Abstract
Juvenile idiopathic arthritis (JIA) encompasses a group of chronic childhood arthritides of unknown etiology. One subtype, systemic JIA (SJIA), is characterized by a combination of arthritis and systemic inflammation. Its systemic nature suggests that clues to SJIA pathogenesis may be found in examination of peripheral blood cells. To determine the immunophenotypic profiles of circulating mononuclear cells in SJIA patients with different degrees of disease activity, we studied PBMC from 31 SJIA patients, 20 polyarticular JIA patients (similar to adult rheumatoid arthritis) and 31 age-matched controls. During SJIA disease flare, blood monocyte numbers were increased, whereas levels of myeloid dendritic cells (DC) and γδ T cells were reduced. At both flare and quiescence, increased levels of CD14 and CD16 were found on SJIA monocytes. Levels of CD16- DC were elevated at SJIA quiescence compared both to healthy controls and to SJIA subjects with active disease. Overall, our findings suggest dysregulation of innate immunity in SJIA and raise the possibility that quiescence represents a state of compensated inflammation.
Keywords: Juvenile arthritis, Inflammation, Innate Immunity, Monocytes, Dendritic cells
Introduction
Juvenile idiopathic arthritis (JIA) comprises a family of childhood-onset, chronic inflammatory arthritides with distinct subtypes. The etiology of JIA is unknown and although new treatments have been identified, many children still suffer significant long term disability [1; 2]. A better understanding of pathogenesis may suggest new therapeutic approaches. In addition, JIA and its subtypes share various features with other inflammatory diseases, potentially broadening the impact of molecular/cellular insights from JIA.
JIA was initially divided into three major subtypes: systemic, polyarticular and pauciarticular (or oligoarticular). The most recent classification scheme for chronic childhood arthritis, developed by the International League of Associations for Rheumatology (ILAR), describes seven subtypes: oligoarticular; polyarticular rheumatoid factor-positive (RF+); polyarticular RF-; systemic; enthesitis-related arthritis; juvenile psoriatic arthritis; and undifferentiated arthritis [3]. The classification of JIA subtypes has been based in large part on clinical observations that reveal heterogeneity in disease presentation, outcome and response to treatment among JIA patients. More recent data on immune parameters and genetic risk factors confirm the notion of JIA subtypes and imply that these subtypes result from different pathogenic processes (reviewed in[4; 5]).
Among the JIA subtypes, polyarticular JIA (polyJIA) is similar to the more prevalent forms of rheumatoid arthritis (RA) in adults. As in adult RA, polyarticular JIA subtypes include RF+ and RF-, the former being associated with more erosive disease and both types being more common in females, similar to the epidemiology of RA in adults [6]. By contrast, systemic juvenile idiopathic arthritis (SJIA) is unique in its combination of systemic features (fever, rash, serositis [e.g., pericarditis, pleuritis]) and arthritis. It also differs from other JIA subtypes in having no predominant age of onset or gender preference, no predisposing HLA susceptibility alleles in Caucasians [7], and no association with common autoantibodies (e.g., ANA, RF) [8; 9]. SJIA represents 10-20% of all JIA, but accounts for more than 2/3 of the mortality [10], in part due to a sometimes fatal complication called macrophage activation syndrome (MAS). A condition similar to SJIA occurs very rarely in adults and is called adult-onset Still's disease (AOSD) [11].
The responses of JIA patients to biologic therapies provide some clues to disease pathophysiology. TNFα blockade is less effective in SJIA compared to polyJIA (and RA) [12]. In contrast, response to IL-1 inhibitors has been more promising in SJIA and AOSD patients than in children with polyJIA or adults with RA [13; 14]. In small studies, treatment with anti-IL6R also has been of benefit in SJIA, consistent with the observation that IL-6 is expressed at high levels in SJIA flare [15; 16; 17; 18]. These drug response patterns implicate IL-1 and IL-6 as important cytokines in SJIA pathophysiology.
The systemic nature of SJIA suggests that clues to pathogenesis will likely be found from examination of peripheral blood cells. However, relatively few studies to date characterize the profiles of circulating cell subsets [19], especially during different stages of the disease. In available studies of levels of T and B lymphocytes, the results have been somewhat variable [19; 20; 21; 22]. There are also conflicting reports on levels of circulating NK cells in SJIA compared to controls [23] [19; 22]. Notably, several investigators have observed reduced NK cytotoxic activity, which is not simply explained by reduced NK cell number[23; 24; 25] [26].
The cytokine profile, evidence for NK cell dysfunction, the lack of HLA association and absence of autoantibodies in SJIA suggest that dysregulation of innate immunity may play a particularly important role in etiology [25]. To test this emerging paradigm, we undertook a comparative analysis of cellular parameters at times of SJIA activity and quiescence using multiparameter flow cytometry. We asked whether there is an imbalance in SJIA in populations of innate immune effector cells (monocytes, DCs, NK cells, γδ T cells) and populations with an immunoregulatory role (T regulatory cells, NKT) in peripheral blood mononuclear cells. We compared our SJIA findings to data from age-matched immunologically normal controls and from patients with polyJIA (mostly RF-), the latter to ask whether our findings were uniquely associated with SJIA or shared with other forms of chronic inflammatory arthritis.
Patients, materials and methods
Subject population and clinical data collection
The Institutional Review Board of Stanford University approved the study. All JIA patients were followed at the Pediatric Rheumatology Clinic at Lucile Packard Children's Hospital. Fifty-one JIA patients were enrolled after consent: 31 SJIA patients (7 children provided samples during both flare and quiescence) and 20 polyJIA patients (2 patients provided samples during both flare and quiescence). Due to limited blood volumes available from pediatric patients, not all samples were used for all studies. Among the polyJIA patients, 14 were RF-negative, and 6 RF-positive. A one-time blood sample was collected after consent from age-matched immunologically healthy children, based on clinical history (n = 31) from the Stanford Endocrinology Clinic. The majority of these children were seen for growth delay (mostly due to constitutional growth delay) or precocious puberty.
Comprehensive clinical information was collected at each JIA patient visit, including history, physical exam (including presence of fever, rash and joint count) and clinical laboratory values [erythrocyte sedimentation rate (ESR), d-dimers, C-reactive protein (CRP), ferritin, complete blood count (CBC) and platelet count]. These data were stored in an ACCESS database, which was designed and maintained by Dr. C. Sandborg and colleagues. Clinical status at each visit was graded according to a scoring system developed by our group to grade severity of systemic disease manifestations or arthritis ([27; 28] and supplementary Tables 1-3). Each sample was classified as “flare” (active disease; score 2 or above) or “quiescence” (inactive disease; score equals to 0), We also developed a treatment intensity score, consisting of the sum of 4 individual scores for use of non-steroidal anti-inflammatory drugs (NSAID), corticosteroids, disease modifying anti-rheumatic drugs (DMARD), and biologic drugs (Supplementary Table 4). Characteristics of the study subjects are shown on Table 1. There was no difference in mean treatment intensity score between the SJIA and polyJIA groups, when both active and quiescence samples were analyzed together. SJIA patients with active disease had higher treatment intensity scores than either SJIA patients without active disease or polyJIA patients with or without active disease (Table 1). However, there was treatment heterogeneity among active disease SJIA subjects and key immunologic findings associated with active disease were not preferentially associated with high treatment intensity scores, as shown in Figures 1-5.
Table 1. Subject characteristics.
| SJIA flare | SJIA quiescence | polyJIA flare | polyJIA quiescence | control | |
|---|---|---|---|---|---|
| n | 16 | 22 | 11 | 11 | 31 |
| F/M(1) | 6/10 | 8/14 | 10/1 | 9/2 | 10/21 |
| African-American | 1 | 2 | 0 | 0 | 0 |
| Asian | 2 | 2 | 0 | 1 | 4 |
| Caucasian | 9 | 8 | 8 | 5 | 17 |
| Caucasian Hispanic | 4 | 4 | 3 | 3 | 10 |
| >1 Ethnicity | 0 | 1 | 0 | 1 | 0 |
| Mean age (yr) at disease onset ±SD (range) | 7.7±5 (2.6-16.1) | 7.3±5 (1.4-15.7) | 8±4.3 (1.2-14) | 6.4±5 (1-15.1) | N/A |
| Mean age (yr) ±SD at sample collection (range) | 10±4.7 (2.7-18.9) | 11.6±3.8 (5.9-17.6) | 11.9±4.1 (5.1-18) | 12.7±5 (2.6-19.5) | 12±5 (3-18) |
| Fever (%) | 50% | 0 | 0 | 0 | N/A |
| Rash (%) | 52% | 0 | 0 | 0 | N/A |
| Mean Joint Count ±SD (, range) | 6.6±9.3 (0-40) | 0.4±1 (0-4) | 17.4±10.6 (2-36) | 0 | N/A |
| WBC (×103/ul) (mean±SD) | 18.8±10 | 7.2±2.8 | 7.3±1.6 | 6.9±1.1 | ND |
| Platelets (×103/ul) (mean±SD) | 554.5±179 | 324±144 | 447±192 | 302±79 | ND |
| Monocytes (×103/ul) (mean±SD) | 0.7±0.35 | 0.52±0.15 | 0.4±0.1 | 0.52±0.2 | ND |
| Lymphocytes (×103/ul) (mean±SD) | 2.2±1.5 | 2.6±1.9 | 2.4±0.5 | 2.9±0.8 | ND |
| Neutrophils (×103/ul) (mean±SD) | 15.4±9.5 | 4±2.3 | 4±0.9 | 3.3±0.8 | ND |
| Mean Treatment intensity score ±SD (range) | 3.3±1.8(2) (0-6) | 1.6±1.5 (0-5) | 1.9±1 (0-3) | 1.8±1.2 (0-4) | N/A |
| Mean Prednisone dose±SD (mg/kg/day) (range) | 0.5±0.9(2) (0-3) | 0.03±0.09 (0-0.4) | 0.001±0.005 (0-0.02) | 0.006±0.02 (0-0.08) | N/A |
differences between SJIA and polyJIA are consistent with known epidemiology of JIA [78]
statistically different from SQ, PF and PQ by ANOVA with Bonferroni correction N/A: not applicable; ND: not done
Figure 1.
