Abstract
The breakdown of polyunsaturated fatty acids (PUFAs) under conditions of oxidative stress results in the formation of lipid peroxidation (LPO) products. These LPO products such as 4-hydroxy-2-nonenal (HNE) and 4-oxo-2-nonenal (ONE) can contribute to the development of cardiovascular and neurodegenerative diseases and cancer. Conjugation with glutathione, followed by further metabolism to mercapturic acid (MA) conjugates, can mitigate the effects of these LPO products in disease development by facilitating their excretion from the body. We have developed a quantitative method to simultaneously assess levels of 4-oxo-2-nonen-1-ol (ONO)-MA, HNE-MA, and 1,4-dihydroxy-2-nonene (DHN)-MA in human urine samples utilizing isotope-dilution mass spectrometry. We are also able to detect 4-hydroxy-2-nonenoic acid (HNA)-MA, 4-hydroxy-2-nonenoic acid lactone (HNAL)-MA, and 4-oxo-2-nonenoic acid (ONA)-MA with this method. The detection of ONO-MA and ONA-MA in humans is significant because it demonstrates that HNE/ONE branching occurs in the breakdown of PUFAs and suggests that ONO may contribute to the harmful effects currently associated with HNE. We were able to show significant decreases in HNE-MA, DHN-MA, and total LPO-MA in a group of seven smokers upon smoking cessation. These data demonstrate the value of HNE and ONE metabolites as in vivo markers of oxidative stress.
Keywords: Lipid peroxidation, quantitation, 4-hydroxy-2-nonenal, 4-oxo-2-nonenal, mass spectrometry, smoking
Introduction
Oxidative degradation of polyunsaturated fatty acids (PUFAs) occurs under conditions of oxidative stress when the cellular antioxidant defense mechanisms are overwhelmed, leading to the formation of electrophilic lipid peroxidation (LPO) products. 4-Hydroxy-2-nonenal (HNE) and 4-oxo-2-nonenal (ONE) are two of the most thoroughly studied LPO products. These reactive aldehydes have been shown to be cytotoxic and genotoxic [1, 2], as well as to contribute to the development and progression of cancer [3], cardiovascular diseases such as atherosclerosis and chronic obstructive pulmonary disease [4-6], and neurodegenerative diseases like Alzheimer’s [7-9]. In biological systems, HNE and ONE undergo phase I metabolism, resulting in their respective oxidation products 4-hydroxy-2-nonenoic acid (HNA) [10] and 4-oxo-2-nonenoic acid (ONA) [11] or reduction products 1,4-dihydroxy-2-nonene (DHN) [12] and 4-oxo-2-nonen-1-ol (ONO) [13-15] (Scheme 1). HNE, ONE, and their phase I metabolites have also been shown to undergo phase II metabolism, forming Michael-type conjugates with glutathione (GSH) [2], a reaction mediated by glutathione S-transferase (GST) [16-18]. Upon conjugation, HNA can form a lactone (HNAL) via spontaneous intramolecular condensation [19]. Further metabolism of these LPO-GSH conjugates in the liver and kidney results in LPO-mercapturic acid (MA) conjugates which are excreted in urine.
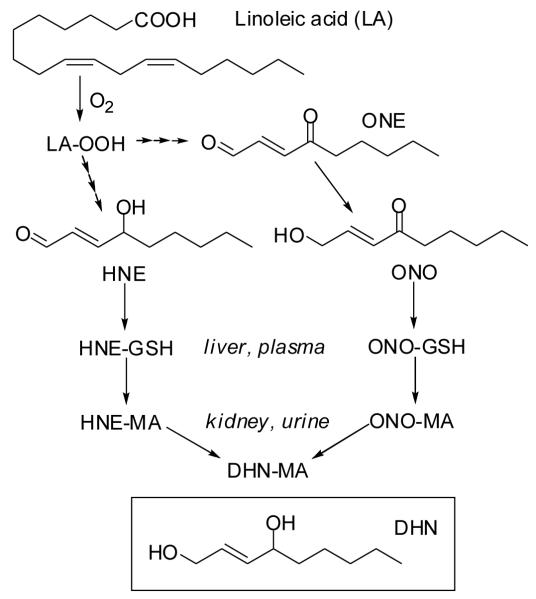
Scheme 1.
