Abstract
It has become increasingly evident that bidirectional (“top-down and bottom-up”) interactions between the brain and peripheral tissues, including the cardiovascular and immune systems, contribute to both mental and physical health. Therapies directed toward addressing functional links between mind/brain and body may be particularly effective in treating the range of symptoms associated with many chronic diseases. In this paper, we describe the basic components of an integrative psychophysiological framework for research aimed at elucidating the underlying substrates of mind-body therapies. This framework recognizes the multiple levels of the neuraxis at which mind-body interactions occur. We emphasize the role of specific fronto-temporal cortical regions in the representation and control of adverse symptoms, which interact reciprocally with subcortical structures involved in bodily homeostasis and responses to stress. Bidirectional autonomic and neuroendocrine pathways transmit information between the central nervous system (CNS) and the periphery and facilitate the expression of affective, autonomic, hormonal, and immune responses. We propose that heart rate variability (HRV) and markers of inflammation are important currently available indices of central-peripheral integration and homeostasis within this homeostatic network. Finally, we review current neuroimaging and psychophysiological research from diverse areas of mind-body medicine that supports the framework as a basis for future research on the specific biobehavioral mechanisms of mind-body therapies.
Keywords: autonomic pathways, neuroendocrine pathways, heart rate, mind-body therapies, sympathetic nervous system, parasympathetic nervous system
Introduction
It has become increasingly evident that bidirectional interactions between the brain and peripheral tissues, including the cardiovascular and immune systems, contribute to both mental and physical health. For instance, interactions of psychological stress, behavioral depression, and peripheral inflammation are now recognized as important contributors leading to chronic conditions such as heart disease. Therapies directed toward addressing functional links between mind/brain and body would be particularly effective in treating the range of symptoms associated with many chronic diseases. Indeed, mind-body therapies (including hypnosis, mental imagery, biofeedback, progressive muscle relaxation, yoga, meditation, and T’ai Chi) have been found effective for reducing depression, insomnia, anxiety, post-traumatic stress, irritable bowel syndrome (IBS), nausea, and acute and chronic pain, and for managing impaired circulation, diabetes, and hypertension (1–10). Furthermore, controlled experimental studies have demonstrated physiological changes during and following mind-body interventions, including enhanced cardiac-vagal tone and cardiovascular function (4, 6, 11); improved glucose tolerance and lipid profiles (4, 5); and modulation of neuroendocrine responses (4, 6, 12), immune responses (13, 14), and inflammatory responses (15, 16). These changes are consistent with the idea that these therapies promote homeostasis, or the maintenance of optimal physiological conditions.
Given the range of potential benefits, it is not surprising that the popularity of complementary and alternative medicine (CAM) is increasing in the United States (17). An earlier prospective survey suggested significantly increased use of mind-body therapies by Americans between 1990 and 1997 (18); recent statistics indicate that between 41% and 59% of a sample of chronically ill adults use CAM (17). Furthermore, our research group reported frequent use of mind-body therapies among samples of clinical outpatients and community members in Charlottesville, Virginia (19, 20). For instance, use of guided imagery was reported by 21% of the patients with chronic pain (n = 63), 27% of the patients with cancer (n = 60), and 29% of the community sample (n = 63). These findings are indicative of an increasing use of mind-body therapies in the management of symptoms accompanying chronic illness.
Although the popularity of mind-body therapies continues to rise among the general public for the self-management of adverse symptoms, and these therapies constitute an integral component of modern psychotherapy, there remains inadequate integration of mind-body therapies into mainstream medicine (3, 21, 22). Because the mechanisms of mind-body therapies are poorly characterized, elucidating the underlying biological substrates should contribute to greater acceptance and integration of these therapies into conventional medical care. This psychophysiological knowledge is necessary to validate subjective clinical endpoints in mind-body research and to refine therapeutic interventions in mind-body medicine for the benefit of public health. Thus, mechanistic studies are currently a research priority identified by the National Center for Complementary and Alternative Medicine (NCCAM) (23). Furthermore, illuminating the biological mechanisms through which the mind and brain influence physiology and health is of fundamental importance for understanding and remediating stress-related morbidity (24, 25).
To advance the physiological knowledge base for mind-body therapies, research should be directed by an empirical framework that integrates knowledge of both established and hypothesized substrates and provides a template useful for evaluating the mechanisms that underlie the efficacy of CAM modalities. Thus, the objective of this paper is to describe the basic components of an integrated psychophysiological research framework that has evolved out of our multidisciplinary research at the University of Virginia on the benefits of mind-body therapies for patients with cancer, depression, diabetes, metabolic syndrome, arthritis, fibromyalgia, and AIDS (3–6, 10, 20, 26–31). This model integrates components of previous models with emerging findings concerning the mechanisms of the bidirectional interaction of mind and body that mediate efficacy of mind-body therapies. The resulting framework recognizes a functional and anatomically interconnected network of brain regions located at every level of the neuraxis, from the spinal and brainstem autonomic nuclei to cortical regions that integrate emotion and cognition with information regarding bodily states. We propose that components of this network mediate the efficacy of mind-body therapies, and we review recent functional magnetic resonance imaging (fMRI) and psychophysiological studies from several areas of mind-body medicine that support the utility of the framework as a basis for future research.
Current Concepts of Neurological Mechanisms in Mind-Body Research
The idea that interactions between mind and body influence physical health has existed since ancient times (32). Over the past several decades, however, modern models have been advanced regarding brain mechanisms and substrates involved in integrating signals from the body with psychological states, such as mood, emotion, and stress. These models have generally focused on specific mechanisms, e.g., inhibition of the sympathetic nervous system (fight or flight responses) or activation of the parasympathetic system, notably via the vagus nerve, and have postulated effects on brain regions that control or respond to activity in these peripheral nerves.
Benson’s (33–35) relaxation response model is an influential and widely cited theoretical framework in mind-body medicine (7). Because increased autonomic arousal from emotional stress has been found to precipitate physical symptoms and exacerbate medical conditions, Benson has suggested that mind-body therapies exert therapeutic effects by eliciting an intrinsic anti-stress response that includes decreased sympathetic and brain cortical activation (7, 34, 36). In contrast, Porges (37, 38) suggests that the myelinated branches of the vagus nerve and specific parasympathetic source nuclei in the medulla are major substrates for the expression and inhibition of the stress response (39), and thus cardiac-vagal activity is an objective physiological marker of stress and homeostasis. Zagon (40) outlined a theory of mind-body communication that implicates vagal afferents in the inhibition of sympathetic activation within the brainstem as well as in the tonic transmission of peripheral visceral information to the forebrain. Moreover, highlighting the signaling role of pro-inflammatory cytokines in psychophysiological regulation, Sternberg (32) and Goehler and colleagues (41) reviewed evidence implicating sensory components of the vagus as a major direct pathway for immune to brain communication in health and illness.
In contrast to models focused on peripheral neural pathways, Thayer and Lane’s (42) recent model of neuro-visceral integration credits the structures of the central autonomic network of brain nuclei (43) with the regulation of emotions and the expression of stress-related pathology. Similarly, building on the cognitive mechanisms of executive control proposed by Hilgard (44, 45) and neuropsychological foundations provided by Pribram (46, 47), Crawford’s neurophysiological model of hypnosis and imagery (48–51) suggests that the fronto-limbic attentional system directs shifts in neural activity, particularly inhibition, during hypnotic/imaginal modulation of perception in responsive individuals. In addition to specific regions of the prefrontal cortex (PFC), this system includes the anterior cingulate cortex (ACC), a midline frontal structure that is also directly implicated in anticipation of stress, pain perception, and clinical depression (52–54). These ideas are consonant with Damasio’s (55, 56) somatic marker hypothesis, which emphasizes hierarchical control and representation within the brain and reciprocal interactions among distributed neural networks. In particular, Damasio’s work and supporting research reviewed by Craig (52) suggest that higher-order representations of visceral states or response patterns encoded in the insular cortex (IC) and PFC (52, 55) and associated physiological responses and thought patterns represented within the ACC (55, 57) may provide accessible substrates for modification using mind-body therapies.
Whereas each of these models has contributed significantly to the understanding of how interactions of mind and body contribute to health and disease, individually each model concerns only a portion of the whole picture of how conditions within the body interact with psychological and environment circumstances and how mind-body oriented therapies may exert beneficial effects. We consider all of these models to be complementary and suggest that an instructive framework for future research on the psychophysiological mechanisms of mind-body therapies should include elements from each. We suggest that a network of central neural structures, which extends well beyond the hypothalamic mechanisms of stress/relaxation described by Benson (7, 33, 34), represents the principal neurophysiological substrate for mind-body therapies (25, 52, 58). In the following sections, we outline the basic components of an integrative theoretical framework, and, based on this framework, we propose that several specific neural mechanisms and related biomarkers may mediate the effects of mind-body therapies on physical and emotional health.
Critical Concepts for Understanding Mechanisms of Mind-Body Therapies
Before we describe our integrated framework, it is first necessary for us to define several concepts that are fundamental to any discussion of mind-body mechanisms. These are detailed below.
Definition of Mind
According to Damasio (55), “having a mind means that an organism forms neural representations that can become images, be manipulated in a process called thought, and eventually influence behavior by helping predict the future, plan accordingly, and choose the next action” (p. 90). Similarly, with regard to mind-body medicine, we define the mind as conscious and unconscious thought patterns, including images, perceptions, and intentions, generated by a functional network of distributed neural centers in the brain and body, including homeostatic representations that provide the context for human self-awareness and emotional experience (25, 39, 52, 56).
Mind-Body Medicine
Astin and colleagues (1) state that mind-body medicine includes “a variety of techniques designed to facilitate the mind’s capacity to affect bodily function and symptoms” (p. 133). More broadly, Spencer and Jacobs (59) defined mind-body therapies as “a group of therapies that emphasizes using the mind or brain in conjunction with the body to assist the healing process” (p. 578). In addition, we have found it useful in our research to draw a heuristic distinction between mind-body interventions that elicit primarily top-down mechanisms (i.e., top-down therapies) from those that elicit mainly bottom-up mechanisms (i.e., bottom-up therapies) (60, 61).
Top-Down and Bottom-Up Mechanisms
Top-down mechanisms are those initiated via mental processing at the level of the cerebral cortex. In the case of clinical hypnosis, imagery, or meditation, for example, we are primarily referring to conscious and intentional mental activities, although unconscious neural processes are also thought to be involved. In contrast, bottom-up mechanisms are initiated by stimulation of various somato-, viscero-, and chemo-sensory receptors (52, 62–64) that influence central neural processing and mental activities via ascending pathways from the periphery to the brainstem and cerebral cortex. However, we also stress that this taxonomy is an oversimplification and that all mind-body therapies actually involve a combination of both top-down and bottom-up mechanisms. Progressive muscle relaxation, for example, involves bottom-up pathways activated by peripheral sensory afferents responding to various visceral activities (e.g., reduced muscle tension and blood pressure) and top-down pathways activated by focused attention and the intention to relax. Similarly, yoga, which incorporates meditation, breathing, and physical practice, affects physiological and emotional states via direct effects on the CNS.
An Integrative Framework for Psychophysiological Aspects of Mind-Body Relationships
Our synthesis of the empirical and theoretical literature suggests that different therapies utilizing top-down and/or bottom-up mind-body mechanisms share the same fundamental set of physiological mechanisms, with bidirectional pathways providing lines of communication between mind/brain and body. In addition, the reciprocal feedback involved in these interactions suggests that corrective effects on clinical symptoms can be initiated by either top-down or bottom-up therapies. The basic components of our framework are summarized below.
