed over all times). PG content and PG and collagen losses were averaged for each treatment and plotted as a function of post-treatment time, with the day −1 and day 0 values used to compare baseline (starting) values for each treatment group. Differences between treatment groups were analyzed using the unpaired Student’s (two-tailed) t-test and analysis of variance (ANOVA) (Systat, SPSS, Chicago, IL; Excel, Microsoft, Redmond, WA). Turkey/Kramer post-hoc analysis was used to determine statistical differences for temporal responses within each treatment group. All data are expressed as mean ± standard deviation unless otherwise indicated. The statistical significance level (α) was set at 0.05.
Results
PROTEOGLYCAN LOSS BY IL-1α AND IL-1β IS DOSE AND TIME DEPENDENT
Cartilage explants were incubated over a six-day period with IL-1α or IL-1β at concentrations of 0, 0.1, 1, 10 or 100 ng/ml and the PG content remaining in each explant normalized by its initial PG content (day −1) (Fig. 3). For the control groups (no IL-1) the mean PG loss per day was 3.4%±2.2 (N=80, r=0.842). Both IL-1α and IL-1β induced almost identical catabolic responses that were dose and time dependent. At 10 ng/ml the PG loss per day due to IL-1α and IL-1β was 10.6%±6.2% (r=0.869) and 11.3%±5.9% (r=0.891), respectively, not significantly different (p>0.5). Thus, for the remainder of the experiments we used IL-1α at a concentration of 10 ng/ml.
Figure 3.
Figures 3a and 3b. Cartilage explants were incubated for 24 hours in IL-1 free media (days −1 to 0) and then were exposed on day 0 to (a) IL-1α and (b) IL-1β at concentrations of 0, 0.1, 1, 10 and 100 ng/ml for six days (days 1–6). The PG content remaining in the explant on each day was determined from the PG released to the media on each day and the PG remaining in the explant on day 6. Media and IL-1 were exchanged on each day. All groups had similar PG contents at the time of IL-1 administration on day 0. The 0 and 10 ng/ml concentrations are highlighted. Data are means ± SEM for n=5 explants.
CELLS REMAIN VIABLE IN RESPONSE TO MECHANICAL LOADING AND IL-1
For each treatment group cell viability was assessed at the end of each experiment using a live/dead cell assay. There was no significant cell death in any treatment group at the time of harvest, on day 0 before the start of the mechanical and IL-1 stimulations, and at the end of each experiment (data not shown). We were thus confident that any PG loss from the explant was due solely to the mechanical or IL-1 stimulations and not cell death.
MECHANICAL LOAD AT 0.2 MPa DID NOT INHIBIT PG LOSS DUE TO IL-1 STIMULATION
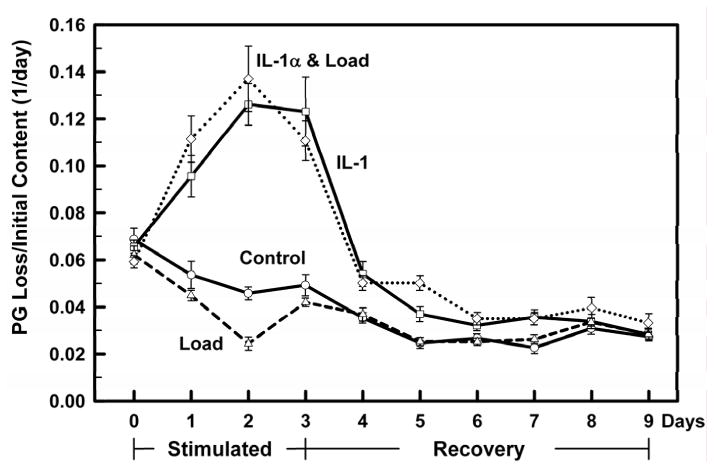
Explants were cyclically loaded for three days with a stress of 0.2 MPa ± 10 ng/ml of IL-1α and compared to control (no load, no IL-1α) and IL-1α stimulated explants. The PG loss on day 0, twenty-four hours prior to mechanical and IL-1α stimulations, was the same for all treatment groups indicating uniformity of PG loss before application of the mechanical and IL-1α stimulations (Fig. 4). PG loss in the Control and Load groups were similar on all days (days 0–9). PG loss in the IL-1 group was elevated on all days and the addition of the 0.2 MPa stress did not effect the PG loss caused by exposure to IL-1α. When the load and IL-1α were removed (day 3), the IL-1α stimulated groups (±load) did not return to the non-IL-1α stimulated PG loss values until day 6 or later.
Figure 4.
All treatment groups (Controls: ○ solid line; IL-1α □ solid line; Load: △ dashed line; Load+ IL-1α ◇ dotted line) were incubated for 24 hours without any treatment, exposed on day 0 (Stimulated) to IL-1α (10 ng/ml), Load (0.2 MPa) and Load+IL-1α for 3 days (days 0–3), and the treatments removed on day 4 (Recovery) for another 6 days (days 4–9). The PG loss to the media per day was normalized to the initial PG content in the explant. All groups had similar initial PG contents at the start of the experiment (day −1, data not shown), and similar PG loss during the 24 hrs prior to treatment (day 0). Load did not affect the IL-1 induced PG catabolism. Data are means ± SEM for n=10 explants.