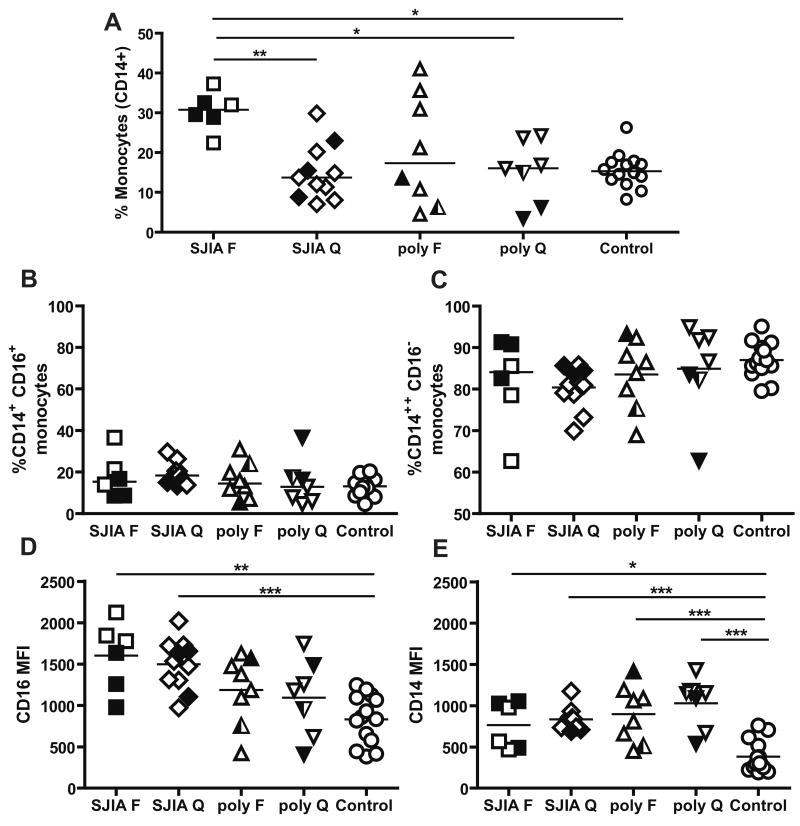
A: Monocyte distribution in SJIA, polyJIA and controls determined by flow cytometric analysis of cell surface staining. Data are expressed as percentage of total mononuclear cells. B and C: Monocyte subsets as determined by CD14 and CD16 surface staining. Data are expressed as percentage of total monocytes. D: Median fluorescent intensity (MFI) for CD16 in CD14+/CD16+ monocytes. E: MFI for CD14 in CD14++/CD16- monocytes. F: flare; Q: quiescence. SJIA F n=6; SJIA Q n=11; polyJIA F n=8; polyJIA Q n=7; Control n=14. Samples with treatment intensity scores > 2 are in black. polyJIA RF+ patients are in half black symbols. Statistical analysis used ANOVA with Bonferroni correction. *: p<0.05; **: p= or <0.01.
Figure 5.
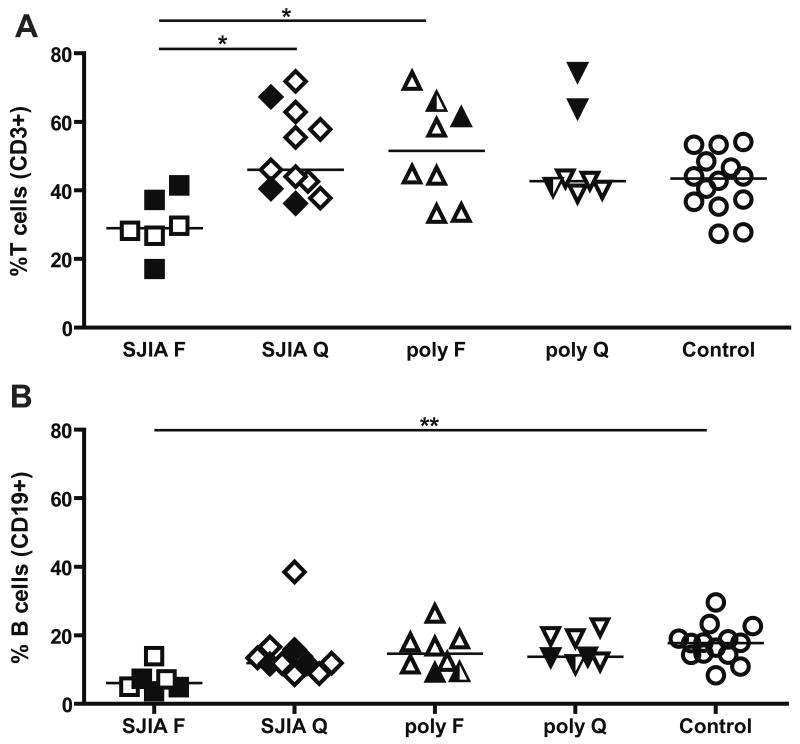
T and B cell distribution in SJIA, polyJIA and controls. Data are expressed as percentage of total mononuclear cells. A: T cells (CD3+ cells). B: B cells (CD19+ cells). F: flare; Q: quiescence. SJIA F n=6; SJIA Q n=11; polyJIA F n=8; polyJIA Q n=7; Control n=14. polyJIA RF+ patients are in half black symbols. Samples with treatment intensity scores > 2 are in black.Statistical analysis used ANOVA with Bonferroni correction. *: p<0.05; **: p= or <0.01.
Sample processing
Venous blood samples from all subjects were obtained after informed consent and treated anonymously throughout the analysis; these samples were obtained only when there was a clinical need for blood tests. 3-4 ml of blood was collected directly in Vacutainer cell preparation tubes (CPT) with sodium citrate (Becton Dickinson, USA). Peripheral blood mononuclear cells (PBMC) were isolated within 3 hours of collection by centrifugation of CPT tubes, per manufacturer's instructions. PBMCs were stored frozen in freezing mix (65% RPMI, 25% HAB, 10% DMSO) in liquid nitrogen until analysis.
PBMC phenotyping
Antibodies, including CD3-PE, CD3-APC, CD14-FITC, CD16-APC, CD19-PerCPCy5.5, and CD56-APC from BD Biosciences (San Jose, CA) and Caltag/Invitrogen (Burlingame, CA) were used for identification of major cell subsets in PBMC: T cells (CD3+ cells), B cells (CD19+), monocytes (CD14+), NK (CD3-, CD56+) and NKT cells (CD3+, CD56+). Monocyte subsets CD14++/CD16- and CD14+/CD16+ [29] were enumerated using HLA-DR-FITC, CD14-PerCP and CD16-APC. γδ T cells were identified in PBMC with a combination of anti-CD3-APC and anti-TCR γδ-PE (BD Biosciences); B cells and monocytes were also stained in the same sample. CD4+ T regulatory cells (Tregs) were identified in total PBMC as CD4+ CD25+ and CD127low [30]. Naïve CD45RA+ Treg cells were identified with CD45RA-FITC, CD127-PE, CD25-Cychrome, and CD4-APC antibodies from BD Biosciences. We considered CD45RA-, CD4+, CD127low, CD25+ cells to be CD45RO+ T reg cells [31], as in humans expression of CD45RA and RO is usually regulated in a reciprocal manner [32].