Specific Cortical Regions Function as Substrates for Mind-Body Therapies
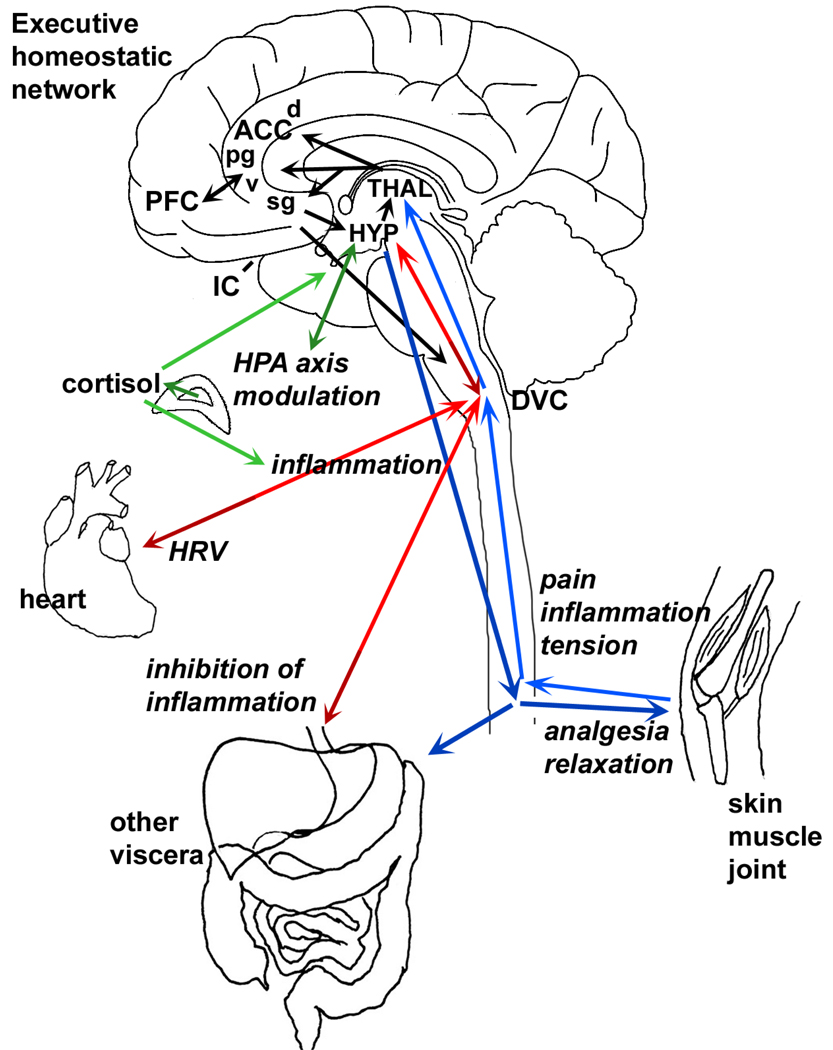
We propose that a functional cortical network of fronto-temporal structures (we refer to this network as the executive homeostatic network, or EHN (see figure 1), which includes the ACC, PFC, and IC, represents the principal neurophysiological substrate for mind-body therapies that involve physiological self-regulation, cognitive control, and/or suggestion (44, 46, 52, 58, 65). These cortical regions integrate information relevant to ongoing social, cognitive, emotional, and personality issues, psychological stress, and homeostatic challenges. The medial PFC, in particular, based on its output to autonomic control regions, is situated to serve as interface for the top-down emotional and social contributions of the ventral medial PFC (66, 67). Together, the structures of the EHN are well suited to mediate effects of mind-body therapies.
Figure 1. Bidirectional connections between “mind” and body.
Bottom-up pathways. Via parallel ascending projections within the spinal cord and brain, vagal sensory pathways (red arrows) and spinal visceral and somatic sensory pathways (blue arrows) provide information regarding inflammation, pain and other important conditions to regulatory brain regions including the hypothalamus (HYP) and thalamus (THAL) that ultimately reach components of the executive homeostatic network, comprised of the anterior cingulate cortex (ACC), prefrontal cortex (PFC) and insular cortex (IC, not visible in this view as it is located deep in the temporal lobe). This information regarding bodily states gives rise to the experience of symptoms associated with illness and injury.
Top-down pathways. The executive homeostatic network influences bodily functions via modulation of neuroendocrine output (green arrow), vagal output (red arrows) and spinal and sympathetic output (blue). The network consists of interconnected loops within and between the multiple levels (e.g., peripheral sensory, brainstem, thalamus, hypothalamus, cortex) and is highly simplified in this depiction. These output pathways provide the means for complementary therapies to ameliorate pain symptoms, inflammation, and other correlates of disease.
Four subregions of the ACC: dACC - dorsal ACC; pgACC - pregenual ACC; vACC - ventral ACC; sgACC - subgenual ACC.
HRV - heart rate variability.
DVC - dorsal vagal complex (comprised of visceral sensory relay and autonomic motor components).
Limbic, Central Autonomic, and Brainstem Viscerosensory Regions Integrate Information about Bodily Conditions and Psychological Challenges
To monitor visceral function and coordinate complex physiological and self-regulatory responses to maintain homeostasis, the structures of the EHN function as part of a reciprocal circuit with limbic areas, central autonomic regions of the thalamus, hypothalamus, and brainstem structures that directly regulate affective, autonomic, endocrine, and immune function (42, 61, 68, 69). These contributing brain regions include (but are not limited to) the hippocampus, septum, amygdala, bed nucleus of the stria terminalis, nucleus accumbens, paraventricular thalamus, paraventricular and perifornical areas of the hypothalamus, periaquiductal grey, locus coeruleus, raphe nuclei, and the dorsal vagal complex. Moreover, it is now well recognized that limbic and brainstem representations of visceral/organ function are important sources of ascending feedback to the executive centers of the brain (e.g., PFC, ACC) that enable these areas to integrate a range of physiological responses with mental activities, thus facilitating complex associative processes such as perception, behavioral intention, and planning (52, 55, 56, 70).
Mind-Body Connections Occur Bidirectionally via Peripheral Nerves and Hormones
Mind-body interactions are mediated by peripheral nerves associated with somatic and autonomic (sympathetic and parasympathetic) neural pathways. Somatic sensory nerves carry tactile, proprioceptive, and pain information from skin, striated muscles, and joints. Vagal viscerosensory nerves (parasympathetic) and spinal viscerosensory nerves (sympathetic) carry signals related to conditions within internal tissues such as metabolic state, infection, and inflammation. Reciprocal innervation by somatic and autonomic motor nerves provides the means by which the mind/brain influences bodily responses to ongoing situations or challenges. Reciprocal vagal pathways support transmission of psychophysiological information from peripheral visceral tissue/organs to the brainstem and upward to the EHN and back down. Optimal health depends upon efficient exchange of information between the periphery and the CNS and is closely associated with efferent vagal tone. Communication along the vagal pathways may be initiated, facilitated, or inhibited by hormones, immune-derived pro-inflammatory cytokines, and/or a variety of brain-derived neuropeptides and neurotransmitters; conversely, changes in mental processing (e.g., focused attention, perceived stress) generated in the EHN can rapidly be expressed in the body via descending modulation of the autonomic, neuroimmune, and neuroendocrine systems (27, 69, 71, 72). Additionally, circulating mediators such as hormones and immune-derived cytokines can influence the brain directly by interfacing with cerebral vasculature and circumventricular organs, where the blood-brain barrier is absent or weak.
Persistent Symptoms Are High-Order Cortical Representations of Dysregulated Physiological/Behavioral Response Patterns Encoded in Specific Areas of the EHN
We propose that clinical symptoms may be modified by mind-body therapies through several interrelated mechanisms, including: (a) activation of specific EHN structures, resulting in functional cortical (and subcortical) reorganization and improved interhemispheric balance; (b) more efficient cortical modulation of limbic and brainstem homeostatic centers and enhanced peripheral-central integration of information, expressed at the periphery as a change in autonomic (sympathovagal) balance and immune (pro-inflammatory cytokine profile) function; (c) re-patterning of primary interoceptive and higher-order homeostatic representations, resulting in more adaptive long-term psychophysiological responses as well as reduced expression of adverse symptoms; and (d) modulation of the epigenetic regulators (e.g., growth factors, hormones, histone function, DNA methylation) that can mediate cellular responses to environmental stress. In this way, mind-body therapies ameliorate symptoms via influence at multiple levels, from gene expression (cellular level) to the interaction of cortical brain regions that mediate systemic responses to internal and external challenges, including stress.
We propose that top-down mind-body therapies facilitate a coordinated shift in cerebral function involving reorganization of neural representations within the CNS (25, 52, 55, 57) and enhanced bidirectional communication between the regions of the cerebral cortex and the limbic and brainstem structures that regulate and pattern autonomic, neuroendocine, emotional, and behavioral activation (73). Conversely, certain bottom-up therapies (involving stimulation of peripheral sensory nerve fibers within the skin, muscle, blood vessels, viscera, and other tissue) may provide corrective feedback to the CNS, resulting in increased heart rate variability (HRV, a marker of autonomic function) reduced expression of pro-inflammatory cytokines, and functional changes in central neural processing. The present framework is a starting point toward elucidating the complex psychophysiological mechanisms of mind-body therapies, and further research is necessary to examine the mechanisms outlined in this paper. In the following section we identify components of the network that may be most amenable to study with current methodologies and pathological conditions in which modulation of the network may be effected via mind-body therapies.
Targets/Objectives for Mind-Body Therapies
The Executive Homeostatic Network (EHN): Shared Substrates of Mind-Body Therapies?
Based on the roles of the PFC, ACC, and IC in executive function and physiological representation, we propose that these areas of the brain operate together as the EHN. Furthermore, we suggest that this network provides the fundamental substrate for the willful self-regulation of physiological function via mind-body therapies (52, 65, 74). Thus, assessment of function in the EHN structures (the PFC, ACC, and IC) is necessary to delineate their role in pathology and in mediating the effects of mind-body therapies.
Prefrontal Cortex (PFC)
The PFC, which accounts for about 30% of the frontal lobe, is a large collection of interconnected subregions that send and receive direct projections from structures throughout the brain (75, 76). The PFC has been described as an important site for integration of mind and body and a basic component of the stress circuit (70, 77). Consistent with the present framework, in their integrative theory of PFC function, Miller and Cohen (76) state that the PFC organizes and executes intentional behavior through top-down processing “in situations when the mapping between sensory inputs, thoughts, and actions either are weakly established relative to other existing ones or are rapidly changing” (p. 168).
Both physical and psychological stressors have been found to impair PFC function (27, 78, 79). There is evidence that the left PFC is preferentially engaged in initial responses to stressors, while the right PFC becomes preferentially activated during prolonged stress (77). Preferential right frontal activation (relative right frontal asymmetry) has been associated with increased pain, depression, anxiety, hostility, and behavioral inhibition in addition to autonomic arousal, immune down-regulation, and activation of the hypothalamic-pituitary-adrenal (HPA) axis (80–84). In contrast, daily left PFC stimulation reduced depressive symptoms in patients with major depression (85). Moreover, chronic stress may alter or reverse normal patterns of PFC activation during cognitive tasks (53, 79, 86–88). Further, it has been proposed that impaired PFC function may constitute a critical link in the well established association of psychological depression and coronary artery disease (89). Thus, certain frontal asymmetries may precipitate and/or maintain stress-related pathology and, as argued by Sullivan and Gratton (77), “normalizing imbalances [authors’ emphasis] of prefrontal function in stress-related psychopathology must be considered central to successful therapies” (p. 107).
Anterior Cingulate Cortex (ACC)
The anterior cingulate cortex (ACC) occupies the long strip of cortex along the corpus callosum in the frontal lobe. The ventral regions of the ACC are also included in descriptions of ventromedial prefrontal cortex (vmPFC) or subgenual PFC (sgPFC). Functions of the ACC differ along its extent such that the dorsal ACC influences cognitive functions whereas ventral (subgenual) areas mediate cortical influences on autonomic and neuroendocrine functions.