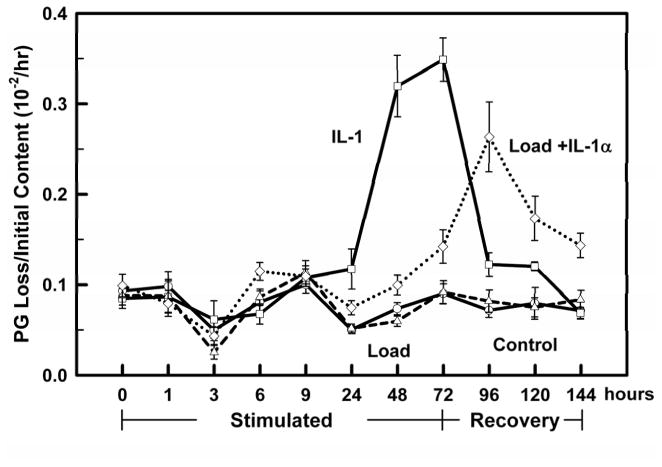
MECHANICAL LOAD AT 0.5 MPa INHIBITS PG LOSS DUE TO IL-1 STIMULATION
A mechanical load of 0.5 MPa was continuously applied to explants for three days (±10 ng/ml of IL-1α) and the PG loss to the media measured after 1, 3, 6, 9, 24, 48 and 72 hours. Thereafter the load and IL-1α stimulations were removed for an additional three days (recovery) and the PG loss measured every 24 hrs. Similar to the 0.2 MPa load, the 0.5 MPa load did not affect the PG loss in non-IL-1α stimulated explants during loading and recovery (Load vs. Control, Fig. 5).
Figure 5.
All treatment groups (Controls: ○ solid line; IL-1α □ solid line; Load: △ dashed line; Load+ IL-1α ◇ dotted line) were incubated for 24 hours without treatment, exposed on day 0 (0 hours) to IL-1α (10 ng/ml), Load (0.5 MPa) and Load+IL-1α for 3 days (0–72 hours), and the treatments removed on day 4 (72 hours) for another 3 days (72–144 hours). PG loss to the media at each time interval (per hr) was normalized to the initial PG content (day −1). All groups had similar PG loss at the start of the experiment (day 0). Load can now clearly be seen to start inhibiting the IL-1 induced PG catabolism by 24 hours, and while still inhibiting the PG catabolism there is a continual increase in the rate of PG loss until the load is removed on day 3. Data are means ± SEM for n=10 explants.
For the first 9 hours of treatment with IL-1α, the PG loss in the IL-1α and Load+IL-1α groups were not different from the Control and Load groups. Over the next 15 hours, however, the PG loss in the IL-1α group significantly increased (p<0.01; see 24 hrs, Fig. 5) while the addition of the mechanical load inhibited the PG loss (Load+IL-1α vs IL-1α, 0.1>p>0.05), though not to the level of the controls (Load+IL-1α vs. Controls, p<0.02). For longer incubation times (≥24 hrs), IL-1α stimulation increased the PG loss from 1.6 fold at 24 hours to 4.3 and 3.7 fold by days 2 and 3, respectively (IL-1α vs. Controls, Fig. 5). Application of the 0.5 MPa mechanical load significantly inhibited the IL-1α induced PG loss on days 2 and 3 by 3.1 and 2.3 fold, respectively, however not to the control levels (Load+IL-1α vs. IL-1α, Fig. 5).
After removing the load and IL-1α stimulations the PG loss for the IL-1α group decreased by almost 3 fold, returning to the control levels on day 6. However the PG loss in the Load+IL-1α group continued to increase (1.9 fold) on day 4 and then slowly decreased over the next two days but never reached the control values (p<0.001, Fig. 5).
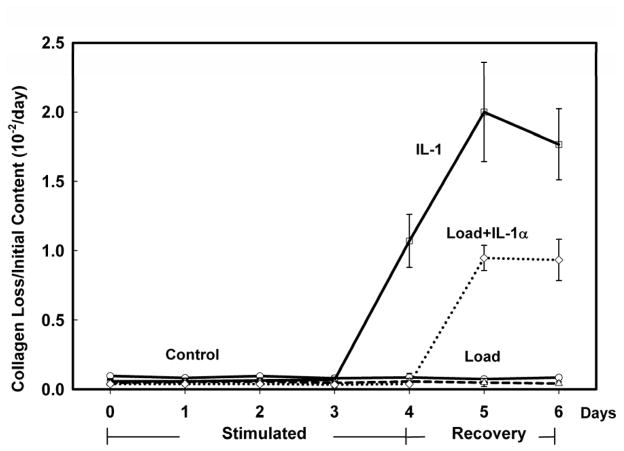
MECHANICAL LOADING AT 0.5 MPa INHIBITED COLLAGEN LOSS DUE TO IL-1 STIMULATION
For explants loaded with 0.5 MPa, collagen loss to the media per day (micrograms) was measured in a subset of explants (N=3 with greatest PG loss) and normalized to the initial collagen content (milligrams, day −1). The collagen loss in the Control and Load groups did not change compared to their baseline loss on day 0 (Fig. 6). In the IL-1α group collagen loss did not change for days 1–3 but significantly increased (~100 to 200 fold) on days 4–6 after IL-1α removal. In the Load+IL-1α group, the collagen loss did not change during stimulation and one day after removal of the IL-1α however it increased on days 5 and 6 but not to the level of the IL-1α group (47% and 53%, respectively).