Dendritic cells (DC) phenotyping
DC were identified in fresh whole blood. We identified plasmacytoid dendritic cells (pDC) and two subpopulations of myeloid dendritic cells (mDC). We used a staining strategy developed by BD Biosciences, based on DC low expression level of lineage markers for monocytes, lymphocytes, and NK cells, and DC high level of expression of HLA-DR. Furthermore, we used two different lineage marker staining cocktails to identify the various lineage-positive cells (non-DC); one staining cocktail [lineage cocktail (Lin)1] includes antibody to CD16 as a marker for neutrophils and NK cells, and the other does not (Lin2, kindly supplied by BD Biosciences), because a subset of mDC also expresses CD16 [33; 34]. The reagents used for DC identification were Lin1-FITC (CD3, CD14, CD16, CD19, CD20, CD56), Lin2-Fitc (all the markers as for Lin1, except CD16), CD123-PE, HLA-DR-PerCP, and CD11c-APC, all from from BD Biosciences. Total CD11c+ mDC including both CD16+ and CD16- DC were identified as Lin2-, HLA-DR+, CD11c+ cells; another mDC subpopulation was defined as Lin1-(CD16-), HLA-DR+, CD11c+, and pDC were identified as Lin1-, HLA-DR+, CD123+ cells.
Flow cytometry
Phenotyping of DC was performed using fresh blood; for all other cell types, frozen PBMCs were used for flow cytometry. Cells were thawed in a 37°C water bath, transferred to 96 well round bottom plates and incubated with antibody cocktails in the dark at room temperature for 30 minutes. After the incubation, cells were washed with PBS + 1% heat inactivated (HI) FCS, fixed with PBS + 1% paraformaldehyde, and stored in polystyrene tubes in the dark at 4°C until analysis. Data were acquired using the FACSCalibur flow cytometer (BD Biosciences, San Jose, CA) and analyzed with FlowJo (Treestar, Ashland, OR). Flow cytometer settings and fluorescence compensation were standardized for each experiment, using CaliBRITE beads and the FACSComp program (BD Immunocytometry Systems). FSC and SSC gates were set to capture the cell population of interest including monocytes and lymphocytes and excluding any contaminating neutrophils and debris. Subsequently, each cell population was identified by the fluorescence associated with surface markers. Cell percentages as well as the median florescence intensity (MFI) were measured.
Statistical analysis of cellular data
Multiple group comparisons were made using ANOVA with Bonferroni's multiple comparison correction. Group to group comparisons were made using Student's t-test. All tests performed with GraphPad Prism (GraphPad Software, San Diego, CA)
Results
Monocytes and monocyte subsets
Monocytes are central effector cells in innate immune responses. Absolute monocyte counts in CBC were generally higher in SJIA flare than in any other patient group (Table 1). Using flow cytometry to analyze isolated PBMCs from SJIA subjects, polyJIA and immunologically healthy control subjects, we found that SJIA flare samples had a statistically significant increase in the percentage of monocytes (CD14+) in total PBMC, in comparison with SJIA quiescence, polyJIA quiescence, and controls (Fig. 1A; data analyzed by multiple group comparisons using ANOVA with Bonferroni's multiple comparison correction). The difference in monocyte abundance was also significant when results were analyzed by group to group Student's t tests (not shown). In polyJIA flare, the proportion of monocytes among PBMC was variable, with only some samples having levels in the SJIA range, and there was no relationship between higher monocyte levels and RF positivity, a marker associated with more severe polyarticular disease (Fig 1A).
We next investigated if the distribution of the monocyte subsets, CD14++/CD16- and CD14+/CD16+, was altered in SJIA. In humans, the CD14++/CD16- subset represents 80 to 90% of circulating monocytes, comprises the high producers of cytokines (IL1, IL6, TNFα) and is highly phagocytic [29]. CD16+ monocytes have been found to be producers of TNFα after LPS stimulation and may be expanded during acute inflammation and infectious diseases, although their precise function remains elusive [35]. We found that the percentage of CD14+/CD16+ monocytes was increased in SJIA quiescent samples compared to controls (p=0.0033 by uncorrected Student's t test), and reciprocally, the percentage of CD14++/CD16- was reduced in these samples compared to controls (p=0.0017, Fig 1 B and C). Although these differences did not remain significant after correction for multiple comparisons, they raised the possibility that immunophenotypic alterations are present in SJIA subjects with clinically inactive disease.
CD16 is the Fc receptor FcγRIII, which functions as an activating Fcγ receptor [36]. CD16 levels by median fluorescence intensity (MFI) in the CD14+/CD16+ cells were highest in SJIA flare and quiescence and significantly higher than controls (Fig. 1D). CD14 can bind LPS and viruses on the cell surface, acting both as a transporter and as an amplifier of TLR-responses [37]. We measured CD14 levels (MFI) in the CD14++/CD16- subset and found CD14 expression was higher in both SJIA and polyJIA samples from subjects with active and inactive disease compared to controls (Fig. 1E). Taken together, the data show that monocytes are increased in number in SJIA flare, but are phenotypically altered in both flare and quiescence.
Dendritic cells and dendritic cell subsets
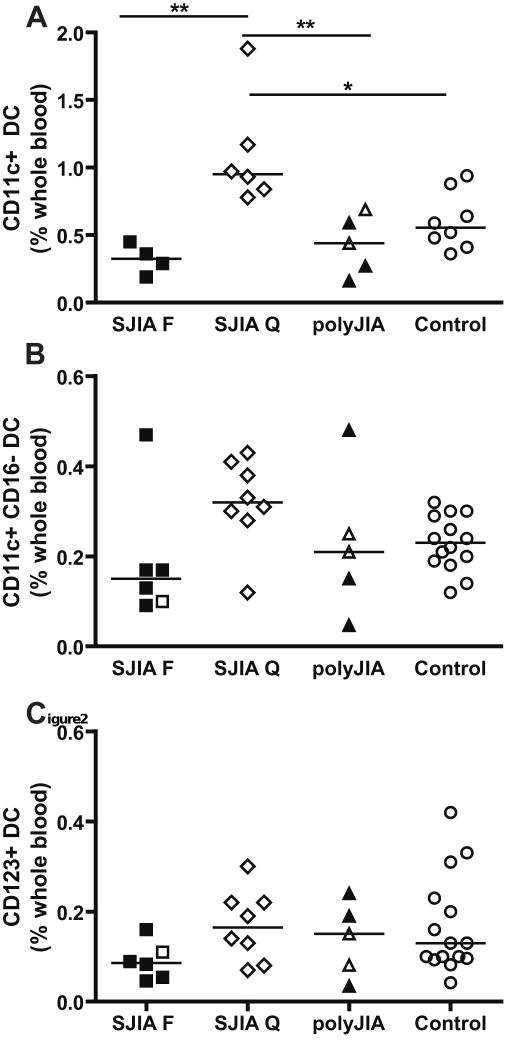
Another potential effector cell population in SJIA is dendritic cells (DC), which play key roles in both innate and adaptive immune responses [38]. We analyzed distribution of the two major blood DC subsets, myeloid (mDC) and plasmacytoid DC (pDC). Within the mDC subsets, 2 subpopulations were analyzed: the total mDC subpopulation (CD11c+), delineated as Lin2-(see Methods), HLA-DR+, CD11c+ cells; this subpopulation includes both CD16- and CD16+ DCs. CD16+ mDC recently have been shown to have strong proinflammatory capacity [39]. The second mDC subpopulation analyzed was defined as Lin1-(CD16-), HLA-DR+, CD11c+ cells. The pDC subset was defined as Lin1-, HLA-DR+, CD123+ cells. Our results showed a significant increase in total myeloid DC in SJIA quiescence compared to controls, and this reflects increases in CD16- mDC (Fig 2A, B and data not shown). These data again suggest that there are immunophenotypic changes detectable during inactive disease. The levels of total mDC were also significantly reduced during SJIA flare in comparison to SJIA quiescence and differences in both subsets of myeloid DC appear to contribute to this change (Fig. 2A and data not shown). In addition, the proportion of plasmacytoid DC (CD123+) was reduced at SJIA flare (p<.05 by Student's t tests, but not significant after correction for multiple group comparisons; Fig. 2C). Thus, proportions of circulating mDC are altered in SJIA, with reciprocal changes in flare compared to quiescence; this may reflect altered generation, life-span, localization or plasticity of phenotype of these cells in association with disease activity. Of note, changes in proportions of DC may not be associated with changes in the absolute DC numbers, and either parameter may have consequences for immune function. Further studies are needed to fully describe the changes in DC subsets.
Figure 2.
