Abstract
Objective
To determine whether obesity related reproductive endocrine abnormalities in ovulatory women are reversible with weight loss
Design
Observational cohort study.
Setting
Healthy volunteers in an academic research environment.
Patients
Women age 18–48 with regular menstrual cycles 21–40 days with a BMI ≥ 35 kg/m2 planning to undergo bariatric surgery were recruited.
Intervention
25 eumenorrheic (non-PCOS) women with a mean BMI of 47.3 +/− 5.2 kg/m2 were sampled with daily menstrual cycle urinary hormones prior to (n=25) and 6 months after (n=9) weight loss surgery resulting in >25% reduction initial body weight. Daily hormones were compared pre- and post-operatively, and to 14 normal weight controls.
Main Outcome Measures
LH, FSH, estradiol and progesterone metabolites measured daily for one menstrual cycle. Group means were compared using t-tests among ovulatory cycles.
Results
Luteal Pdg increased from 32.8 ± 10.9 to 73.7 ± 30.5 ug/mgCr (p<0.001) and whole cycle LH increased from 168.8 ± 24.2 to 292.1 ± 79.6 mIU/mgCr (p<0.001) after surgically induced weight loss. Luteal Pdg remained lower than normal weight controls (151.7 ± 111.1 ug/mgCr). Obese women took longer to attain a postovulatory Pdg rise >2mcg/mg creatinine than controls (3.91 ± 1.51 versus 1.71 ± 1.59 days); this improved post-operatively (2.4 ± 1.82 days, p=0.046). Whole cycle E1c was similar to controls at baseline, but decreased after weight loss (from 1026.7 ± 194.2 to 605.4 ± 167.1 ng/mgCr, p<0.001). FSH did not relate to body size in this sample.
Conclusions
Women of very high BMI have deficient luteal LH and Pdg excretion and a delayed ovulatory Pdg rise compared to normal weight women. Although all of these parameters improved with weight loss, Pdg did not approach levels seen in normal weight women. LH may be less effective in stimulating the corpus luteum in obesity. The large postoperative decrease in E1c may reflect the loss of estrone-producing adipose tissue after weight loss.
Keywords: obesity, weight loss, luteal function, LH, FSH, E1c, Pdg, fertility
INTRODUCTION
The expanding obesity epidemic in the United States has negative repercussions for the reproductive health of both men and women(1–3). Overweight women experience more menstrual cycle abnormalities, anovulation, infertility, and fetal loss than their normal weight counterparts(4–5). Decreased levels of sex steroids and altered pulsatile gonadotropin secretion have also been observed in obese women(6–8). The reproductive deficits associated with obesity do not appear limited to the hypothalamic-pituitary axis. Obese women have higher in vitro fertilization cycle cancellation rates, despite administration of more exogenous gonadotropin(9). We recently reported longer and more irregular menstrual cycles, and decreased urinary LH, FSH, estrogen metabolite and luteal progesterone metabolite excretion among overweight women in a large cohort of ovulatory women aged 43–55(10). Moreover, there appeared to be a progressive relationship between increasing obesity and decreasing reproductive hormone excretion. This growing body of evidence for decreased corpus luteum function in obese women suggests that it plays a role in their relative infertility(6–8).
Weight loss in obese women improves menstrual cycle dysfunction, infertility, and pregnancy outcomes(3,10,11). Infertility is improved both through the resumption of spontaneous ovulation and in an improved response to ovulation stimulation during infertility treatment(12,13). However, not all studies demonstrate improvements in fertility outcomes with weight loss(14,15). The mechanisms for improved reproductive function with weight loss are not well understood and most prior studies have not distinguished between ovulatory and anovulatory obese women.
We hypothesized that obesity related reproductive endocrine abnormalities in ovulatory women are reversible with weight loss. Since there are currently no effective medical therapies for long-term weight loss for obesity and dietary restriction is ineffective(16), we addressed this issue by investigating a group of women planning to undergo weight loss surgery. We studied regularly-cycling morbidly obese women (baseline BMI>35 kg/m2) scheduled to undergo bariatric surgery before weight loss and after loss of at least 25% initial body weight and compared menstrual cycle urinary hormone excretion before and after weight loss. Results from normal weight controls were included to help interpret the changes in hormone excretion.