Figure 6.
Collagen loss to the media on each day (Controls: ○ solid line; IL-1α □ solid line; Load: △ dashed line; Load+IL-1α ◇ dotted line) was normalized to the initial collagen content (day −1). There was minimal collagen loss in all groups during the treatment interval (Stimulated, days 0–3). After treatment removal (Recovery, days 4–6) there was an increase in collagen loss in the IL-1α group starting on day 4 while in the Load+IL-1α group an increase in collagen loss was delayed until day 5. Data are means ± SEM for n=10 explants.
MECHANICAL LOADING INHIBITED AGGRECAN CLEAVAGE BY MMPs AND AGGRECANASES
The aggrecan released into the media was analyzed using Western blots to determine the enzyme specific site of aggrecan cleavage. Cleavage within the interglobular domain between G1 and G2 was analyzed using BC-3 Mab to detect aggrecanase generated cleavage and AF 28 and BC14 Mab to detect MMP cleavage. In addition G1 Mab was used to recognize the G1 domain at the N terminal of the aggrecan. Two explants were analyzed from each treatment group having similar responses as shown in Figs. 5 and 6, and having the greatest PG loss. The later criteria was chosen to maximize the amount of aggrecan fragmentation for detection of the specific cleavage sites. To further confirm the aggrecan cleavage results from this subset, additional Western blot analyses were performed on a separate set of explants similarly stimulated (0.5 MPa, IL-1α 10 ng/ml) for three days plus one day of recovery (data not shown). A minimum of two positive specimens out of four analyses were used to validate specificity of aggrecan cleavage sites.
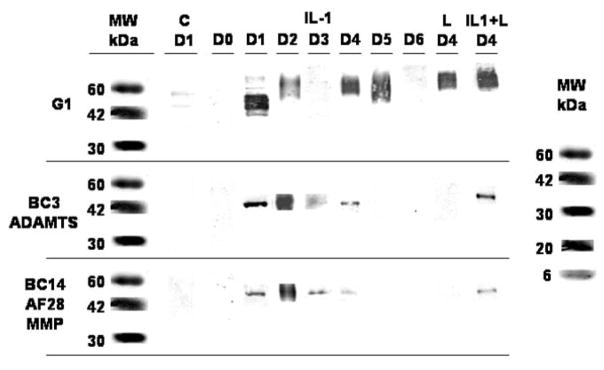
A representative Western blot for all days is shown in Fig. 7. There were no aggrecan fragments (cleavage products) found in the media of the Control group on any of the days. In the IL-1α treated group, aggrecan fragments where released to the media on days 1–3, when the IL-1α was present within the culture medium, and one day after IL-1α removal from the media. Further aggrecan cleavage by either enzyme was not detected (days 5 and 6). The fragments on days 1–4 were generated by aggrecanase and MMP cleavage within the aggrecan’s interglobular domain at the Asn373-Ala374 and Asn341-Phe342 sites, respectively. The greatest amount of fragments by both enzymes occurred on day 2.
Figure 7.
Aggrecan fragments released into the media before (day 0, D0) and during treatment (days 1–3, D1–D3) and recovery (days 4–6, D4–D6) were measured by Western blot analysis using antibodies which recognize aggrecan cleavage sites within the interglobular (G1–G2) domain (BC-3 and AF28/BC14 for aggrecanase and MMP generated cleavage, respectively; G1 recognizes the G1 domain). No aggrecan fragments were found in the Control group (C) on any day (only D1 is shown). In the Load (L) group the G1 domain fragment was detected only on day 4 (only D4 is shown). In the IL-1α (IL-1) group aggrecan cleavage by both enzymes was detected on days 1–4, with the greatest amount of fragments occurring on day 2. Load inhibited IL-1-induced aggrecan cleavage by both enzymes except on day 4 were both enzyme cleavage sites were detected (L+IL-1; only D4 is shown).
No aggrecan fragments were found in the media of the Load group on any day nor for the Load+IL-1α group on days 0–3 (Fig. 7). However aggrecan fragments were found in the media of the Load+IL-1α group one day after load and IL-1α removal (day 4). These fragments were generated by aggrecanase and MMP cleavage in about equal amounts.
Aggrecan cleavage (nonspecific) was further confirmed using G1 Mab (Fig. 7). Aggrecan fragments were found in the media for the IL-1 α group on days 1–5, and the Load and Load+IL-1α groups on day 4. No aggrecan fragments were detected for the Control group on any day nor for the Load and Load+IL-1α groups on days 0–3 and 5–6.