Percentage of dendritic cells (DC) in whole blood in SJIA, polyJIA and controls. Data are expressed as percentage of DC in whole blood. A: Total myeloid (CD11c+), including both CD16- and CD16+ DC; SJIA F n=4; SJIA Q n=6; polyJIA n=5; Control n=8. B: Myeloid (CD11c+, CD16-) DC. C: Plasmacytoid (CD123+) DC. F: flare; Q: quiescence. For B and C: SJIA F n=6; SJIA Q n=8; polyJIA n=5; Control n=14. All polyJIA patients are RF- (3 poly F, 2 poly Q). Samples with treatment intensity scores > 2 are in black. Statistical analysis used ANOVA with Bonferroni correction. *: p<0.05; **: p= or <0.01.
NK cells and γδT cells
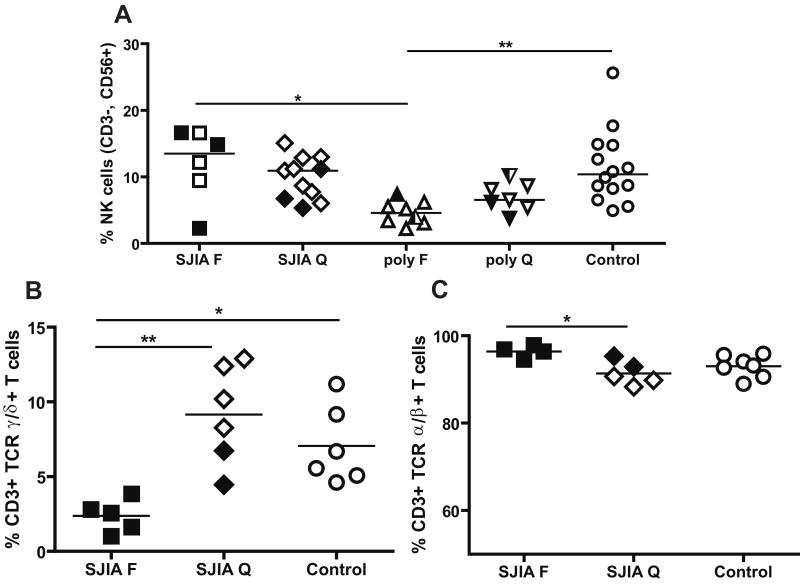
NK cells (CD3-CD56+) play key roles in innate immune responses, including killing of target cells and production of cytokines like IFNγ [40]. In our cohort, the percentage of NK cells in SJIA was similar to controls both at flare and quiescence (Fig 3A). However, we observed a significantly decreased percentage of NK cells in polyJIA flare, in comparison to SJIA flare and controls, after correction for multiple group comparisons (Fig 3A). We also measured levels of γδT cells, another innate effector population that like NK cells is both cytotoxic and a potent source of cytokines [41; 42] [43]. We observed that the percentage of γδT cells was decreased during SJIA flare in comparison to SJIA quiescence and controls (Fig 3B); polyJIA samples were not tested. Reciprocally, the percentage of αβT cells was increased in SJIA flare in comparison to SJIA quiescence (Fig 3C).
Figure 3.
A: Percentage of NK cells (CD3-, CD56+ cells) in SJIA, polyJIA and controls. Data are expressed as percentage of total mononuclear cells. F: flare; Q: quiescence. SJIA F n=6; SJIA Q n=11; polyJIA F n=8; polyJIA Q n=7; Control n=14. polyJIA RF+ patients are in half black symbols. Samples with treatment intensity scores > 2 are in black. B: Percentage of CD3+ T cells expressing TCR γδ as a percentage of total CD3+ T cells in PBMC in SJIA and controls. SJIA F n=5; SJIA Q n=6; Control n=6. C: Percentage of CD3+ TCR αβ T cells. SJIA F N=4; SJIA Q N=5; Control N=7. Samples with treatment intensity scores > 2 are in black. Statistical analysis used ANOVA with Bonferroni correction. *: p<0.05; **: p= or <0.01.
T regulatory cells and NKT cells
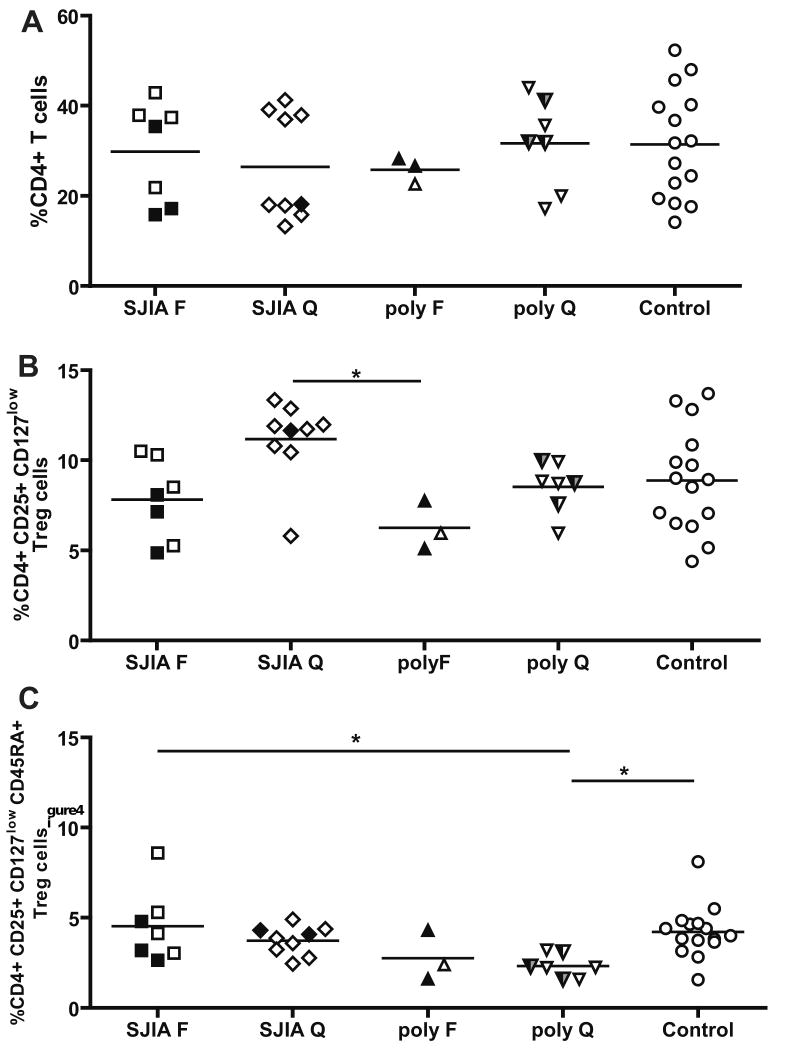
Alterations in immune status can derive from changes in cell populations that regulate the effectors. We next investigated the distribution of CD4+ T regulatory cells, which have been shown to regulate the activity of both adaptive and innate effectors [44]. To analyze T regulatory cell numbers, we first measured the percentage of CD4+ T cells in SJIA and polyJIA, and these were not different from controls (Fig 4A). Analysis of total Treg cells (CD4+ CD25+ CD127low) suggested an increase (using uncorrected Student's t-test) in quiescent compared to active SJIA; levels during active disease resembled those of healthy controls (Fig 4B). The increase in total Tregs in SJIA quiescence apparently derived from the RA-(RO+) subset, which is the memory T reg subpopulation [31], as there is no increase in the percentage of CD45RA+ Treg cells in SJIA quiescence (Fig. 4C). This finding suggests a role for Tregs in controlling SJIA disease activity during quiescence, as has been proposed by others [45]. For polyJIA, the percentage of naïve Tregs (CD45RA+ [31]) was significantly decreased in quiescence in comparison to controls (Fig 4C). The number of CD45RA+ Tregs declines with age [31]; however, age distribution was similar among the groups we tested (Table 1).
Figure 4.
Percentage of T regulatory (T regs) in in SJIA, polyJIA and controls A: Percentage of CD4+ T cells. Data are expressed as percentage of total mononuclear cells. B: Percentage of total Tregs, identified as CD4+, CD25+, CD127low, among CD4+ T cells. C: Percentage of CD45RA+Tregs among CD4+ T cells. F: flare; Q: quiescence. SJIA F n=7; SJIA Q n=9; polyJIA F n=3; polyJIA Q n=7; Control n=15. polyJIA RF+ patients are in half black symbols. Samples with treatment intensity scores > 2 are in black. polyJIA RF+ patients with treatment intensity scores > 2 are in half black and half grey symbols. Statistical analysis used ANOVA with Bonferroni correction. * p<0.05; **: p= or <0.01.
NKT cells are a subset of innate immune cells that can produce large amounts of cytokines shortly after antigenic stimulation; they also have cytolytic activity [46]. Percentage of NKT cells, identified as CD3+ CD56+ cells did not differ between SJIA and polyJIA subjects either at flare or quiescence and did not differ from the control group (not shown).