MATERIALS AND METHODS
Participants
Thirty-three participants were recruited through a weight loss surgery support group from the surgical practice of one of the authors (KG) at the Montefiore Medical Center in the Bronx, NY. Participants who were planning to undergo bariatric surgery in the near future were enrolled and had to meet the following criteria: age 18–48; regular menstrual cycles 21–40 days long; BMI≥35 kg/m2; presence of a uterus and at least one ovary; no evidence of renal, hepatic or systemic disease that might affect gonadotropin or sex steroid production or clearance; and no exogenous hormones for at least 3 months prior to study entry. Participants were screened with a fasting glucose, prolactin, TSH, reverse T3, and urine dipstick analysis. Patients with diabetes, alcoholism, or any screening laboratory test abnormality were excluded. Postoperative participants had to achieve loss of at least 25% initial body weight within 6–12 months after surgery to have their data included in the final analysis. Institutional Review Board approval was obtained for this study. There are no conflicts of interest to report.
Controls
A control group of normal weight, eumenorrheic women was used. Fourteen normal weight (BMI=21.5 ± 3.4 kg/m2) women aged 26–39 (mean 31.7 ± 4.6 years) who underwent the same daily urine collection methods and some of whose data have been previously reported were used as controls(17,18). These women were euthyroid, normoprolactinemic, free of systemic illness and had no history of excessive exercise (>4hrs/wk).
Protocol
First-morning voided urine was collected into polypropylene test tubes pre-filled with glycerol to give a final concentration of 7%. Details on specimen collection procedures have been published previously(19). Women were instructed to collect their first-morning voided urine daily with the onset of their menstrual period and to continue collection until the onset of their next menstrual period. The first collection was performed pre-operatively and the second collection was performed 6–12 months post-op, after weight stabilization and at least 25% initial body weight reduction. Serum was collected at baseline screening and at 6 months post-operatively for adipocytokine and lipoprotein analysis (not reported in this communication). Weight and cycle lengths were recorded in the pre-operative period as well as for 6–12 months post-operatively. Dietary habits were recorded for a 24 hour period pre- and post-operatively and reviewed to assess nutritional intake.
Hormone Assays
Urine was assayed for LH, FSH, estrone conjugates (E1c) and pregnanediol glucuronide (Pdg). LH and FSH were measured using a solid-phase, two-site fluoroimmunometric assay (DELFIA, PerkinElmer, Turku, Finland) and validated using previously described methods(17,18,20). For urinary LH, inter-assay coefficient of variation (CV) and intra-assay CVs were 13.7% and 5.0% respectively, and 16.4% and 7.6% for FSH respectively. E1c and Pdg were measured in duplicate by enzyme-linked immunosorbent assay using antibodies and conjugate tracers provided by Dr. Bill Lasley (University of California- Davis). Inter-assay CV for E1c was 10.1% and intra-assay CV was 8.4%. Corresponding CVs for Pdg were 15.0% and 14.0%, respectively. Hormone concentrations were adjusted for glycerol and normalized to creatinine(20).
Data analysis
Day of ovulation was determined using a previously published algorithm validated in prior studies(17). A significant rise in Pdg was considered evidence of luteal activity (ELA) which correlates with presumed ovulation. The algorithm used locates the 5 nadir days in the follicular phase using moving averages throughout the cycle(21). A 3-fold increase in Pdg concentrations above this nadir for at least 3 consecutive days was considered ELA. Determination of the presumed day of ovulation was based on the day of the LH and FSH peak, the day of or day after E1c peak in proximity to the Pdg rise defining ovulation. Whole cycle LH, FSH, E1c and luteal Pdg were estimated by computing sums of individual hormones. Prior to analysis, cycle length was normalized to 28 days and cycle days were aligned to the day of ovulation. LH and FSH for cycle days 1–7 were estimated by computing transverse sums. Mean peak LH was calculated. Comparisons between groups were performed using t-tests and p<0.05 was considered statistically significant.
