Abstract
The repertoire of signal transduction pathways activated by dopamine in brain includes the increase of intracellular calcium. However the mechanism(s) by which dopamine activated this important second messenger system was unknown. Although we showed that activation of the D5 dopamine receptor increased calcium concentrations, the restricted anatomic distribution of this receptor made this unlikely to be the major mechanism in brain. We have identified novel heteromeric dopamine receptor complexes that are linked to calcium signaling. The calcium pathway activated through the D1–D2 receptor heteromer involved coupling to Gq, through phospholipase C and IP3 receptors to result in a rise in intracellular calcium. The calcium rise activated through the D2–D5 receptor heteromer involved a small rise in intracellular calcium through the Gq pathway that triggered a store operated channel mediated influx of extracellular calcium. These novel receptor heteromeric complexes, for the first time, establish the link between dopamine action and rapid calcium signaling.
Introduction
Dopamine is involved in the regulation of various physiological functions including locomotion, behavior, learning and emotion. In a variety of diseases, such as Alzheimer’s disease, schizophrenia, Parkinson’s disease and drug addiction, the pathophysiology has been linked to dysfunctional dopaminergic signaling [1–3]. The genes for five dopamine receptors (D1R–D5R) have been cloned and the receptors shown to belong to the G-protein-coupled receptor (GPCR) superfamily, which based on their sequence homology, pharmacology and the modulation of cyclic AMP, were divided into two major subclasses, the D1-like and D2-like receptors [1–2]. D1-like receptors (D1R, D5R) activate, whereas D2-like receptors (D2R, D3R, D4R) inhibit adenylyl cyclase (AC) activity, resulting in opposite modulation of cyclic AMP through Gs/olf or Gi/o proteins, respectively [2]. The modulation of this pathway and related proteins, protein kinase A (PKA) and DARPP32 (dopamine and PKA regulated protein) represents the most studied dopamine signaling pathway [4], but other signaling cascades have been reported, including the modulation of the Akt-GSK3 pathway [5] and the activation of the PAR4 signaling pathway [6]. We have reported a new calcium-CaMKII (calcium-calmodulin kinase) signaling pathway activated through heteromerization between D1R and D2R creating D1–D2 heteromers [reviewed in 7–8]. We have also reported another heteromerization involving D2R and D5R, leading to the formation of D2–D5 heteromeric complexes [9], which also signal via calcium. These novel pathways, for the first time, show a link between dopamine action and rapid calcium signaling. We will describe these two heteromeric receptor complexes and their signaling, and discuss their differences along with their respective putative physiological relevance.
D1–D2 heteromers and intracellular calcium mobilization
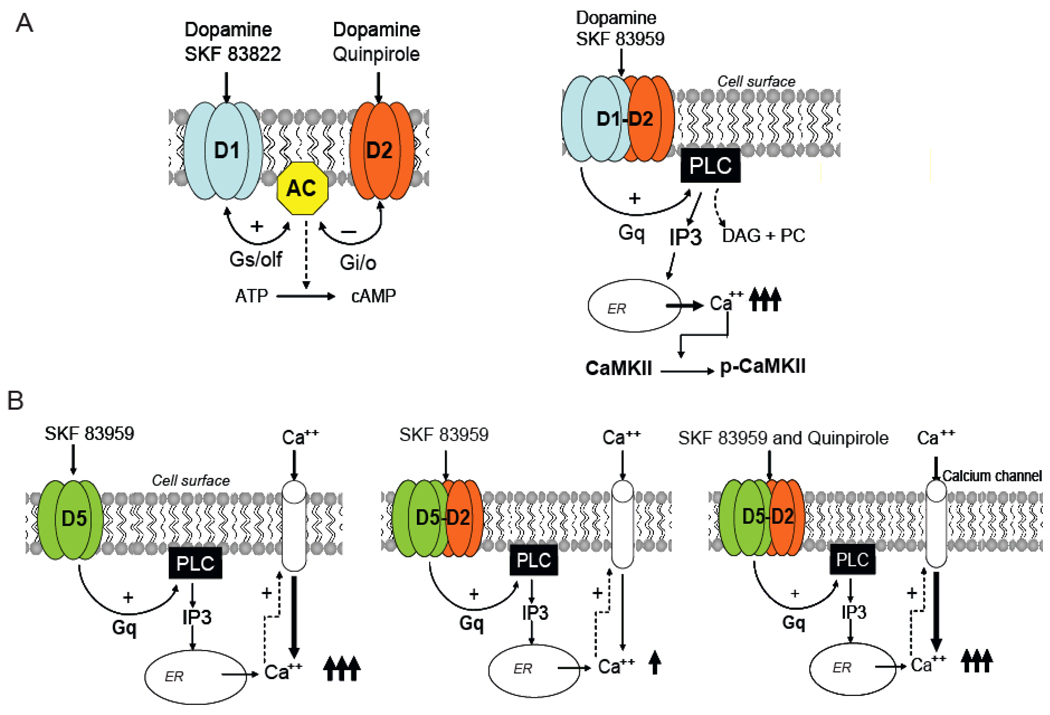
Evidence of a D1-like receptor activating IP3 production and/or increasing intracellular calcium has been described to occur in slices from different brain regions, such as striatum, hippocampus and cortex [10–12]. It was also shown in striatal neurons in culture that the activation of a D1-like receptor resulted in a rise in intracellular calcium, which was mobilized through both extracellular influx and from intracellular compartments [13]. However, no such effects were observed when the cloned D1R was expressed in different host cells [reviewed in 7]. Further investigations led us to the discovery that the mobilization of intracellular calcium was in fact a new signaling pathway generated by the activation of a D1–D2 heteromeric receptor complex [14–16]. Such heteromers were demonstrated by co-immunoprecipitating both receptors from rat striatum, as well as from cells coexpressing D1R and D2R [14], and by the fluorescence resonance energy transfer (FRET) technique [16–17]. The calcium signal generated by the activation of the D1–D2 heteromer was rapid, transient, independent of extracellular calcium influx, and involved the activation of Gq protein, phospholipase C (PLC), protein kinase C (PKC) and inositol triphosphate receptors (IP3-R) [14–15]. Interestingly, the specific activation of the D1–D2 receptor heteromer induced an increase in the phosphorylated activated form of CaMKIIα in rat striatum, more precisely in the nucleus accumbens [15] (Fig.1A). This latter effect, as well as the Gq activation, was absent in gene deleted D1−/− and D2−/− mice, indicating that the described signaling pathway exclusively involved both D1R and D2R within a functional complex [15].
