Abstract
Although older age is the greatest risk factor for OA, OA is not an inevitable consequence of growing old. Radiographic changes of OA, particularly osteophytes, are common in the aged population but symptoms of joint pain may be independent of radiographic severity in many older adults. Aging changes in the musculoskeletal system increase the propensity to OA but the joints affected and the severity of disease are most closely related to other OA risk factors such as joint injury, obesity, genetics, and anatomical factors that affect joint mechanics. The aging changes in joint tissues that contribute to the development of OA include cell senescence that results in development of the senescent secretory phenotype and aging changes in the matrix, including formation of advanced glycation end-products that affect the mechanical properties of joint tissues. An improved mechanistic understanding of joint aging will likely reveal new therapeutic targets to slow or halt disease progression. The ability to slow progression of OA in older adults will have enormous public health implications given the aging of our population and the increase in other OA risk factors such as obesity.
Keywords: aging, osteoarthritis, elderly, cell senescence, oxidative stress, articular cartilage, menisci
Introduction
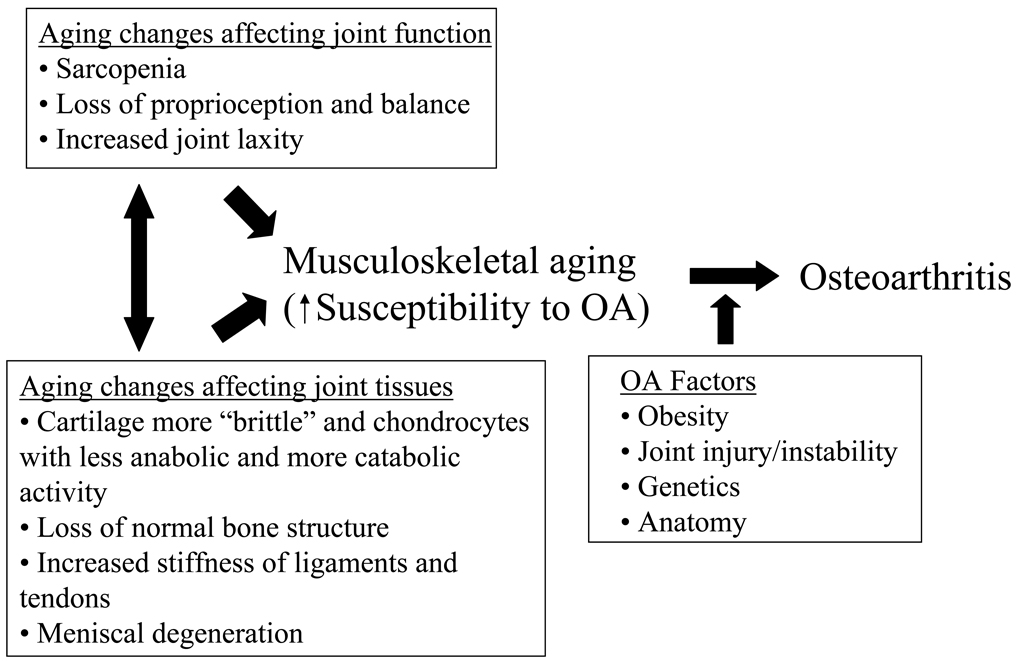
Osteoarthritis (OA) is a classic age-related disorder. It is often described as a chronic degenerative disease and thought by many to be an inevitable consequence of growing old. In OA, degradation and loss of the articular cartilage is a central feature that is sometimes attributed to "wear and tear". However, unlike an automobile tire that wears thin over time, the tissues affected by OA contain living cells that respond to mechanical stimulation and function to maintain joint homeostasis. Rather than OA being a simple consequence of joint aging and repeated "wear and tear", the current conceptual framework for the relationship between aging and OA is that aging of the musculoskeletal system increases the susceptibility to OA but alone does not cause it. Changes outside the joint (including sarcopenia and reduced proprioception) and within the joint (including cell and matrix changes in joint tissues) contribute to the development of OA, when other OA risk factors are also present (Figure 1). The concept that aging contributes to, but does not directly cause OA, is consistent with the multifactorial nature of OA and the knowledge that not all older adults develop OA and not all joints in the body are affected to the same degree. In this review, we will discuss the relationship between aging and the development of OA from both an epidemiological perspective and from a biological perspective with the goal of answering the question of why OA is an age-related disease.
Figure 1. Relationship between musculoskeletal aging and the development of osteoarthritis.
Changes that affect joint structure and function with aging increase the susceptibility to developing osteoarthritis but additional factors (OA factors) are usually also present which lead to the development of symptomatic OA. Reproduced with permission from Hazzard's Geriatric Medicine and Gerontology, Sixth Edition, McGraw Hill Medical, 2009.
Epidemiology of OA Relevant to Aging
OA is the most common joint disorder in the world and one of the most common sources of pain and disability in the elderly [1, 2]. While there remains considerable heterogeneity in defining OA among epidemiological studies, the evidence is conclusive that age remains the single greatest risk factor for the development of OA in susceptible joints. Radiographic changes, in particular osteophytosis, are very common in the aging population and when used alone may provide an overestimation of the true prevalence of symptomatic OA. Defining OA solely as joint pain occurring in an older adult without evidence for another form of arthritis is also inaccurate as there are many causes of nonarticular pain, such as bursitis, that are common in older adults. In a study of 480 adults over the age of 65 years who reported chronic knee pain, only about 50% had radiographic evidence of knee OA[3]. A recent systematic review [4] comparing the prevalence of knee pain and radiographic knee OA found considerable discordance between the two, adding further evidence that joint pain and severity of radiographic changes of OA do not correlate. However, Duncan et al have shown through their work with the Knee Clinical Assessment Study cohort that as the severity and persistence of knee pain increases, the degree of discordance between symptoms and radiography diminishes [5, 6].
Due to the discrepancies between pain and radiographic evidence of OA, most current epidemiological studies define OA by a combination of clinical and radiographic criteria. The most often used system for defining symptomatic OA is the American College of Rheumatology (ACR) OA criteria. The ACR OA criteria were developed to standardize the definition of hip, knee, and hand OA and are comprised of joint symptoms, exclusion of inflammatory conditions, and positive radiography [7–9]. The Kellgren-Lawrence (K-L) system for radiographic grading of OA has been the standard for several decades and is based upon the presence and severity of certain defined radiographic features including osteophytosis, joint space narrowing, joint line sclerosis, and subchondral cysts [10]. These radiographic features are used to the grade the severity of OA from O (normal joint) to 4 (complete joint space loss).