T and B cells
Reported data on levels of circulating CD3+ T cells and B cells (CD19+ or CD20+) in SJIA have been conflicting [19; 20; 21; 22]. We found that the percentage of T cells (CD3+) was significantly decreased in SJIA flare group in comparison to SJIA quiescence and polyJIA flare in multiple group comparisons (Fig 5A); using group to group Student's t tests, the percentage of CD3+ T cells in SJIA flare was significantly lower than in any other group. Total lymphocyte counts in CBC tend to be lower in SJIA flare in comparison to SJIA and polyJIA (Table 1). The percentage of B cells (CD19+) was decreased significantly in SJIA flare, compared to the control after correction for multiple comparisons (Fig 5B) and compared to all other groups, using uncorrected t tests. Notably, increased spontaneous lymphocyte apoptosis has been reported in active SJIA [47].
Discussion
Here we report cellular studies of PBMC obtained from children with SJIA and, for comparison, children with polyJIA and immunologically healthy age-matched controls. Given the systemic nature of SJIA, we reasoned that analyses of blood would be informative. The value of investigating PBMCs in inflammatory arthritis also has been highlighted recently by studies of gene expression in PBMC in active adult RA [48; 49], polyJIA [50; 51] and SJIA [16; 22; 52]. Notably, these studies often evaluate whole PBMC; our analyses of cell type distribution provide important complementary information. A limitation of our study is that, for cell subpopulations besides monocytes and lymphocytes, we have only determined changes in their proportion among total PBMC; changes in one cell population may produce a relative change in other populations without affecting absolute cell numbers. A distinctive cellular feature of SJIA flare revealed in our studies is an increase in monocytes. Both by CBC count and as a percentage of PBMC by flow-cytometric analysis, we observed an expansion of the monocytes in SJIA flare samples compared to the other subject groups studied. An increase in monocytes has also been described in Kawasaki disease (KD) [53], a condition that can resemble SJIA at presentation. However, in contrast to SJIA, CD19+ B cells also are increased in KD subjects [53].
Both monocyte subsets, CD14++/CD16- and CD14+/CD16+, contribute to the increase in monocytes in SJIA flare, as the proportions of monocyte subsets did not differ significantly among the groups of subjects studied. The number and percentage of total monocytes normalized during disease quiescence. However, the percentage of CD14+/CD16+ monocytes was higher in SJIA quiescence compared to controls (significantly, using uncorrected Student's t tests). The CD14+/CD16+ subset has been observed to expand in inflammatory conditions, and this population has been suggested in some studies to produce high levels of TNF [54]. This monocyte subset has also been described to have characteristics of tissue macrophages [55]. Thus, our data raise the possibility of altered monocyte homeostasis in SJIA at quiescence, perhaps reflecting an underlying abnormality in SJIA-susceptible individuals. We found that the expression of surface CD16 was increased in CD14+/CD16+ monocytes in SJIA compared to controls, both during flare and quiescence, but not in polyJIA Increased expression of CD16 in CD14+/CD16+ monocytes during SJIA quiescence suggests that this may be an intrinsic characteristic of SJIA monocytes. Ligation of CD16 induces cytokine production in monocytes [56], and the increase in CD16 expression may be involved in maintaining a pro-inflammatory environment even in the absence of clinical manifestations of disease. Increased levels of circulating monocytes and elevated surface levels of CD16 on the CD16+ monocyte subset also can be observed in response to infection [57; 58]. An infectious trigger for SJIA, though suspected in part due to reports of seasonal incidence of SJIA, has not been identified [59; 60; 61].
Another monocyte-related finding was that the expression level of surface CD14 was increased in CD14++/CD16- monocytes in both SJIA and polyJIA. Increased expression of surface CD14 has been associated with resistance to apoptosis [62]. Dysregulation of apoptosis may contribute to the accumulation of monocytes during SJIA flare (S. Srivastava, C. Macaubas, unpublished data).
The role of monocyte/macrophages in SJIA in comparison to other JIA subsets appears to be unique. Monocyte-derived cytokines, particularly IL-1 and IL-6, appear to be key mediators of SJIA [18; 52; 63], and activation of macrophages (MAS) is a critical complication that may be more common than previously thought [64]. Monocyte infiltration into skin is observed in rash biopsies in SJIA [65]. Microarray data from Fall et al [22] suggested that an increase in monocyte differentiation into macrophages may contribute to SJIA immunopathology.
In addition to monocytes, myeloid dendritic cells are a potential source of pro-inflammatory cytokines, especially IL-1, IL-6 and TNF [34; 66]. Interestingly, in our study, the levels of total mDC are increased in samples from SJIA quiescence, compared to the other subject groups, due to changes in the CD16- DC subset. We also observed significant reductions in mDC (from quiescent levels) at disease flare. Our study has analyzed only DC percentage, not absolute numbers. However, Smolewska et al[67], analyzing JIA subtypes (SJIA, polyJIa and oligoJIA) as a single group in comparison to healthy controls, reported a small decrease in absolute numbers of total blood DC in JIA children with higher disease activity. Furthermore, they observed a reduction in both the percentage and absolute number of circulating DC subtypes (myeloid DC1 (mDC1), mDC2 and pDC), and higher DC counts in the synovial fluid of active joints. The number of DCs in blood correlated with clinical status, with lower DC numbers being associated with poorer response to treatment and abnormal clinical laboratory values [67]. DC migration from circulation to the joints may occur during period of active disease. High dose glucocorticoid has been reported to induce a decrease in circulating mDC and especially pDC [68; 69]. However, in our study, reduced DC levels did not correlate with presence of glucocorticoid therapy (not shown).
We found that the percentage of NK cells during active SJIA was similar to that in healthy controls. This corroborates results of Yabuhara et al [23], although others have reported reduced numbers of NK cells in SJIA [19] [22]. One reason for the normal percentage of NK cells in our cohort may be their older age and their longer duration of disease, as reduced NK cell levels were observed in subjects with new onset disease [22] or in younger patients [19]. As we did not evaluate the cytotoxic function of NK cells, our data do not exclude the possibility that there is a functional defect in NK cells in our SJIA samples. We did not observe a significant reduction in the expression of perforin in SJIA NK cells (A. Pashine, E. Mellins et al, unpublished observations). However, NK cell dysfunction in SJIA does not appear to be strictly related to the amount of granzyme A, or perforin [24; 25; 70].
We found the percentage of γδ T cells to be decreased during SJIA flare. Here, we cannot exclude a possible effect of treatment, as the flare samples tested had higher treatment intensity scores compared to quiescence samples. Nonetheless, our finding is in agreement with observations of Wouters et al [19] in a mixed group of SJIA patients with and without disease activity. In contrast, an increase in γδ T cells during active disease was reported in patients with adult Still's disease [71]. Also, Black et al [72] observed an increase in the percentage of γδT cells in synovial fluid compared to peripheral blood in JIA (mostly oligoarthritis cases), suggesting that these cells may leave the circulation during JIA flare and contribute to disease at tissue sites.
We did not find alterations in the proportions of Treg cells in SJIA. Reductions in the percentage of Treg cells, and naïve, natural Tregs (CD45RA+) in particular, were associated with polyJIA. These results suggest that Treg cells may play a more important role in polyJIA than in SJIA, and a more detailed investigation in the different polyJIA subsets (RF+ and RF-) is warranted.
In our study, the percentages of T and B cells were decreased during active SJIA. These decreases do not appear to be due to treatment, as values for samples with low treatment intensity score are similar to those for samples with higher treatment intensity score. In prior studies, percentages of CD3+ T cells and of CD4+ T cells sometimes have been found to be similar in SJIA and healthy controls [20; 22], while others report increases in these cell types in SJIA peripheral blood [19]. However, these prior studies either did not use age-matched controls [20] or did not distinguish samples based on clinical status [19]. As we find some samples from SJIA quiescence with percentages of T cells that are higher than the controls, combined analysis of samples from active and inactive patients may alter the percentage of CD3+ T cells compared to controls. Our finding of decreased proportions of B cells (defined as CD19+ cells) corroborates the report of Wouters et al [19], but differs from other studies that found that percentages of B cells were comparable in SJIA and controls [21; 22]. Higher rates of spontaneous lymphocyte apoptosis have been observed in SJIA, in association with disease activity [47]. Using an antibody against activated caspase-3, we also detected an increased level of spontaneous apoptosis in peripheral blood CD3+ T cells in SJIA (data not shown). Reduced T and B cells in SJIA flare could thus be a result of increased cell death during active disease. Of note, studies in active adult-onset Still's disease, the probable adult form of SJIA, also show increased spontaneous apoptosis of peripheral lymphocytes and provide evidence implicating the pro-inflammatory monokine IL-18 as a key mediator in this process [73]; high serum levels of IL-18 have been associated with SJIA flare [74]. An alternative, though not mutually exclusive, possibility is that reduced levels of circulating lymphocytes reflect their egress from the circulation into the joints during disease activity [75].