Figure 1.
A. Dopamine D1 and D2 receptor homooligomers modulate adenylyl cyclase activity and cAMP accumulation in opposite ways through Gs/olf and Gi/o proteins, respectively (left panel). D1–D2 receptor heteromer activation leads to a novel signaling pathway mediated through Gq/11 protein activation, to a rapid, transient, intracellular calcium mobilization from endoplasmic reticulum (right panel). This calcium signal involves phospholipase C (PLC) and inositol-triphosphate (IP3) receptors and leads to CaMKIIα activation.
B. Activation of dopamine D5 receptor homooligomers results in the mobilization of intracellular calcium involving Gq/11 and PLC activation, and extracellular calcium influx leading to a robust rise in intracellular calcium (left panel). D5-D2 receptor heteromer formation confers two roles to D2 receptor, one is the inhibition exerted by D2R on D5R-induced rise in calcium when only D5 receptor is activated (middle panel), and the ability of D2R to participate in the increase in calcium mobilization when both D5 and D2 receptors forming the heteromer are co-stimulated (right panel)
D2–D5 heteromers and calcium signaling
Due to the high sequence homology (~80%) between the two dopamine D1-like class receptors, D1R and D5R, and the absence of specific agonists and antagonists able to discriminate between these two receptors, we postulated that the occurrence of cooperativity and synergism between D2-like and D1-like receptors may also involve the D2R and D5R pair. We therefore investigated the possibility of heteromerization between these two receptors. FRET analysis showed that D2R and D5R formed heteromeric complexes [9]. Interestingly, while the D2–D5 receptor heteromer showed preserved affinities for the ligands, the calcium signal differed in cells expressing the D2–D5 heteromer from cells expressing D5R alone. Activation of D5R expressed alone resulted in a robust calcium increase, highly dependent on calcium influx and also on intracellular calcium mobilization, via a mechanism involving Gq, PLC, PKC and IP3. The formation of the D2–D5 receptor heteromer resulted in an attenuation of the D5R-induced calcium signal, which was restored only when both receptors were concomitantly activated [9] (Fig.1B).
Differences in calcium signaling between D1–D2 and D2–D5 receptor heteromers
It is interesting to note that by forming heteromers with the same receptor, namely D2R, two related receptors, D1R and D5R, with very similar pharmacological properties, showed different patterns of dopamine-induced calcium regulation. These two different effects, when considered together with the known signaling of the receptor homooligomers through the modulation of adenylyl cyclase and cAMP, identify multiple pathways of signal transduction by dopamine (Fig. 1). The effects of receptor heteromerization add completely novel mechanisms by which the calcium signal can be finely tuned by dopamine. D1–D2 receptor heteromer activation led to a rapid, transient, intracellular calcium mobilization that was not observed with either D1R or D2R alone, and this suggests that the heteromerization has conferred a new stimulatory signaling pathway accessible to both receptors (Fig. 1A). In contrast, the formation of the D2–D5 receptor heteromer has conferred two other distinct roles, one being the inhibition exerted by D2R on the D5R-induced rise in calcium and secondly, the possibility for D2R to participate in the increase in calcium mobilization when co-stimulated with D5R (Fig. 1B).
The formation of the D1–D2 receptor heteromer may have switched the D1R into a conformation more favorable for interaction with Gq, as was shown by selective GTPγS incorporation into Gq protein, together with attenuated Gs-mediated signaling [15]. On the other hand, D2–D5 receptor heteromer formation may have attenuated the D5R-induced calcium signal by switching the conformation of D5R toward a Gs favorable conformation and attenuated its affinity toward Gq protein, and the concomitant activation of D2R and D5R in the complex may re-switch the conformation and affinity of D5R toward the two G proteins. This may explain the increase in cAMP accumulation observed when D2R and D5R were coexpressed [18–19] and the inhibition of the D5R-induced calcium increase [9].