LPO-induced degradation of linoleic acid. HNE and ONE undergo phase I and phase II metabolism, resulting in the excretion of MA conjugates including HNE-MA, ONO-MA, and DHN-MA. DHN-MA may originate from DHN-GSH and possibly ONO-GSH, but it is shown as a metabolite of HNE-MA and ONO-MA for simplicity.
We have previously reported that HNE and ONE metabolite levels are significantly increased in rats after an acute oxidative stress insult [20]. In that study we were able to differentiate between HNE-MA and its isomer ONO-MA which had not been previously demonstrated. This is an important distinction because previous analyses have likely attributed the effects of ONO to HNE. These metabolites also form by different pathways, so being able to distinguish between the two could provide insight into the mechanisms of oxidative stress in biological systems. Previous studies have focused on the quantitation of DHN-MA [21-24].
Here we report the quantitation of HNE-MA and ONO-MA, as well as DHN-MA in human urine. The phase I metabolites of HNE-MA and ONE-MA represent biologically relevant pathways for the elimination of these LPO products in a rat model of oxidative stress [20]. We have detected HNE-MA, DHN-MA, HNA-MA, HNAL-MA, ONO-MA, and ONA-MA in human samples and are able to quantitate the HNE-MA, ONO-MA, and DHN-MA metabolites in smokers. Twelve weeks of smoking cessation resulted in a significant decrease in the levels of urinary HNE-MA, DHN-MA, and overall LPO-MA. These results demonstrate the potential utility of these metabolites as non-invasive diagnostic tools for assessing oxidative stress in vivo.
Materials and Methods
Materials
[2H]Chloroform was purchased from Cambridge Isotope Laboratories (Andover, MA). HPLC-grade formic acid (0.1 %) in water was purchased from Honeywell Burdick and Jackson (Muskegon, MI). 3-Chloroperoxybenzoic acid and dithiothreitol were purchased from TCI America (Portland, OR). HNE-MA (1 mg in 100 μl ethanol) was purchased from Cayman Chemical (Ann Arbor, MI). Cotinine was purchased from Alfa Aesar (Ward Hill, MA) and cotinine-d3 (99 atom % D, 1 mg/ml in methanol) was from Sigma-Aldrich (St. Louis, MO). All other chemicals were purchased from Sigma-Aldrich (St. Louis, MO).
Synthesis
LPO products
HNE, HNA, ONE, ONO, and ONA and their MA conjugates were prepared and chemically characterized as described in our previous work [20].
Deuterium labeled MA
N-(acetyl-d3)-L-cysteine (MAd3) was prepared using the method of Slatter et al. [25]. Briefly, cystine (5.3 mmol) was added to 13 ml of a 1.5 M NaOH solution and the mixture was cooled in an ice bath with stirring. [2H6]Acetic anhydride (10.6 mmol) was added dropwise over 20 min, the ice bath was removed, and the reaction continued stirring at room temperature for 1 h. 1,4-Dithiothreitol (10.6 mmol) was added, and the reaction mixture was stirred continuously at room temperature for 1 h, concentrated in vacuo, washed with ether, frozen, and lyophilized. Purification was performed on a 52 × 2.5 cm Sephadex LH-20 column using methanol as the eluting solvent. Fraction purity was verified by LC-MS analysis in negative ion mode. Only fractions containing a peak at m/z 165 (MAd3) were carried forward. Further purification was carried out by acidification to pH 3 with 1 M HCl and extraction with ethyl acetate in order to remove any remaining cystine or cysteine. The organic layer was concentrated in vacuo. The resulting white residue was characterized by LC-MS/MS analysis and found to be free of cystine, cysteine, and unlabeled mercapturic acid.
Preparation of LPO-MAd3 conjugates
MAd3 was used in place of MA for LPO-MA adduct formation [20] for use as internal standards. Briefly, a 20 mM solution of MAd3 was prepared in 0.1 M sodium phosphate buffer, pH 8. To 50 μl of this solution was added 450 μl of the same phosphate buffer and 400 μl of water. A 1.0 mM solution of the LPO product of interest was made up in ethanol and 100 μl was added to the MAd3 solution (10-fold molar excess). The reaction was stirred at 37 °C for 2 h and then acidified to pH 3 with 1 N HCl. It was then extracted with ethyl acetate, 3 × 1 ml, evaporated under nitrogen using a Zymark TurboVap LV (Caliper Life Sciences, Hopkinton, MA), and reconstituted in 1.0 ml of 2:8 acetonitrile-H2O containing 0.1 % formic acid, yielding a nominal LPO-MAd3 concentration of 100 μM. LC-MS/MS analyses were used to verify conjugate formation.