RESULTS
Participant Disposition
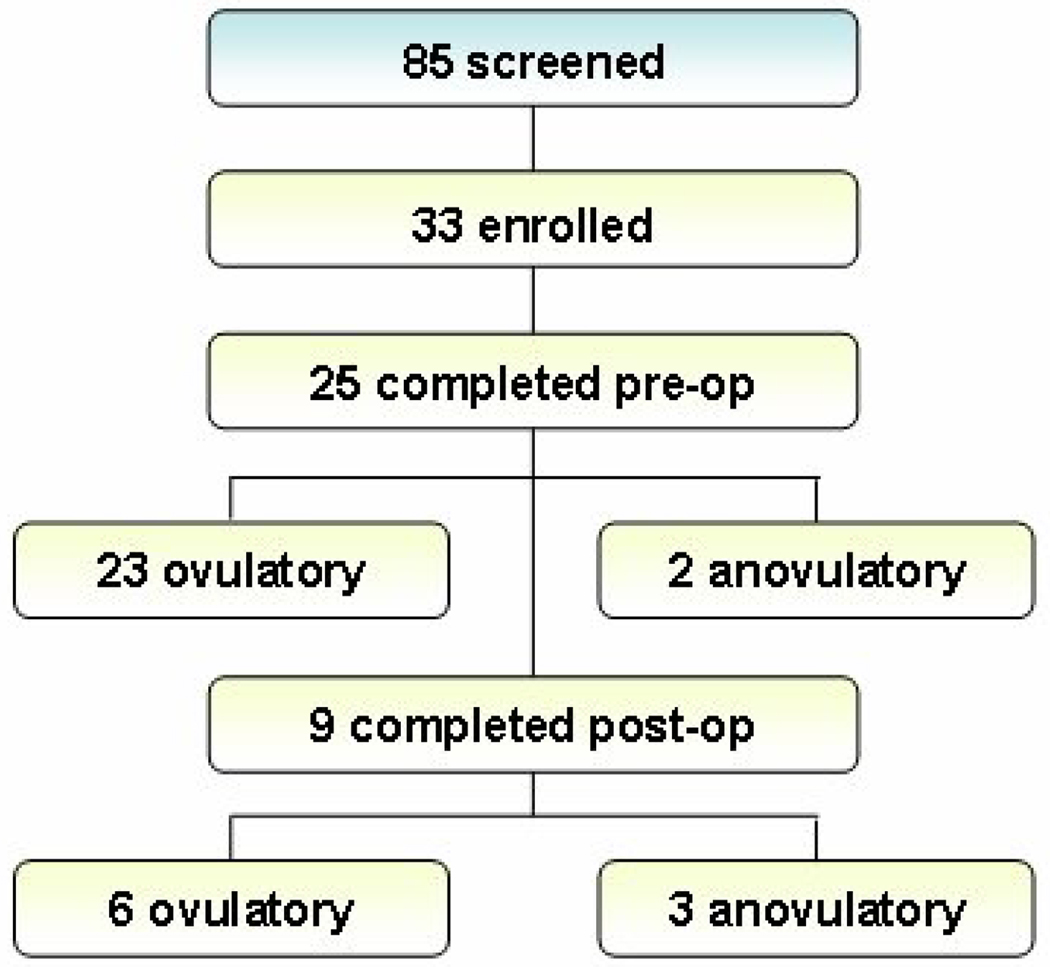
Thirty-three normally cycling, morbidly obese patients who met the inclusion and exclusion criteria were enrolled. Twenty-five patients completed a pre-weight loss surgery baseline hormone cycle collection and serum collection, but two demonstrated anovulatory cycles based on urine assay results and were excluded from pre-operative data analysis. Eight patients withdrew from the study before completing a pre-operative urine collection. At the time of data analysis nine patients had not yet undergone bariatric surgery. Six of these patients were denied insurance coverage for their intended surgery and three patients were awaiting scheduling at the time of last contact. After an informed discussion with the attending surgeon on the type of surgical procedure available, 6 patients elected to undergo a lap-banding procedure, and 10 patients underwent gastric bypass.
Of the sixteen patients who underwent bariatric surgery, fourteen were available for post-operative studies. Of these, three were lost to follow-up, one began treatment with oral-contraceptive pills, and one lost only 3.3% initial body weight at 6 months follow-up (from a lap-banding procedure). Of the nine patients who completed a second, post-operative menstrual cycle urinary hormone collection and serum collection, three had anovulatory cycles based on urine assay results and were excluded from post-operative data analysis. Anovulatory cycles did not display any consistent pattern and formal analyses were not performed. Two of these three had been anovulatory on pre-operative evaluation, despite having reported regular menstrual cycles. These three patients lost a mean of 21.6% initial body weight, and two of these patients had undergone a banding procedure. Of the six patients who had ovulatory post-operative cycles, only one had a banding procedure. See Figure 1 for participant attrition. Weight loss was stable during post-op collections (mean weight loss was 1.3 pounds during collection).
Figure 1.