The compartmentalization of a particular GPCR with individual G-proteins, effectors, and other signaling or regulatory proteins, seems to be another attractive idea to explain the different cellular, biochemical or physiological responses mediated by the same receptor or by different receptors through activation of a same G-protein (for review 20–22). For dopamine receptors, we have shown through sucrose gradient membrane fractionation that D1R, when expressed alone, was localized in the lipid raft/caveolae fraction (23). Caveolae are now considered to be a subset of lipid rafts, sharing some qualities but with some differences in the associated proteins such as G-proteins or other signaling proteins (24–25; 20). For example, it has been shown that different G-proteins were differentially segregated between caveolae versus lipid rafts (20), with Gq preferentially localized in caveolae, while Gs and Gi localized in lipid rafts (24). A possible explanation for the differences in the signaling between D1R and the D1–D2 heteromer may lie within the differences of their compartmentalization in membrane microdomains. Along the same lines, differences in the compartmentalization and co-localization with signaling proteins may also play a role in the differences observed in the calcium signal triggered by D5R, versus that seen with D2–D5 heteromers or D1–D2 heteromers. Although the differential partitioning of homooligomers and heteromers may represent an attractive hypothesis, there is no immediate evidence, except that the D1R has been localized in lipid raft/caveolae (23). Further studies are needed to precisely localize D1R, D2R, the D1–D2 receptor oligomer, and the D2–D5 receptor heteromer within these membrane microdomains.
Another difference between the D1–D2 and D2–D5 receptor heteromers is the dependence of the calcium signals generated by these two heteromeric complexes on extracellular calcium influx. This reveals that while the D1–D2 heteromer-generated signal is not dependent on calcium influx from extracellular sources, the D2–D5 heteromer calcium signal is tightly linked to the regulation of store-operated calcium channels as well as to the intracellular pools of calcium. The D2–D5 heteromer exhibited the unusual phenomenon of intracellular calcium release triggered calcium influx [9].
Another level of interpretation is related to the regional expression of each type of dopamine receptor. For example, in the striatum, it has been shown that D1 and D2 receptors are expressed in the most representative population of neurons in the striatum (~95%), the medium spiny neurons (MSNs) [26]. Using bacterial artificial chromosome (BAC) transgenic mice, where the expression of the fluorescent protein, EGFP was driven by D1 or D2 receptor promoters [26–31], it has been confirmed that D1 and D2 receptors were largely expressed in two separate populations of MSNs, with co-localization of these two receptors in ~20% of neurons [30–31]. Other studies, using different techniques, such as electrophysiology, immunocytochemistry, immunohistochemistry, RT-PCR, electron microscopy, have indicated co-localization of D1R and D2R in striatal neurons in culture [32–35] as well as in striatal tissue [36–40]. A discrepancy exists however, between the levels of D1R and D2R co-localization reported in the cultured neurons and those observed in tissue, with a much higher prevalence in cultured neurons [32], which may be explained either by the lack of afferents in the cultured systems and/or could be due to a developmental regulation of expression of the receptors, leading to different levels of co-localization. According to our investigations, in adult rat brain D1R and D2R are co-localized in a significant proportion of nucleus accumbens MSNs and are scarce in caudate nucleus MSNs [14; and unpublished data].
In contrast to D1R expression, the homologous receptor, D5R, was not found in MSNs, but was expressed in the cholinergic interneurons, which represent only ~1–2% of neurons in the striatum [41]. Interestingly, these cholinergic interneurons were shown to express D2R and D5R mRNA and protein, but not D1R [41–47]. This suggests that these interneurons are functionally different from, or complementary to, the MSNs in striatum due to the different ways of transducing dopaminergic signals. The co-localization of D2R and D5R is not restricted to the cholinergic interneurons in the striatum, but co-localization also occurs in cholinergic neurons in other brain regions, such as hippocampus, amygdala, cerebral cortex [45], rat forebrain and diencephalon [41]. This suggests that D2R and D5R may regulate the dopamine-induced regulation of acetylcholine release. The cholinergic interneurons, although representing only a fraction of the striatal neuronal population, possess a dense and widespread dendritic and axonal arborization [41]. Recent studies tend to show a major role for these interneurons that co-express D2R and D5R, in different dopaminergic influences. In the striatum it has been shown that these interneurons play a key role in the D2R-mediated long-term depression (LTD), by a sophisticated mechanism in which cholinergic interneurons act as an intermediate between dopamine neurons and the MSNs to transduce the signal [48].