The clinical presentation of SJIA, the absence of HLA association and major autoantibodies, and the important role played by IL-1β, based on the clinical response to IL-1β inhibition in ∼50% of SJIA patients [52], suggest that SJIA may belong to the group of autoinflammatory diseases, although the criteria for inclusion in this group are still evolving [76]. Our data support this possibility: we observed an increase in monocyte number, especially CD16+ monocytes and expansion of the mDC dendritic cell subset. In autoinflammatory diseases, fever and other inflammatory symptoms develop in the absence of concurrent infection or signs of adaptive autoimmune responses [76]. Interestingly, level of CD16, but not the absolute numbers of CD14+/CD16+ monocytes, is elevated in the autoinflammatory disease, tumor necrosis factor (TNF) receptor-associated periodic syndrome (TRAPS), which is caused by mutations in the p55 TNF receptor (TNFRSF1A). Similar to our findings with SJIA, elevated CD16 is observed even in the absence of disease activity in TRAPS [77]. Future studies comparing SJIA to periodic fevers may clarify the extent of overlap between SJIA and these diseases.
In summary, we observed changes in distribution and phenotype among peripheral blood mononuclear cells consistent with activity of the innate immune system during SJIA flare. Further, detection of immunophenotypic changes during quiescent SJIA implies that inflammatory activity is more likely compensated rather than absent. Further studies will be needed to clarify the relevance of these findings to disease immunopathology and treatment.
Supplementary Material
Acknowledgments
We would like to thank the patients, their families and the medical staff of Pediatric Rheumatology and Pediatric Endocrinology Clinics at Lucile Packard Hospital for Children. This work was supported by the Dana Foundation, The Wasie Foundation and the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bowyer SL, Roettcher PA, Higgins GC, Adams B, Myers LK, Wallace C, Rennebohm R, Moore TL, Pepmueller PH, Spencer C, Wagner-Weiner L, Rabinovich E, Passo M, Lovell DJ, Madson K, McCurdy D, Zemel L, Schikler KN, Szer I, Kurtin P, Lindsley C. Health status of patients with Juvenile Rheumatoid Arthritis at 1 and 5 years after diagnosis. J Rheumatology. 2003;30:394–400. [PubMed] [Google Scholar]
- 2.Adib N, Silman A, Thomson W. Outcome following onset of juvenile idiopathic inflammatory arthritis: I. Frequency of different outcomes. Rheumatology. 2005;44:995–1001. doi: 10.1093/rheumatology/keh620. [DOI] [PubMed] [Google Scholar]
- 3.Petty RE, Southwood TR, Manners P, Baum J, Glass DN, Goldenberg J, He X, Maldonado-Coco J, Orozco-Alcada J, Prieur AM, Suarez-Almazor ME, Woo P, Rheumatology ILoAf. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatology. 2004;31:390–392. [PubMed] [Google Scholar]
- 4.Borchers AT, Selmi C, Cheema G, Keen CL, Shoenfeld Y, Gershwin ME. Juvenile idiopathic arthritis. Autoimmun Rev. 2006;5:279–298. doi: 10.1016/j.autrev.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 5.Macaubas C, Nguyen K, Milojevic D, Park JL, Mellins ED. Oligoarticular and polyarticular JIA: epidemiology and pathogenesis. Nat Rev Rheumatology. 2009;5 doi: 10.1038/nrrheum.2009.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schaller JG. Juvenile rheumatoid arthritis. Pediatrics in review. 1997;18:337–49. doi: 10.1542/pir.18-10-337. [DOI] [PubMed] [Google Scholar]
- 7.Prahalad S, Glass DN. A comprehensive review of the genetics of juvenile idiopathic arthritis. Pediatric rheumatology online journal. 2008;6:11. doi: 10.1186/1546-0096-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walker SM, Shaham B, McCrudy DK, Wietting H, Arora YK, Hanson V, Bernstein B. Prevalence and concentration of IgM rheumatoid factor in polyarticular onset disease as compared to systemic or pauciarticular onset disease in active juvenile rheumatoid arthritis as measured by ELISA. The Journal of rheumatology. 1990;17:936–40. [PubMed] [Google Scholar]
- 9.Oen K, Malleson P, Cabral D, Rosenberg A, Petty R, Reed M, Schroeder M, Cheang M. Early predictors of longterm outcome in patients with juvenile rheumatoid arthritis: subset-specific correlations. The Journal of rheumatology. 2003;30:585–93. [PubMed] [Google Scholar]
- 10.Ramanan AV, Grom AA. Does systemic-onset juvenile idiopathic arthritis belong under juvenile idiopathic arthritis? Rheumatology. 2005;44:1350–1353. doi: 10.1093/rheumatology/keh710. [DOI] [PubMed] [Google Scholar]
- 11.Efthimiou P, Georgy S. Pathogenesis and management of adult-onset Still's disease. Semin Arthritis Rheum. 2006;36:144–152. doi: 10.1016/j.semarthrit.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 12.Quartier P, Taupin P, Bourdeaut F, Lemelle I, Pillet P, Bost M, Sibilia J, Koné-Paut I, Gandon-Laloum S, LeBideau M, Bader-Meunier B, Mouy R, Debré M, Landais p, Prier AM. Efficacy of etanercept for the treatment of juvenile idiopathic arthritis according to the onset type. Arthritis Rheum. 2003;48:1093–1101. doi: 10.1002/art.10885. [DOI] [PubMed] [Google Scholar]
- 13.Reiff A. The use of anakinra in juvenile arthritis. Curr Rheumatol Rep. 2005;7:434–440. doi: 10.1007/s11926-005-0047-2. [DOI] [PubMed] [Google Scholar]
- 14.Lequerre T, Quartier P, Rosellini D, Alaoui F, De Bandt M, Mejjad O, Kone-Paut I, Michel M, Dernis E, Khellaf M, Limal N, Job-Deslandre C, Fautrel B, Le Loet X, Sibilia J. Interleukin-1 receptor antagonist (anakinra) treatment in patients with systemic-onset juvenile idiopathic arthritis or adult onset Still disease: preliminary experience in France. Annals of the rheumatic diseases. 2008;67:302–8. doi: 10.1136/ard.2007.076034. [DOI] [PubMed] [Google Scholar]
- 15.de Benedetti F, Massa M, Robbioni P, Ravelli A, Burgio GR, Martini A. Correlation of serum interleukin-6 levels with joint involvement and thrombocytosis in systemic juvenile rheumatoid arthritis. Arthritis Reumathism. 1991;34:1158–1163. doi: 10.1002/art.1780340912. [DOI] [PubMed] [Google Scholar]
- 16.Ogilvie EM, Khan A, Hubank M, Kellam P, Woo P. Specific gene expression profiles in systemic juvenile idiopathic arthritis. Arthritis Rheum. 2007;56:1954–1965. doi: 10.1002/art.22644. [DOI] [PubMed] [Google Scholar]
- 17.Woo P, Wilkinson N, Prieur AM, Southwood T, Leone V, Livermore P, Wythe H, Thomson D, Kishimoto T. Open label phase II trial of single, ascending doses of MRA in Caucasian children with severe systemic juvenile idiopathic arthritis: proof of principle of the efficacy of IL-6 receptor blockade in this type of arthritis and demonstration of prolonged clinical improvement. Arthritis Res Ther. 2005;7:R1281–R1288. doi: 10.1186/ar1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yokota S, Miyamae T, Imagawa T, Iwata N, Katakura S, mori M, Woo P, Nishimoto N, Yoshizaki K, Kishimoto T. Therapeutic efficacy of humanized recombinant anti-interleukin-6 receptor antibody in children with systemic-onset juvenile idiopathic arthritis. Arthritis Rheum. 2005;52:818–825. doi: 10.1002/art.20944. [DOI] [PubMed] [Google Scholar]
- 19.Wouters CH, Ceuppens JL, Stevens EA. Different circulating lymphocyte profiles in patients with different subtypes of juvenile idiopathic arthritis. Clin Exp Rheumatol. 2002;20:239–248. [PubMed] [Google Scholar]
- 20.Tsokos GC, Mavridis A, Inghirami G, Pillemer SR, Emery HM, Magilavy DB. Cellular immunity in patients with systemic juvenile rheumatoid arthritis. Clinical immunology and immunopathology. 1987;42:86–92. doi: 10.1016/0090-1229(87)90175-9. [DOI] [PubMed] [Google Scholar]
- 21.Martini A, Massa M, De Benedetti F, Viola S, Neirotti G, Burgio RG. CD5 positive B lymphocytes in seronegative juvenile arthritis. The Journal of rheumatology. 1990;17:932–5. [PubMed] [Google Scholar]