BMI Changes After Weight Loss
Participant and control group characteristics are shown in Table 1. By design, BMI was greater in the pre-operative group compared to both post-operative and control groups. Participants lost an average of 27.7% (range 25–37% at 6 months post-op) of initial body weight within the first six months post-operatively but did not attain a body weight comparable to the control group (p<0.01).
Table 1.
Age and BMI of study sample
| Normal Weight | High BMI Pre-OP | High BMI Post-Op | |
|---|---|---|---|
| Age (y) | 31.7 +/−4.6 | 36.6 +/−4.7* | 38.0 +/− 8.0 |
| BMI (kg/m2) | 21.5 +/−3.4 | 47.3 +/−5.2** | 32.0 +/−2.9 |
p=0.03 pre-op vs. control;
p<0.01 pre-op vs. control and pre-op vs. post-op
p<0.05 considered significant
The participant group was significantly older than the control group (p=0.03) TSH, prolactin, and rT3 were not significantly different between groups (data not shown).
Menstrual Cycle and Hormone Outcomes
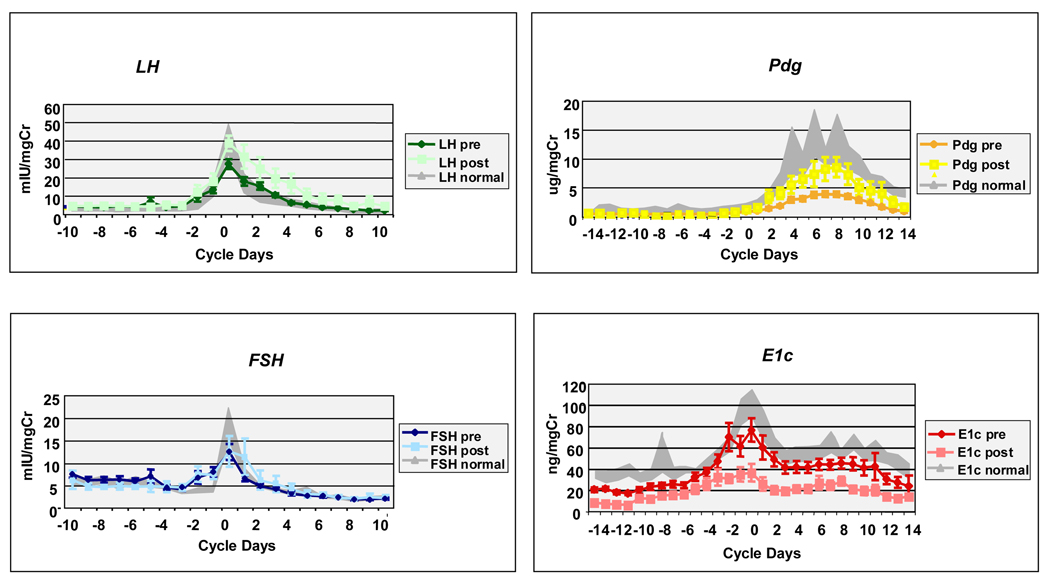
Table 2 shows the menstrual cycle parameters pre- and post-operatively. Mean cycle length did not significantly differ between groups. Whole cycle LH significantly (p<0.001) increased in the post-operative group as did peak LH (p=0.007). FSH did not differ between groups. Gonadotropin levels for the first seven cycle days did not change post-operatively. Luteal Pdg excretion was significantly increased in the post-operative group (p<0.001) but did not approach the levels of the normal weight control group. The lag in the number of days after ovulation until Pdg rose to a level >2 ug/mgCr improved significantly after post-operative weight loss (p=0.046) and no longer differed from the control group. The slope of the rise was greater in the post-operative group. Estrone conjugate excretion was similar in the control and pre-operative group, but significantly decreased in the post-operative group (p<0.001). A paired analysis of the 6 women who completed the study similarly showed a significant increase in whole cycle (p=0.009) and peak LH (p=0.04), a decrease in whole cycle E1c (p=0.01) and an increase in luteal Pdg (p=0.006) (data not shown). Figure 2 demonstrates whole-cycle urine menstrual hormone excretion in the control, pre-operative and post-operative groups.
Table 2.