Physiological relevance
It is well established that D1-like and D2-like receptors can mediate opposing as well as synergistic effects depending on cellular location, the brain region involved [reviewed in 49] and also on the local dopamine concentrations [50]. For example, it has been reported that dopamine attenuates glutamatergic input in the nucleus accumbens through D1-like receptors, whereas, no direct effects on postsynaptic glutamatergic NMDARs and AMPARs were observed in the dorsal striatum. In the latter case, dopamine seems to indirectly regulate NMDAR- or AMPAR-mediated responses through the regulation of voltage-activated ion channels localized in dendritic spines (e.g. L-type Ca2+ channels) [reviewed in 49; 51–52]. In the analysis of the dopamine-modulated calcium signals generated by the D1–D2 or D2–D5 receptor heteromers, it has to be noted that IP3 accumulation triggered by D1-like agonists in the striatum of D1−/− mice was reported, suggesting another D1-like, i.e the D5 receptor, was involved in this effect [53]. However, these results are in contradiction with another study, which showed that the D1-like receptor antagonist [3H]-SCH 23390 showed no significant binding to preparations from the striatum of D1−/− mice [54], suggesting that D5 receptors were undetectable in the striatum of these animals. In the same line of evidence, we showed that in D1−/− mice, as well as in D2−/− mice, no significant incorporation of GTPγS into Gq/11 occurred [15]. Another possibility to be considered is that these two methods were not sensitive enough to detect the activation of Gq and the expression of D5R, since any D5R-induced changes would be limited to only a small proportion of neurons (1–2%). Furthermore, in the two gene-deleted D1−/− and D2−/− mice, no activation of CaMKIIα was detected [15], which clearly indicates the necessary mutual presence of D1, from D1-like subclass, and only D2, from D2-like subclass, to activate CAMKIIα through a specific signaling pathway involving the mobilization of intracellular calcium. This activation of CaMKIIα is therefore a specific feature of the activation of the D1–D2 receptor heteromer. Accumulating evidence show the important role played by the activation of CaMKII, all forms included, but especially the CaMKIIα form, in different physiological or pathophysiological conditions, such as drug addiction [55], schizophrenia [56] and other important brain functions [57]. The fact that the D1-D2 receptor heteromer was shown to activate CaMKIIα in the nucleus accumbens [15] may be related to the involvement of dopamine in drug addiction [58]. Another aspect of the D1–D2 heteromer relevance may be linked to schizophrenia. The components of the D1–D2-induced signaling pathway have been reported to be affected in the brain of schizophrenics: 1) A link between D1R and D2R has been reported to be missing [59]; 2) The levels and/or the activation of Gq, PLC, IP3 and CaMKII have all been reported to be affected [60]. Some of these components, such as Gq/PLC/IP3, constitute integral partners in the transduction of the calcium signal by the D2–D5 receptor heteromers as well [9]. In regard to the differences that we showed between the D1–D2 versus the D2–D5 calcium signaling pathways, namely the notable difference in their dependence on extracellular calcium, the nucleus accumbens CaMKIIα activation, the regional localization (MSNs versus cholinergic interneurons), and the pharmacological differences (D5R and D5-D2 have higher affinity for dopamine than D1R or D1–D2), it is highly likely that the two different receptor heteromers, D1–D2 and D2–D5, may play different or complementary roles in dopamine-induced calcium signal transduction. It is possible that the signaling pathway triggered by the D1–D2 heteromer may be of high importance in LTP, schizophrenia and drug addiction, whereas the D2–D5 heteromer signaling pathway may be important, as was recently reported, in acetylcholine release [42], LTD [61], motor activity [61], coordination and exploration [19], and possibly other regulatory roles such as the modulation of calcium channels. Local dopamine concentration may be a determinant factor in the level of activation of one type of receptor and/or one type of receptor heteromer. For example, it has been reported that dopamine acts through activation of both D1- and D2-class receptors to promote LTP at low concentrations, whereas, it facilitates LTD at high concentrations [62; 50]. In the striatum, the predominant form of plasticity is mediated by LTD, which seems to involve the activation of both D1- and D2-class receptors [63; 49]. These differences also emphasize that the D1–D2 and D2–D5 receptor heteromers represent distinct mechanisms by which dopamine can fine tune the levels of intracellular calcium and regulate rapid calcium-mediated effects in the brain. This underlines the potential for new therapeutic strategies specifically targeting one signaling pathway among the variety of pathways that dopamine receptors and their oligomerization offer.
Acknowledgements
The work on dopamine receptors in our laboratory is supported by a grant from the National Institute on Drug Abuse. SRG holds the Canada Research Chair in Molecular Neuroscience.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Missale C, Nash SR, Robinson SW, Jaber M, Caron MG. Dopamine receptors: from structure to function. Physiol Rev. 1998;78:189–225. doi: 10.1152/physrev.1998.78.1.189. [DOI] [PubMed] [Google Scholar]
- 2.Neve KA, Seamans JK, Trantham-Davidson H. Dopamine receptor signaling. J. Recept. Signal Transduct. Res. 2004;24:165–205. doi: 10.1081/rrs-200029981. [DOI] [PubMed] [Google Scholar]
- 3.Pivonelli Pivonello R, Ferone D, Lombardi G, Colao A, Lamberts SW, Hofland LJ. Novel insights in dopamine receptor physiology. Eur J Endocrinol. 2007;(S13) Suppl1:S21. doi: 10.1530/eje.1.02353. Erratum in: Eur J Endocrinol. 157(4): 543. [DOI] [PubMed] [Google Scholar]
- 4.Greengard P, Allen PB, Nairn AC. Beyond the dopamine receptor: the DARPP-32/protein phosphatase-1 cascade. Neuron. 1999;23:435–447. doi: 10.1016/s0896-6273(00)80798-9. [DOI] [PubMed] [Google Scholar]
- 5.Beaulieu JM, Sotnikova TD, Marion S, Lefkowitz RJ, Gainetdinov RR, Caron MG. An Akt/beta-arrestin 2/PP2A signaling complex mediates dopaminergic neurotransmission and behavior. Cell. 2005;122:261–273. doi: 10.1016/j.cell.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 6.Park SK, Nguyen MD, Fischer A, Luke MP, Affar el B, et al. Par-4 links dopamine signaling and depression. Cell. 2005;122:275–287. doi: 10.1016/j.cell.2005.05.031. [DOI] [PubMed] [Google Scholar]
- 7.George SR, O'Dowd BF. A novel dopamine receptor signaling unit in brain: heterooligomers of D1 and D2 dopamine receptors. Scientific World Journal. 2007;7:58–63. doi: 10.1100/tsw.2007.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rashid AJ, O’Dowd BF, Verma V, George SR. Neuronal Gq/11-coupled dopamine receptors: an uncharted role for dopamine. Trends Pharmacol Sci. 2007;28:551–555. doi: 10.1016/j.tips.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 9.So CH, Verma V, Alijaniaram M, Cheng R, Rashid AJ, O'Dowd BF, George SR. Calcium signaling by dopamine D5 receptor and D5-D2 receptor hetero-oligomers occurs by a mechanism distinct from that for dopamine D1–D2 receptor hetero-oligomers. Mol Pharmacol. 2009;75:843–854. doi: 10.1124/mol.108.051805. *In this article the authors show that activation of the D5 receptor can trigger a calcium response and by the FRET technique that D2 and D5 receptors form heterooligomers. The D2–D5 heteromerization led to an inhibition of the robust, D5R-mediated calcium increase. This attenuation was inhibited when both D2R and D5R were concomitantly activated.