Discussion
Previously we used our in vitro mechanical explant test system (METS) to produce an OA-like cellular response and ECM damage in mature bovine articular cartilage when loaded with a stress ≥1 MPa for ≤24 hours4,12,23. Our METS cyclically loads the ECM in confined compression, a configuration that simulates one aspect of the functional environment within a joint where the compressive stress applied to the articular surface causes fluid (water) and solute transport (i.e., IL-1, aggrecan fragments, nutrients) across the surface. In this study we loaded explants with smaller stresses (0.2 and 0.5 MPa) for longer times (three days) and studied the cellular response to these loads individually and when applied simultaneously with an inflammatory cytokine (IL-1). We hypothesized that administration of both stimuli would result in an accelerated degradation process. On the contrary, we found that the 0.5 MPa stress inhibited the IL-1- induced cellular catabolism of the ECM while the 0.2 MPa stress had no effect. To our knowledge this is the first study to evaluate the coupled effects of IL-1 and mechanical load on ECM degradation in full-thickness, native, mature articular cartilage.
Similar to other studies3,5,29 we found that exposure of unloaded, free-swelling articular cartilage to IL-1 over a six-day interval resulted in aggrecan cleavage and loss to the media (Figs. 3–5). Western blot analysis of aggrecan fragments revealed that cleavage within the IGD was at two different sites attributed to aggrecanase and MMP activity (Asn373-Ala374 and Asn341-Phe342 bonds, respectively). Both cleavage-site fragments appeared at equivalent times after IL-1 administration (Fig. 7), which differs from previous findings in which aggrecan was initially cleaved by aggrecanases and at a later time by MMPs3,29,30. While we cannot explain this difference, it is most likely due to differences in specie (bovine vs. human), age (immature vs. mature), joint (knee vs. ankle), tissue type (articular vs. nasal), explant geometry (full thickness vs. partial), stimulus interval, and even the test configuration (confined vs. unconfined).
Application of a cyclic mechanical load of 0.2 or 0.5 MPa for 3 days did not cause ECM degradation compared to unloaded, free-swelling controls. This result is not unique as similar findings have been reported for loads ≤0.5 MPa under a variety of different loading regimes (unconfined, intermittent, duration)31–34. Based on these and other studies35,36 we can hypothesize that a cyclic stress of ≤0.5 MPa applied to the ECM (equivalent static stress of 0.35 MPa) is probably below the level needed to cause a significant catabolic response by the chondrocytes. However, this hypothesis needs to be validated especially considering the inhomogeneous and anisotropic properties of the ECM where local deformations are significantly greater at the articular surface then in the deeper zones36,37.
One of the most interesting findings of our study was that a 0.2 MPa cyclic stress applied concurrently with IL-1α (10 ng/ml) had no effect on ECM degradation while a 0.5 MPa stress significantly inhibited the IL-1α-induced ECM degradation. Similar to our lower stress, Shin et al.38 reported that IL-1-induced (1 ng/ml) PG loss in meniscal fibrocartilage explants was not inhibited by cyclic compression (0.1 MPa, 0.5 Hz, 24 hrs). On the contrary, our higher stress almost completely inhibited the increased PG loss caused by the IL-1 stimulation (Fig. 5) while completely inhibiting aggrecan cleavage within the IGD as no fragments were found in the media until after load removal on day 4 (Fig. 6). The absence of aggrecan cleavage products within the ECM was later confirmed by Western blot analyses (data not shown). This unique finding has not been previously reported and indicates that mechanical loads >0.2 MPa applied to native articular cartilage may have an anti-inflammatory role in counteracting the degradative affects of IL-1. At present the exact mechanism for the inhibition is not known but probably involves preservation of the mechanobiological signal transduction pathways between the ECM and the chondrocyte.
Several other studies have reported similar inhibitory affects of a mechanical stimulus on chondrocyte response to IL-1 (10 ng/ml) stimulation. However different from our METS configuration which applied a cyclic load (stress) to native cartilage in confined compression, these studies applied a cyclic deformation (strain) to isolated chondrocytes (monolayer tensile) or to chondrocyte-seeded agarose gels (unconfined compression). While different these cell-based configurations can help explain possible inhibitory mechanisms when loading intact cartilage using our METS. Agarwal et al.16,19–21,39 reported that tensile strain (3%, 20%) inhibited or reduced IL-1-induced increases in gene expression (mRNA) for nitric oxide (NO), inducible nitric oxide synthase (iNOS), tumor necrosis factor (TNF)-α, cyclo-oxygenase (COX)-2, and MMP-3, 7, 8, 9, 13, 16, 17 and 19, had no affect on MMP-2, 11 and 14 and TIMP-1, 2 and 3, and reversed IL-1 inhibition of aggrecan synthesis. They concluded that the tensile strain acts at multiple sites within the NF-κB signaling pathway. Likewise, Chowdbury et al.17,18,40 found a compressive strain (15%) inhibited IL-1-induced increases in synthesis of NO and prostaglandin-E2 and mRNA for iNOS and COX-2, and that the mechanical affect involved the p38 mitgen-activated protein kinase (MAPK) dependent pathway. Contrary to these inhibitory effects, Lima et al.41 reported that a compressive strain (10%±5%) did not counteract the catabolic effects of IL-1-induced loss of GAG content and mechanical properties in long-term (≤45 days) chondrocyte-gel cultures. However this result was confounded by the presence in the culture media of dexamethasone (0.1 uM) and transforming growth factor-beta (10 ng/ml), two cytokines that can be mutually antagonistic, regulate the gene expression for MMPs, and inhibit IL-1-induced ECM degradation42–46. In either case, these results indicate that under a variety of loading conditions and configurations, a mechanical stimulus of sufficient magnitude and duration can counteract the IL-1-induced catabolic degradation of the cartilage extracellular matrix.