Joint Specific Prevalence and Incidence of OA in the Elderly
Knee
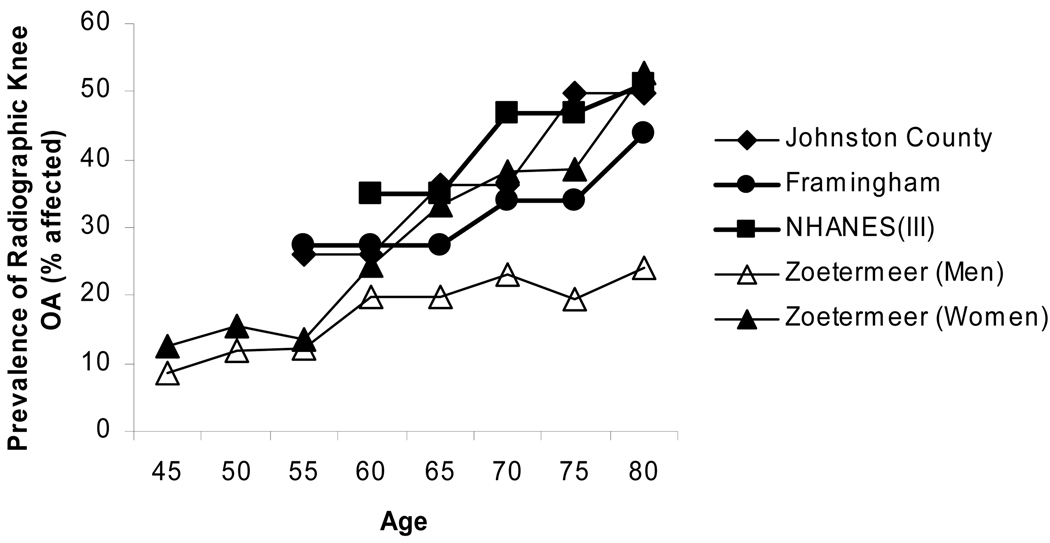
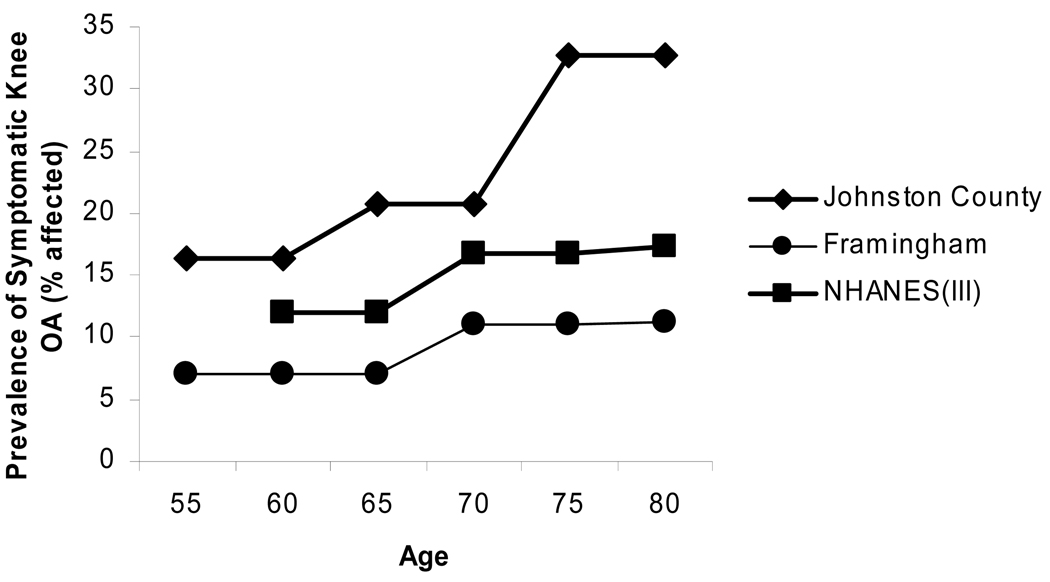
The knee is commonly affected by OA and is thought to account for the majority of disability from OA. The Framingham Osteoarthritis Study evaluated the prevalence of knee osteoarthritis in 1420 subjects aged 60 and higher [11]. OA was defined as the presence of knee symptoms in a patient with ipsilateral (KL) grade 2 or greater radiographic changes. The prevalence of radiographic OA increased with each decade of life from 33% among those aged 60–70 to 43.7% among those over 80 years of age (Figure 2). The prevalence of symptomatic knee OA in all subjects was 9.5% and increased with age in women but not men (Figure 3). The Johnson Country Osteoarthritis Project is a population-based cohort of knee and hip OA based in a rural county in North Carolina [12]. Over 3,000 study participants were involved with almost one-third being African-Americans. Radiographic knee OA (RKOA) was considered a KL score of 2 or greater and symptomatic OA was defined as knee symptoms in at least one knee with corresponding radiographic OA. The prevalence of RKOA rose from 26.2% in the 55–64 year range to nearly half of participants in the 75+ group. The prevalence of symptomatic knee OA likewise increased from 16.3% to 32.8% between these groups [12].
Figure 2. Relationship between age in years and the prevalence of radiographic knee OA.
Data was extracted from the following studies: Johnston County[12], Framingham[11], NHANES (III)[13], and Zoetermeer[14].
Figure 3. Relationship between age in years and the prevalence of symptomatic knee OA.
Data was extracted from the following studies: Johnston County[12], Framingham[11], and NHANES(III)[13].
The National Health and Nutrition Examination Survey (NHANES) III reported the prevalence of RKOA in 2415 persons and symptomatic knee OA in 2394 persons over the age of 60 [13]. Only single anterior-posterior (AP) non weight-bearing images were obtained therefore RKOA was defined as osteophytosis and sclerosis. The prevalence of K-L grade II or greater RKOA in at least one knee was 37.4%. The prevalence of symptomatic RKOA was 12.1% [13]. The Zoetermeer survey, a cohort of over 6500 participants, evaluated the prevalence of knee OA from a suburban area near The Hague [14]. All participants over the age of 45 received standing AP knee films. The prevalence of K-L 2+ knee OA (average of both knees) increased sequentially through each age group [14]. The increase in prevalence was more prominent in women. No symptom survey was included. Prevalence figures from both The Netherlands and the US appear to be higher than that reported from Greece [15] but lower than what was reported from the recent Japanese Research on Osteoarthritis Against Disability (ROAD) study [16].
The largest study to date on incident knee OA comes from the Fallon Community Health Plan which is a heath maintenance organization in the United States which provides services to some 130,000 members. Using the organization’s database, the authors in this study were able to report a yearly incidence of symptomatic knee OA of >1% and >0.8% in women and men respectively over the age of 70 [17]. The Framingham Study detected a comparable yearly incidence of symptomatic knee OA for both women and men [18].
Hip
Hip OA appears to be somewhat less common in the aging population than knee OA but is still quite prevalent. A recent systematic review of the prevalence of primary hip OA detected a clear trend toward increasing prevalence with age [19]. The prevalence of primary radiographic hip OA increased from 0.7% in the 40–44 age group to 14% in the 85+ age group [19]. Analysis of symptomatic hip OA from the Johnston County group published just after the systematic review by Dagenais et al, reported a higher prevalence of symptomatic hip OA in their population of 5.9% in the 45–54 age group increasing to 17% in the 75+ age group [20]. Symptomatic hip OA appeared to be more common in African-Americans and women. The prevalence and incidence of hip OA in women over age 65 has now been well defined with the recent analysis from the Study of Osteoporotic Fractures cohort [21]. Supine AP radiographs of the pelvis were obtained in 5839 women at baseline and (on average) at 8 years of follow up. Eleven different definitions of hip OA were reported and the prevalence and incidence varied accordingly. Excluding minimum joint space of less than 2.5mm as a definition, the prevalence ranged from 1.8 to 9.4% and the incidence from 3.6 to 8.9% [21].
Hand
The hand is the appendicular joint most commonly affected by OA in the aging population and although it often not as disabling as OA of the knee or hip, it can interfere with hand function. Estimates from the Zoetermeer survey found that radiographic involvement of the distal interphalangeal joint (DIP) affected more than half of men over the age of 65 and more than half of women over the age of 55 [14]. Thirteen percent of men and 26% of women over the age of 70 were found to have symptomatic hand OA involving at least one joint in the Framingham study [22]. Yearly incidence rates from the Fallon Community Health Plan for hand OA were 0.35% and 0.21% for men and women over the age of 60 respectively [17]. The incidence rates for those younger than 60 were dramatically lower.
A less common form of hand OA that is found mainly in the older adult population is erosive OA. Erosive OA is characterized by central erosions in distal and/or proximal interphalangeal joints accompanied by other typical changes of OA such as joint space loss, osteophytosis, and subchondral sclerosis [23]. It is thought that calcium crystals, such as hydroxyapatite and calcium pyrophosphate, may play a role in erosive OA. Erosive OA is considered to be much more inflammatory than non-erosive OA, a finding confirmed by a recent ultrasound study [24].