- 10.Jin LQ, Goswami S, Cai G, Zhen X, Friedman E. SKF83959 selectively regulates phosphatidylinositol-linked D1 dopamine receptors in rat brain. J Neurochem. 2003;85:378–386. doi: 10.1046/j.1471-4159.2003.01698.x. [DOI] [PubMed] [Google Scholar]
- 11.Undie AS, Friedman E. Stimulation of a dopamine D1 receptor enhances inositol phosphates formation in rat brain. J Pharmacol Exp Ther. 1990;253:987–992. [PubMed] [Google Scholar]
- 12.Lezcano N, Bergson C. D1/D5 dopamine receptors stimulate intracellular calcium release in primary cultures of neocortical and hippocampal neurons. J Neurophysiol. 2002;87:2167–2175. doi: 10.1152/jn.00541.2001. [DOI] [PubMed] [Google Scholar]
- 13.Tang TS, Bezprozvanny I. Dopamine receptor-mediated Ca2+ signaling in striatal medium spiny neurons. J Biol Chem. 2004;279:42082–42094. doi: 10.1074/jbc.M407389200. [DOI] [PubMed] [Google Scholar]
- 14.Lee SP, So CH, Rashid AJ, Varghese G, Cheng R, Lanca AJ, O’Dowd BF, George SR. Dopamine D1 and D2 receptor co-activation generates a novel phospholipase C-mediated calcium signal. J Biol Chem. 2004;279:35671–35678. doi: 10.1074/jbc.M401923200. [DOI] [PubMed] [Google Scholar]
- 15.Rashid AJ, So CH, Kong MM, Furtak T, El-Ghundi M, Cheng R, O'Dowd BF, George SR. D1–D2 dopamine receptor heterooligomers with unique pharmacology are coupled to rapid activation of Gq/11 in the striatum. Proc Natl Acad Sci USA. 2007;104:654–659. doi: 10.1073/pnas.0604049104. *In this article, authors showed the activation of a signaling complex in brain through the co-stimulation of D1R and D2R in the heteromeric D1–D2 receptor complex. They also identified the D1 agonist SKF 83959 as a specific agonist for the heteromer, functioning as a full agonist for the D1R and a partial agonist for a pertussis toxin-resistant D2R in the heteromer. The activation of the D1–D2 heteromer incresed calcium through Gq, PLC and IP3R and led to increased levels of phospho-CaMKIIα in the nucleus accumbens, a mechanism by which dopamine may contribute to synaptic plasticity.
- 16.So CH, Varghese G, Curley KJ, Kong MM, Alijaniaram M, Ji X, Nguyen T, O'Dowd BF, George SR. D1 and D2 dopamine receptors form heterooligomers and co-internalize after selective activation of either receptor. Mol Pharmacol. 2005;68:568–578. doi: 10.1124/mol.105.012229. [DOI] [PubMed] [Google Scholar]
- 17.Dziedzicka-Wasylewska M, Faron-Górecka A, Andrecka J, Polit A, Kuœmider M, Wasylewski Z. Fluorescence studies reveal heterodimerization of dopamine D1 and D2 receptors in the plasma membrane. Biochemistry. 2006;45:8751–8759. doi: 10.1021/bi060702m. [DOI] [PubMed] [Google Scholar]
- 18.Lee D, Huang W, Lim AT. Dopamine induces a biphasic modulation of hypothalamic ANF neurons: a ligand concentration-dependent effect involving D5 and D2 receptor interaction. Mol Psychiatry. 2000;5:39–48. doi: 10.1038/sj.mp.4000601. [DOI] [PubMed] [Google Scholar]
- 19.O’Sullivan GJ, Kinsella A, Sibley DR, Tighe O, Croke DT, Waddington JL. Ethological resolution of behavioural topography and D1-like versus D2-like agonist responses in congenic D5 dopamine receptor mutants: identification of D5:D2-like interactions. Synapse. 2005;55:201–211. doi: 10.1002/syn.20107. [DOI] [PubMed] [Google Scholar]
- 20.Ostrom RS, Insel PA. The evolving role of lipid rafts and caveolae in G protein-coupled receptor signaling: implications for molecular pharmacology. Br J Pharmacol. 2004;143:235–245. doi: 10.1038/sj.bjp.0705930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Insel PA, Head BP, Patel HH, Roth DM, Bundey RA, Swaney JS. Compartmentation of G-protein-coupled receptors and their signalling components in lipid rafts and caveolae. Biochem Soc Trans. 2005;33:1131–1134. doi: 10.1042/BST20051131. [DOI] [PubMed] [Google Scholar]