A number of investigators have suggested that the ACC functions as a convergence zone for attentional and motivational information that is critical for self-regulation (42, 56). Research by Critchley and colleagues (25, 65) on the cortical representations of anticipation and self-directed relaxation (with “cognitive intent” and biofeedback) suggests that ACC activation reflects an “integration of cognitive states with adaptive changes in bodily states mediated by the autonomic nervous system” (p. 541) (25). Other studies have found ACC activation associated with perceived effort during exercise (90, 91) and cognitively driven increases and decreases in arousal (25, 92). Moreover, Davidson and colleagues (80) suggest that ACC activation may be a mechanism for effortful control leading to further processing by other brain regions. Indeed, there is evidence that the ACC and PFC operate jointly to modulate brain dynamics and recruit other brain regions to meet the attentional, emotional, and behavioral demands of various mind-body states (93, 94). For instance, Capuron and colleagues (95) have reported that, in immunotherapy patients receiving the cytokine interferon-α, challenge with a cognitive task was associated with activation of the dorsal ACC, which was not seen in the controls. Although the patients in the study reported fatigue and impaired concentration, they performed similarly to the controls, suggesting that activation of the ACC may have been recruited to compensate for a need to exert greater mental effort.
The ventral and perigenual region of the ACC, including parts of Brodman areas 24, 25 and 33, seems to process information related to autonomic and emotional functioning (96), based on its connections with autonomic and stress-related brain regions, and the success of deep brain stimulation (DBS) of the area for treatment-resistant depression (97). The convergence of autonomic and mood-related processing implicates the ventral ACC (along with adjacent areas of vmPFC) as a key nodal brain region integrating functions of “mind” with the control of bodily functions. That is: this area constitutes a potentially critical neurological target through which mind-body therapies can exert influence on health-related outcomes.
Insular Cortex (IC)
The IC is involved in the integration and representation of sensory and visceral information and higher-order representations of homeostasis, which have been proposed as a basis for self-awareness (52). Interestingly, the IC plays a key role in brain mechanisms that mediate empathy, in that it serves as an interface between the “mirror neurons” that subserve imitation, and the limbic areas that provide emotional content (98). Thus the insula may contribute to other-awareness as well.
Along with the ACC, the IC is believed to provide an interface between autonomic arousal and cognitive-motivational (volitional) behavior (92). Moreover, the IC has significance within the current framework because it functions as a relatively direct cortical target for central viscero-sensory pathways and, with the sg ACC, contributes to aspects of both vagal modulation and immune regulation (41, 69, 73). For example, patients with irritable bowel syndrome (IBS) are hypersensitive to visceral stimulation, and the anterior IC and ACC of IBS patients show increased activation during rectal distension, suggesting that plasticity in pain responses could be associated with enhanced responses in the IC and ACC (96). Further, an analysis of the pattern of activation within the brain networks that process viscerosensory stimuli and expectation in male and female IBS patients revealed gender differences in the activation of emotion/arousal networks, suggesting that modulation of these identified circuits could ameliorate symptoms, especially in women (99). Finally, recent findings suggest that hyperactivity of the IC may be a common feature of anxiety-prone individuals (100), further implicating the IC as a key interface between body states and mood.
Vagal Pathways of Mind-Body Communication
Positive associations between measures of cardiac-vagal tone (HRV) and adaptive physiological, emotional, and behavioral responses support (6, 27, 37, 42, 101) the idea that vagus nerve function may constitute a productive target for mind-body therapies (27). Although the parasympathetic nervous system (PNS) has traditionally been viewed as a motor (effector) or reflexive system, recent neurophysiological evidence implicates bidirectional vagal pathways in the expression of arousal and stress (27, 37, 42) and in immune-brain communication (41). Indeed, the vagus (10th cranial nerve) is composed of over 80% sensory (afferent) fibers that continuously relay information regarding peripheral visceral sensation, organ function, and immune-inflammatory status to the nucleus tractus solitarius (NTS), which in turn projects to various higher limbic and cortical centers (27, 41, 63, 69, 102, 103). The vagal system functions via ascending projections from peripheral receptors to limbic and anterior cortical structures and descending projections that modulate autonomic, visceral, and immune activation. Thus, the bidirectional vagal system provides an important pathway through which mind-body therapies may diminish stress-related symptoms encoded in the brain and expressed at the body (6, 40).
Inflammation
Inflammation occurs as a result of tissue damage, infection with pathogens, or by exposure to irritants. It is characterized by activation of immune cells that release chemical mediators, such as prostaglandins, cytokines, and chemokines, which coordinate local tissue responses and, via interaction with peripheral nerves or by transport in the general circulation, influence the brain to induce physiological and behavioral responses supportive of host defense. In the acute phase, these responses, which can involve fever, metabolic alterations, and mood symptoms, are beneficial, but, when chronic, inflammation becomes detrimental. Chronic inflammation is involved in the pathophysiology of many diseases, including heart disease, diabetes, autoimmunity, allergy, inflammatory bowel diseases, neurodegenerative disease, stroke, and epilepsy (104). Thus, mechanisms of inflammation are important targets for mind-body therapies.
Stress and Stress-Related Pathology
Based on the type of situation and the neural substrates involved, stress is conceptualized as two categories—psychological and systemic (104–106). Psychological (also termed “processive” or “exteroceptive”) stress reflects the objective demands on the individual in relation to his/her coping abilities as well as the perception of such demands and coping resources (107). In contrast, stress following physiological challenges such as injury, infection, or inflammation, is termed “systemic” or “interoceptive” stress. Both types of stressors can evoke similar patterns of physiological and behavioral responses, consistent with the goal of maintaining homeostasis (106). For instance, anticipation of threat, a mental activity initiated in the brain’s EHN, is generally accompanied by activation of the HPA axis and the sympathetic-adrenal (SA) system, resulting in the secretion of the stress hormones corticotropin-releasing hormone (CRH), cortisol, and norepinephrine (NE), and decrease in HRV (27, 37, 71, 108, 109). Although necessary in the short term to cope with stressors, over time, these responses, collectively termed the “fight or flight,” or stress response, can lead to multiple negative down-stream effects on the brain and body and contribute to a recurrent cycle of dysfunction (24, 27, 110). The cost to the body resulting from coping with stressors has been termed allostatic load (111, 112), the consequences of which can include autonomic dysfunction, neurodegeneration, depression, and cognitive impairment (27). Indeed, stress impairs adult neurogenesis in the hippocampus, aw well as mobilization and survival of new neurons, which is thought to contribute to neuropathologies including psychological depression, schizophrenia, and brain consequences of diabetes mellitus (113). McEwen (111) emphasizes, however, that effects of allostatic load are reversible if intervention occurs early enough. Reduction of allostatic load thus constitutes an important objective for mind-body therapies.
Neurological substrates of stress-related pathology map to the brain regions and pathways that constitute our proposed framework. Individuals with stress-related pathology, including depression, post-traumatic stress disorder (PTSD), anxiety, hypertension, IBS, and fibromyalgia, often exhibit disturbances in cerebral blood flow in the frontal and temporal regions of the brain, along with dysfunctional vagal, hormonal (i.e., HPA), and pro-inflammatory cytokine responses (24, 53, 108, 114–122). Moreover, there are data linking immune dysfunction to dysregulation of the PNS, functional imbalances in the PFC, and alterations in the cytokine network (69, 77, 123, 124). Further, though more speculatively, Craig (52) has suggested that mysterious pain syndromes such as fibromyalgia could be a result of dysfunction in the neural representations within a system responsible for homeostasis and associated with stress.
Experimental Approaches to Assess Mechanisms and Efficacy of Mind-Body Therapies
Evaluation of the efficacy of mind-body therapies requires objective outcome variables that reflect presence or amelioration of symptoms. Hence, due to the connection with the putative pathways of mind-body communication and mechanisms of mind-body therapies, we propose that functional mapping (e.g., EEG, fMRI, positron-emission tomography [PET]) of the EHN structures (i.e., PFC, ACC, and IC), HRV analysis, inflammatory marker profiles (e.g., pro-inflammatory cytokines), and endocrine markers (cortisol, adrenal monoamines) represent promising biomarkers for future research seeking to examine specific physiological mechanisms of mind-body therapies. We review evidence that mind-body therapies can be associated with functional changes in the brain, as assessed with functional neuroimaging techniques such as PET, EEG, and fMRI, and evidence supporting the utility of HRV and pro-inflammatory markers as objective indices of mind-body integration within the network. The significance of these psychophysiological measures lies in these measures’ status as important risk factors for many clinical symptoms and diseases, association with the proposed mechanisms of stress-related illness, and functional role in mind-body communication via the vagus nerve.
Functional Neuroimaging as a Window to Brain Function
Neuroimaging studies of top-down mind-body therapies have consistently demonstrated changes in the pattern of activation in the PFC, ACC, and IC associated with the therapies. Using PET, two studies (94, 125) found increased activation in the left PFC and right ACC following typical hypnotic inductions. Similar results were found in meditation studies using fMRI (126) and single photon emission computerized tomography (SPECT) (127) and in two biofeedback studies using fMRI (65, 92). Furthermore, deactivation of the ACC was reported in neurophysiological research using hypnotic analgesia to reduce pain (94, 128, 129) and following hypnotic suggestions to reduce the feelings of effort associated with physical and mental stressors (91). Activation of the PFC and IC are consistent with cerebral reorganization in response to engagement of effortful attention and imagery representation in working memory, while ACC activation is likely to reflect enhanced modulation of limbic and brainstem centers that directly regulate emotion-motivation and autonomic function (125).
As noted previously, imbalances in PFC activation are associated with affective symptoms and inadequate responses to stress. Many studies have reported shifts in frontal hemispheric EEG dominance during meditation, hypnosis, and imagery, although the specific regional patterns of EEG activation depend upon the particular components of the therapeutic intervention (49, 130–134). Relative decreases in right-sided PFC activation were also found among patients with major depression following treatment with interpersonal psychotherapy (135), sessions of psychotherapy (136), and with neurofeedback (brainwave biofeedback) (137). Finally, studies by Jones and colleagues (138, 139) have demonstrated functional alterations in frontal EEG hemispheric asymmetry following massage therapy. Specifically, decreased right-sided EEG activation was found following massage sessions in both infants and adolescents, indicating that massage can elicit cortical reorganization (140). These studies, taken together, support the idea that the efficacy of mind-body therapies may involve improvement in PFC asymmetry.
Using quantitative EEG, another window into functional brain dynamics, studies have also observed increased frontal and prefrontal alpha- and theta-wave activity during hypnosis (141) and meditation (127, 142–144). This can be interpreted to represent activation in brainstem-limbic (145) and ACC-PFC (93) networks, respectively. Interestingly, Kubota and colleagues (144) found a meditative state marked by frontal midline theta activity associated with significantly increased HRV, a marker of vagal tone (see next section). Further, Travis and colleagues (146) show that in college students (presumed to be a stressed population) meditation training improved some measures of stress, including sleep and EEG findings, indicative of increased efficiency of frontal lobe processing. In addition, another recent study found increased frontal EEG activation associated with changes in HRV during hypnotic relaxation and anxiety (147), underscoring the association between frontal activation and autonomic function.
Whereas subcortical structures form the connection between brainstem autonomic structures and the EHN, few studies have assessed the response of subcortical structures to mind-body therapies. However, a recent study by Ouchi and colleagues (148) reported a correlation between regional cerebral blood flow (assessed by PET) in the amygdala, basal forebrain, and hypothalamus, and increased parasympathetic activity (HRV) in the context of back massage. These findings buttress the idea that central autonomic regions play an important role in efficacy of mind-body therapies and underline the importance of further research into subcortical constituents of the homeostatic network that we propose mediates the effects of mind-body therapies.
Heart Rate Variability as a Marker of Vagal Tone
Standardized measures of HRV are considered valid and reliable makers of vagal modulation of the cardiac SA node (37, 149) and have gained acceptance as markers of stress and stress vulnerability (37, 101, 150). By definition, HRV is the cyclic variation in beat-to-beat interval (duration between successive heartbeats). This variability is influenced by multiple inputs, including the firing of cardiac-vagal efferents originating in the medulla nucleus ambiguus (37, 149, 150), as well as by sympathetic and endocrine influences on intrinsic cardiac neurons. In general, increased vagal activity is associated with increased HRV, whereas sympathetic activity and stress are associated with reduced HRV. Studies have consistently observed reduced HRV during and following acute and chronic stress (109, 151–153), and investigators have reported diminished HRV among men and women exhibiting depression (154), anxiety (155), PTSD (116), chronic fatigue syndrome (156), and hypertension (121). Additionally, prospective studies have found measures of HRV to be predictive of cardiovascular disease, diabetes, and sudden death (6, 27, 149, 150, 157, 158). Because cardiac-vagal tone is generated by the structures of the central autonomic network (including the PFC, ACC, IC) (38, 42, 68, 159), Thayer and Lane (42) propose that HRV is a marker of central-peripheral neural integration and homeostasis.