Risk Factors for Development of OA in the Elderly
The common risk factors for OA such as obesity, joint injury, genetics, and anatomical abnormalities are important in the elderly just as they are in younger adult populations. There is some evidence to suggest that after an acute joint injury, such as an anterior cruciate ligament tear, that older adults will develop OA faster than younger adults [25]. Some contributing factors to the development of OA, including degenerative changes in the meniscus and joint ligaments, increased bone turnover, as well as calcification of joint tissues appear to be more common in older adult populations. These contributing factors will be discussed further.
Meniscal damage is increasingly being appreciated as a major risk factor for the development of OA. Results from the Multicenter Osteoarthritis Study (MOST) study showed an odds ratio of 7.4 for the development of RKOA, after 30 months, in symptomatic subjects with significant meniscal damage [26]. Englund et al also recently reported that incidental meniscal damage on MRI is quite common in the elderly [27]. In subjects ranging from 50 to 90 years of age, the prevalence of meniscal tears was lowest (19%) in women aged 50–59 and was highest (56%) in men in the 70–90 age group. The prevalence increased to 63% in symptomatic subjects with KL >2 RKOA [27]. These studies suggest that age-related changes in the meniscus may contribute to meniscal degeneration that in turn may contribute to the development and progression of knee OA.
Anterior cruciate ligament (ACL) injury is also known to be a risk factor for the development of knee OA and is a common cause of post-traumatic OA developing in young adults as a result of sports injuries. The prevalence of acute ACL injury in the elderly is not well known and although the incidence is reported to be low, the latter likely represents a bias towards reporting athletic injuries [28]. A recent MRI study in people without a known history of an acute injury found that ACL disruption associated with OA may be more common than appreciated [29]. The changes which occur in aging ligaments are not entirely known, though increasing stiffness from collagen crosslinking combined with decreasing fibril diameter may increase the risk for ACL tears [30]. Studies are needed to determine if aging changes in joint ligaments are important contributors to the development of OA in older adults.
Bone is clearly involved in the development of OA, although the mechanism by which changes in bone influence the development or progression of OA is not clear [31, 32]. An increase in either bone turnover or regional bone remodeling may be a factor in OA progression and these processes are potentially affected by aging. Bone marrow lesions detected by MRI may represent areas of localized abnormal bone remodeling [33]. These lesions are associated with knee pain, limb mal-alignment, and meniscal derangement, and may predict OA progression [34–36]. Recently, increasing age has been shown to be a risk factor for the development of bone marrow lesions in asymptomatic individuals [37]. This is another area where future research may help elucidate how aging changes in a tissue outside of cartilage contributes to the risk of OA progression in older adults.
Calcification and crystal formation within joint tissues, most notably the cartilage and menisci, are known to increase with age. Calcium pyrophosphate’s association with the presence of radiographic osteoarthritis has been well established [38, 39]. Although it is known that the prevalence of chondrocalcinosis increases with age as does OA, the role of calcium crystals in the progression of OA is less clear [39, 40]. Some investigators believe that they are common but separate age-related conditions and others believe that the two are closely connected [39–41]. Since OA and calcium pyrophosphate are equally associated with osteophyte formation, it has been suggested that mechanical stress may induce release of chemokines which encourage both proliferative bone changes and calcium pyrophosphate formation [42, 43]. Further work is ongoing to determine additional mechanisms by which calcium crystals contribute to the development and potentially progression of OA. In the next section we will discuss how aging at the biological level may contribute to the development of OA.
Cell and Tissue Aging and the Development of OA
Most of the conditions associated with aging, OA included, result from an age-related loss in the ability of cells and tissues in the body to maintain homeostasis, particularly when put under stress [44]. In OA, excessive or abnormal mechanical stresses clearly play a key role in the development of the disease [45]. Under conditions where an anatomically normal joint is stressed, the joint tissues appear to be capable of adapting to stress without resulting in OA. As an example of successful adaptation, the chronic repetitive loads endured by long distance runners do not appear to result in OA later in life [46, 47]. But joint stress resulting from abnormal load distribution or abnormal joint anatomy clearly contributes to OA. In the knee, a varus alignment is strongly associated with the development of medial compartment disease while valgus alignment predisposes to lateral compartment disease [48]. Malalignment appears to be particularly important in individuals who are also overweight or obese [49].
Because OA is rare in young adults and even serious joint injuries usually don't manifest as OA until years later [50], it appears that young joint tissues can compensate, to some degree, to abnormal mechanical stress. But with aging the ability to compensate to stress declines. As noted above, older adults who experience a joint injury develop OA much more rapidly than younger adults with a similar injury [25]. Likewise, older adults who develop inflammatory arthritis, such as rheumatoid arthritis, exhibit more rapid joint destruction relative to younger adults [51]. If the basic cellular mechanisms that maintain tissue homeostasis decline with aging, then the response to stress or joint injury will not be adequate and joint tissue destruction and loss will be the result.
There is mounting evidence that the changes that occur in the articular cartilage during the development of OA are the result of a loss in normal homeostasis. The chondrocyte is the one cell type present in articular cartilage and therefore is responsible for both the synthesis and the breakdown of the cartilaginous extracellular matrix [52]. Signals generated by cytokines, growth factors, and the matrix regulate chondrocyte metabolic activity. In OA cartilage, it appears that the inflammatory and catabolic signals are in excess of the anti-inflammatory and anabolic signals. This signaling imbalance promotes increased production of matrix degrading enzymes by the chondrocyte, including matrix metalloproteinases (MMPs), aggrecanases and other proteases that degrade the cartilage matrix. Aging changes that occur in the chondrocyte appear to contribute to the loss in homeostasis and will be discussed next.
Chondrocyte Senescence
Chondrocytes are very unique cells that may be particularly prone to the development of aging-related changes. The chondrocytes present in the cartilage of an 80 year-old are likely to be the very same cells that were present at age 25 years. There is little to no cell division or cell death in normal adult articular cartilage [53] and there does not appear to be a ready supply of progenitor cells to replace chondrocytes if they do die. Although recent studies have challenged the notion that cartilage does not contain progenitor cells, these studies were performed with either bovine tissue from very young animals [54] or OA tissue [55], that latter of which can include cells from the synovium and bone marrow.
Adult articular chondrocytes are capable of cell division. When chondrocytes are removed from the joint and placed in tissue culture they do divide and after multiple passages will exhibit telomere shortening [56]. Proliferation of chondrocytes is a common feature of OA where clusters of chondrocyte "clones" can be seen in the regions of cartilage where matrix loss has occurred [57]. But without disease, aging itself is not associated with chondrocyte proliferation but rather with a loss in the normal mitogenic response of isolated chondrocytes to growth factor stimulation [58].
The fact that with normal aging chondrocytes rarely divide suggests that classic replicative senescence, which requires over 30–40 population doublings [59], would not be the form of senescence affecting chondrocytes. Replicative senescence or "intrinsic senescence" is thought to be related to shortened telomeres accompanied by telomere dysfunction [60]. Evidence of telomere shortening in chondrocytes from older adults has been reported [61]. However, extrinsic or stress-induced senescence that occurs from diverse stimuli including oxidative damage, activated oncogenes, and inflammation can also damage telomeres [60, 62] and is a much more likely mechanism for senescence in cartilage [63].
Besides limiting cell replication, changes that occur in aging cells can result in the senescent secretory phenotype [62, 64]. This phenotype is characterized by the increased production of cytokines including IL-6 and IL-1, matrix metalloproteinases, and growth factors such as EGF. The accumulation of cells expressing the senescent secretory phenotype can contribute to tissue aging [62, 64]. The senescent secretory phenotype has some features in common with the OA chondrocyte phenotype, including increased production of cytokines and MMPs, suggesting an important role for chondrocyte senescence in the development of OA (Table 1).
Table 1.