- 22.Chini B, Parenti M. G-protein-coupled receptors, cholesterol and palmitoylation: facts about fats. J Mol Endocrinol. 2009;42:371–379. doi: 10.1677/JME-08-0114. [DOI] [PubMed] [Google Scholar]
- 23.Kong MM, Hasbi A, Mattocks M, Fan T, O'Dowd BF, George SR. Regulation of D1 dopamine receptor trafficking and signaling by caveolin-1. Mol Pharmacol. 2007;72:1157–1170. doi: 10.1124/mol.107.034769. [DOI] [PubMed] [Google Scholar]
- 24.Oh P, Schnitzer JE. Immunoisolation of caveolae with high affinity antibody binding to the oligomeric caveolin cage. Toward understanding the basis of purification. J Biol Chem. 1999;274(33):23144–23154. doi: 10.1074/jbc.274.33.23144. Erratum in: J Biol Chem 274(41): 29582. [DOI] [PubMed] [Google Scholar]
- 25.Patel HH, Murray F, Insel PA. G-protein-coupled receptor-signaling components in membrane raft and caveolae microdomains. Handb Exp Pharmacol. 2008;186:167–184. doi: 10.1007/978-3-540-72843-6_7. [DOI] [PubMed] [Google Scholar]
- 26.Lee KW, Kim Y, Kim AM, Helmin K, Nairn AC, Greengard P. Cocaine-induced dendritic spine formation in D1 and D2 dopamine receptor-containing medium spiny neurons in nucleus accumbens. Proc Natl Acad Sci. 2006;103:3399–3404. doi: 10.1073/pnas.0511244103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gong S, Zheng C, Doughty ML, Losos K, Didkovsky N, Schambra UB, Nowak NJ, Joyner A, Leblanc G, Hatten ME, Heintz N. A gene expression atlas of the central nervous system based on bacterial artificial chromosomes. Nature. 2003;425:917–925. doi: 10.1038/nature02033. [DOI] [PubMed] [Google Scholar]
- 28.Lobo MK, Karsten SL, Gray M, Geschwind DH, Yang XW. FACS-array profiling of striatal projection neuron subtypes in juvenile and adult mouse brains. Nat Neurosci. 2006;9:443–452. doi: 10.1038/nn1654. [DOI] [PubMed] [Google Scholar]
- 29.Shuen JA, Chen M, Gloss B, Calakos N. Drd1a-tdTomato BAC transgenic mice forsimultaneous visualization of medium spiny neurons in the direct and indirect pathways of the basal ganglia. J Neurosci. 2008;28:2681–2685. doi: 10.1523/JNEUROSCI.5492-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bertran-Gonzalez J, Bosch C, Maroteaux M, Matamales M, Hervé D, et al. Opposing patterns of signaling activation in dopamine D1 and D2 receptor-expressing striatal neurons in response to cocaine and haloperidol. J Neurosci. 2008;28:5671–5685. doi: 10.1523/JNEUROSCI.1039-08.2008. *The authors used transgenic mice, in which EGFP expression was driven by D1R or D2R promoters in BAC (bacterial artificial chromosome) constructs. They confirmed the expression of D1R in striatonigral and D2R in striatopallidal neurons, with co-localization of D1 and D2R in up to 17% of neurons. D2R was also shown to be expressed in cholinergic interneurons, whereas no expression of either promoter was detected in GABAergic interneurons. They also demonstrated that cocaine and haloperidol used selective mechanisms to exert their short and long-term effects by specifically activating signaling pathways in the two segregated populations of striatal output neurons.
- 31.Matamales M, Bertran-Gonzalez J, Salomon L, Degos B, Deniau JM, Valjent E, Hervé D, Girault JA. Striatal medium-sized spiny neurons: identification by nuclear staining and study of neuronal subpopulations in BAC transgenic mice. PLoS One. 2009;4:e4770. doi: 10.1371/journal.pone.0004770. *The authors used bacterial artificial chromosome (BAC) transgenic mice expressing EGFP driven by D1R or D2R promoters and performed a quantitative analysis of striatal neurons in the drd1a-EGFP and drd2-EGFP mice. They showed that nuclear labeling was a simple method for identifying MSNs, and they confirmed that D1R and D2R were largely expressed in two populations of striatal neurons, however with an expression of both types of receptors, D1R and D2R in 5%, 6%, and 17%, in the dorsal striatum, core, and shell, respectively.