Sample Collection
This study protocol #4312 was approved by Oregon State University’s and Samaritan Health Systems’ Institutional Review Boards. Participants were recruited by newspaper advertisements in the Corvallis, Oregon area. Participants who responded were enrolled because they met our study participation criteria: age 18-65 years, healthy, current smoker motivated to quit for one arm of the study, nonsmoker with minimal exposure to second-hand smoke in the control group, BMI < 35 kg/m2, subjects may not be taking any prescription, no over-the-counter or herbal medications that induce or inhibit the liver enzymes involved in drug metabolism (CYP P450 3A4, 2D6), no known active liver disease (hepatitis, cirrhosis), no excessive alcohol use defined as > 1 drink per day for women and > 2 drinks per day for men. Nonsmokers were enrolled into the study and matched by age and BMI to one of the smokers already enrolled in the study (Table 1). Each subject signed an informed consent statement and completed a questionnaire that provided the following information: age, sex, weight, height, history of tobacco use, and health status prior to enrollment in the study.
TABLE 1.
Study participant characteristics
| All enrolled Participants | Cessation Studyb | |||
|---|---|---|---|---|
| Parameter | Nonsmokers (n = 23) |
Smokers (n = 23) |
Nonsmokers (n = 7) |
Smokers (n = 7) |
| Gendera | 10 Male, | 9 Male, | 4 Male, | 4 Male, |
| 13 Female | 14 Female | 3 Female | 3 Female | |
| Age (years)a | 42.7 ± 12.0 | 42.5 ± 10.8 | 37.4 ± 10.7 | 38.7 ± 8.6 |
| BMI (kg/m2)a | 26.8 ± 4.5 | 27.0 ± 4.6 | 25.9 ± 4.1 | 26.0 ± 4.1 |
| Years Smoked | 0 | 19.5 ± 12.5 | 0 | 16.2 ± 7.5 |
No significant difference between the two groups.
Nonsmokers were matched by age and BMI to one of the smokers.
Urine samples were collected from smokers and nonsmokers at Good Samaritan Hospital (Corvallis, OR). On the day of the study, 23 smokers and 23 nonsmokers provided at least 10 ml of clean catch urine. A second urine sample was collected at least 12 weeks after smoking cessation from the seven smokers who successfully quit smoking and their nonsmoking counterparts. Upon collection, samples were frozen and stored at -80 °C until analysis. Smoking cessation was carried out using either Chantix® (varenicline tartrate, which blocks nicotine receptors in the brain), Zyban® (bupropion hydrochloride, presumably acting by modulation of noradrenergic and dopaminergic receptors in the brain), or quitting ‘cold turkey’. Support was provided during the cessation process through a smoking cessation class and phone calls. Self reporting and cotinine levels were used to verify the success of smoking cessation.
Urine Samples
A volume of 0.2 ml of human urine was acidified with 20 μl of 1 N HCl to pH 3. To the urine was added 5 μl of a 100 μM solution of DHN-MAd3, 10 μl of a 10 μM solution of HNE-MAd3, and 10 μl of a 10 μM solution of ONO-MAd3 as internal standards. The samples were extracted with ethyl acetate (2 × 700 μl). The ethyl acetate layers were combined and evaporated under nitrogen. Samples were then reconstituted in 100 μl of 2:8 acetonitrile:water containing 0.1 % formic acid and analyzed by LC-MS/MS.
Urinary creatinine was measured using a Creatinine Assay Kit, catalog no. 500701 (Cayman Chemical). The assay was performed according to the manufacturer’s directions. There was no significant difference between smoker and non-smoker creatinine levels (p = 0.37).
Urinary cotinine was analyzed by LC-MS/MS. To a volume of 0.2 ml of human urine was added 5 μl of 10 μM cotinine-d3 as an internal standard. Proteins were precipitated by the addition of 795 μl of MeCN containing 0.1 % formic acid and centrifugation. The supernatant was analyzed by LC-MS/MS.