Once the load and IL-1α stimulations were removed (recovery >72 hrs) the ECM degradation was found to increase dramatically for the next 24 hours (96 hrs, Fig. 5; day 4, Fig. 7) and then gradually decrease over the next 48 hours (120 and 144 hrs, Fig. 5). What caused the large increase in PG release after the load and IL-1α were removed is not known. Several mechanisms are possible: a cellular catabolic response due to the removal of the load and IL-1α; residual IL-1α in the ECM after load removal; release of trapped aggrecan fragments within the compressed ECM; or continued catabolism from the previous 3 days. It is unlikely that a catabolic response resulted from the simultaneous removal of both stimuli since a similar response was not observed in the Load or IL-1 groups. On the other hand, aggrecan entrapment within the ECM probably occurred during cyclic loading as we have previously reported on this mechanism47 Even though aggrecan entrapment may have occurred in both loaded groups, it can only partially explain the increase in aggrecan release in the Load+IL-1 group since this did not occur in the Load group (Fig. 5). In addition, only intact aggrecan was released to the media during loading in both groups while aggrecan fragments were released only in the Load+IL-1 group on day 4 (Fig. 7). Finally aggrecan fragments were not found within the ECM of any group on day 3, thus ruling out fragment entrapment (Western blot analysis; data not shown).
The most likely mechanism responsible for the increased aggrecan release after both stimuli were removed appears to be from residual IL-1α in the ECM. Since aggrecan release in the IL-1 group significantly decreased after IL-1α removal, it may be that the concentration of residual IL-1α in the Load+IL-1 group was greater than in the IL-1 group, causing continued aggrecan degradation. However, a delayed or inhibited cellular response cannot be ruled out.
Concomitant with aggrecan proteolysis in the later stages of degeneration is collagen cleavage by collagenases (MMP-1 and 13)3,29,30,48,49. Similar to others we found a delay in collagen loss (days 4–6) in response to IL-1 stimulation (Fig. 6). On the other hand, the mechanical load did not affect the collagen loss during or after stimulation nor during stimulation when combined with IL-1. Similar to the aggrecan loss, we found increased collagen loss after both stimuli were removed but delayed one day (day 5) compared to the aggrecan loss (day 4). Whether the mechanical load inhibited the catabolism of the collagen during loading cannot be assessed, however it does appear that the loading did delay the collagen loss.
In summary, we examined the effect of mechanical load on the degradation of the extracellular matrix by IL-1 and found that a 0.2 MPa stress had no effect while 0.5 MPa almost completely inhibited the IL-1-induced degradation during loading. The mechanism responsible for the inhibition appears to be at the cellular level from an as yet unknown mechanochemical signal-transduction pathway. Further studies will be required to fully understand how different magnitudes, durations and patterns of mechanical load affect cartilage health and degradation in the presence of inflammatory cytokines such as IL-1 and TNF-α.
Acknowledgments
Support for this study was made possible by Grant Number AR45748 (PAT) from the National Institutes of Health - National Institute of Arthritis and Musculoskeletal and Skin Diseases. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH-NIAMS. This investigation was conducted in a facility constructed with support from Research Facilities Improvement Program Grant Number C06-RR12538-01 from the National Center for Research Resources, National Institutes of Health.