Several studies investigating the mechanisms of top-down interventions have revealed increased HRV during and following these therapies. Increases in HRV were found following autogenic training (160), progressive muscle relaxation (11), and yoga practice (4), although some studies also have found increased sympathetic tone (144, 161). In addition, several studies have reported increased HRV following hypnotic relaxation (162–164), and Carney and colleagues (165) observed increased HRV among clinically depressed patients with coronary heart disease following 16 sessions of individual cognitive-behavioral psychotherapy.
Therapies using bottom-up pathways have also been shown to positively influence autonomic (e.g., vagal) tone. For instance, various types of bodywork and massage have been found to increase vagal activity and HRV (166–168). Delaney and colleagues (169) reported increased HRV following myofascial trigger point therapy in a healthy sample of men and women. Similarly, two recent studies reported increased HRV in healthy men following treatment with needle acupuncture (170, 171). In preliminary research, direct electrical vagal stimulation has been found effective for the treatment of clinical pain and depression (103, 172, 173), although Rush and colleagues (174) reported that their study did not yield definitive evidence of short-term efficacy for adjunctive vagus nerve stimulation in treatment-resistant depression. Fallen and colleagues (175) have demonstrated increased HRV following stimulation of several different branches of the vagus nerve. Finally, animal studies suggest that various types of tactile stimulation can facilitate the expression and release of neuropeptides (e.g., oxytocin, nitric oxide, opioids) implicated in the central and peripheral modulation of relaxation and cardiac-vagal tone (161, 168, 176).
Taken together, these studies support the idea that mind-body therapies modulate autonomic function and indicate the utility of HRV in assessing potential mechanisms by which the therapies exert beneficial effects on stress and, potentially, inflammation.
Assessment of Markers of Inflammation
Pro-inflammatory cytokines, including interleukin-1β (IL-1β), interleukin-6 (IL-6), and tumor necrosis factor (TNF-α), are large peptide signaling molecules that are secreted by immune and neural cells in response to both physical (i.e., pathogens) and psychological stressors (177, 178). These cytokines coordinate peripheral immune responses, acute phase responses, and hematopoiesis; promote the release of various neurotransmitters (178); and are implicated in the pathogenesis of cardiovascular disease, diabetes, neurodegenerative disease, chronic pain, and depression (108, 178). The pro-inflammatory cytokines IL-1β, IL-6, and TNF-α rapidly signal the brain regarding immune activity in the periphery via multiple mechanisms, including the vagus nerve (41, 179); are able to induce glucocorticoid resistance (180); and are potent stimulators of the HPA (71, 181). These pro-inflammatory cytokines also contribute to central autonomic dysfunction, endothelial damage, and joint degeneration (71, 108, 182, 183). Peripheral and brain cytokines are thought to play a critical role in mood-related symptoms (especially depression), which are frequently associated with acute infections and chronic illnesses, including cancer, heart disease, diabetes, and autoimmunity (6, 123, 184–186).
Because pro-inflammatory cytokines are signaling molecules that provide feedback to the CNS, we propose that excessive expression and secretion of these cytokines suggest impairment in central to peripheral integration and balance (32). An inverse relationship has been found between HRV and both TNF-α and IL-6 (187, 188), which suggests an inhibitory relationship between vagal tone and the production of pro-inflammatory cytokines. This is also consistent with animal studies that found vagus stimulation to inhibit cytokine production and cytokine-mediated immune and inflammatory responses (189–191) and raises the possibility that therapies that increase vagal tone may exert beneficial effects on inflammation. To date, however, the effects of mind-body therapies on cytokine function are not well documented, although a few small studies have reported evidence of reduced production of pro-inflammatory cytokines following top-down interventions. For instance, Weber and colleagues (12) found reduced TNF-α following 10 weeks of relaxation therapy among patients with tinnitus, and other studies have reported reduced IL-1 (14, 81) following sessions of hypnosis and relaxation. Although a few studies have shown that massage therapy can alter some immune parameters (167, 192), the authors are unaware of any human research studies that have assessed the effects of massage on circulating pro-inflammatory cytokines. Thus, given the prominent role of inflammation in pathology within the body and brain, studies investigating the influence of mind-body therapies on cytokine expression are needed.
Based on the association of stress- and inflammation-related pathology and the EHN, we suggest that these disorders are associated with functional disturbances at multiple levels of the neuraxis; that adverse emotional and physical response patterns (symptoms) associated with these disorders are represented in the structures of the EHN and associated brain regions; and that HRV and pro-inflammatory cytokine profiles provide markers of homeostasis in the context of these systems. It is important to emphasize, however, that mind-body therapies include a wide variety of techniques that range from simple muscular relaxation to diverse combinations of muscle relaxation, controlled respiration as in yoga practice, imagery, visualization, and suggestion, to different types of visceral stimulation. Therefore, although the present framework describes a set of structures and mechanisms associated with homeostasis and balance that appear to represent the major common substrate for mind-body therapies, specific mind-body interventions and treatment strategies will have somewhat differing effects on cortical and subcortical activation, autonomic regulation of the organs (e.g., sympathovagal balance at cardiac SA node), and cellular immunity (134, 193, 194). Taken together, the findings noted here provide ample evidence supporting the idea that mind-body therapies can influence parameters of autonomic, brain, and immune function, and that established outcome measures can provide critical validation of the efficacy and mechanisms of mind-body therapies.
Conclusion
In this paper we have noted current research approaches that we view as useful for research on neural and biological substrates of therapeutic efficacy, and we have indicated specific targets (i.e., the EHN) that are particularly amenable to assessment of a number of roles in brain and physiological responses to mind-body therapies. We suggest that symptoms of stress are manifested as functional disturbances within the structures of the EHN (e.g., imbalances in PFC activation and higher-order visceral representations encoded in the PFC, IC, and ACC) and that these central disturbances are expressed at the periphery as reduced HRV and increased expression of pro-inflammatory cytokines. Conversely, we suggest that effective mind-body interventions should induce demonstrable functional changes such as shifts in regional EHN activation or increased HRV and reduced cytokine production. However, it is basic to our framework that the underlying substrates of mind-body therapies manifest at multiple levels of the neuraxis, including specific fronto-temporal cortical regions (EHN) that interact reciprocally with subcortical structures involved in bodily homeostasis and responses to stress. Thus, we advocate further research attention into activity of subcortical constituents of the network in the context of mind-body therapies. It is emphasized that bidirectional autonomic and neuroendocrine pathways serve as mind-body pathways between the CNS and the periphery and facilitate the expression of affective, autonomic, hormonal, and immune responses challenges and therapies. In this way, the theoretical framework presented here represents an integrative model to use in investigating and seeking to understand the mechanisms by which mind-body therapies enhance mental and physiological functioning.
Acknowledgments
This publication was made possible by grant numbers 5-T32-AT000052, 5-K30-AT000060, and 5-K07- AT002943 from the National Center for Complementary and Alternative Medicine (NCCAM) and grant number 5-R01-MH68832 from the National Institute of Mental Health (NIMH) at the National Institutes of Health. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NCCAM or NIMH.
References
- 1.Astin JA, Shapiro SL, Eisenberg DM, Forys KL. Mind-body medicine: state of the science, implications for practice. J Am Board Fam Pract. 2003;16(2):131–147. doi: 10.3122/jabfm.16.2.131. [DOI] [PubMed] [Google Scholar]
- 2.Carlson CR, Hoyle RH. Efficacy of abbreviated progressive muscle relaxation training: a quantitative review of behavioral medicine research. J Consult Clin Psychol. 1993;61(6):1059–1067. doi: 10.1037//0022-006x.61.6.1059. [DOI] [PubMed] [Google Scholar]
- 3.Galper DI, Taylor AG, Cox DJ. Current status of mind-body interventions for vascular complications of diabetes. Fam Community Health. 2003;26(1):34–40. doi: 10.1097/00003727-200301000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Innes KE, Bourguignon C, Taylor AG. Risk indices associated with the insulin resistance syndrome, cardiovascular disease, and possible protection with yoga: a systematic review. J Am Board Fam Pract. 2005;18(6):491–519. doi: 10.3122/jabfm.18.6.491. [DOI] [PubMed] [Google Scholar]
- 5.Innes KE, Vincent HK. The influence of yoga-based programs on risk profiles in adults with type 2 diabetes mellitus: a systematic review. Evid Based Complement Alternat Med. 2007;4(4):469–486. doi: 10.1093/ecam/nel103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Innes KE, Vincent HK, Taylor AG. Chronic stress and insulin resistance-related indices of cardiovascular disease risk, part I: neurophysiological responses and pathological sequelae. Altern Ther Health Med. 2007;13(4):46–52. [PubMed] [Google Scholar]
- 7.Jacobs GD. The physiology of mind-body interactions: the stress response and the relaxation response. J Altern Complement Med. 2001;7 Suppl 1:S83–S92. doi: 10.1089/107555301753393841. [Erratum appears in J Altern Complement Med 2002 Apr;8(2):219.] [DOI] [PubMed] [Google Scholar]