Calibration Curves
A calibration curve was constructed from standard solutions of HNE-MA, DHN-MA, and ONO-MA in 2:8 acetontrile:water (both with 0.1 % formic acid). The ONO-MA concentrations ranged from 10 nM to 1.0 μM and included six points, while the DHN-MA and HNE-MA concentrations ranged from 50 nM to 5.0 μM and included seven points. HNE-MAd3 (10 μl of a 10 μM solution), DHN-MAd3 (5 μl of a 100 μM solution), and ONO-MAd3 (10 μl of a 10 μM solution) were added as internal standards. The final volume of each standard solution was 100 μl.
A second calibration curve was prepared for the analysis of urinary cotinine. The curve, prepared in ethanol, included seven points with concentrations ranging from 0.1 nM to 1 μM. Cotinine-d3 (5 μl of 10 μM) was used as the internal standard.
Standard Addition Curves
The standard addition curves were prepared by adding synthetic HNE-MA, DHN-MA, or ONO-MA and the corresponding internal standard to urine samples. One milliliter aliquots of urine were used for each point on the curve. The urine was acidified to pH 3 with 1 N HCl and spiked with varying concentrations of HNE-MA (0.5-5.0 μM), DHN-MA (0.1-5.0 μM), or ONO-MA (0.25-1.0 μM) and a fixed amount of internal standard, i.e., HNE-MAd3 (20 μl of a 10 μM solution), DHN-MAd3 (10 μl of a 100 μM solution), or ONO-MAd3 (20 μl of a 10 μM solution). Samples were then vortex mixed and extracted with ethyl acetate (2 × 2 ml). The combined ethyl acetate layers were evaporated under N2 and reconstituted in 200 μl of 2:8 acetonitrile:water containing 0.1 % formic acid.
HPLC
A Shimadzu Prominence HPLC system (Shimadzu, Columbia, MD) consisting of four LC-20AD pumps, a DQU-20A5 degasser, and an SIL-HTc autosampler, equipped with switching valves, was used for all chromatography. For LPO product analyses, the HPLC column used was a 250 × 2 mm Synergi Max RP C12 column (Phenomenex, Torrance, CA). The mobile phase consisted of Solvent A, 0.1 % (v/v) formic acid in water, and Solvent B, acetonitrile containing 0.1 % (v/v) formic acid. The flow rate was 0.2 ml/min. A linear solvent gradient was used, running from 20 to 50 % B in 10 min, 50 to 90 % B over the next 2 min, held constant at 90 % B for 7 min, returned to 20 % B over 1 min, and equilibrated at 20 % B for 5 min. For analysis of cotinine, the HPLC column used was a 150 × 2 mm Synergi Hydro RP C18 column (Phenomenex). The mobile phase consisted of Solvent A, 0.1 % (v/v) formic acid in water, and Solvent B, acetonitrile containing 0.1 % (v/v) formic acid. The flow rate was 0.2 ml/min. Separations were carried out by isocratic elution at 5 % B with a run time of 5 min. Cotinine eluted at 2.33 minutes.
Mass Spectrometry
An Applied Biosystems MDS Sciex hybrid triple quadrupole/linear ion trap mass spectrometer (4000 QTrap) equipped with a TurboV electrospray source (Concord, Canada) was used for these analyses. The TurboV source was maintained at 400 °C. The ionspray voltage was 4500 V and the declustering potential was 40 V. Nitrogen was used as the source gas, curtain gas, and collision gas. Various scanning techniques, all run in negative ion mode, were used for the characterization and detection of LPO-MA products, including Q1, product ion scanning, and selected reaction monitoring (SRM). All SRM transitions and collision energies for the LPO-MA conjugates are shown in Table 2. SRM in positive ion mode was used for the quantitation of cotinine. The transitions used were m/z 177 → 80 as the quantifier and m/z 177 → 98 as the qualifier.
TABLE 2.