Footnotes
Conflict of interest
All authors declare there is no financial interests or personal relationships with other people or organizations, direct or indirect, that could inappropriately influence the conduct or reporting of this work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tortorella MD, Malfait AM, Deccico C, Arner E. The role of ADAM-TS4 (aggrecanase-1) and ADAM-TS5 (aggrecanase-2) in a model of cartilage degradation. Osteoarthritis Cartilage. 2001;9:539–552. doi: 10.1053/joca.2001.0427. [DOI] [PubMed] [Google Scholar]
- 2.Tortorella MD, Liu RQ, Burn T, Newton RC, Arner E. Characterization of human aggrecanase 2 (ADAM-TS5): substrate specificity studies and comparison with aggrecanase 1 (ADAM-TS4) Matrix Biol. 2002;21:499. doi: 10.1016/s0945-053x(02)00069-0. [DOI] [PubMed] [Google Scholar]
- 3.Little CB, Hughes CE, Curtis CL, Janusz MJ, Bohne R, Wang-Weigand S, et al. Matrix metalloproteinases are involved in C-terminal and interglobular domain processing of cartilage aggrecan in late stage cartilage degradation. Matrix Biol. 2002;21:271–288. doi: 10.1016/s0945-053x(02)00004-5. [DOI] [PubMed] [Google Scholar]
- 4.Lin PM, Chen CT, Torzilli PA. Increased stromelysin-1 (MMP-3), proteoglycan degradation (3B3- and 7D4) and collagen damage in cyclically load-injured articular cartilage. Osteoarthritis Cartilage. 2004;12:485–496. doi: 10.1016/j.joca.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 5.Arner EC. Aggrecanase-mediated cartilage degradation. Curr Opin Pharmacol. 2002;2:322–329. doi: 10.1016/s1471-4892(02)00148-0. [DOI] [PubMed] [Google Scholar]
- 6.Malfait AM, Liu RQ, Ijiri K, Komiya S, Tortorella MD. Inhibition of ADAM-TS4 and ADAM-TS5 prevents aggrecan degradation in osteoarthritic cartilage. J Biol Chem. 2002;277:22201–22208. doi: 10.1074/jbc.M200431200. [DOI] [PubMed] [Google Scholar]
- 7.Song RH, Tortorella MD, Malfait AM, Alston JT, Yang Z, Arner EC, et al. Aggrecan degradation in human articular cartilage explants is mediated by both ADAMTS-4 and ADAMTS-5. Arthritis Rheum. 2007;56:575–585. doi: 10.1002/art.22334. [DOI] [PubMed] [Google Scholar]
- 8.Honda K, Ohno S, Tanimoto K, Ijuin C, Tanaka N, Doi T, et al. The effects of high magnitude cyclic tensile load on cartilage matrix metabolism in cultured chondrocytes. Eur J Cell Biol. 2000;79:601–609. doi: 10.1078/0171-9335-00089. [DOI] [PubMed] [Google Scholar]
- 9.Jin G, Sah RL, Li YS, Lotz M, Shyy JY, Chien S. Biomechanical regulation of matrix metalloproteinase-9 in cultured chondrocytes. J Orthop Res. 2000;18:899–908. doi: 10.1002/jor.1100180608. [DOI] [PubMed] [Google Scholar]
- 10.Patwari P, Fay J, Cook MN, Badger AM, Kerin AJ, Lark MW, et al. In vitro models for investigation of the effects of acute mechanical injury on cartilage. Clin Orthop. 2001:S61–71. doi: 10.1097/00003086-200110001-00007. [DOI] [PubMed] [Google Scholar]
- 11.Patwari P, Cook MN, DiMicco MA, Blake SM, James IE, Kumar S, et al. Proteoglycan degradation after injurious compression of bovine and human articular cartilage in vitro: interaction with exogenous cytokines. Arthritis Rheum. 2003;48:1292–1301. doi: 10.1002/art.10892. [DOI] [PubMed] [Google Scholar]
- 12.Chen CT, Bhargava M, Lin PM, Torzilli PA. Time, stress, and location dependent chondrocyte death and collagen damage in cyclically loaded articular cartilage. J Orthop Res. 2003;21:888–898. doi: 10.1016/S0736-0266(03)00050-0. [DOI] [PubMed] [Google Scholar]
- 13.DiMicco MA, Patwari P, Siparsky PN, Kumar S, Pratta MA, Lark MW, et al. Mechanisms and kinetics of glycosaminoglycan release following in vitro cartilage injury. Arthritis Rheum. 2004;50:840–848. doi: 10.1002/art.20101. [DOI] [PubMed] [Google Scholar]
- 14.Kuroki K, Cook JL, Stoker AM, Turnquist SE, Kreeger JM, Tomlinson JL. Characterizing osteochondrosis in the dog: potential roles for matrix metalloproteinases and mechanical load in pathogenesis and disease progression. Osteoarthritis Cartilage. 2005;13:225–234. doi: 10.1016/j.joca.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 15.Lee JH, Fitzgerald JB, Dimicco MA, Grodzinsky AJ. Mechanical injury of cartilage explants causes specific time-dependent changes in chondrocyte gene expression. Arthritis Rheum. 2005;52:2386–2395. doi: 10.1002/art.21215. [DOI] [PubMed] [Google Scholar]
- 16.Gassner R, Buckley MJ, Georgescu H, Studer R, Stefanovich-Racic M, Piesco NP, et al. Cyclic tensile stress exerts antiinflammatory actions on chondrocytes by inhibiting inducible nitric oxide synthase. J Immunol. 1999;163:2187–2192. [PMC free article] [PubMed] [Google Scholar]