- 8.Manber R, Allen JJ, Morris MM. Alternative treatments for depression: empirical support and relevance to women. J Clin Psychiatry. 2002;63(7):628–640. doi: 10.4088/jcp.v63n0716. [DOI] [PubMed] [Google Scholar]
- 9.Montgomery GH, DuHamel KN, Redd WH. A meta-analysis of hypnotically induced analgesia: how effective is hypnosis? Int J Clin Exp Hypn. 2000;48(2):138–153. doi: 10.1080/00207140008410045. [DOI] [PubMed] [Google Scholar]
- 10.Taylor AG, Galper DI, D'Huyvetter K, Bourguignon C, Lyon DE. Pain. In: Spencer JW, Jacobs JJ, editors. Complementary and Alternative Medicine: an Evidenced-Based Approach. 2nd ed. St. Louis, MO: Mosby, Inc; 2003. pp. 311–408. [Google Scholar]
- 11.Lucini D, Covacci G, Milani R, Mela GS, Malliani A, Pagani M. A controlled study of the effects of mental relaxation on autonomic excitatory responses in healthy subjects. Psychosom Med. 1997;59(5):541–552. doi: 10.1097/00006842-199709000-00012. [DOI] [PubMed] [Google Scholar]
- 12.Weber C, Arck P, Mazurek B, Klapp BF. Impact of a relaxation training on psychometric and immunologic parameters in tinnitus sufferers. J Psychosom Res. 2002;52(1):29–33. doi: 10.1016/s0022-3999(01)00281-1. [DOI] [PubMed] [Google Scholar]
- 13.Kiecolt-Glaser JK, Marucha PT, Atkinson C, Glaser R. Hypnosis as a modulator of cellular immune dysregulation during acute stress. J Consult Clin Psychol. 2001;69(4):674–682. doi: 10.1037//0022-006x.69.4.674. [DOI] [PubMed] [Google Scholar]
- 14.Wood GJ, Bughi S, Morrison J, Tanavoli S, Tanavoli S, Zadeh HH. Hypnosis, differential expression of cytokines by T-cell subsets, and the hypothalamo-pituitary-adrenal axis. Am J Clin Hypn. 2003;45(3):179–196. doi: 10.1080/00029157.2003.10403525. [DOI] [PubMed] [Google Scholar]
- 15.Lutgendorf S, Logan H, Kirchner HL, et al. Effects of relaxation and stress on the capsaicin-induced local inflammatory response. Psychosom Med. 2000;62(4):524–534. doi: 10.1097/00006842-200007000-00011. [DOI] [PubMed] [Google Scholar]
- 16.Zachariae R, Jorgensen MM, Egekvist H, Bjerring P. Skin reactions to histamine of healthy subjects after hypnotically induced emotions of sadness, anger, and happiness. Allergy. 2001;56(8):734–740. doi: 10.1034/j.1398-9995.2001.056008734.x. [DOI] [PubMed] [Google Scholar]
- 17.Saydah SH, Eberhardt MS. Use of complementary and alternative medicine among adults with chronic diseases: United States 2002. J Altern Complement Med. 2006;12(8):805–812. doi: 10.1089/acm.2006.12.805. [DOI] [PubMed] [Google Scholar]
- 18.Eisenberg DM, Davis RB, Ettner SL, et al. Trends in alternative medicine use in the United States, 1990–1997: results of a follow-up national survey. JAMA. 1998;280(18):1569–1575. doi: 10.1001/jama.280.18.1569. [DOI] [PubMed] [Google Scholar]
- 19.Cuellar N, Galper DI, Taylor AG, D'Huyvetter K, Miederhoff P, Stubbs P. Restless legs syndrome. J Altern Complement Med. 2004;10(3):422–423. doi: 10.1089/1075553041323786. [DOI] [PubMed] [Google Scholar]
- 20.Owens JE, Taylor AG, Degood D. Complementary and alternative medicine and psychologic factors: toward an individual differences model of complementary and alternative medicine use and outcomes. J Altern Complement Med. 1999;5(6):529–541. doi: 10.1089/acm.1999.5.529. [DOI] [PubMed] [Google Scholar]
- 21.NIH Technology Assessment Panel on Integration of Behavioral and Relaxation Approaches into the Treatment of Chronic Pain and Insomnia. Integration of behavioral and relaxation approaches into the treatment of chronic pain and insomnia. JAMA. 1996;276(4):313–318. doi: 10.1001/jama.1996.03540040057033. [DOI] [PubMed] [Google Scholar]
- 22.Watkins AD. Contemporary context of complementary and alternative medicine: integrated mind-body medicine. In: Micozzi MS, editor. Fundamentals of Complementary and Alternative Medicine. New York: Churchill Livingston; 1996. pp. 49–63. [Google Scholar]
- 23.Barnes P, Powell-Griner E, McFann K, Nahin R. CDC Advance Data Report #343. Complementary and Alternative Medicine Use among Adults: United States, 2002. [Accessed. July 26, 2008];2004 May 27; Available at: http://nccam.nih.gov/news/camstats.htm. [PubMed]
- 24.Bremner JD. Understanding Trauma-Related Disorders from a Mind-Body Perspective. New York: WW Norton; 2002. Does Stress Damage the Brain? [Google Scholar]
- 25.Critchley HD, Mathias CJ, Dolan RJ. Neural activity in the human brain relating to uncertainty and arousal during anticipation. Neuron. 2001;29(2):537–545. doi: 10.1016/s0896-6273(01)00225-2. [DOI] [PubMed] [Google Scholar]
- 26.Fiero PL, Galper DI, Cox DJ, Phillips LH, 2nd, Fryburg DA. Thermal biofeedback and lower extremity blood flow in adults with diabetes: is neuropathy a limiting factor? Appl Psychophysiol Biofeedback. 2003;28(3):193–203. doi: 10.1023/a:1024681113746. [DOI] [PubMed] [Google Scholar]
- 27.Innes KE, Vincent HK, Taylor AG. Chronic stress and insulin resistance-related indices of cardiovascular disease risk, part 2: a potential role for mind-body therapies. Altern Ther Health Med. 2007;13(5):44–51. [Erratum appears in Altern Ther Health Med 2007 Nov–Dec;13(6):15.] [PubMed] [Google Scholar]
- 28.Menzies V, Taylor AG, Bourguignon C. Effects of guided imagery on outcomes of pain, functional status, and self-efficacy in persons diagnosed with fibromyalgia. J Altern Complement Med. 2006;12(1):23–30. doi: 10.1089/acm.2006.12.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Menzies V, Taylor AG, Bourguignon C. Absorption: an individual difference to consider in mind-body interventions. J Holist Nurs. 2008;26(4):297–302. doi: 10.1177/0898010107307456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taibi DM, Bourguignon C. The role of complementary and alternative therapies in managing rheumatoid arthritis. Fam Community Health. 2003;26(1):41–52. doi: 10.1097/00003727-200301000-00006. [DOI] [PubMed] [Google Scholar]
- 31.Taylor AG, Galper DI, Taylor P, et al. Effects of adjunctive Swedish massage and vibration therapy on short-term postoperative outcomes: a randomized, controlled trial. J Altern Complement Med. 2003;9(1):77–89. doi: 10.1089/107555303321222964. [DOI] [PubMed] [Google Scholar]
- 32.Sternberg EM. The Balance within: the Science Connecting Health and Emotions. New York: WH Freeman; 2001. [Google Scholar]
- 33.Benson H. The relaxation response: its subjective and objective historical precedents and physiology. Trends Neurosci. 1983;6:281–284. [Google Scholar]
- 34.Benson H, Klipper MZ. The Relaxation Response. New York: HarperCollins; 2000. [Google Scholar]
- 35.Benson H, Beary J, Carol MP. The relaxation response. Psychiatry. 1974;37:37–46. doi: 10.1080/00332747.1974.11023785. [DOI] [PubMed] [Google Scholar]
- 36.Hoffman JW, Benson H, Arns PA, et al. Reduced sympathetic nervous system responsivity associated with the relaxation response. Science. 1982;(215):190–192. doi: 10.1126/science.7031901. [DOI] [PubMed] [Google Scholar]
- 37.Porges SW. Cardiac vagal tone: a physiological index of stress. Neurosci Biobehav Rev. 1995;19(2):225–233. doi: 10.1016/0149-7634(94)00066-a. [DOI] [PubMed] [Google Scholar]
- 38.Porges SW. The polyvagal theory: phylogenetic substrates of a social nervous system. Int J Psychophysiol. 2001;42(2):123–146. doi: 10.1016/s0167-8760(01)00162-3. [DOI] [PubMed] [Google Scholar]
- 39.LeDoux JE. Synaptic Self: How Our Brains Become Who We Are. New York: Viking; 2002. [Google Scholar]
- 40.Zagon A. Does the vagus nerve mediate the sixth sense? Trends Neurosci. 2001;24(11):671–673. doi: 10.1016/s0166-2236(00)01929-9. [DOI] [PubMed] [Google Scholar]
- 41.Goehler LE, Gaykema RP, Hansen MK, Anderson K, Maier SF, Watkins LR. Vagal immune-to-brain communication: a visceral chemosensory pathway. Auton Neurosci. 2000;85(1–3):49–59. doi: 10.1016/S1566-0702(00)00219-8. [DOI] [PubMed] [Google Scholar]
- 42.Thayer JF, Lane RD. A model of neurovisceral integration in emotion regulation and dysregulation. J Affect Disord. 2000;61(3):201–216. doi: 10.1016/s0165-0327(00)00338-4. [DOI] [PubMed] [Google Scholar]
- 43.Benarroch EE. Central Autonomic Network: Functional Organization and Clinical Correlations. Armonk, NY: Futura Pub.; 1997. [Google Scholar]
- 44.Hilgard ER. Hypnotic Susceptibility. New York: Harcourt, Brace & World; 1965. [Google Scholar]
- 45.Hilgard ER. Divided Consciousness: Multiple Controls in Human Thought and Action. New York: Wiley; 1977. [Google Scholar]
- 46.Pribram KH. Brain and Perception: Holonomy and Structure in Figural Processing. Hillsdale, NJ: Lawrence Erlbaum Associates; 1991. [Google Scholar]
- 47.Pribram KH, McGuinness D. Arousal, activation, and effort in the control of attention. Psychol Rev. 1975;82(2):116–149. doi: 10.1037/h0076780. [DOI] [PubMed] [Google Scholar]
- 48.Crawford HJ. Brain dynamics and hypnosis: attentional and disattentional processes. Int J Clin Exp Hypn. 1994;42(3):204–232. doi: 10.1080/00207149408409352. [DOI] [PubMed] [Google Scholar]
- 49.Crawford HJ. Cerebral brain dynamics of mental imagery: evidence and issues for hypnosis. In: Kunzendorf RJ, Spanos M, Wallace B, editors. Hypnosis and Imagination. Amityville, NY: Baywood Publishing; 1996. pp. 253–283. [Google Scholar]
- 50.Crawford HJ, Brown AM, Moon CE. Sustained attentional and disattentional abilities: differences between low and highly hypnotizable persons. J Abnorm Psychol. 1993;102(4):534–543. doi: 10.1037//0021-843x.102.4.534. [DOI] [PubMed] [Google Scholar]
- 51.Crawford HJ, Gur RC, Skolnick B, Gur RE, Benson DM. Effects of hypnosis on regional cerebral blood flow during ischemic pain with and without suggested hypnotic analgesia. International Journal of Psychophysiology. 1993;15(3):181–195. doi: 10.1016/0167-8760(93)90002-7. [DOI] [PubMed] [Google Scholar]
- 52.Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci. 2002;3(8):655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- 53.Naliboff BD, Derbyshire SW, Munakata J, et al. Cerebral activation in patients with irritable bowel syndrome and control subjects during rectosigmoid stimulation. Psychosom Med. 2001;63(3):365–375. doi: 10.1097/00006842-200105000-00006. [DOI] [PubMed] [Google Scholar]
- 54.Pizzagalli D, Pascual-Marqui RD, Nitschke JB, et al. Anterior cingulate activity as a predictor of degree of treatment response in major depression: evidence from brain electrical tomography analysis. Am J Psychiatry. 2001;158(3):405–415. doi: 10.1176/appi.ajp.158.3.405. [DOI] [PubMed] [Google Scholar]
- 55.Damasio AR. Descartes' Error: Emotion, Reason, and the Human Brain. New York: Putnam; 1994. [Google Scholar]
- 56.Damasio AR. The Feeling of What Happens: Body and Emotion in the Making of Consciousness. New York: Harcourt Brace; 1999. [Google Scholar]