Aging Changes in Joint Tissues and the Contribution of Aging to the Development of OA.
| Aging Change | Contribution to OA |
|---|---|
| Accumulation of cells exhibiting the senescent secretory phenotype |
Increased cytokine and MMP production stimulates matrix degradation |
| Oxidative stress/damage | Increased susceptibility to cell death and reduced matrix synthesis |
| Decreased levels of growth factors and decreased growth factor responsiveness |
Reduced matrix synthesis and repair |
| Increased AGE formation | Brittle tissue with increased fatigue failure |
| Reduced aggrecan size and cartilage hydration and increased collagen cleavage |
Reduced resiliency and tensile strength |
| Increased matrix calcification | Altered mechanical properties and potential activation of inflammatory signaling |
MMP=Matrix Metalloproteinases; AGE=Advanced Glycation End-products
There is evidence to suggest senescent chondrocytes adopt a secretory phenotype, in particular an increased production of MMPs. Studies have shown increased immunostaining for MMP-3 and MMP-13 in cartilage with aging [65] as well as an age-related accumulation of collagen neoepitopes representing denatured or cleaved collagen [66, 67]. Cleavage of type II collagen by MMPs has been noted in cartilage from hip joints of older individuals [66] as well as in “normal appearing” knee cartilage taken at autopsy [65]. However, since these joints are commonly affected by OA, it is not clear if the collagen damage represents aging changes, early OA, or a continuum from aging to OA.
A potential contributor to the age-related increase in cartilage catabolism may be the finding that chondrocyte anabolic activity goes down with age. Growth factors including IGF-I, OP-1 (BMP-7), and TGF-β are important cartilage anabolic factors. There is substantial evidence for a decline in the chondrocyte response to IGF-I with aging [68–70]. A reduced anabolic response to IGF-I has also been noted in chondrocytes isolated from OA cartilage [69, 71]. It has been shown that the expression and amount of OP-1 present in cartilage declines with age [72] and levels of TGF-β2 and TGF-β3 (but not TGF-β1) decline with age as does the level of the TGF-β receptor I and II [73]. The results of the studies of the effects of aging on IGF-I, OP-1, and TGF-β activity in cartilage suggest that an age-related decline in anabolic activity could tip the balance towards increased catabolic activity and play a key role in the increased susceptibility to OA.
There appears to a reduction in the number of chondrocytes in normal cartilage with aging and perhaps a greater loss of cells in OA cartilage but the extent of cell death is debated [74–76]. A 30% fall in cell density between the ages of 30 and 70 years has been described in human hip specimens [77]. However, a study of human knees found less than 5% cell loss with aging [53]. Although many studies have reported apoptotic chondrocytes in OA cartilage [76] few have examined apoptosis in cartilage with normal aging with the exception of a study in rat cartilage [78].
The mechanisms that might contribute to cell death in cartilage include the decline in growth factor activity, loss of survival promoting matrix proteins, and oxidative damage. Insulin-like growth factors are important autocrine survival factors in cartilage [79] and as mentioned above the ability of chondrocytes to respond to IGF-I declines with age. A recent study suggested that an age-related decline in levels of the high-mobility group box (HMGB) protein 2, which is expressed in the superficial zone of cartilage, might contribute to an increase in chondrocyte death [80]. HMGB2 is a nonhistone chromatin protein that can serve as a transcriptional regulator. Deletion of HMGB2 in transgenic mice was found to cause an early onset of OA-like changes in the superficial zone of cartilage that were associated with an increase in susceptibility of chondrocytes to cell death. Levels of reactive oxygen species (ROS) increase in cartilage with aging and chondrocytes from older adults are more susceptible to ROS-mediated cell death [81]. The role of ROS and oxidative stress in cartilage aging will be discussed further below.
The Aging Cartilage Matrix
In addition to age-related changes in chondrocytes, age-related changes that occur in the cartilage matrix have been described that could also contribute to the development of OA. MRI studies have shown that knee cartilage thins with aging, particularly at the femoral side of the joint [82] and at the patella [83] suggesting a gradual loss of cartilage matrix with aging. One of the best studied aging-related matrix protein modification in cartilage is the formation of advanced glycation end-products (AGEs). AGEs are produced from the spontaneous nonenzymatic glycation of proteins that occurs when reducing sugars such as glucose, fructose or ribose, react with lysine or arginine residues [84]. Because the articular cartilage has a relatively low turnover rate, it is particularly susceptible to AGE formation. Type II collagen, the most abundant matrix protein in cartilage, has a half-life that has been calculated to be over 100 years [85]. The accumulation of AGEs in cartilage has been suggested to play a role in the development of osteoarthritis and has been found in both knee [84, 86] and ankle cartilage (Cole and DeGroot, unpublished observation).
AGE formation could contribute to OA through effects on the mechanical properties of cartilage as well effects on the cells. Modification of collagen by AGE formation results in increased cross-linking of collagen molecules. The most common AGE-related cross link is pentosidine which has been found to be present in cartilage in increasing amounts with age [85, 87, 88]. Formation of excessive collagen cross-links affects the biomechanical properties of cartilage resulting in increased stiffness making the cartilage more brittle [89] and increasing the susceptibility of the tissue to fatigue failure [87]. Increased levels of AGEs in cartilage have also been associated with a decline in anabolic activity [90].
After type II collagen, aggrecan is the second most abundant cartilage matrix protein. Aggrecan is a large “aggregating” proteoglycan that consists of a core protein to which numerous highly sulfated glycosaminoglycan chains are covalently attached. Because of the hydrophilic nature of aggrecan’s negatively charged sulfates, articular cartilage is about 70–80% water and is very resilient. Age-related changes in the size, structure, and sulfation of aggrecan have been reported [91–94] which affected cartilage resiliency and hydration [95]. When aggrecan is degraded, a fragment which contains the binding region for hyaluronic acid can be left behind and appears to accumulate in cartilage with aging due to a low turnover rate with an estimated aggrecan half-life in cartilage of 25 years [96]. The aggrecan fragment which remains bound to hyaluronic acid can occupy the space where a newly synthesized complete aggrecan molecule would bind and thus result in smaller proteoglycan aggregates being present with increasing age.
Aging, Oxidative Stress and OA
Oxidative damage from the chronic production of endogenous reactive oxygen species (ROS) or “free radicals” has been associated with aging in various human tissues and in animal models [97] and has long been thought to play a central role in the aging process [98]. Increased production of ROS leads to oxidative stress, a condition within cells where the amount of ROS exceeds the anti-oxidant capacity of the cell. Human articular chondrocytes can actively produce ROS including superoxide, hydroxyl radical, hydrogen peroxide, as well as reactive nitrogen species, most notably nitric oxide [99–101]. Increased levels of intracellular ROS were recently detected in cartilage from old rats when compared to young adults [102]. Glutathione is an important intracellular anti-oxidant and one measure of oxidative stress is the ratio of oxidized to reduced glutathione. This ratio was measured in chondrocytes isolated from normal ankle tissue and found to increase directly with age [81]. Further evidence for oxidative damage in articular cartilage was provided by a study showing increased nitrotyrosine (a measure of oxidative damage to proteins) with aging, as well as with OA [103].
The aging-related increase in ROS levels could play an important role in OA [104]. The various inflammatory mediators found to be increased in OA, including IL-1, IL-6, IL-8, TNF-α, and other cytokines can all stimulate the further production of ROS. Excess production of ROS by chondrocytes can directly damage both intracellular proteins and the extracellular matrix. Increased levels of ROS can also result in DNA damage which has been noted in OA cartilage [105]. Since ROS are also involved in the stimulation of MMP production and activity [106], they could play an important role in stimulating cartilage degradation in OA.