- 17.Chowdhury TT, Bader DL, Lee DA. Dynamic compression counteracts IL-1 beta-induced release of nitric oxide and PGE2 by superficial zone chondrocytes cultured in agarose constructs. Osteoarthritis Cartilage. 2003;11:688–696. doi: 10.1016/s1063-4584(03)00149-3. [DOI] [PubMed] [Google Scholar]
- 18.Chowdhury TT, Appleby RN, Salter DM, Bader DA, Lee DA. Integrin-mediated mechanotransduction in IL-1 beta stimulated chondrocytes. Biomech Model Mechanobiol. 2006;5:192–201. doi: 10.1007/s10237-006-0032-3. [DOI] [PubMed] [Google Scholar]
- 19.Deschner J, Rath-Deschner B, Agarwal S. Regulation of matrix metalloproteinase expression by dynamic tensile strain in rat fibrochondrocytes. Osteoarthritis Cartilage. 2006;14:264–272. doi: 10.1016/j.joca.2005.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Madhavan S, Anghelina M, Rath-Deschner B, Wypasek E, John A, Deschner J, et al. Biomechanical signals exert sustained attenuation of proinflammatory gene induction in articular chondrocytes. Osteoarthritis Cartilage. 2006;14:1023–1032. doi: 10.1016/j.joca.2006.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dossumbekova A, Anghelina M, Madhavan S, He L, Quan N, Knobloch T, et al. Biomechanical signals inhibit IKK activity to attenuate NF-kappaB transcription activity in inflamed chondrocytes. Arthritis Rheum. 2007;56:3284–3296. doi: 10.1002/art.22933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levin AS, Chen CT, Torzilli PA. Effect of tissue maturity on cell viability in load-injured articular cartilage explants. Osteoarthritis Cartilage. 2005;13:488–496. doi: 10.1016/j.joca.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 23.Torzilli PA, Grigiene R, Huang C, Friedman SM, Doty SB, Boskey AL, et al. Characterization of cartilage metabolic response to static and dynamic stress using a mechanical explant test system. Journal of Biomechanics. 1997;30:1–9. doi: 10.1016/s0021-9290(96)00117-0. [DOI] [PubMed] [Google Scholar]
- 24.Lucchinetti E, Adams CS, Horton WE, Jr, Torzilli PA. Cartilage viability after repetitive loading: a preliminary report. Osteoarthritis Cartilage. 2002;10:71–81. doi: 10.1053/joca.2001.0483. [DOI] [PubMed] [Google Scholar]
- 25.Farndale RW, Buttle DJ, Barrett AJ. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim Biophys Acta. 1986;883:173–177. doi: 10.1016/0304-4165(86)90306-5. [DOI] [PubMed] [Google Scholar]
- 26.Edwards CA, O’Brien WD., Jr Modified assay for determination of hydroxyproline in a tissue hydrolyzate. Clin Chim Acta. 1980;104:161–167. doi: 10.1016/0009-8981(80)90192-8. [DOI] [PubMed] [Google Scholar]
- 27.Hughes CE, Caterson B, Fosang AJ, Roughley PJ, Mort JS. Monoclonal antibodies that specifically recognize neoepitope sequences generated by ‘aggrecanase’ and matrix metalloproteinase cleavage of aggrecan: application to catabolism in situ and in vitro. Biochem J. 1995;305 (Pt 3):799–804. doi: 10.1042/bj3050799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fosang AJ, Last K, Gardiner P, Jackson DC, Brown L. Development of a cleavage-site-specific monoclonal antibody for detecting metalloproteinase-derived aggrecan fragments: detection of fragments in human synovial fluids. Biochem J. 1995;310 (Pt 1):337–343. doi: 10.1042/bj3100337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Little CB, Flannery CR, Hughes CE, Goodship A, Caterson B. Cytokine induced metalloproteinase expression and activity does not correlate with focal susceptibility of articular cartilage to degeneration. Osteoarthritis Cartilage. 2005;13:162–170. doi: 10.1016/j.joca.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 30.Sumer EU, Sondergaard BC, Rousseau JC, Delmas PD, Fosang AJ, Karsdal MA, et al. MMP and non-MMP-mediated release of aggrecan and its fragments from articular cartilage: a comparative study of three different aggrecan and glycosaminoglycan assays. Osteoarthritis Cartilage. 2007;15:212–221. doi: 10.1016/j.joca.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 31.Koob T, Clark P, Hernandez D, Thurmond F, Vogel K. Compression loading in vitro regulates proteoglycan synthesis by tendon fibrocartilage. Archives of Biochemistry and Biophysics. 1992;298:303–312. doi: 10.1016/0003-9861(92)90127-i. [DOI] [PubMed] [Google Scholar]
- 32.Sauerland K, Raiss RX, Steinmeyer J. Proteoglycan metabolism and viability of articular cartilage explants as modulated by the frequency of intermittent loading. Osteoarthritis Cartilage. 2003;11:343–350. doi: 10.1016/s1063-4584(03)00007-4. [DOI] [PubMed] [Google Scholar]
- 33.Wolf A, Raiss RX, Steinmeyer J. Fibronectin metabolism of cartilage explants in response to the frequency of intermittent loading. J Orthop Res. 2003;21:1081–1089. doi: 10.1016/S0736-0266(03)00064-0. [DOI] [PubMed] [Google Scholar]