- 22.Fall N, Barnes M, T S, Luyrink L, Olson J, Ilowite NT, Gottlieb BS, Griffin T, Sherry DD, Thompson S, Glass DN, Colbert RA, Grom AA. Gene expression profiling of peripheral blood from patients with untreated new-onset systemic juvenile idiopathic arthritis reveals molecular heterogeneity that may predict macrophage activation syndrome. Arthritis Reumathism. 2007;56:3793–3804. doi: 10.1002/art.22981. [DOI] [PubMed] [Google Scholar]
- 23.Yabuhara A, Yang FC, Nakazawa T, Iwasaki Y, Mori T, Koike K, Kawai H, Komiyama A. A killing defect of natural killer cells as an underlying immunologic abnormality in childhood systemic lupus erythematosus. The Journal of rheumatology. 1996;23:171–7. [PubMed] [Google Scholar]
- 24.Grom AA, Villanueva J, Lee S, Goldmuntz EA, Passo MH, Filipovich A. Natural killer cell dysfunction in patients with systemic-onset juvenile rheumatoid arthritis and macrophage activation syndrome. J Pediatr. 2003;142:292–296. doi: 10.1067/mpd.2003.110. [DOI] [PubMed] [Google Scholar]
- 25.Villanueva J, Lee S, Giannini EH, Graham TB, Passo MH, Filipovich A, Grom AA. Natural killer cell dysfunction is a distinguishing feature of systemic onset juvenile rheumatoid arthritis and macrophage activation syndrome. Arthritis Res Ther. 2005;7:R30–37. doi: 10.1186/ar1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hazen MM, Woodward AL, Hofmann I, Degar BA, Grom A, Filipovich AH, Binstadt BA. Mutations of the hemophagocytic lymphohistiocytosis-associated gene UNC13D in a patient with systemic juvenile idiopathic arthritis. Arthritis and rheumatism. 2008;58:567–70. doi: 10.1002/art.23199. [DOI] [PubMed] [Google Scholar]
- 27.Sandborg CI, Nepom BS, Mellins E. Juvenile Arthritis. In: Rich TFR, Kotzin B, Shearer W, S H, editors. Clinical Immunology, Harcourt Health Sciences, London. 2001. [Google Scholar]
- 28.Sandborg C, Holmes TH, Lee T, Biederman K, Bloch DA, Emery H, McCurdy D, Mellins ED. Candidate early predictors for progression to joint damage in systemic juvenile idiopathic arthritis. J Rheumatology. 2006;33:2322–2329. [PubMed] [Google Scholar]
- 29.Passlick B, Flieger D, Ziegler-Heitbrock HW. Identification and characterization of a novel monocyte subpopulation in human peripheral blood. Blood. 1989;74:2527–2534. [PubMed] [Google Scholar]
- 30.Liu W, Putnam AL, Xu-Yu Z, Szot GL, Lee MR, Zhu S, Gottlieb PA, Kapranov P, Gingeras TR, Fazekas de St Groth B, Clayberger C, Soper DM, Ziegler SF, Bluestone JA. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med. 2006;203:1701–1711. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seddiki N, Santner-Nanan B, Martinson J, Zaunders J, Sasson S, Landay A, Solomon M, Selby W, Alexander SI, Nanan R, Kelleher A, Fazekas de St Groth B. Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J Exp Med. 2006;203:1693–1700. doi: 10.1084/jem.20060468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seddiki N, Santner-Nanan B, Tangye SG, Alexander SI, Solomon M, Lee S, Nanan R, Fazekas de St Groth B. Persistence of naive CD45RA+ regulatory T cells in adult life Blood. 2006;107:2830–2838. doi: 10.1182/blood-2005-06-2403. [DOI] [PubMed] [Google Scholar]
- 33.MacDonald KPA, Munster DJ, Clark GJ, Dzionek A, Schmitz J, Hart DNJ. Characterization of human blood dendritic cell subsets. Blood. 2002;100:4512–4520. doi: 10.1182/blood-2001-11-0097. [DOI] [PubMed] [Google Scholar]
- 34.Drohan L, Harding JJ, Holm B, Cordoba-Tongson E, Dekker CL, Holmes T, Maecker H, Mellins ED. Selective developmental defects of cord blood antigen-presenting cell subsets Hum Immunol. 2004;65:1356–1369. doi: 10.1016/j.humimm.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 35.Grage-Griebenow E, Flad HD, E M. Heterogeneity of human peripheral blood monocyte subsets. J Leukoc Biol. 2001;69:11–20. [PubMed] [Google Scholar]
- 36.Ravetch JV, Bolland S. IgG Fc receptors. Annual Review of Immunology. 2001;19:275–90. doi: 10.1146/annurev.immunol.19.1.275. [DOI] [PubMed] [Google Scholar]
- 37.Akashi-Takamura S, Miyake K. TLR accessory molecules. Current opinion in immunology. 2008;20:420–5. doi: 10.1016/j.coi.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 38.Steinman R. Linking innate to adaptive immunity through dendritic cells. Novartis Foundation symposium. 2006;279:101–9. discussion 109. [PubMed] [Google Scholar]
- 39.Piccioli D, Tavarini S, Borgogni E, Steri V, Nuti S, Sammicheli C, Bardelli M, Montagna D, Locatelli F, Wack A. Functional specialization of human circulating CD16 and CD1c myeloid dendritic-cell subsets. Blood. 2007;109:5371–9. doi: 10.1182/blood-2006-08-038422. [DOI] [PubMed] [Google Scholar]
- 40.Farag SS, Caligiuri MA. Human natural killer cell development and biology. Blood Reviews. 2005;20:123–137. doi: 10.1016/j.blre.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 41.Nakata M, Smyth MJ, Norihisa Y, Kawasaki A, Shinkai Y, Okumura K, Yagita H. Constitutive expression of pore-forming protein in peripheral blood gamma/delta T cells: implication for their cytotoxic role in vivo. The Journal of experimental medicine. 1990;172:1877–80. doi: 10.1084/jem.172.6.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Koizumi H, Liu CC, Zheng LM, Joag SV, Bayne NK, Holoshitz J, Young JD. Expression of perforin and serine esterases by human gamma/delta T cells. The Journal of experimental medicine. 1991;173:499–502. doi: 10.1084/jem.173.2.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Beetz S, Wesch D, Marischen L, Welte S, Oberg HH, Kabelitz D. Innate immune functions of human gammadelta T cells. Immunobiology. 2008;213:173–182. doi: 10.1016/j.imbio.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 44.Milojevic D, Nguyen KD, Wara D, Mellins ED. Regulatory T cells and their role in rheumatic diseases: a potential target for novel therapeutic development. Pediatric rheumatology online journal. 2008;6:20. doi: 10.1186/1546-0096-6-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Roord ST, de Jager W, Boon L, Wulffraat N, Martens A, Prakken B, van Wijk F. Autologous bone marrow transplantation in autoimmune arthritis restores immune homeostasis through CD4+CD25+Foxp3+ regulatory T cells. Blood. 2008;111:4838–4839. doi: 10.1182/blood-2007-12-128488. [DOI] [PubMed] [Google Scholar]
- 46.Linsen L, Somers V, Stinissen P. Immunoregulation of autoimmunity by natural killer T cells. Human immunology. 2005;66:1193–202. doi: 10.1016/j.humimm.2006.02.020. [DOI] [PubMed] [Google Scholar]
- 47.Smolewska E, Brozik H, Smolewski P, Biernacka-Zielinska M, Darzynkiewicz Z, Stanczyk J. Apoptosis of peripheral blood lymphocytes in patients with juvenile idiopathic arthritis. Annals of the rheumatic diseases. 2003;62:761–3. doi: 10.1136/ard.62.8.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Olsen N, Sokka T, Seehorn CL, Kraft B, Maas K, Moore J, Aune TM. A gene expression signature for recent onset rheumatoid arthritis in peripheral blood mononuclear cells. Ann Rheum Dis. 2004;63:1387–1392. doi: 10.1136/ard.2003.017194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Batliwalla FM, Baechler EC, Xiao X, Li W, Balasubramanian S, Khalili H, Damle A, Ortmann WA, Perrone A, Kantor AB, Gulko PS, Kern M, Furie R, Behrens TW, Gregersen PK. Peripheral blood gene expression profiling in rheumatoid arthritis. Genes Immun. 2005;6:388–397. doi: 10.1038/sj.gene.6364209. [DOI] [PubMed] [Google Scholar]