Summary
Osteoarthritis is a multi-factorial condition for which aging is the major risk factor. However, there are important differences between an aged joint and one with OA. The aging changes observed in the cells and extracellular matrix of joint tissues likely increase the susceptibility of older adults to OA when other OA risk factors are also present. OA is characterized by an imbalance between catabolic and anabolic activity in the joint and aging likely contributes to this imbalance. Aged chondrocytes respond poorly to growth factor stimulation and so are unable to maintain homeostasis in the articular cartilage. A loss of chondrocytes due to an increased susceptibility to cell death appears to be important as well. Much of the research to date has focused on aging changes in the articular cartilage but recent clinical studies, particularly those that have used MRI to evaluate joint structure, suggest that aging changes that can contribute to OA are also found in other joint tissues including bone, ligaments, and, in the knee, the meniscus. Further work is needed to understand the basic biology of aging in the various joint tissues affected by OA in order to determine if targeting aging-changes in the joint might prevent or slow the development and progression of OA.
Practice Points
OA is not simply mechanical wear and tear but is an active biological process of matrix degradation mediated by cells within the joint. Future therapies for OA will target the processes driving matrix degradation.
Joint pain in older adults is not always due to OA, even when radiographs show OA changes since these changes are common in older adults. Findings of OA on plain radiographs do not correlate well with symptoms.
Erosive OA is a less common form of OA most often seen in distal and proximal phalengeal joints of older women.
Age-related oxidative stress and damage likely play a role in OA but to date anti-oxidant therapies for OA have not been well tested and may need to be more targeted than the currently available general anti-oxidants.
Research Agenda
Better define basic mechanisms by which aging contributes to OA, in particular the role of oxidative stress.
Determine mechanisms of age-related degeneration in ligaments and menisci and their contribution to OA.
Study the oldest old who have not developed OA and discover what factors may be protective.
When using animal models to study OA consider the importance of age and design studies to include older animals when possible.
Acknowledgements
Dr. Loeser work was supported by the National Institute on Aging (RO1 AG16697 and the Wake Forest University Claude D. Pepper Older Americans Independence Center P30 AG021332), the National Institute on Arthritis, Musculoskeletal and Skin Diseases (RO1 AR49003), the American Federation for Aging Research, and the Dorothy Rhyne Kimbrell and Willard Duke Kimbrell Professorship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Arden N, Nevitt MC. Osteoarthritis: epidemiology. Best practice & research. 2006 Feb;20:3–25. doi: 10.1016/j.berh.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 2.Lawrence RC, Felson DT, Helmick CG, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States: Part II. Arthritis Rheum. 2008 Jan;58:26–35. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miller ME, Rejeski WJ, Messier SP, et al. Modifiers of change in physical functioning in older adults with knee pain: the Observational Arthritis Study in Seniors (OASIS) Arthritis Rheum. 2001 Aug;45:331–339. doi: 10.1002/1529-0131(200108)45:4<331::AID-ART345>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 4.Bedson J, Croft PR. The discordance between clinical and radiographic knee osteoarthritis: a systematic search and summary of the literature. BMC musculoskeletal disorders. 2008;9:116. doi: 10.1186/1471-2474-9-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duncan R, Peat G, Thomas E, et al. Symptoms and radiographic osteoarthritis: not as discordant as they are made out to be? Ann Rheum Dis. 2007 Jan;66:86–91. doi: 10.1136/ard.2006.052548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duncan R, Peat G, Thomas E, et al. How do pain and function vary with compartmental distribution and severity of radiographic knee osteoarthritis? Rheumatology (Oxford) 2008 Nov;47:1704–1707. doi: 10.1093/rheumatology/ken339. [DOI] [PubMed] [Google Scholar]
- 7.Altman R, Asch E, Bloch D, et al. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum. 1986 Aug;29:1039–1049. doi: 10.1002/art.1780290816. [DOI] [PubMed] [Google Scholar]
- 8.Altman R, Alarcon G, Appelrouth D, et al. The American College of Rheumatology criteria for the classification and reporting of osteoarthritis of the hand. Arthritis Rheum. 1990 Nov;33:1601–1610. doi: 10.1002/art.1780331101. [DOI] [PubMed] [Google Scholar]
- 9.Altman R, Alarcon G, Appelrouth D, et al. The American College of Rheumatology criteria for the classification and reporting of osteoarthritis of the hip. Arthritis Rheum. 1991 May;34:505–514. doi: 10.1002/art.1780340502. [DOI] [PubMed] [Google Scholar]
- 10.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Annals of the Rheumatic Diseases. 1957;16:494–502. doi: 10.1136/ard.16.4.494. 1957/// [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Felson DT, Naimark A, Anderson J, et al. The prevalence of knee osteoarthritis in the elderly. The Framingham Osteoarthritis Study. Arthritis Rheum. 1987 Aug;30:914–918. doi: 10.1002/art.1780300811. [DOI] [PubMed] [Google Scholar]
- 12.Jordan JM, Helmick CG, Renner JB, et al. Prevalence of knee symptoms and radiographic and symptomatic knee osteoarthritis in African Americans and Caucasians: the Johnston County Osteoarthritis Project. J Rheumatol. 2007 Jan;34:172–180. [PubMed] [Google Scholar]
- 13.Dillon CF, Rasch EK, Gu Q, et al. Prevalence of knee osteoarthritis in the United States: arthritis data from the Third National Health and Nutrition Examination Survey 1991–94. J Rheumatol. 2006 Nov;33:2271–2279. [PubMed] [Google Scholar]
- 14.van Saase JL, van Romunde LK, Cats A, et al. Epidemiology of osteoarthritis: Zoetermeer survey. Comparison of radiological osteoarthritis in a Dutch population with that in 10 other populations. Ann Rheum Dis. 1989 Apr;48:271–280. doi: 10.1136/ard.48.4.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andrianakos AA, Kontelis LK, Karamitsos DG, et al. Prevalence of symptomatic knee, hand, and hip osteoarthritis in Greece. The ESORDIG study. J Rheumatol. 2006 Dec;33:2507–2513. [PubMed] [Google Scholar]
- 16.Muraki S, Oka H, Akune T, et al. Prevalence of radiographic knee osteoarthritis and its association with knee pain in the elderly of Japanese population-based cohorts: The ROAD study. Osteoarthritis Cartilage. 2009 Apr 17; doi: 10.1016/j.joca.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 17.Oliveria SA, Felson DT, Reed JI, et al. Incidence of symptomatic hand, hip, and knee osteoarthritis among patients in a health maintenance organization. Arthritis Rheum. 1995 Aug;38:1134–1141. doi: 10.1002/art.1780380817. [DOI] [PubMed] [Google Scholar]
- 18.Felson DT, Zhang Y, Hannan MT, et al. The incidence and natural history of knee osteoarthritis in the elderly. The Framingham Osteoarthritis Study. Arthritis Rheum. 1995 Oct;38:1500–1505. doi: 10.1002/art.1780381017. [DOI] [PubMed] [Google Scholar]