Menstrual Cycle Hormone Parameters Pre and Post Weight Loss Surgery
| Controls (n=14) |
High BMI Pre-Op (n=23) |
High BMI Post-Op (n=6) |
P Value control vs. pre-op |
P Value pre-op vs. post-op |
|
|---|---|---|---|---|---|
| Cycle Length (d) |
27.6 +/−3.4 | 29.5 +/−4.0 | 27.2+/−2.8 | 0.15 | 0.20 |
| Peak LH (mIU/mg Cr) |
40.8+/−29.4 | 27.6+/−12.4 | 43.7+/−10.6 | 0.06 | 0.007* |
| Whole cycle LH (mIU/mg Cr) |
166.6+/− 146.0 |
168.8+/− 102.7 |
292.1+/− 112.6 |
0.96 | <0.001* |
| Whole cycle FSH (mIU/mg Cr) |
141.3+/− 63.1 |
149.4+/−57.9 | 178.4+/− 118.9 |
0.69 | 0.12 |
| 1st 7 days LH (mIU/mg Cr) |
26.4+/−23.6 | 22.0+/−23.0 | 36.2+/−16.7 | 0.58 | 0.17 |
| 1st 7 days FSH (mIU/mg Cr) |
44.5+/−16.6 | 47.4+/−19.7 | 42.0+/−17.4 | 0.65 | 0.55 |
| Whole cycle E1c (ng/mg Cr) |
1278.2+/− 475.0 |
1026+/− 336.3 |
605.4+/− 438.3 |
0.07 | <0.001* |
| Luteal Pdg (mcg/mg Cr) |
151.7+/− 111.1 |
32.8+/−10.9 | 73.7+/−30.5 | <0.001* | <0.001* |
| Days until Pdg >2 |
1.7+/−1.6 | 3.9+/−1.5 | 2.4+/−1.8 | <0.001* | 0.046* |
p<0.05 considered significant
Figure 2.
Pre (n=23; diamonds) versus post (n=6; squares) operative LH, FSH, Pdg and E1c concentrations in daily urine samples compared to 14 normal weight controls +/−SEM (shaded background). Error bars indicate standard error of the mean in pre and post-operative groups.
DISCUSSION
We demonstrate in this study that the large deficit in luteal Pdg excretion seen in morbidly obese, ovulatory women significantly improves after surgically-induced weight loss but does not completely recover to levels seen in normal weight women. We also found a slowing of the ovulatory Pdg rise which improved post-operatively, but again did not match that of the controls. Urinary LH increased post-operatively to levels consistent with those seen in normal weight women and no between group differences were seen in urinary FSH levels. There was a large decrease in E1c after weight loss to levels well below those observed in normal weight women.
We posited in an earlier study that the reduced luteal Pdg in large BMI women was due to impaired central LH drive as demonstrated by decreased LH pulse amplitude in these women8. We presented data showing that high BMI women secreted LH pulses with significantly smaller amplitudes, but similar pulse frequency to normal weight women. These findings are similar to the inverse relationship in LH pulse amplitude described in PCOS women with increasing body size(22). It is possible that alterations in adipocytokine levels and actions in obesity are responsible for a dampened pituitary response to endogenous GnRH. The pituitary contains both insulin and leptin receptors(23,24) and other adipokines such as tumor necrosis-alpha and IL-1 beta, have been shown to alter pituitary production of LH(25).
Herein we demonstrate that this deficiency in luteal Pdg significantly improves with weight loss. We were unable to gather sufficient data on minute-to-minute post-operative changes in pulsatile LH secretion to demonstrate improvements in LH pulse amplitude postoperatively. This limitation was due to the large proportion of our initial sample that was unable to follow through on surgery. However the large increase in luteal Pdg found after significant weight loss makes this an important area to pursue. While luteal Pdg levels improved post-operatively, there was still a deficit compared to normal weight controls. The study patients had not attained ideal body weight at the time of data analysis. It is possible that further weight loss could result in a normalization of LH pulse amplitude and luteal Pdg.
The lack of normalization of luteal progesterone, in the face of improved daily LH profiles after substantial weight loss, implies that there may be a defect at the level of the corpus luteum. Adipocytokines have been shown to negatively affect corpus luteum function(26,27) and thus the altered hormonal milieu of obesity may directly affect the production of progesterone.
We found that urinary LH levels significantly increased after surgical weight loss. This may reflect a restoration of LH pulse amplitude and further investigation of our subjects is needed to confirm this.
It is also possible that the increase in urinary LH we observed is related to loss of feedback inhibition of estrogen on pulsatile LH release. Such an effect has been observed in a prior study of 15 women with PCOS who lost 10% of their body weight on a very low calorie diet(28). In this study, reductions in estradiol predicted an increase in LH and the initiation of ovulatory cycling in the PCOS women studied. The significantly lower estrogen excretion we observed post-operatively may have led to increased pituitary LH production via a similar mechanism.