- 50.Jarvis JN, Dozmorov I, Jiang K, Frank MB, Szodoray P, Alex P, Centola M. Novel approaches to gene expression analysis of active polyarticular juvenile rheumatoid arthritis. Arthritis Res Ther. 2004;6:R15–R32. doi: 10.1186/ar1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Barnes MG, Aronow BJ, Luyrink LK, Moroldo MB, Pavlidis P, Passo MH, Grom AA, Hirsch R, Giannini EH, Colbert RA, Glass DN, Thompson SD. Gene expression in juvenile arthritis and spondyloarthropathy: pro-angiogenic ELR+ chemokine genes relate to course of arthritis. Rheumatology. 2004;43:973–979. doi: 10.1093/rheumatology/keh224. [DOI] [PubMed] [Google Scholar]
- 52.Pascual V, Allantaz F, Arce E, Punaro M, Banchereau J. Role of interleukin-1 (IL-1) in the pathogenesis of systemic onset juvenile idiopathic arthritis and clinical response to IL-1 blockade. J Exp Med. 2005;201:1479–1486. doi: 10.1084/jem.20050473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Furukawa S, Ichiyama T, Matsubara T. Immunological profile of peripheral blood lymphocytes and monocytes/macrophages in Kawasaki disease. Clinical and experimental immunology. 2005;141:381–7. doi: 10.1111/j.1365-2249.2005.02821.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Belge KU, Dayyani F, Horelt A, Siedlar M, Frankenberger M, Frankenberger B, Espevik T, Ziegler-Heitbrock L. The proinflammatory CD14+CD16+DR++ monocytes are a major source of TNF. J Immunol. 2002;168:3536–3542. doi: 10.4049/jimmunol.168.7.3536. [DOI] [PubMed] [Google Scholar]
- 55.Ziegler-Heitbrock HW, Fingerle G, Strobel M, Schraut W, Stelter F, Schutt C, Passlick B, Pforte A. The novel subset of CD14+/CD16+ blood monocytes exhibits features of tissue macrophages. European journal of immunology. 1993;23:2053–8. doi: 10.1002/eji.1830230902. [DOI] [PubMed] [Google Scholar]
- 56.Guan G, Kramer SF, Kramer PR. 17 beta-estradiol regulates cytokine release through modulation of CD16 expression in monocytes and monocyte-derived macrophages. Arthritis and rheumatism. 2004;50:1967–75. doi: 10.1002/art.20309. [DOI] [PubMed] [Google Scholar]
- 57.Fernandez G, Ramos M, Gomez S, Dran G, Exeni R, Alduncin M, Grimoldi I, Vallejo G, Elias-Costa C, Isturiz M, Palermo M. Differential expression of function-related antigens on blood monocytes in children with hemolytic uremic syndrome. Journal of leukocyte biology. 2005;78:853–61. doi: 10.1189/jlb.0505251. [DOI] [PubMed] [Google Scholar]
- 58.Soares G, Barral A, Costa J, Barral-Netto M, Van Weyenbergh J. CD16+ monocytes in human cutaneous leishmaniasis: increased ex vivo levels and correlation with clinical data. Journal of leukocyte biology. 2006;79:36–9. doi: 10.1189/jlb.0105040. [DOI] [PubMed] [Google Scholar]
- 59.Oen K, Fast M, Postl B. Epidemiology of juvenile rheumatoid arthritis in Manitoba, Canada, 1975-92: cycles in incidence. The Journal of rheumatology. 1995;22:745–50. [PubMed] [Google Scholar]
- 60.Lindsley CB. Seasonal variation in systemic onset juvenile rheumatoid arthritis. Arthritis and rheumatism. 1987;30:838–9. doi: 10.1002/art.1780300719. [DOI] [PubMed] [Google Scholar]
- 61.Uziel Y, Pomeranz A, Brik R, Navon P, Mukamel M, Press J, Barash J, Tauber T, Harel L, Virgilis D, Bibi H, Heldenberg D, Wolach B. Seasonal variation in systemic onset juvenile rheumatoid arthritis in Israel. The Journal of rheumatology. 1999;26:1187–9. [PubMed] [Google Scholar]
- 62.Heidenreich S. Monocyte CD14: a multifunctional receptor engaged in apoptosis from both sides. J Leukoc Biol. 1999;65:737–743. doi: 10.1002/jlb.65.6.737. [DOI] [PubMed] [Google Scholar]
- 63.de Benedetti F, Massa M, Robbioni P, Ravelli A, Burgio GR, Martini A. Correlation of serum interleukin-6 levels with joint involvement and thrombocytosis in systemic juvenile rheumatoid arthritis. Arthritis and rheumatism. 1991;34:1158–63. doi: 10.1002/art.1780340912. [DOI] [PubMed] [Google Scholar]
- 64.Behrens EM, Beukelman T, Paessler M, Cron RQ. Occult macrophage activation syndrome in patients with systemic juvenile idiopathic arthritis. The Journal of rheumatology. 2007;34:1133–8. [PubMed] [Google Scholar]
- 65.Frosch M, Metze D, Foell D, Vogl T, Sorg C, Sunderkotter C, Roth J. Early activation of cutaneous vessels and epithelial cells is characteristic of acute systemic onset juvenile idiopathic arthritis. Experimental dermatology. 2005;14:259–65. doi: 10.1111/j.0906-6705.2005.00271.x. [DOI] [PubMed] [Google Scholar]
- 66.Blanco P, Palucka AK, Pascual V, Banchereau J. Dendritic cells and cytokines in human inflammatory and autoimmune diseases. Cytokine Growth Factor Rev. 2008;19:41–52. doi: 10.1016/j.cytogfr.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Smolewska E, Stanczyk J, Brozik H, Biernacka-Zielinska M, Cebula B, Robak T, Smolewski P. Distribution and clinical significance of blood dendritic cells in children with juvenile idiopathic arthritis. Annals of the rheumatic diseases. 2008;67:762–8. doi: 10.1136/ard.2007.077669. [DOI] [PubMed] [Google Scholar]
- 68.Shodell M, Shah K, Siegal FP. Circulating human plasmacytoid dendritic cells are highly sensitive to corticosteroid administration. Lupus. 2003;12:222–230. doi: 10.1191/0961203303lu362xx. [DOI] [PubMed] [Google Scholar]
- 69.Rozkova D, Horvath R, Bartunkova J, Spisek R. Glucocorticoids severely impair differentiation and antigen presenting function of dendritic cells despite upregulation of Toll-like receptors. Clinical immunology. 2006;120:260–71. doi: 10.1016/j.clim.2006.04.567. [DOI] [PubMed] [Google Scholar]
- 70.Wulffraat NM, Rijkers GT, Elst E, Brooimans R, Kuis W. Reduced perforin expression in systemic juvenile idiopathic arthritis is restored by autologous stem-cell transplantation. Rheumatology. 2003;42:375–9. doi: 10.1093/rheumatology/keg074. [DOI] [PubMed] [Google Scholar]
- 71.Hoshino T, Ohta A, Nakao M, Ota T, Inokuchi T, Matsueda S, Gouhara R, Yamada A, Itoh K, Oizumi K. TCR gamma delta + T cells in peripheral blood of patients with adult Still's disease. J Rheumatology. 1996;23:124–129. [PubMed] [Google Scholar]
- 72.Black APB, Bhayani H, Ryder CAJ, Pugh MT, Gardner-Medwin JMM, Southwood TR. An association between the acute phase response and patterns of antigen induced T cell proliferation in juvenile idiopathic arthritis. Arthritis research & therapy. 2003;5:R277–84. doi: 10.1186/ar791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chen DY, Hsieh TY, Hsieh CW, Lin FJ, Lan JL. Increased apoptosis of peripheral blood lymphocytes and its association with interleukin-18 in patients with active untreated adult-onset Still's disease. Arthritis and rheumatism. 2007;57:1530–8. doi: 10.1002/art.23088. [DOI] [PubMed] [Google Scholar]
- 74.Jelusic M, Lukic IK, Tambic-Bukovac L, Dubravcic K, Malcic I, Rudan I, Batinic D. Interleukin-18 as a mediator of systemic juvenile idiopathic arthritis. Clinical rheumatology. 2007;26:1332–4. doi: 10.1007/s10067-006-0474-0. [DOI] [PubMed] [Google Scholar]
- 75.Murray KJ, Luyrink L, Grom AA, Passo MH, Emery H, Witte D, Glass DN. Immunohistological characteristics of T cell infiltrates in different forms of childhood onset chronic arthritis. The Journal of rheumatology. 1996;23:2116–24. [PubMed] [Google Scholar]
- 76.Masters S, Simon A, Aksentijevich I, Kastner D. Horror autoinflammaticus: the molecular pathophysiology of autoinflammatory disease (*) Annual Review of Immunology. 2009;27:621–68. doi: 10.1146/annurev.immunol.25.022106.141627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tighe P, Powell R, Ghaemmaghami A, Ziegler-Heitbrock L, Radford P, Todd I. Elevated CD16 expression by monocytes from patients with tumor necrosis factor receptor-associated periodic syndrome. Arthritis and rheumatism. 2007;56:4182–8. doi: 10.1002/art.23133. [DOI] [PubMed] [Google Scholar]
- 78.Ravelli A, Martini A. Juvenile idiopathic arthritis. The Lancet. 2007;369:767–78. doi: 10.1016/S0140-6736(07)60363-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.