- 19.Dagenais S, Garbedian S, Wai EK. Systematic review of the prevalence of radiographic primary hip osteoarthritis. Clinical orthopaedics and related research. 2009 Mar;467:623–637. doi: 10.1007/s11999-008-0625-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jordan JM, Helmick CG, Renner JB, et al. Prevalence of hip symptoms and radiographic and symptomatic hip osteoarthritis in African Americans and Caucasians: the Johnston County Osteoarthritis Project. J Rheumatol. 2009 Apr;36:809–815. doi: 10.3899/jrheum.080677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arden NK, Lane NE, Parimi N, et al. Defining incident radiographic hip osteoarthritis for epidemiologic studies in women. Arthritis Rheum. 2009 Apr;60:1052–1059. doi: 10.1002/art.24382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Y, Niu J, Kelly-Hayes M, et al. Prevalence of symptomatic hand osteoarthritis and its impact on functional status among the elderly: The Framingham Study. American journal of epidemiology. 2002 Dec 1;156:1021–1027. doi: 10.1093/aje/kwf141. [DOI] [PubMed] [Google Scholar]
- 23.Punzi L, Ramonda R, Sfriso P. Erosive osteoarthritis. Best practice & research. 2004 Oct;18:739–758. doi: 10.1016/j.berh.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 24.Vlychou M, Koutroumpas A, Malizos K, et al. Ultrasonographic evidence of inflammation is frequent in hands of patients with erosive osteoarthritis. Osteoarthritis Cartilage. 2009 May 7; doi: 10.1016/j.joca.2009.04.020. [DOI] [PubMed] [Google Scholar]
- 25.Roos H, Adalberth T, Dahlberg L, et al. Osteoarthritis of the knee after injury to the anterior cruciate ligament or meniscus: the influence of time and age. Osteoarthitis Cartilage. 1995 Dec;3:261–267. doi: 10.1016/s1063-4584(05)80017-2. [DOI] [PubMed] [Google Scholar]
- 26.Englund M, Guermazi A, Roemer FW, et al. Meniscal tear in knees without surgery and the development of radiographic osteoarthritis among middle-aged and elderly persons: The Multicenter Osteoarthritis Study. Arthritis Rheum. 2009 Mar;60:831–839. doi: 10.1002/art.24383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Englund M, Guermazi A, Gale D, et al. Incidental meniscal findings on knee MRI in middle-aged and elderly persons. N Engl J Med. 2008 Sep 11;359:1108–1115. doi: 10.1056/NEJMoa0800777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clayton RA, Court-Brown CM. The epidemiology of musculoskeletal tendinous and ligamentous injuries. Injury. 2008 Dec;39:1338–1344. doi: 10.1016/j.injury.2008.06.021. [DOI] [PubMed] [Google Scholar]
- 29.Hill CL, Seo GS, Gale D, et al. Cruciate ligament integrity in osteoarthritis of the knee. Arthritis Rheum. 2005 Mar;52:794–799. doi: 10.1002/art.20943. [DOI] [PubMed] [Google Scholar]
- 30.Strocchi R, De Pasquale V, Facchini A, et al. Age-related changes in human anterior cruciate ligament (ACL) collagen fibrils. Italian journal of anatomy and embryology = Archivio italiano di anatomia ed embriologia. 1996 Oct–Dec;101:213–220. [PubMed] [Google Scholar]
- 31.Burr DB. The importance of subchondral bone in the progression of osteoarthritis. J Rheumatol Suppl. 2004 Apr;70:77–80. [PubMed] [Google Scholar]
- 32.Felson DT, Neogi T. Osteoarthritis: is it a disease of cartilage or of bone? Arthritis Rheum. 2004 Feb;50:341–344. doi: 10.1002/art.20051. [DOI] [PubMed] [Google Scholar]
- 33.Hunter DJ, Gerstenfeld L, Bishop G, et al. Bone marrow lesions from osteoarthritis knees are characterized by sclerotic bone that is less well mineralized. Arthritis Res Ther. 2009;11:R11. doi: 10.1186/ar2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Felson DT, Chaisson CE, Hill CL, et al. The Association of Bone Marrow Lesions with Pain in Knee Osteoarthritis. Ann Intern Med. 2001;134:541–549. doi: 10.7326/0003-4819-134-7-200104030-00007. [DOI] [PubMed] [Google Scholar]
- 35.Felson DT, McLaughlin S, Goggins J, et al. Bone marrow edema and its relation to progression of knee osteoarthritis. Ann Intern Med. 2003 Sep 2;139:330–336. doi: 10.7326/0003-4819-139-5_part_1-200309020-00008. [DOI] [PubMed] [Google Scholar]
- 36.Lo GH, Hunter DJ, Nevitt M, et al. Strong association of MRI meniscal derangement and bone marrow lesions in knee osteoarthritis: data from the osteoarthritis initiative. Osteoarthritis Cartilage. 2009 Jun;17:743–747. doi: 10.1016/j.joca.2008.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baranyay FJ, Wang Y, Wluka AE, et al. Association of bone marrow lesions with knee structures and risk factors for bone marrow lesions in the knees of clinically healthy, community-based adults. Semin Arthritis Rheum. 2007 Oct;37:112–118. doi: 10.1016/j.semarthrit.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 38.Felson DT, Anderson JJ, Naimark A, et al. The prevalence of chondrocalcinosis in the elderly and its association with knee osteoarthritis: the Framingham Study. J Rheumatol. 1989 Sep;16:1241–1245. [PubMed] [Google Scholar]
- 39.Rosenthal AK. Calcium crystal deposition and osteoarthritis. Rheum Dis Clin North Am. 2006 May;32:401–412. doi: 10.1016/j.rdc.2006.02.004. vii. [DOI] [PubMed] [Google Scholar]
- 40.Richette P, Bardin T, Doherty M. An update on the epidemiology of calcium pyrophosphate dihydrate crystal deposition disease. Rheumatology (Oxford) 2009 Apr 27; doi: 10.1093/rheumatology/kep081. [DOI] [PubMed] [Google Scholar]
- 41.Doherty M, Dieppe P. Clinical aspects of calcium pyrophosphate dihydrate crystal deposition. Rheum Dis Clin North Am. 1988 Aug;14:395–414. [PubMed] [Google Scholar]
- 42.Neame RL, Carr AJ, Muir K, et al. UK community prevalence of knee chondrocalcinosis: evidence that correlation with osteoarthritis is through a shared association with osteophyte. Ann Rheum Dis. 2003 Jun;62:513–518. doi: 10.1136/ard.62.6.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nalbant S, Martinez JA, Kitumnuaypong T, et al. Synovial fluid features and their relations to osteoarthritis severity: new findings from sequential studies. Osteoarthritis Cartilage. 2003 Jan;11:50–54. doi: 10.1053/joca.2002.0861. [DOI] [PubMed] [Google Scholar]
- 44.Ferrucci L, Cavazzini C, Corsi A, et al. Biomarkers of frailty in older persons. Journal of endocrinological investigation. 2002;25:10–15. [PubMed] [Google Scholar]
- 45.Andriacchi TP, Mundermann A, Smith RL, et al. A framework for the in vivo pathomechanics of osteoarthritis at the knee. Ann Biomed Eng. 2004 Mar;32:447–457. doi: 10.1023/b:abme.0000017541.82498.37. [DOI] [PubMed] [Google Scholar]
- 46.Lane NE, Bloch DA, Wood PD, et al. Aging, long-distance running, and the development of musculoskeletal disability. A controlled study. Am J Med. 1987 Apr;82:772–780. doi: 10.1016/0002-9343(87)90014-3. [DOI] [PubMed] [Google Scholar]
- 47.Lane NE, Michel B, Bjorkengren A, et al. The risk of osteoarthritis with running and aging: a 5-year longitudinal study. J Rheumatol. 1993 Mar;20:461–468. [PubMed] [Google Scholar]
- 48.Sharma L, Song J, Felson DT, et al. The role of knee alignment in disease progression and functional decline in knee osteoarthritis. JAMA. 2001;286:188–195. doi: 10.1001/jama.286.2.188. 2001/07/11/ [DOI] [PubMed] [Google Scholar]