Weight loss in anovulatory obese women can restore ovulatory cycles(29). Modest weight loss of only ~10% has been shown to normalize menstrual cycles, improve hormonal profiles, and improve pregnancy rates in one study(30). However reproductive parameters are not completely normalized after weight loss. A recent study looking at the effect of very low-calorie diets before and during IVF in obese women found that despite significant short-term weight loss, there was not a clear benefit in IVF outcomes(15).
Due to the nature of this study there were several limitations. We enrolled the intended number of subjects into the pre-surgical group, however only two-thirds actually underwent weight loss surgery, and only one-third of enrolled subjects completed a post-operative urine collection. While the longitudinal nature of the study resulted in a small number of data points for post-operative analysis, we were still able to gather sufficient post-operative data to determine significant between group differences. Additionally not all subjects had the same surgical procedure performed and lap-banding tends to result in less weight loss than gastric bypass(31). The underlying physiological mechanisms mediating weight loss may differ based on the type of the surgery. An example of this is fasting ghrelin secretion, which is reduced in obese and post-bariatric surgery patients compared to normal weight individuals, but which is about half again reduced in patients undergoing Roux en Y procedures compared to laparoscopic banding techniques(32). While we had intended for the patients to serve as their own controls post-operatively, only 36% of the enrolled subjects completed the study and while they lost a substantial amount of weight they did not attain an ideal weight at the time of post-operative data analysis. Additional follow-up of our study subjects would potentially yield further data of interest as weight-loss surgery patients have been shown to have maximal weight loss at 12 months post-operatively(33). Nonetheless, the likelihood that this model will result in attainment of ideal body weight is remote. There are currently no known experimental models of weight loss that have a high probability of taking a woman from a morbidly obese state to an ideal body weight. The large loss to follow up also caused us to seek out a historical normal weight controls to clarify the direction of the hormone changes associated with obesity. The available controls were significantly younger than the obese women, and this age difference may have led to a lack of ability to determine that LH and FSH were relatively suppressed in the pre-op obese women, as we and others have previously shown (7,8).
In summary, we found that the deficit in luteal Pdg in obese, ovulatory women significantly improves with weight loss, but does not return to the levels seen in normal weight ovulatory women. These findings may be due to central factors causing dampened LH pulses and possible peripheral factors related to obesity which negatively impact corpus luteum function and the action of LH on the corpus luteum. Maintaining a near-ideal weight range while attempting pregnancy appears to be important for attainment of normal reproductive endocrine cycle parameters and may be important for optimal fecundity.
Acknowledgments
Supported by DK069349 and HD041978 (to NS), RR012248 (GCRC) and NOVO Nordisk.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Poster presented at Endo 2007, The Endocrine Society’s Annual Meeting June 2-June 5 2007 Toronto, Canada.
REFERENCES
- 1.Sallmen M, Sandler DP, Hoppin JA, Blair A, Baird DD. Reduced fertility among overweight and obese men. Epidemiology(Cambridge, Mass. 2006;17:520–523. doi: 10.1097/01.ede.0000229953.76862.e5. [DOI] [PubMed] [Google Scholar]
- 2.Davies MJ. Evidence for effects of weight on reproduction in women. Reproductive biomedicine online. 2006;12(5):552–561. doi: 10.1016/s1472-6483(10)61180-7. [DOI] [PubMed] [Google Scholar]