- 57.Critchley HD, Mathias CJ, Dolan RJ. Neuroanatomical basis for first- and second-order representations of bodily states. Nat Neurosci. 2001;4(2):207–212. doi: 10.1038/84048. [DOI] [PubMed] [Google Scholar]
- 58.Frith CD, Friston K, Liddle PF, Frackowiak RS. Willed action and the prefrontal cortex in man: a study with PET. Proc R Soc Lond B Bio Sci. 1991;244(1311):241–246. doi: 10.1098/rspb.1991.0077. [DOI] [PubMed] [Google Scholar]
- 59.Spencer JW, Jacobs JJ. Complementary and Alternative Medicine: an Evidence-Based Approach. St. Louis, MO: Mosby; 2003. [Google Scholar]
- 60.Hugdahl K. Psychophysiology: The Mind-Body Perspective. Cambridge, MA: Harvard University Press; 1995. [Google Scholar]
- 61.Mayberg HS, Liotti M, Brannan SK, et al. Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. Am J Psychiatry. 1999;156(5):675–682. doi: 10.1176/ajp.156.5.675. [DOI] [PubMed] [Google Scholar]
- 62.Berthoud HR, Neuhuber WL. Functional and chemical anatomy of the afferent vagal system. Auton Neurosci. 2000;85(1–3):1–17. doi: 10.1016/S1566-0702(00)00215-0. [DOI] [PubMed] [Google Scholar]
- 63.Janig W. Neurobiology of visceral afferent neurons: neuroanatomy, functions, organ regulations and sensations. Biol Psychol. 1996;42(1–2):29–51. doi: 10.1016/0301-0511(95)05145-7. [DOI] [PubMed] [Google Scholar]
- 64.Verberne AJ, Saita M, Sartor DM. Chemical stimulation of vagal afferent neurons and sympathetic vasomotor tone. Brain Res Brain Res Rev. 2003;41(2–3):288–305. doi: 10.1016/s0165-0173(02)00269-2. [DOI] [PubMed] [Google Scholar]
- 65.Critchley HD, Melmed RN, Featherstone E, Mathias CJ, Dolan RJ. Brain activity during biofeedback relaxation: a functional neuroimaging investigation. Brain. 2001;124(Pt 5):1003–1012. doi: 10.1093/brain/124.5.1003. [DOI] [PubMed] [Google Scholar]
- 66.van den Bos W, McClure SM, Harris LT, Fiske ST, Cohen JD. Dissociating affective evaluation and social cognitive processes in the ventral medial prefrontal cortex. Cogn Affect Behav Neurosci. 2007;7(4):337–346. doi: 10.3758/cabn.7.4.337. [DOI] [PubMed] [Google Scholar]
- 67.Wong SW, Masse N, Kimmerly DS, Menon RS, Shoemaker JK. Ventral medial prefrontal cortex and cardiovagal control in conscious humans. Neuroimage. 2007;35(2):698–708. doi: 10.1016/j.neuroimage.2006.12.027. [DOI] [PubMed] [Google Scholar]
- 68.Drevets WC. Prefrontal cortical-amygdalar metabolism in major depression. Ann N Y Acad Sci. 1999;877:614–637. doi: 10.1111/j.1749-6632.1999.tb09292.x. [DOI] [PubMed] [Google Scholar]
- 69.Haas HS, Schauenstein K. Neuroimmunomodulation via limbic structures--the neuroanatomy of psychoimmunology. Prog Neurobiol. 1997;51(2):195–222. doi: 10.1016/s0301-0082(96)00055-x. [DOI] [PubMed] [Google Scholar]
- 70.Van Eden CG, Buijs RM. Functional neuroanatomy of the prefrontal cortex: autonomic interactions. Prog Brain Res. 2000;126:49–62. doi: 10.1016/S0079-6123(00)26006-8. [DOI] [PubMed] [Google Scholar]
- 71.Gold PW, Chrousos GP. Organization of the stress system and its dysregulation in melancholic and atypical depression: high vs low CRH/NE states. Mol Psychiatry. 2002;7(3):254–275. doi: 10.1038/sj.mp.4001032. [DOI] [PubMed] [Google Scholar]
- 72.Janig W, Habler HJ. Specificity in the organization of the autonomic nervous system: a basis for precise neural regulation of homeostatic and protective body functions. Prog Brain Res. 2000;122:351–367. doi: 10.1016/s0079-6123(08)62150-0. [DOI] [PubMed] [Google Scholar]
- 73.Saper CB. The central autonomic nervous system: conscious visceral perception and autonomic pattern generation. Annu Rev Neurosci. 2002;25:433–469. doi: 10.1146/annurev.neuro.25.032502.111311. [DOI] [PubMed] [Google Scholar]
- 74.Beauregard M, Levesque J, Bourgouin P. Neural correlates of conscious self-regulation of emotion. J Neurosci. 2001;21(18):R165. doi: 10.1523/JNEUROSCI.21-18-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Buijs RM, Van Eden CG. The integration of stress by the hypothalamus, amygdala and prefrontal cortex: balance between the autonomic nervous system and the neuroendocrine system. Prog Brain Res. 2000;126:117–132. doi: 10.1016/S0079-6123(00)26011-1. [DOI] [PubMed] [Google Scholar]
- 76.Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- 77.Sullivan RM, Gratton A. Prefrontal cortical regulation of hypothalamic-pituitary-adrenal function in the rat and implications for psychopathology: side matters. Psychoneuroendocrinology. 2002;27(1–2):99–114. doi: 10.1016/s0306-4530(01)00038-5. [DOI] [PubMed] [Google Scholar]
- 78.Arnsten AF. Stress impairs prefrontal cortical function in rats and monkeys: role of dopamine D1 and norepinephrine alpha-1 receptor mechanisms. Prog Brain Res. 2000;126:183–192. doi: 10.1016/S0079-6123(00)26014-7. [DOI] [PubMed] [Google Scholar]
- 79.Papousek I, Schulter G. Associations between EEG asymmetries electrodermal lability in low vs. high depressive and anxious normal individuals. Int J Psychophysiol. 2001;41(2):105–117. doi: 10.1016/s0167-8760(01)00131-3. [DOI] [PubMed] [Google Scholar]
- 80.Davidson RJ, Pizzagalli D, Nitschke JB, Putnam K. Depression: perspectives from affective neuroscience. Annu Rev Psychol. 2002;53:545–574. doi: 10.1146/annurev.psych.53.100901.135148. [DOI] [PubMed] [Google Scholar]
- 81.Gruzelier J, Clow A, Evans P, Lazar I, Walker L. Mind-body influences on immunity: lateralized control, stress, individual differences predictors, and prophylaxis. Ann N Y Acad Sci. 1998;851:487–494. doi: 10.1111/j.1749-6632.1998.tb09027.x. [DOI] [PubMed] [Google Scholar]
- 82.Pauli P, Wiedemann G, Nickola M. Pain sensitivity, cerebral laterality, and negative affect. Pain. 1999;80(1–2):359–364. doi: 10.1016/s0304-3959(98)00231-0. [DOI] [PubMed] [Google Scholar]
- 83.Wittling W. The right hemisphere and the human stress response. Acta Physiol Scand. 1997 Suppl 640:55–59. [PubMed] [Google Scholar]
- 84.Wittling W, Block A, Schweiger E, Genzel S. Hemisphere asymmetry in sympathetic control of the human myocardium. Brain Cogn. 1998;38(1):17–35. doi: 10.1006/brcg.1998.1000. [DOI] [PubMed] [Google Scholar]
- 85.Bortolomasi M, Minelli A, Fuggetta G, et al. Long-lasting effects of high frequency repetitive transcranial magnetic stimulation in major depressed patients. Psychiatry Res. 2007;150(2):181–186. doi: 10.1016/j.psychres.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 86.Davidson RJ. Affective style, psychopathology, and resilience: brain mechanisms and plasticity. Am Psychol. 2000;55(11):1196–1214. doi: 10.1037//0003-066x.55.11.1196. [DOI] [PubMed] [Google Scholar]
- 87.Jennings JR, Muldoon MF, Ryan CM, et al. Cerebral blood flow in hypertensive patients: an initial report of reduced and compensatory blood flow responses during performance of two cognitive tasks. Hypertension. 1998;31(6):1216–1222. doi: 10.1161/01.hyp.31.6.1216. [DOI] [PubMed] [Google Scholar]
- 88.Soufer R, Bremner JD, Arrighi JA, et al. Cerebral cortical hyperactivation in response to mental stress in patients with coronary artery disease. Proc Natl Acad Sci U S A. 1998;95(11):6454–6459. doi: 10.1073/pnas.95.11.6454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Irani SR. A novel neurological mechanism to explain the adverse effect of depression on coronary artery disease. Med Hypotheses. 2005;64(2):284–287. doi: 10.1016/j.mehy.2004.06.033. [DOI] [PubMed] [Google Scholar]
- 90.Williamson JW, McColl R, Mathews D, Ginsburg M, Mitchell JH. Activation of the insular cortex is affected by the intensity of exercise. J Appl Physiol. 1999;87(3):1213–1219. doi: 10.1152/jappl.1999.87.3.1213. [DOI] [PubMed] [Google Scholar]
- 91.Williamson JW, McColl R, Mathews D, Mitchell JH, Raven PB, Morgan WP. Brain activation by central command during actual and imagined handgrip under hypnosis. J Appl Physiol. 2002;92(3):1317–1324. doi: 10.1152/japplphysiol.00939.2001. [DOI] [PubMed] [Google Scholar]
- 92.Critchley HD, Melmed RN, Featherstone E, Mathias CJ, Dolan RJ. Volitional control of autonomic arousal: a functional magnetic resonance study. Neuroimage. 2002;16(4):909–919. doi: 10.1006/nimg.2002.1147. [DOI] [PubMed] [Google Scholar]
- 93.Asada H, Fukuda Y, Tsunoda S, Yamaguchi M, Tonoike M. Frontal midline theta rhythms reflect alternative activation of prefrontal cortex and anterior cingulate cortex in humans. Neurosci Lett. 1999;274(1):29–32. doi: 10.1016/s0304-3940(99)00679-5. [DOI] [PubMed] [Google Scholar]
- 94.Rainville P, Hofbauer RK, Paus T, Duncan GH, Bushnell MC, Price DD. Cerebral mechanisms of hypnotic induction and suggestion. J Cogn Neurosci. 1999;11(1):110–125. doi: 10.1162/089892999563175. [DOI] [PubMed] [Google Scholar]
- 95.Capuron L, Pagnoni G, Demetrashvili M, et al. Anterior cingulate activation and error processing during interferon-alpha treatment. Biol Psychiatry. 2005;58(3):190–196. doi: 10.1016/j.biopsych.2005.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rosenkranz MA, Davidson RJ. Affective neural circuitry and mind–body influences in asthma. Neuroimage. 2009;47:972–980. doi: 10.1016/j.neuroimage.2009.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hamani C, Mayberg H, Snyder B, Giacobbe P, Kennedy S, Lozano AM. Deep brain stimulation of the subcallosal cingulate gyrus for depression: anatomical location of active contacts in clinical responders and a suggested guideline for targeting. J Neurosurg. 2009:1–7. doi: 10.3171/2008.10.JNS08763. DOI: 10.3171/2008.10JNS08763. [DOI] [PubMed] [Google Scholar]
- 98.Carr L, Iacoboni M, Dubeau MC, Mazziotta JC, Lenzi GL. Neural mechanisms of empathy in humans: a relay from neural systems for imitation to limbic areas. Proc Natl Acad Sci U S A. 2003;100(9):5497–5502. doi: 10.1073/pnas.0935845100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Labus JS, Naliboff BN, Fallon J, et al. Sex differences in brain activity during aversive visceral stimulation and its expectation in patients with chronic abdominal pain: a network analysis. Neuroimage. 2008;41(3):1032–1043. doi: 10.1016/j.neuroimage.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Simmons A, Strigo I, Matthews SC, Paulus MP, Stein MB. Anticipation of aversive visual stimuli is associated with increased insula activation in anxiety-prone subjects. Biol Psychiatry. 2006;60(4):402–409. doi: 10.1016/j.biopsych.2006.04.038. [DOI] [PubMed] [Google Scholar]
- 101.El-Sheikh M. Parental drinking problems and children's adjustment: vagal regulation and emotional reactivity as pathways and moderators of risk. J Abnorm Psychol. 2001;110(4):499–515. doi: 10.1037//0021-843x.110.4.499. [DOI] [PubMed] [Google Scholar]
- 102.Cameron OG. Visceral Sensory Neuroscience: Interoception. New York: Oxford University Press; 2002. [Google Scholar]
- 103.Henry TR. Therapeutic mechanisms of vagus nerve stimulation. Neurology. 2002;59(6 Suppl 4):S3–S14. doi: 10.1212/wnl.59.6_suppl_4.s3. [DOI] [PubMed] [Google Scholar]