- 49.Felson DT, Goggins J, Niu J, et al. The effect of body weight on progression of knee osteoarthritis is dependent on alignment. Arthritis Rheum. 2004 Dec;50:3904–3959. doi: 10.1002/art.20726. [DOI] [PubMed] [Google Scholar]
- 50.Gelber AC, Hochberg MC, Mead LA, et al. Joint injury in young adults and risk for subsequent knee and hip osteoarthritis. Ann Intern Med. 2000;133:321–328. doi: 10.7326/0003-4819-133-5-200009050-00007. [DOI] [PubMed] [Google Scholar]
- 51.Bukhari M, Lunt M, Barton A, et al. Increasing age at symptom onset is associated with worse radiological damage at presentation in patients with early inflammatory polyarthritis. Ann Rheum Dis. 2007 Mar;66:389–393. doi: 10.1136/ard.2006.055657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Goldring MB, Goldring SR. Osteoarthritis. J Cell Physiol. 2007 Dec;213:626–634. doi: 10.1002/jcp.21258. [DOI] [PubMed] [Google Scholar]
- 53.Aigner T, Hemmel M, Neureiter D, et al. Apoptotic cell death is not a widespread phenomenon in normal aging and osteoarthritis human articular knee cartilage: a study of proliferation, programmed cell death (apoptosis), and viability of chondrocytes in normal and osteoarthritic human knee cartilage. Arthritis Rheum. 2001;44:1304–1312. doi: 10.1002/1529-0131(200106)44:6<1304::AID-ART222>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 54.Dowthwaite GP, Bishop JC, Redman SN, et al. The surface of articular cartilage contains a progenitor cell population. J Cell Sci. 2004 Feb 29;117:889–897. doi: 10.1242/jcs.00912. [DOI] [PubMed] [Google Scholar]
- 55.Alsalameh S, Amin R, Gemba T, et al. Identification of mesenchymal progenitor cells in normal and osteoarthritic human articular cartilage. Arthritis Rheum. 2004 May;50:1522–1532. doi: 10.1002/art.20269. [DOI] [PubMed] [Google Scholar]
- 56.Parsch D, Brummendorf TH, Richter W, et al. Replicative aging of human articular chondrocytes during ex vivo expansion. Arthritis Rheum. 2002 Nov;46:2911–2916. doi: 10.1002/art.10626. [DOI] [PubMed] [Google Scholar]
- 57.Loeser RF, Shakoor N. Aging or osteoarthritis: which is the problem? Rheum Dis Clin North Am. 2003 Nov;29:653–673. doi: 10.1016/s0889-857x(03)00062-0. [DOI] [PubMed] [Google Scholar]
- 58.Guerne PA, Blanco F, Kaelin A, et al. Growth factor responsiveness of human articular chondrocytes in aging and development. Arthritis Rheum. 1995;38:960–968. doi: 10.1002/art.1780380712. [DOI] [PubMed] [Google Scholar]
- 59.Hayflick L. Intracellular determinants of cell aging. Mech Ageing Dev. 1984 Dec;28:177–185. doi: 10.1016/0047-6374(84)90018-6. [DOI] [PubMed] [Google Scholar]
- 60.Itahana K, Campisi J, Dimri GP. Mechanisms of cellular senescence in human and mouse cells. Biogerontology. 2004;5:1–10. doi: 10.1023/b:bgen.0000017682.96395.10. [DOI] [PubMed] [Google Scholar]
- 61.Martin JA, Buckwalter JA. Telomere erosion and senescence in human articular cartilage chondrocytes. J Gerontol A Biol Sci Med Sci. 2001 Apr;56:B172–B179. doi: 10.1093/gerona/56.4.b172. [DOI] [PubMed] [Google Scholar]
- 62.Campisi J. Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell. 2005 Feb 25;120:513–522. doi: 10.1016/j.cell.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 63.Dai SM, Shan ZZ, Nakamura H, et al. Catabolic stress induces features of chondrocyte senescence through overexpression of caveolin 1: possible involvement of caveolin 1-induced down-regulation of articular chondrocytes in the pathogenesis of osteoarthritis. Arthritis Rheum. 2006 Mar;54:818–831. doi: 10.1002/art.21639. [DOI] [PubMed] [Google Scholar]
- 64.Campisi J, d'Adda di Fagagna F. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol. 2007 Sep;8:729–740. doi: 10.1038/nrm2233. [DOI] [PubMed] [Google Scholar]
- 65.Wu W, Billinghurst RC, Pidoux I, et al. Sites of collagenase cleavage and denaturation of type II collagen in aging and osteoarthritic articular cartilage and their relationship to the distribution of matrix metalloproteinase 1 and matrix metalloproteinase 13. Arthritis Rheum. 2002 Aug;46:2087–2094. doi: 10.1002/art.10428. [DOI] [PubMed] [Google Scholar]
- 66.Hollander AP, Pidoux I, Reiner A, et al. Damage to type II collagen in aging and osteoarthritis starts at the articular surface, originates around chondrocytes, and extends into the cartilage with progressive degeneration. J Clin Invest. 1995 Dec;96:2859–2869. doi: 10.1172/JCI118357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Aurich M, Poole AR, Reiner A, et al. Matrix homeostasis in aging normal human ankle cartilage. Arthritis Rheum. 2002 Nov;46:2903–2910. doi: 10.1002/art.10611. [DOI] [PubMed] [Google Scholar]
- 68.Martin JA, Ellerbroek SM, Buckwalter JA. Age-related decline in chondrocyte response to insulin-like growth factor-I: the role of growth factor binding proteins. J Orthop Res. 1997;15:491–498. doi: 10.1002/jor.1100150403. [DOI] [PubMed] [Google Scholar]
- 69.Loeser RF, Shanker G, Carlson CS, et al. Reduction in the chondrocyte response to insulin-like growth factor 1 in aging and osteoarthritis: studies in a non-human primate model of naturally occurring disease. Arthritis Rheum. 2000;43:2110–2120. doi: 10.1002/1529-0131(200009)43:9<2110::AID-ANR23>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 70.Messai H, Duchossoy Y, Khatib A, et al. Articular chondrocytes from aging rats respond poorly to insulin-like growth factor-1: an altered signaling pathway. Mech Ageing Dev. 2000;115:21–37. doi: 10.1016/s0047-6374(00)00107-x. [DOI] [PubMed] [Google Scholar]
- 71.Dore S, Pelletier JP, DiBattista JA, et al. Human osteoarthritic chondrocytes possess an increased number of insulin-like growth factor 1 binding sites but are unresponsive to its stimulation. Possible role of IGF-1-binding proteins. Arthritis Rheum. 1994;37:253–263. doi: 10.1002/art.1780370215. [DOI] [PubMed] [Google Scholar]
- 72.Chubinskaya S, Kumar B, Merrihew C, et al. Age-related changes in cartilage endogenous osteogenic protein-1 (OP-1) Biochim Biophys Acta. 2002 Nov 20;1588:126–134. doi: 10.1016/s0925-4439(02)00158-8. [DOI] [PubMed] [Google Scholar]
- 73.Blaney Davidson EN, Scharstuhl A, Vitters EL, et al. Reduced transforming growth factor-beta signaling in cartilage of old mice: role in impaired repair capacity. Arthritis Res Ther. 2005;7:R1338–R1347. doi: 10.1186/ar1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Horton WE, Jr, Feng L, Adams C. Chondrocyte apoptosis in development, aging and disease. Matrix Biol. 1998;17:107–115. doi: 10.1016/s0945-053x(98)90024-5. [DOI] [PubMed] [Google Scholar]
- 75.Aigner T, Kim HA, Roach HI. Apoptosis in osteoarthritis. Rheum Dis Clin North Am. 2004 Aug;30:639–653. doi: 10.1016/j.rdc.2004.04.002. xi. [DOI] [PubMed] [Google Scholar]
- 76.Kuhn K, D'Lima DD, Hashimoto S, et al. Cell death in cartilage. Osteoarthritis Cartilage. 2004 Jan;12:1–16. doi: 10.1016/j.joca.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 77.Vignon E, Arlot M, Patricot LM, et al. The cell density of human femoral head cartilage. Clin Orthop. 1976;121:303–308. [PubMed] [Google Scholar]