- 3.Pasquali R. Obesity, fat distribution and infertility. Maturitas. 2006;54:363–371. doi: 10.1016/j.maturitas.2006.04.018. [DOI] [PubMed] [Google Scholar]
- 4.Rowland AS, Baird DD, Long S, Wegienka G, Harlow SD, Alavanja M, et al. Influence of medical conditions and lifestyle factors on the menstrual cycle. Epidemiology. 2002;13:668–674. doi: 10.1097/00001648-200211000-00011. [DOI] [PubMed] [Google Scholar]
- 5.Grodstein F, Goldman MB, Cramer DW. Body mass index and ovulatory infertility. Epidemiology. 1994;5:247–250. doi: 10.1097/00001648-199403000-00016. [DOI] [PubMed] [Google Scholar]
- 6.Sherman BM, Korenman SG. Measurement of plasma LH, FSH, estradiol and progesterone in disorders of the human menstrual cycle: the short luteal phase. The Journal of clinical endocrinology and metabolism. 1974;38:89–93. doi: 10.1210/jcem-38-1-89. [DOI] [PubMed] [Google Scholar]
- 7.Grenman S, Ronnemaa T, Irjala K, Kaihola HL, Gronroos M. Sex steroid, gonadotropin, cortisol, and prolactin levels in healthy, massively obese women: correlation with abdominal fat cell size and effect of weight reduction. The Journal of clinical endocrinology and metabolism. 1986;63:1257–1261. doi: 10.1210/jcem-63-6-1257. [DOI] [PubMed] [Google Scholar]
- 8.Jain A, Polotsky AJ, Rochester D, Berga S, Loucks T, Zeitlan G, et al. Pulsatile luteinizing hormone amplitude and progesterone metabolite excretion are reduced in obese women. The Journal of clinical endocrinology and metabolism. 2007;92:2468–2473. doi: 10.1210/jc.2006-2274. [DOI] [PubMed] [Google Scholar]
- 9.Dokras A, Baredziak L, Blaine J, Syrop C, VanVoorhis BJ, Sparks A. Obstetric outcomes after in vitro fertilization in obese and morbidly obese women. Obstetrics and gynecology. 2006;108:61–69. doi: 10.1097/01.AOG.0000219768.08249.b6. [DOI] [PubMed] [Google Scholar]
- 10.Santoro N, Lasley B, McConnell D, Allsworth J, Crawford S, Gold E, et al. Body size and ethnicity are associated with menstrual cycle alterations in women in the early menopausal transition: The Study of Women's Health across the Nation (SWAN) Daily Hormone Study. The Journal of clinical endocrinology and metabolism. 2004;89:2622–2631. doi: 10.1210/jc.2003-031578. [DOI] [PubMed] [Google Scholar]
- 11.Dixon JB, O'Brien PE. Changes in comorbidities and improvements in quality of life after LAP-BAND placement. American journal of surgery. 2002;184:51S–54S. doi: 10.1016/s0002-9610(02)01181-9. [DOI] [PubMed] [Google Scholar]
- 12.Norman RJ, Noakes M, Wu R, Davies MJ, Moran L, Wang JX. Improving reproductive performance in overweight/obese women with effective weight management. Human reproduction update. 2004;10:267–280. doi: 10.1093/humupd/dmh018. [DOI] [PubMed] [Google Scholar]
- 13.Clark AM, Ledger W, Galletly C, Tomlinson L, Blaney F, Wang X, et al. Weight loss results in significant improvement in pregnancy and ovulation rates in anovulatory obese women. Human reproduction (Oxford, England) 1995;10:2705–2712. doi: 10.1093/oxfordjournals.humrep.a135772. [DOI] [PubMed] [Google Scholar]
- 14.Moran LJ, Noakes M, Clifton PM, Tomlinson L, Galletly C, Norman RJ. Dietary composition in restoring reproductive and metabolic physiology in overweight women with polycystic ovary syndrome. The Journal of clinical endocrinology and metabolism. 2003;88:812–819. doi: 10.1210/jc.2002-020815. [DOI] [PubMed] [Google Scholar]
- 15.Tsagareli V, Noakes M, Norman RJ. Effect of a very-low-calorie diet on in vitro fertilization outcomes. Fertility and sterility. 2006;86:227–229. doi: 10.1016/j.fertnstert.2005.12.041. [DOI] [PubMed] [Google Scholar]
- 16.Scheen AJ. Results of obesity treatment. Annales d'endocrinologie. 2002;63:163–170. [PubMed] [Google Scholar]
- 17.Santoro N, Brown JR, Adel T, Skurnick JH. Characterization of reproductive hormonal dynamics in the perimenopause. The Journal of clinical endocrinology and metabolism. 1996;81:1495–501. doi: 10.1210/jcem.81.4.8636357. [DOI] [PubMed] [Google Scholar]
- 18.Brown J, Skurnick J, Sharma N, Santoro N. Frequent intermittent ovarian function in women with premature menopause: a longitudinal study. Endocrine J. 1993;1:467–474. [Google Scholar]