- 32.Aizman O, Brismar H, Uhlen P, Zettergren E, Levey AI, et al. Anatomical and physiological evidence for D1 and D2 dopamine receptor colocalization in neostriatal neurons. Nat Neurosci. 2000;3:226–230. doi: 10.1038/72929. [DOI] [PubMed] [Google Scholar]
- 33.Shetreat ME, Lin L, Wong AC, Rayport S. Visualization of D1 dopamine receptors on living nucleus accumbens neurons and their colocalization with D2 receptors. J Neurochem. 1996;66:1475–1482. doi: 10.1046/j.1471-4159.1996.66041475.x. [DOI] [PubMed] [Google Scholar]
- 34.Wong AC, Shetreat ME, Clarke JO, Rayport S. D1- and D2-like dopamine receptors are colocalized on the presynaptic varicosities of striatal and nucleus accumbens neurons in vitro. Neuroscience. 1999;89:221–233. doi: 10.1016/s0306-4522(98)00284-x. [DOI] [PubMed] [Google Scholar]
- 35.Iwatsubo K, Suzuki S, Li C, Tsunematsu T, Nakamura F, et al. Dopamine induces apoptosis in young, but not in neonatal, neurons via Ca2+-dependent signal. Am. J. Physiol. Cell Physiol. 2007;293:C1498–C1508. doi: 10.1152/ajpcell.00088.2007. *The authors established a method for primary culture of striatal neurons from 2- to 3-wk-old mice. They also demonstrated that the effect of dopamine on apoptosis varied at different developmental stages with an induction of apoptosis in young, but not neonatal, striatal neurons. The apoptosis was most likely regulated through the activation of a dopamine-PLC-Ca2+ signaling mechanism, which changed during the postnatal period.
- 36.Levey AI, Hersch SM, Rye DB, Sunahara RK, Niznik HB, et al. Localization of D1 and D2 dopamine receptors in brain with subtype-specific antibodies. Proc Nat Acad Sci USA. 1993;90:8861–8865. doi: 10.1073/pnas.90.19.8861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Deng YP, Lei WL, Reiner A. Differential perikaryal localization in rats of D1 and D2 dopamine receptors on striatal projection neuron types identified by retrograde labeling. J Chem Neuroanat. 2006;32:101–116. doi: 10.1016/j.jchemneu.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 38.Hersch SM, Ciliax BJ, Gutekunst CA, Rees HD, Heilman CJ, Yung KK, et al. Electron microscopic analysis of D1 and D2 dopamine receptor proteins in the dorsal striatum and their synaptic relationships with motor corticostriatal afferents. J Neurosci. 1995;15:5222–5237. doi: 10.1523/JNEUROSCI.15-07-05222.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Capper-Loup C, Canales JJ, Kadaba N, Graybiel AM. Concurrent activation of dopamine D1 and D2 receptors is required to evoke neural and behavioral phenotypes of cocaine sensitization. J Neurosci. 2002;22:6218–6227. doi: 10.1523/JNEUROSCI.22-14-06218.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harsing LG, Jr, Zigmond MJ. Influence of dopamine on GABA release in striatum: Evidence for D1–D2 interactions and non-synaptic influences. Neuroscience. 1997;77:419–429. doi: 10.1016/s0306-4522(96)00475-7. [DOI] [PubMed] [Google Scholar]
- 41.Berlanga ML, Simpson TK, Alcantara AA. Dopamine D5 receptor localization on cholinergic neurons of the rat forebrain and diencephalon: a potential neuroanatomical substrate involved in mediating dopaminergic influences on acetylcholine release. J Comp Neurol. 2005;492(1):34–49. doi: 10.1002/cne.20684. [DOI] [PubMed] [Google Scholar]
- 42.Bergson C, Mrzljak L, Smiley JF, Pappy M, Levenson R, Goldman-Rakic PS. Regional, cellular, and subcellular variations in the distribution of D1 and D5 dopamine receptors in primate brain. J Neurosci. 1995;15:7821–7836. doi: 10.1523/JNEUROSCI.15-12-07821.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ciliax BJ, Nash N, Heilman C, Sunahara R, Hartney A, Tiberi M, Rye DB, Caron MG, Niznik HB, Levey AI. Dopamine D(5) receptor immunolocalization in rat and monkey brain. Synapse. 2000;2:125–145. doi: 10.1002/1098-2396(200008)37:2<125::AID-SYN7>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 44.Rappaport MS, Sealfon SC, Prikhozhan A, Huntley GW, Morrison JH. Heterogeneous distribution of D1, D2 and D5 receptor mRNAs in monkey striatum. Brain Res. 1993;616:242–250. doi: 10.1016/0006-8993(93)90215-9. [DOI] [PubMed] [Google Scholar]
- 45.Yan Z, Song W-J, Surmeier DJ. D2 dopamine receptors reduce N-type Ca2+ currents in rat neostriatal cholinergic interneurons through a membrane-delimited, protein kinase C-insensitive pathway. J Neurophysiol. 1997;77:1003–1015. doi: 10.1152/jn.1997.77.2.1003. [DOI] [PubMed] [Google Scholar]
- 46.Alcantara AA, Chen V, Herring BH, Mendenhall JM, Berlanga ML. Localization of dopamine D2 receptors on cholinergic interneurons of the dorsal striatum and nucleus accumbens of the rat. Brain Res. 2003;986:22–29. doi: 10.1016/s0006-8993(03)03165-2. [DOI] [PubMed] [Google Scholar]
- 47.Nicola SM, Surmeier DJ, Malenka RC. Dopaminergic modulation of neuronal excitability in the striatum and nucleus accumbens. Annu Rev Neurosci. 2000;23:185–215. doi: 10.1146/annurev.neuro.23.1.185. [DOI] [PubMed] [Google Scholar]
- 48.Wang Z, Kai L, Day M, Ronesi J, Yin HH, Ding J, et al. Dopaminergic control of corticostriatal long-term synaptic depression in medium spiny neurons is mediated by cholinergic interneurons. Neuron. 2006;50:443–452. doi: 10.1016/j.neuron.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 49.Yao W-D, Spealman RD, Zhang J. Dopaminergic signaling in dendritic spines. Biochem. pharmacol. 2008;75:2055–2069. doi: 10.1016/j.bcp.2008.01.018. *This is an excellent review, which presents an overview of dopaminergic system signaling and details the dopaminergic signaling in dendritic spines.