LC-MS/MS properties of ONE and HNE metabolites detected in the urine of human smokers and nonsmokers
| Analyte | MW | SRM Transition |
Collision energy (eV) |
Retention Time (min.) |
Figure | Concentration (nM)# |
|---|---|---|---|---|---|---|
| ONE-MA | 317 | 316 → 162 | 25 | Peak not found | ||
| ONE-MA-d3 | 320 | 319 → 165 | 25 | Peak not found | ||
| HNE-MA | 319 | 318 → 189* | 25 | 10.5, 10.8 | 2 | 7.4-225 |
| 318 → 171 | 25 | 10.5, 10.8 | ||||
| HNE-MA-d3 | 322 | 321 → 189 | 25 | 10.5, 10.8 | ||
| 321 → 171 | 25 | 10.5, 10.8 | ||||
| ONO-MA | 319 | 318 → 162* | 25 | 11.3 | 2 | 1.7-177 |
| ONO-MA-d3 | 322 | 321 → 165 | 25 | 11.3 | ||
| DHN-MA | 321 | 320 → 191* | 25 | 9.8 | 2,3 | 6.6-316 |
| 321 | 320 → 143 | 25 | 9.8 | |||
| DHN-MA-d3 | 324 | 323 → 191 | 25 | 9.8 | ||
| 324 | 323 → 143 | 25 | 9.8 | |||
| ONA-MA | 333 | 332 → 169 | 25 | 12.2 | 2 | |
| 333 | 332 → 162 | 25 | 12.2 | |||
| ONA-MA-d3 | 336 | 335 → 169 | 25 | 12.2 | ||
| 336 | 335 → 162 | 25 | 12.2 | |||
| HNA-MA | 335 | 334 → 162 | 25 | 10.2 | 2 | |
| 335 | 334 → 143 | 25 | 10.2 | |||
| HNA-MA-d3 | 338 | 337 → 165 | 25 | 10.2 | ||
| 338 | 337 → 143 | 25 | 10.2 | |||
| HNAL-MA | 317 | 316 → 162 | 25 | 13.2-13.6 | 2 | |
| 317 | 316 → 143 | 25 | 13.2-13.6 | |||
| HNAL-MA-d3 | 320 | 319 → 165 | 25 | 13.2-13.6 | ||
| 320 | 319 → 143 | 25 | 13.2-13.6 |
Quantifying transition. Other transitions used as qualifiers.
Concentration is shown as a range encompassing the levels found for all study participants.
Data Analysis
Peak area analysis was performed using Analyst 1.4.1 (Applied Biosystems). Analyte peak areas were normalized for the internal standard peak area, a 2-fold sample concentration, and for the creatinine concentration in mg/ml. Thus, all data are represented as mg/g creatinine unless otherwise stated. The standard addition curve samples were concentrated 5-fold during sample preparation and this was taken into account for the calculation of endogenous metabolite levels using these curves. LPO-MA is the sum of HNE-MA, DHN-MA, and ONO-MA concentrations represented as mg/g creatinine. Statistical comparisons were performed with GraphPad (San Diego, CA) using a paired or unpaired Student’s t test as appropriate. Data are shown either as a range of concentrations or as mean ± S.D.
Results
Quantitation of LPO products in human urine
We have developed an LC-MS/MS method for the simultaneous quantitation of HNE-MA, ONO-MA, and DHN-MA in human urine. Previous human studies have focused only on the quantitation of DHN-MA [22]. A study in rats by Mally et al. [26] quantified both HNE-MA and DHN-MA, however they did not account for ONO-MA in their analysis.
Quantitation of HNE-MA
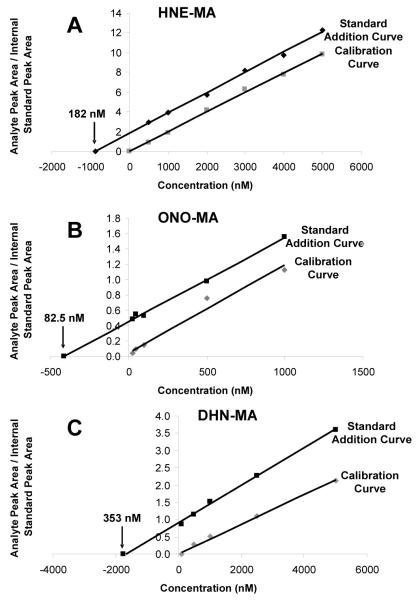
A calibration curve was constructed, using standard solutions containing varying concentrations of HNE-MA and a fixed concentration of HNE-MAd3 (1.0 μM) as the internal standard. We also prepared a standard addition curve in order to assess the accuracy of our method and to investigate the possibility of matrix effects. Urine aliquots were spiked with a fixed amount of internal standard (10 μl of a 10 μM solution) and varying amounts (0.5-5.0 μM) of HNE-MA. Both curves were analyzed by LC-MS/MS (Fig. 1A). Extrapolation of the standard addition curve (R2 ≥ 0.998) to y = 0 gave a sample concentration of 910 nM HNE-MA. However, it was also necessary to account for concentration (5x) of the sample during preparation. Thus, the calculated 910 nM concentration divided by 5 gave the endogenous urinary HNE-MA concentration of 182 nM. This urinary concentration, calculated by the standard addition method, corresponded to a concentration of 203 ± 4.5 nM HNE-MA in aliquots (n = 3) of the same urine sample calculated using the calibration curve (R2 ≥ 0.998, Fig. 1A). The lower limit of quantitation was determined to be 5 nM (S/N = 10) for these analyses, allowing for detection of 2.5 nM concentrations of HNE-MA since smoker and nonsmoker samples were concentrated 2x during sample preparation.