- 78.Adams CS, Horton WE., Jr Chondrocyte apoptosis increases with age in the articular cartilage of adult animals. Anat Rec. 1998;250:418–425. doi: 10.1002/(SICI)1097-0185(199804)250:4<418::AID-AR4>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 79.Loeser RF, Shanker G. Autocrine stimulation by insulin-like growth factor 1 and insulin-like growth factor 2 mediates chondrocyte survival in vitro. Arthritis Rheum. 2000;43:1552–1559. doi: 10.1002/1529-0131(200007)43:7<1552::AID-ANR20>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 80.Taniguchi N, Carames B, Ronfani L, et al. Aging-related loss of the chromatin protein HMGB2 in articular cartilage is linked to reduced cellularity and osteoarthritis. Proc Natl Acad Sci U S A. 2009 Jan 27;106:1181–1186. doi: 10.1073/pnas.0806062106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Del Carlo M, Jr, Loeser RF. Increased oxidative stress with aging reduces chondrocyte survival: Correlation with intracellular glutathione levels. Arthritis Rheum. 2003 Dec;48:3419–3430. doi: 10.1002/art.11338. [DOI] [PubMed] [Google Scholar]
- 82.Hudelmaier M, Glaser C, Hohe J, et al. Age-related changes in the morphology and deformational behavior of knee joint cartilage. Arthritis Rheum. 2001 Nov;44:2556–2561. doi: 10.1002/1529-0131(200111)44:11<2556::aid-art436>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 83.Ding C, Cicuttini F, Scott F, et al. Association between age and knee structural change: a cross sectional MRI based study. Ann Rheum Dis. 2005 Apr;64:549–555. doi: 10.1136/ard.2004.023069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Verzijl N, Bank RA, TeKoppele JM, et al. AGEing and osteoarthritis: a different perspective. Curr Opin Rheumatol. 2003 Sep;15:616–622. doi: 10.1097/00002281-200309000-00016. [DOI] [PubMed] [Google Scholar]
- 85.Verzijl N, DeGroot J, Thorpe SR, et al. Effect of collagen turnover on the accumulation of advanced glycation endproducts. J Biol Chem. 2000;275:39027–39031. doi: 10.1074/jbc.M006700200. [DOI] [PubMed] [Google Scholar]
- 86.DeGroot J, Verzijl N, Wenting-van Wijk MJ, et al. Accumulation of advanced glycation end products as a molecular mechanism for aging as a risk factor in osteoarthritis. Arthritis Rheum. 2004 Apr;50:1207–1215. doi: 10.1002/art.20170. [DOI] [PubMed] [Google Scholar]
- 87.Bank RA, Bayliss MT, Lafeber FP, et al. Ageing and zonal variation in post-translational modification of collagen in normal human articular cartilage. The age-related increase in non-enzymatic glycation affects biomechanical properties of cartilage. Biochem J. 1998 Feb 15;330(Pt 1):345–351. doi: 10.1042/bj3300345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Verzijl N, DeGroot J, Ben ZC, et al. Crosslinking by advanced glycation end products increases the stiffness of the collagen network in human articular cartilage: a possible mechanism through which age is a risk factor for osteoarthritis. Arthritis Rheum. 2002;46:114–123. doi: 10.1002/1529-0131(200201)46:1<114::AID-ART10025>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 89.Chen AC, Temple MM, Ng DM, et al. Induction of advanced glycation end products and alterations of the tensile properties of articular cartilage. Arthritis Rheum. 2002 Dec;46:3212–3217. doi: 10.1002/art.10627. [DOI] [PubMed] [Google Scholar]
- 90.DeGroot J, Verzijl N, Bank RA, et al. Age-related decrease in proteoglycan synthesis of human articular chondrocytes: the role of nonenzymatic glycation. Arthritis Rheum. 1999;42:1003–1109. doi: 10.1002/1529-0131(199905)42:5<1003::AID-ANR20>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 91.Buckwalter JA, Roughley PJ, Rosenberg LC. Age-related changes in cartilage proteoglycans: quantitative electron microscopic studies. Microsc Res Tech. 1994 Aug 1;28:398–408. doi: 10.1002/jemt.1070280506. [DOI] [PubMed] [Google Scholar]
- 92.Dudhia J, Davidson CM, Wells TM, et al. Age-related changes in the content of the C-terminal region of aggrecan in human articular cartilage. Biochem J. 1996 Feb 1;313(Pt 3):933–940. doi: 10.1042/bj3130933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bayliss MT, Osborne D, Woodhouse S, et al. Sulfation of chondroitin sulfate in human articular cartilage. The effect of age, topographical position, and zone of cartilage on tissue composition. J Biol Chem. 1999 May 28;274:15892–15900. doi: 10.1074/jbc.274.22.15892. [DOI] [PubMed] [Google Scholar]
- 94.Wells T, Davidson C, Morgelin M, et al. Age-related changes in the composition, the molecular stoichiometry and the stability of proteoglycan aggregates extracted from human articular cartilage. Biochem J. 2003 Feb 15;370:69–79. doi: 10.1042/BJ20020968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Grushko G, Schneiderman R, Maroudas A. Some biochemical and biophysical parameters for the study of the pathogenesis of osteoarthritis: a comparison between the processes of ageing and degeneration in human hip cartilage. Connect Tissue Res. 1989;19:149–176. doi: 10.3109/03008208909043895. [DOI] [PubMed] [Google Scholar]
- 96.Maroudas A, Bayliss MT, Uchitel-Kaushansky N, et al. Aggrecan turnover in human articular cartilage: use of aspartic acid racemization as a marker of molecular age. Arch Biochem Biophys. 1998;350:61–71. doi: 10.1006/abbi.1997.0492. [DOI] [PubMed] [Google Scholar]
- 97.Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 98.Harman D. Aging: A theory based on free radical and radiation chemistry. J Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 99.Studer R, Jaffurs D, Stefanovic-Racic M, et al. Nitric oxide in osteoarthritis. Osteoarthritis Cartilage. 1999;7:377–379. doi: 10.1053/joca.1998.0216. [DOI] [PubMed] [Google Scholar]
- 100.Hiran TS, Moulton PJ, Hancock JT. Detection of superoxide and NADPH oxidase in porcine articular chondrocytes. Free Radic Biol Med. 1997;23:736–743. doi: 10.1016/s0891-5849(97)00054-3. [DOI] [PubMed] [Google Scholar]
- 101.Tiku ML, Shah R, Allison GT. Evidence linking chondrocyte lipid peroxidation to cartilage matrix protein degradation: Possible role in cartilage aging and the pathogenesis of osteoarthritis. J Biol Chem. 2000;275:20069–20076. doi: 10.1074/jbc.M907604199. [DOI] [PubMed] [Google Scholar]
- 102.Jallali N, Ridha H, Thrasivoulou C, et al. Vulnerability to ROS-induced cell death in ageing articular cartilage: the role of antioxidant enzyme activity. Osteoarthritis Cartilage. 2005 Jul;13:614–622. doi: 10.1016/j.joca.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 103.Loeser RF, Carlson CS, Carlo MD, et al. Detection of nitrotyrosine in aging and osteoarthritic cartilage: Correlation of oxidative damage with the presence of interleukin-1beta and with chondrocyte resistance to insulin-like growth factor 1. Arthritis Rheum. 2002 Sep;46:2349–2357. doi: 10.1002/art.10496. [DOI] [PubMed] [Google Scholar]
- 104.Henrotin YE, Bruckner P, Pujol JP. The role of reactive oxygen species in homeostasis and degradation of cartilage. Osteoarthritis Cartilage. 2003 Oct;11:747–755. doi: 10.1016/s1063-4584(03)00150-x. [DOI] [PubMed] [Google Scholar]
- 105.Davies CM, Guilak F, Weinberg JB, et al. Reactive nitrogen and oxygen species in interleukin-1-mediated DNA damage associated with osteoarthritis. Osteoarthritis Cartilage. 2008 May;16:624–630. doi: 10.1016/j.joca.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Nelson KK, Melendez JA. Mitochondrial redox control of matrix metalloproteinases. Free Radic Biol Med. 2004 Sep 15;37:768–784. doi: 10.1016/j.freeradbiomed.2004.06.008. [DOI] [PubMed] [Google Scholar]