- 104.Lucas SM, Rothwell NJ, Gibson RM. The role of inflammation in CNS injury and disease. Br J Pharmacol. 2006;147:232–240. doi: 10.1038/sj.bjp.0706400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Emmert MH, Herman JP. Differential forebrain c-fos mRNA induction by ether inhalation and novelty: evidence for distinctive stress pathways. Brain Res. 1999;845(1):60–67. doi: 10.1016/s0006-8993(99)01931-9. [DOI] [PubMed] [Google Scholar]
- 106.Sawchenko PE, Li HY, Ericsson A. Circuits and mechanisms governing hypothalamic responses to stress: a tale of two paradigms. Prog Brain Res. 2000;122:61–78. doi: 10.1016/s0079-6123(08)62131-7. [DOI] [PubMed] [Google Scholar]
- 107.Lazarus RS. Stress and Emotion: A New Synthesis. New York: Springer Pub. Co; 1999. [Google Scholar]
- 108.Kronfol Z, Remick DG. Cytokines and the brain: implications for clinical psychiatry. Am J Psychiatry. 2000;157(5):683–694. doi: 10.1176/appi.ajp.157.5.683. [DOI] [PubMed] [Google Scholar]
- 109.Lucini D, Mela GS, Malliani A, Pagani M. Impairment in cardiac autonomic regulation preceding arterial hypertension in humans: insights from spectral analysis of beat-by-beat cardiovascular variability. Circulation. 2002;106(21):2673–2679. doi: 10.1161/01.cir.0000039106.89299.ab. [DOI] [PubMed] [Google Scholar]
- 110.Hosoi T, Okuma Y, Nomura Y. Electrical stimulation of afferent vagus nerve induces IL-1beta expression in the brain and activates HPA axis. Am J Physiol Regul Integr Comp Physiol. 2000;279(1):R141–R147. doi: 10.1152/ajpregu.2000.279.1.R141. [DOI] [PubMed] [Google Scholar]
- 111.McEwen BS. Sex, stress and the hippocampus: allostasis, allostatic load and the aging process. Neurobiol Aging. 2002;23(5):921–939. doi: 10.1016/s0197-4580(02)00027-1. [DOI] [PubMed] [Google Scholar]
- 112.McEwen BS. Protection and damage from acute and chronic stress: allostasis and allostatic overload and relevance to the pathophysiology of psychiatric disorders. Ann N Y Acad Sci. 2004;1032:1–7. doi: 10.1196/annals.1314.001. [DOI] [PubMed] [Google Scholar]
- 113.Balu DT, Lucki I. Adult hippocampal neurogenesis: regulation, functional implications, and contribution to disease pathology. Neurosci Biobehav Rev. 2009;33(3):232–252. doi: 10.1016/j.neubiorev.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Buckley TC, Kaloupek DG. A meta-analytic examination of basal cardiovascular activity in posttraumatic stress disorder. Psychosom Med. 2001;63(4):585–594. doi: 10.1097/00006842-200107000-00011. [DOI] [PubMed] [Google Scholar]
- 115.Chang L. Brain responses to visceral and somatic stimuli in irritable bowel syndrome: a central nervous system disorder? Gastroenterol Clin North Am. 2005;34(2):271–279. doi: 10.1016/j.gtc.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 116.Cohen H, Kotler M, Matar MA, et al. Analysis of heart rate variability in posttraumatic stress disorder patients in response to a trauma-related reminder. Biol Psychiatry. 1998;44(10):1054–1059. doi: 10.1016/s0006-3223(97)00475-7. [DOI] [PubMed] [Google Scholar]
- 117.Cohen H, Neumann L, Alhosshle A, Kotler M, Abu-Shakra M, Buskila D. Abnormal sympathovagal balance in men with fibromyalgia. J Rheumatol. 2001;28(3):581–589. [PubMed] [Google Scholar]
- 118.Ehlert U, Gaab J, Heinrichs M. Psychoneuroendocrinological contributions to the etiology of depression, posttraumatic stress disorder, and stress-related bodily disorders: the role of the hypothalamus-pituitary-adrenal axis. Biol Psychol. 2001;57(1–3):141–152. doi: 10.1016/s0301-0511(01)00092-8. [DOI] [PubMed] [Google Scholar]
- 119.Herbert TB, Cohen S. Stress and immunity in humans: a meta-analytic review. Psychosom Med. 1993;55(4):364–379. doi: 10.1097/00006842-199307000-00004. [DOI] [PubMed] [Google Scholar]
- 120.Larson MR, Ader R, Moynihan JA. Heart rate, neuroendocrine, and immunological reactivity in response to an acute laboratory stressor. Psychosom Med. 2001;63(3):493–501. doi: 10.1097/00006842-200105000-00020. [DOI] [PubMed] [Google Scholar]
- 121.Lucini D, Norbiato G, Clerici M, Pagani M. Hemodynamic and autonomic adjustments to real life stress conditions in humans. Hypertension. 2002;39(1):184–188. doi: 10.1161/hy0102.100784. [DOI] [PubMed] [Google Scholar]
- 122.Zubieta JK, Chinitz JA, Lombardi U, Fig LM, Cameron OG, Liberzon I. Medial frontal cortex involvement in PTSD symptoms: a SPECT study. J Psychiatr Res. 1999;33(3):259–264. doi: 10.1016/s0022-3956(98)00060-0. [DOI] [PubMed] [Google Scholar]
- 123.Dantzer R. Somatization: a psychoneuroimmune perspective. Psychoneuroendocrinology. 2005;30(10):947–952. doi: 10.1016/j.psyneuen.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 124.Sternberg EM. Neural-immune interactions in health and disease. J Clin Invest. 1997;100(11):2641–2647. doi: 10.1172/JCI119807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Maquet P, Faymonville ME, Degueldre C, et al. Functional neuroanatomy of hypnotic state. Biol Psychiatry. 1999;45(3):327–333. doi: 10.1016/s0006-3223(97)00546-5. [DOI] [PubMed] [Google Scholar]
- 126.Lazar SW, Bush G, Gollub RL, Fricchione GL, Khalsa G, Benson H. Functional brain mapping of the relaxation response and meditation. Neuroreport. 2000;11(7):1581–1585. [PubMed] [Google Scholar]
- 127.Newberg A, Alavi A, Baime M, Pourdehnad M, Santanna J, d'Aquili E. The measurement of regional cerebral blood flow during the complex cognitive task of meditation: a preliminary SPECT study. Psychiatry Res. 2001;106(2):113–122. doi: 10.1016/s0925-4927(01)00074-9. [DOI] [PubMed] [Google Scholar]
- 128.Kropotov JD, Crawford HJ, Polyakov YI. Somatosensory event-related potential changes to painful stimuli during hypnotic analgesia: anterior cingulate cortex and anterior temporal cortex intracranial recordings. Int J Psychophysiol. 1997;27(1):1–8. doi: 10.1016/s0167-8760(97)00785-x. [DOI] [PubMed] [Google Scholar]
- 129.Rainville P, Duncan GH, Price DD, Carrier B, Bushnell MC. Pain affect encoded in human anterior cingulate but not somatosensory cortex. Science. 1997;277(5328):968–971. doi: 10.1126/science.277.5328.968. [DOI] [PubMed] [Google Scholar]
- 130.Brauchli P, Michel CM, Zeier H. Electrocortical, autonomic, and subjective responses to rhythmic audio-visual stimulation. Int J Psychophysiol. 1995;19(1):53–66. doi: 10.1016/0167-8760(94)00074-o. [DOI] [PubMed] [Google Scholar]
- 131.De Pascalis V, Perrone M. EEG asymmetry and heart rate during experience of hypnotic analgesia in high and low hypnotizables. Int J Psychophysiol. 1996;21(2–3):163–175. doi: 10.1016/0167-8760(95)00050-x. [DOI] [PubMed] [Google Scholar]
- 132.De Pascalis V, Ray WJ, Tranquillo I, D'Amico D. EEG activity and heart rate during recall of emotional events in hypnosis: relationships with hypnotizability and suggestibility. Int J Psychophysiol. 1998;29(3):255–275. doi: 10.1016/s0167-8760(98)00009-9. [DOI] [PubMed] [Google Scholar]
- 133.Isotani T, Tanaka H, Lehmann D, et al. Source localization of EEG activity during hypnotically induced anxiety and relaxation. Int J Psychophysiol. 2001;41(2):143–153. doi: 10.1016/s0167-8760(00)00197-5. [DOI] [PubMed] [Google Scholar]
- 134.Jasiukaitis P, Nouriani B, Hugdahl K, Spiegel D. Relateralizing hypnosis: or, have we been barking up the wrong hemisphere? Int J Clin Exp Hypn. 1997;45(2):158–177. doi: 10.1080/00207149708416116. [DOI] [PubMed] [Google Scholar]
- 135.Brody AL, Saxena S, Stoessel P, et al. Regional brain metabolic changes in patients with major depression treated with either paroxetine or interpersonal therapy: preliminary findings. Arch Gen Psychiatry. 2001;58(7):631–640. doi: 10.1001/archpsyc.58.7.631. [DOI] [PubMed] [Google Scholar]
- 136.Rosenfeld JP, Baehr E, Baehr R, Gotlib IH, Ranganath C. Preliminary evidence that daily changes in frontal alpha asymmetry correlate with changes in affect in therapy sessions. Int J Psychophysiol. 1996;23(1–2):137–141. doi: 10.1016/0167-8760(96)00037-2. [DOI] [PubMed] [Google Scholar]
- 137.Allen JJ, Harmon-Jones E, Cavender JH. Manipulation of frontal EEG asymmetry through biofeedback alters self-reported emotional responses and facial EMG. Psychophysiology. 2001;38(4):685–693. [PubMed] [Google Scholar]
- 138.Jones NA, Field T. Massage and music therapies attenuate frontal EEG asymmetry in depressed adolescents. Adolescence. 1999;34(135):529–534. [PubMed] [Google Scholar]
- 139.Jones NA, Field T, Davalos M. Massage therapy attenuates right frontal EEG asymmetry in one-month-old infants of depressed mothers. Infant Behav Dev. 1998;21(3):527. [Google Scholar]
- 140.Field TM. Massage therapy effects. Am Psychol. 1998;53(12):1270–1281. doi: 10.1037//0003-066x.53.12.1270. [DOI] [PubMed] [Google Scholar]
- 141.Williams JD, Gruzelier JH. Differentiation of hypnosis and relaxation by analysis of narrow band theta and alpha frequencies. Int J Clin Exp Hypn. 2001;49(3):185–206. doi: 10.1080/00207140108410070. [DOI] [PubMed] [Google Scholar]
- 142.Delmonte MM. Electrocortical activity and related phenomena associated with meditation practice: a literature review. Int J Neurosci. 1984;24(3–4):217–231. doi: 10.3109/00207458409089810. [DOI] [PubMed] [Google Scholar]
- 143.Jevning R, Wallace RK, Beidebach M. The physiology of meditation: a review. A wakeful hypometabolic integrated response. Neurosci Biobehav Rev. 1992;16(3):415–424. doi: 10.1016/s0149-7634(05)80210-6. [DOI] [PubMed] [Google Scholar]
- 144.Kubota Y, Sato W, Toichi M, et al. Frontal midline theta rhythm is correlated with cardiac autonomic activities during the performance of an attention demanding meditation procedure. Brain Res Cogn Brain Res. 2001;11(2):281–287. doi: 10.1016/s0926-6410(00)00086-0. [DOI] [PubMed] [Google Scholar]
- 145.Sadato N, Nakamura S, Oohashi T, et al. Neural networks for generation and suppression of alpha rhythm: a PET study. Neuroreport. 1998;9(5):893–897. doi: 10.1097/00001756-199803300-00024. [DOI] [PubMed] [Google Scholar]
- 146.Travis F, Haaga DAF, Hagelin J, et al. Effects of transcendental meditation practice on brain functioning and stress reactivity in college students. Int J Psychophysiol. 2009;71(2):170–176. doi: 10.1016/j.ijpsycho.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 147.Gemignani A, Santarcangelo E, Sebastiani L, et al. Changes in autonomic and EEG patterns induced by hypnotic imagination of aversive stimuli in man. Brain Res Bull. 2000;53(1):105–111. doi: 10.1016/s0361-9230(00)00314-2. [DOI] [PubMed] [Google Scholar]
- 148.Ouchi Y, Kanno T, Okada H, et al. Changes in cerebral blood flow under the prone condition with and without massage. Neurosci Lett. 2006;407(2):131–135. doi: 10.1016/j.neulet.2006.08.037. [DOI] [PubMed] [Google Scholar]