- 19.Santoro N, Crawford SL, Allsworth JE, Gold E, Greendale G, Korenman S, et al. Assessing menstrual cycles with urinary hormone assays. American journal of physiology. 2003;284:E521–E530. doi: 10.1152/ajpendo.00381.2002. [DOI] [PubMed] [Google Scholar]
- 20.Taussky H. A microcolorimetric determination of creatinine in urine using the Jaffe reaction. J Biol Chem. 1954;208:835–861. [PubMed] [Google Scholar]
- 21.Kassam A, Overstreet JW, Snow-Harter C, De Souza MJ, Gold EB, Lasley BL. Identification of anovulation and transient luteal function using a urinary pregnanediol-3-glucuronide ratio algorithm. Environmental health perspectives. 1996;104:408–413. doi: 10.1289/ehp.96104408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taylor AE, McCourt B, Martin KA, Anderson E, Adams J, Schoenfeld D, et al. Determinants of abnormal gonadotropin secretion in clinically defined women with polycystic ovary syndrome. The Journal of clinical endocrinology and metabolism. 1997;82:2248–2256. doi: 10.1210/jcem.82.7.4105. [DOI] [PubMed] [Google Scholar]
- 23.Pagan YL, Srouji SS, Jimenez Y, Emerson A, Gill S, Hall JE. Inverse relationship between luteinizing hormone and body mass index in polycystic ovarian syndrome: investigation of hypothalamic and pituitary contributions. The Journal of clinical endocrinology and metabolism. 2006;91:1309–1316. doi: 10.1210/jc.2005-2099. [DOI] [PubMed] [Google Scholar]
- 24.Moschos S, Chan JL, Mantzoros CS. Leptin and reproduction: a review. Fertility and sterility. 2002;77:433–444. doi: 10.1016/s0015-0282(01)03010-2. [DOI] [PubMed] [Google Scholar]
- 25.Rivier C, Vale W. Cytokines act within the brain to inhibit luteinizing hormone secretion and ovulation in the rat. Endocrinology. 1990;127:849–856. doi: 10.1210/endo-127-2-849. [DOI] [PubMed] [Google Scholar]
- 26.Machelon V, Gougeon A, Duquenne C, Testart J, Wallon C, Delattre RM, et al. Ovarian production of IL6 and its potential inhibitory effect on progesterone secretion in Cynomolgus fascicularis. Comptes rendus de l'Academie des sciences. 1995;318:1111–1118. [PubMed] [Google Scholar]
- 27.Brannian JD, Zhao Y, McElroy M. Leptin inhibits gonadotrophin-stimulated granulosa cell progesterone production by antagonizing insulin action. Human reproduction (Oxford, England) 1999;14:1445–1448. doi: 10.1093/humrep/14.6.1445. [DOI] [PubMed] [Google Scholar]
- 28.van Dam EW, Roelfsema F, Veldhuis JD, Hogendoorn S, Westernberg J, Helmerhorst F, et al. Retention of estradiol negative feedback relationship to LH predicts ovulation in response to caloric restriction and weight loss in obese patients with polycystic ovary syndrome. American journal of physiology. 2004;286:E615–E620. doi: 10.1152/ajpendo.00377.2003. [DOI] [PubMed] [Google Scholar]
- 29.Mitchell GW, Jr, Rogers J. The influence of weight reduction on amenorrhea in obese women. The New England journal of medicine. 1953;249:835–837. doi: 10.1056/NEJM195311192492102. [DOI] [PubMed] [Google Scholar]
- 30.Clark AM, Thornley B, Tomlinson L, Galletley C, Norman RJ. Weight loss in obese infertile women results in improvement in reproductive outcome for all forms of fertility treatment. Human reproduction (Oxford, England) 1998;13:1502–1505. doi: 10.1093/humrep/13.6.1502. [DOI] [PubMed] [Google Scholar]
- 31.Buchwald H, Avidor Y, Braunwald E, Jensen M, Pories W, Fahrbach K, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292:1724–1737. doi: 10.1001/jama.292.14.1724. [DOI] [PubMed] [Google Scholar]
- 32.Leonetti F, Silecchia G, Iacobellis G, Ribaudo M, Zappaterreno A, Tiberti C, et al. Different plasma ghrelin levels after laparoscopic gastric bypass and adjustable gastric banding in morbid obese subjects. The Journal of clinical endocrinology and metabolism. 2003;88:4227–4231. doi: 10.1210/jc.2003-030133. [DOI] [PubMed] [Google Scholar]
- 33.Sjostrom L, Lindroos AK, Peltonen M, Torgerson J, Bouchard C, Carlsson B, et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. The New England journal of medicine. 2004;351:2683–2693. doi: 10.1056/NEJMoa035622. [DOI] [PubMed] [Google Scholar]