- 50.Sajikumar S, Frey JU. Resetting of 'synaptic tags' is time- and activity-dependent in rat hippocampal CA1 in vitro. Neuroscience. 2004;129:503–507. doi: 10.1016/j.neuroscience.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 51.Seamans JK, Yang CR. The principal features and mechanisms of dopamine modulation in the prefrontal cortex. Prog Neurobiol. 2004;74:1–58. doi: 10.1016/j.pneurobio.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 52.Calabresi P, Picconi B, Tozzi A, Di Filippo M. Dopamine mediated regulation of corticostriatal synaptic plasticity. Trends Neurosci. 2007;30:211–219. doi: 10.1016/j.tins.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 53.Friedman E, Jin LQ, Cai GP, Hollon TR, Drago J, Sibley DR, Wang HY. D1-like dopaminergic activation of phosphoinositide hydrolysis is independent of D1A dopamine receptors: evidence from D1A knockout mice. Mol. Pharmacol. 1997;51:6–11. doi: 10.1124/mol.51.1.6. [DOI] [PubMed] [Google Scholar]
- 54.Montague DM, Striplin CD, Overcash JS, Drago J, Lawler CP, Mailman RB. Quantification of D1B(D5) receptors in dopamine D1A receptor-deficient mice. Synapse. 2001;39:319–322. doi: 10.1002/1098-2396(20010315)39:4<319::AID-SYN1015>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 55.Lowetha JA, Bakerb LK, Guptaab T, Guillorya AM, Vezina P. Inhibition of CaMKII in the nucleus accumbens shell decreases enhanced amphetamine intake in sensitized rats. Neuroscience Letters. 2008;444:157–160. doi: 10.1016/j.neulet.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mouri A, Noda Y, Noda A, Nakamura T, Tokura T, Yura Y, Nitta A, Furukawa H, Nabeshima T. Involvement of a dysfunctional dopamine-D1/N-methyl-D-aspartate-NR1 and Ca2+/calmodulin-dependent protein kinase II pathway in the impairment of latent learning in a model of schizophrenia induced by phencyclidine. Mol Pharmacol. 2007;71:1598–1609. doi: 10.1124/mol.106.032961. [DOI] [PubMed] [Google Scholar]
- 57.Rushlow WJ, Seah C, Sutton LP, Bjelica A, Rajakumar N. Antipsychotics affect multiple calcium calmodulin dependent proteins. Neuroscience. 2009;161:877–886. doi: 10.1016/j.neuroscience.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 58.Anderson SM, Famous KR, Sadri-Vakili G, Kumaresan V, Schmidt HD, Bass CE, Terwilliger EF, Cha JH, Pierce RC. CaMKII: a biochemical bridge linking accumbens dopamine and glutamate systems in cocaine seeking. Nat Neurosci. 2008;3:344–353. doi: 10.1038/nn2054. Erratum in: Nat Neurosci. 5: 617. *In this article, the authors showed that the activation of D1-like dopamine receptors in the nucleus accumbens shell led to reinstatement of cocaine seeking in a rat model, by activating L-type-Ca2+ channels and CaMKII. They concluded that CaMKII may represent an essential link between shell dopaminergic and glutamatergic signaling and may be an important element in the neuronal plasticity involved in cocaine craving and relapse.
- 59.Seeman P, Niznik HB, Guan HC, Booth G, Ulpian C. Link between D1 and D2 dopamine receptors is reduced in schizophrenia and Huntington diseased brain. Proc Natl Acad Sci USA. 1989;86:10156–10160. doi: 10.1073/pnas.86.24.10156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lidow MS. Calcium signaling dysfunction in schizophrenia: a unifying approach. Brain Res Brain Res Rev. 2003;43:70–84. doi: 10.1016/s0165-0173(03)00203-0. [DOI] [PubMed] [Google Scholar]
- 61.Centonze D, Grande C, Usiello A, Gubellini P, Erbs E, Martin AB, Pisani A, Toqnazzi N, Bernardi G, Moratalla R, et al. Receptor subtypes involved in the presynaptic and postsynaptic actions of dopamine on striatal interneurons. J Neurosci. 2003;23:6245–6254. doi: 10.1523/JNEUROSCI.23-15-06245.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Otani S, Daniel H, Roisin MP, Crepel F. Dopaminergic modulation of long-term synaptic plasticity in rat prefrontal neurons. Cereb Cortex. 2003;13:1251–1256. doi: 10.1093/cercor/bhg092. [DOI] [PubMed] [Google Scholar]
- 63.Kreitzer AC, Malenka RC. Endocannabinoid-mediated rescue of striatal LTD and motor deficits in Parkinson’s disease models. Nature. 2007;445:643–647. doi: 10.1038/nature05506. [DOI] [PubMed] [Google Scholar]