- 34.Ackermann B, Steinmeyer J. Collagen biosynthesis of mechanically loaded articular cartilage explants. Osteoarthritis Cartilage. 2005;13:906–914. doi: 10.1016/j.joca.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 35.Durrant LA, Archer CW, Benjamin M, Ralphs JR. Organisation of the chondrocyte cytoskeleton and its response to changing mechanical conditions in organ culture. J Anat. 1999;194:343–353. doi: 10.1046/j.1469-7580.1999.19430343.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Torzilli PA, Deng XH, Ramcharan M. Effect of compressive strain on cell viability in statically loaded articular cartilage. Biomech Model Mechanobiol. 2006;5:123–132. doi: 10.1007/s10237-006-0030-5. [DOI] [PubMed] [Google Scholar]
- 37.Schinagl RM, Gurskis D, Chen AC, Sah RL. Depth-dependent confined compression modulus of full-thickness bovine articular cartilage. J Orthop Res. 1997;15:499–506. doi: 10.1002/jor.1100150404. [DOI] [PubMed] [Google Scholar]
- 38.Shin SJ, Fermor B, Weinberg JB, Pisetsky DS, Guilak F. Regulation of matrix turnover in meniscal explants: role of mechanical stress, interleukin-1, and nitric oxide. J Appl Physiol. 2003;95:308–313. doi: 10.1152/japplphysiol.00131.2003. [DOI] [PubMed] [Google Scholar]
- 39.Deschner J, Rath-Deschner B, Wypasek E, Anghelina M, Sjostrom D, Agarwal S. Biomechanical strain regulates TNFR2 but not TNFR1 in TMJ cells. J Biomech. 2007;40:1541–1549. doi: 10.1016/j.jbiomech.2006.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chowdhury TT, Arghandawi S, Brand J, Akanji OO, Bader DL, Salter DM, et al. Dynamic compression counteracts IL-1beta induced inducible nitric oxide synthase and cyclo-oxygenase-2 expression in chondrocyte/agarose constructs. Arthritis Res Ther. 2008;10:R35. doi: 10.1186/ar2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lima EG, Tan AR, Tai T, Bian L, Ateshian GA, Cook JL, et al. Physiologic deformational loading does not counteract the catabolic effects of interleukin-1 in long-term culture of chondrocyte-seeded agarose constructs. J Biomech. 2008;41:3253–3259. doi: 10.1016/j.jbiomech.2008.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Arner EC, Hughes CE, Decicco CP, Caterson B, Tortorella MD. Cytokine-induced cartilage proteoglycan degradation is mediated by aggrecanase. Osteoarthritis Cartilage. 1998;6:214–228. doi: 10.1053/joca.1998.0114. [DOI] [PubMed] [Google Scholar]
- 43.Sadowski T, Steinmeyer J. Effects of non-steroidal antiinflammatory drugs and dexamethasone on the activity and expression of matrix metalloproteinase-1, matrix metalloproteinase-3 and tissue inhibitor of metalloproteinases-1 by bovine articular chondrocytes. Osteoarthritis Cartilage. 2001;9:407–415. doi: 10.1053/joca.2000.0406. [DOI] [PubMed] [Google Scholar]
- 44.Tamura T, Kosaka N, Ishiwa J, Sato T, Nagase H, Ito A. Rhein, an active metabolite of diacerein, down-regulates the production of pro-matrix metalloproteinases-1, -3, -9 and -13 and up-regulates the production of tissue inhibitor of metalloproteinase-1 in cultured rabbit articular chondrocytes. Osteoarthritis Cartilage. 2001;9:257–263. doi: 10.1053/joca.2000.0383. [DOI] [PubMed] [Google Scholar]
- 45.Lima EG, Tan AR, Tai T, Bian L, Stoker AM, Ateshian GA, et al. Differences in interleukin-1 response between engineered and native cartilage. Tissue Eng Part A. 2008;14:1721–1730. doi: 10.1089/ten.tea.2007.0347. [DOI] [PubMed] [Google Scholar]
- 46.Takahashi N, Rieneck K, van der Kraan PM, van Beuningen HM, Vitters EL, Bendtzen K, et al. Elucidation of IL-1/TGF-beta interactions in mouse chondrocyte cell line by genome-wide gene expression. Osteoarthritis Cartilage. 2005;13:426–438. doi: 10.1016/j.joca.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 47.Torzilli PA, Grigiene R. Continuous cyclic load reduces proteoglycan release from articular cartilage. Osteoarthritis Cartilage. 1998;6:260–268. doi: 10.1053/joca.1998.0119. [DOI] [PubMed] [Google Scholar]
- 48.Janusz MJ, Little CB, King LE, Hookfin EB, Brown KK, Heitmeyer SA, et al. Detection of aggrecanase- and MMP-generated catabolic neoepitopes in the rat iodoacetate model of cartilage degeneration. Osteoarthritis Cartilage. 2004;12:720–728. doi: 10.1016/j.joca.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 49.Lohmander LS, Atley LM, Pietka TA, Eyre DR. The release of crosslinked peptides from type II collagen into human synovial fluid is increased soon after joint injury and in osteoarthritis. Arthritis Rheum. 2003;48:3130–3139. doi: 10.1002/art.11326. [DOI] [PubMed] [Google Scholar]