- 149.Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability: standards of measurement, physiological interpretation and clinical use. Circulation. 1996;93(5):1043–1065. [PubMed] [Google Scholar]
- 150.Berntson GG, Bigger JT, Jr, Eckberg DL, et al. Heart rate variability: origins, methods, and interpretive caveats. Psychophysiology. 1997;34(6):623–648. doi: 10.1111/j.1469-8986.1997.tb02140.x. [DOI] [PubMed] [Google Scholar]
- 151.Dishman RK, Nakamura Y, Garcia ME, Thompson RW, Dunn AL, Blair SN. Heart rate variability, trait anxiety, and perceived stress among physically fit men and women. Int J Psychophysiol. 2000;37(2):121–133. doi: 10.1016/s0167-8760(00)00085-4. [DOI] [PubMed] [Google Scholar]
- 152.Huang JL, Chiou CW, Ting CT, Chen YT, Chen SA. Sudden changes in heart rate variability during the 1999 Taiwan earthquake. Am J Cardiol. 2001;87(2):245–248. doi: 10.1016/s0002-9149(00)01331-x. [DOI] [PubMed] [Google Scholar]
- 153.Vrijkotte TG, van Doornen LJ, de Geus EJ. Effects of work stress on ambulatory blood pressure, heart rate, and heart rate variability. Hypertension. 2000;35(4):880–886. doi: 10.1161/01.hyp.35.4.880. [DOI] [PubMed] [Google Scholar]
- 154.Hughes JW, Stoney CM. Depressed mood is related to high-frequency heart rate variability during stressors. Psychosom Med. 2000;62(6):796–803. doi: 10.1097/00006842-200011000-00009. [DOI] [PubMed] [Google Scholar]
- 155.Friedman BH, Thayer JF. Anxiety and autonomic flexibility: a cardiovascular approach. Biol Psychol. 1998;47(3):243–263. doi: 10.1016/s0301-0511(97)00027-6. [Republished in BiolPsychol 1998 Nov;49(3):303–;23; PMID: 9858059] [DOI] [PubMed] [Google Scholar]
- 156.Freeman R, Komaroff AL. Does the chronic fatigue syndrome involve the autonomic nervous system? Am J Med. 1997;102(4):357–364. doi: 10.1016/s0002-9343(97)00087-9. [DOI] [PubMed] [Google Scholar]
- 157.Buch AN, Coote JH, Townend JN. Mortality, cardiac vagal control and physical training--what's the link? Exp Physiol. 2002;87(4):423–435. doi: 10.1111/j.1469-445x.2002.tb00055.x. [DOI] [PubMed] [Google Scholar]
- 158.Tsuji H, Larson MG, J VF, Jr, et al. Impact of reduced heart rate variability on risk for cardiac events. The framingham heart study. Circulation. 1996;94(11):2850–2855. doi: 10.1161/01.cir.94.11.2850. [DOI] [PubMed] [Google Scholar]
- 159.Lane RD, Reiman EM, Ahern GL, Thayer JF. Activity in medial prefrontal cortex correlates with vagal component of heart rate variability during emotion. Brain Cogn. 2001;47(1–2):54–135. [Google Scholar]
- 160.Sakakibara M, Takeuchi S, Hayano J. Effect of relaxation training on cardiac parasympathetic tone. Psychophysiology. 1994;31(3):223–228. doi: 10.1111/j.1469-8986.1994.tb02210.x. [DOI] [PubMed] [Google Scholar]
- 161.Stefano GB, Fricchione GL, Slingsby BT, Benson H. The placebo effect and relaxation response: neural processes and their coupling to constitutive nitric oxide. Brain Res Brain Res Rev. 2001;35(1):1–19. doi: 10.1016/s0165-0173(00)00047-3. [DOI] [PubMed] [Google Scholar]
- 162.DeBenedittis G, Cigada M, Bianchi A, Signorini MG, Cerutti S. Autonomic changes during hypnosis: a heart rate variability power spectrum analysis as a marker of sympatho-vagal balance. Int J Clin Exp Hypn. 1994;42(2):140–152. doi: 10.1080/00207149408409347. [DOI] [PubMed] [Google Scholar]
- 163.Hippel CV, Hole G, Kaschka WP. Autonomic profile under hypnosis as assessed by heart rate variability and spectral analysis. Pharmacopsychiatry. 2001;34(3):111–113. doi: 10.1055/s-2001-14279. [DOI] [PubMed] [Google Scholar]
- 164.Santarcangelo EL, Emdin M, Picano E, Raciti M. Can hypnosis modify the sympathetic-parasympathetic balance at heart level? Journal of Ambulatory Monitoring. 1992;5(2):191. [Google Scholar]
- 165.Carney RM, Freedland KE, Stein PK, Skala JA, Hoffman P, Jaffe AS. Change in heart rate and heart rate variability during treatment for depression in patients with coronary heart disease. Psychosom Med. 2000;62(5):639–647. doi: 10.1097/00006842-200009000-00007. [DOI] [PubMed] [Google Scholar]
- 166.Cottingham JT, Porges SW, Richmond K. Shifts in pelvic inclination angle and parasympathetic tone produced by rolfing soft tissue manipulation. Phys Ther. 1988;68(9):1364–1370. doi: 10.1093/ptj/68.9.1364. [DOI] [PubMed] [Google Scholar]
- 167.Field T. Massage therapy. Med Clin North Am. 2002;86(1):163–171. doi: 10.1016/s0025-7125(03)00078-6. [DOI] [PubMed] [Google Scholar]
- 168.Uvnas-Moberg K. Oxytocin may mediate the benefits of positive social interaction and emotions. Psychoneuroendocrinology. 1998;23(8):819–835. doi: 10.1016/s0306-4530(98)00056-0. [DOI] [PubMed] [Google Scholar]
- 169.Delaney JP, Leong KS, Watkins A, Brodie D. The short-term effects of myofascial trigger point massage therapy on cardiac autonomic tone in healthy subjects. J Adv Nurs. 2002;37(4):364–371. doi: 10.1046/j.1365-2648.2002.02103.x. [DOI] [PubMed] [Google Scholar]
- 170.Haker E, Egekvist H, Bjerring P. Effect of sensory stimulation (acupuncture) on sympathetic and parasympathetic activities in healthy subjects. J Auton Nerv Syst. 2000;79(1):52–59. doi: 10.1016/s0165-1838(99)00090-9. [DOI] [PubMed] [Google Scholar]
- 171.Wang JD, Kuo TB, Yang CC. An alternative method to enhance vagal activities and suppress sympathetic activities in humans. Auton Neurosci. 2002;100(1–2):90–95. doi: 10.1016/s1566-0702(02)00150-9. [DOI] [PubMed] [Google Scholar]
- 172.Rush AJ, George MS, Sackeim HA, et al. Vagus nerve stimulation (VNS) for treatment-resistant depressions: a multicenter study. Biol Psychiatry. 2000;47(4):276–286. doi: 10.1016/s0006-3223(99)00304-2. [DOI] [PubMed] [Google Scholar]
- 173.Schachter SC, Saper CB. Vagus nerve stimulation. Epilepsia. 1998;39(7):677–686. doi: 10.1111/j.1528-1157.1998.tb01151.x. [DOI] [PubMed] [Google Scholar]
- 174.Rush AJ, Marangell LB, Sackeim HA, et al. Vagus nerve stimulation for treatment-resistant depression: a randomized, controlled acute phase trial. Biol Psychiatry. 2005;58(5):347–354. doi: 10.1016/j.biopsych.2005.05.025. [DOI] [PubMed] [Google Scholar]
- 175.Fallen EL, Kamath MV, Tougas G, Upton A. Afferent vagal modulation. Clinical studies of visceral sensory input. Auton Neurosci. 2001;90(1–2):35–40. doi: 10.1016/S1566-0702(01)00265-X. [DOI] [PubMed] [Google Scholar]
- 176.Chowdhary S, Vaile JC, Fletcher J, Ross HF, Coote JH, Townend JN. Nitric oxide and cardiac autonomic control in humans. Hypertension. 2000;36(2):264–269. doi: 10.1161/01.hyp.36.2.264. [DOI] [PubMed] [Google Scholar]
- 177.Anisman H, Merali Z. Cytokines, stress, and depressive illness. Brain Behav Immun. 2002;16(5):513–524. doi: 10.1016/s0889-1591(02)00009-0. [DOI] [PubMed] [Google Scholar]
- 178.Irwin M. Psychoneuroimmunology of depression: clinical implications. Brain Behav Immun. 2002;16(1):1–16. doi: 10.1006/brbi.2001.0654. [DOI] [PubMed] [Google Scholar]
- 179.Goehler LE, Gaykema RP, Nguyen KT, et al. Interleukin-1beta in immune cells of the abdominal vagus nerve: a link between the immune and nervous systems? J Neurosci. 1999;19(7):2799–2806. doi: 10.1523/JNEUROSCI.19-07-02799.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Capuron L, Dantzer R. Cytokines and depression: the need for a new paradigm. Brain Behav Immun. 2003;17 Suppl 1:S119–S124. doi: 10.1016/s0889-1591(02)00078-8. [DOI] [PubMed] [Google Scholar]
- 181.Rivest S. How circulating cytokines trigger the neural circuits that control the hypothalamic-pituitary-adrenal axis. Psychoneuroendocrinology. 2001;26(8):761–788. doi: 10.1016/s0306-4530(01)00064-6. [DOI] [PubMed] [Google Scholar]
- 182.Penninx BW, Kritchevsky SB, Yaffe K, et al. Inflammatory markers and depressed mood in older persons: results from the health, aging and body composition study. Biol Psychiatry. 2003;54(5):566–572. doi: 10.1016/s0006-3223(02)01811-5. [DOI] [PubMed] [Google Scholar]
- 183.Ter Horst GJ, Postema F. Forebrain parasympathetic control of heart activity: retrograde transneuronal viral labeling in rats. Am J Physiol. 1997;273(6 Pt 2):H2926–H2930. doi: 10.1152/ajpheart.1997.273.6.H2926. [DOI] [PubMed] [Google Scholar]
- 184.Gold SM, Irwin MR. Depression and immunity: inflammation and depressive symptoms in multiple sclerosis. Neurol Clin. 2006;24(3):507–519. doi: 10.1016/j.ncl.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 185.Heesen C, Nawrath L, Reich C, Bauer N, Schulz KH, Gold SM. Fatigue in multiple sclerosis: an example of cytokine mediated sickness behaviour? J Neurol Neurosurg Psychiatry. 2006;77(1):34–39. doi: 10.1136/jnnp.2005.065805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Schiepers OJ, Wichers MC, Maes M. Cytokines and major depression. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29(2):201–217. doi: 10.1016/j.pnpbp.2004.11.003. [Erratum appears in Prog Neuropsychopharmacol Biol Psychiatry 2005 May;29(4):637–638.] [DOI] [PubMed] [Google Scholar]
- 187.Aronson D, Mittleman MA, Burger AJ. Interleukin-6 levels are inversely correlated with heart rate variability in patients with decompensated heart failure. J Cardiovasc Electrophysiol. 2001;12(3):294–300. doi: 10.1046/j.1540-8167.2001.00294.x. [DOI] [PubMed] [Google Scholar]
- 188.Malave HA, Taylor AA, Nattama J, Deswal A, Mann DL. Circulating levels of tumor necrosis factor correlate with indexes of depressed heart rate variability: a study in patients with mild-to-moderate heart failure. Chest. 2003;123(3):716–724. doi: 10.1378/chest.123.3.716. [DOI] [PubMed] [Google Scholar]
- 189.Bernik TR, Friedman SG, Ochani M, et al. Cholinergic antiinflammatory pathway inhibition of tumor necrosis factor during ischemia reperfusion. J Vasc Surg. 2002;36(6):1231–1236. doi: 10.1067/mva.2002.129643. [DOI] [PubMed] [Google Scholar]
- 190.Borovikova LV, Ivanova S, Zhang M, et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405(6785):458–462. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- 191.Floto RA, Smith KG. The vagus nerve, macrophages, and nicotine. Lancet. 2003;361(9363):1069–1070. doi: 10.1016/S0140-6736(03)12902-9. [DOI] [PubMed] [Google Scholar]
- 192.Zeitlin D, Keller SE, Shiflett SC, Schleifer SJ, Bartlett JA. Immunological effects of massage therapy during academic stress. Psychosom Med. 2000;62(1):83–84. doi: 10.1097/00006842-200001000-00012. [DOI] [PubMed] [Google Scholar]
- 193.Danziger N, Fournier E, Bouhassira D, et al. Different strategies of modulation can be operative during hypnotic analgesia: a neurophysiological study. Pain. 1998;75(1):85–92. doi: 10.1016/S0304-3959(97)00208-X. [DOI] [PubMed] [Google Scholar]
- 194.D'Esposito M, Detre JA, Aguirre GK, et al. A functional MRI study of mental image generation. Neuropsychologia. 1997;35(5):725–730. doi: 10.1016/s0028-3932(96)00121-2. [DOI] [PubMed] [Google Scholar]