Figure 1.
Calibration curve and standard addition curve plots for HNE-MA (A), ONO-MA (B), and DHN-MA (C). The calibration curves were derived from the analysis of synthetic standards ranging in concentration from 0.5 to 5.0 μM for HNE-MA, 0.25 to 1.0 μM for ONO-MA, and 0.1 to 5.0 μM for DHN-MA. All curves were constructed using a fixed concentration of internal standard (1.0 μM HNE-MAd3, 1.0 μM ONO-MAd3, and 5.0 μM DHN-MAd3). The standard addition curves were prepared by spiking 1.0 ml aliquots of urine with a fixed amount of internal standard and known amounts of LPO-MA conjugate. Samples were concentrated five times during preparation. Extrapolation of the standard addition curve to y = 0 and division by 5 to account for concentration of the sample during preparation, gave endogenous urinary concentrations of 182 nM HNE-MA, 82.5 nM of ONO-MA, and 353 nM of DHN-MA.
Validation of HNE-MA Standard
A HNE-MA standard (1 mg in 100 μl ethanol) was purchased from Cayman Chemical for use to validate the concentration of our synthetically prepared material. In order to do this, a calibration curve was prepared using varying amounts (0.5-5.0 μM) of the Cayman HNE-MA standard and a fixed amount of HNE-MAd3 (10 μl of a 10 μM solution). This curve was analyzed by LC-MS/MS and compared to the calibration curve prepared using HNE-MA synthesized in our lab. The curve prepared from HNE-MA prepared in our lab had a slope of 0.0020 and R2 ≥ 0.997, while the Cayman HNE-MA resulted in a curve with a slope of 0.0023 and R2 ≥ 0.994, demonstrating that our method of HNE-MA synthesis provided comparable results to those obtained with the commercial material.
Quantitation of ONO-MA
Varying concentrations of ONO-MA and a fixed concentration of internal standard ONO-MAd3 (1.0 μM) were used to prepare a calibration curve. A standard addition curve was also prepared by spiking urine aliquots with a fixed amount of internal standard (10 μl of a 10 μM solution) and varying concentrations of ONO-MA (0.25-1.0 μM). Both curves were analyzed by LC-MS/MS (Fig. 1B). Extrapolation of the standard addition curve (R2 ≥ 0.998) to y = 0 (calculated concentration 412.5 nM) and accounting for sample concentration (5x) gave an endogenous ONO-MA concentration of 82.5 nM. This urinary concentration, calculated by the standard addition method, corresponded to a concentration of 67.1 ± 7.9 nM ONO-MA in aliquots (n = 3) of the same urine sample analyzed using the calibration curve (R2 ≥ 0.975, Fig. 1B). The lower limit of quantitation was determined to be 0.5 nM (S/N = 10) for these analyses, allowing for detection of 0.25 nM concentrations of ONO-MA since smoker and nonsmoker samples were concentrated 2x during sample preparation.
Quantitation of DHN-MA
A calibration curve was constructed, using varying amounts of DHN-MA and a fixed amount of DHN-MAd3 (5.0 μl of a 100 μM solution) as the internal standard. We also prepared a standard addition curve, spiking urine aliquots with a fixed amount of internal standard (5.0 μl of a 100 μM solution) and varying concentrations of DHN-MA (0.1-5.0 μM). Both curves were analyzed by LC-MS/MS (Fig. 1C). Extrapolation of the standard addition curve (R2 ≥ 0.998) to y = 0 (calculated concentration 1765 nM) and accounting for sample concentration (5x) gave an endogenous DHN-MA concentration of 353 nM. This urinary concentration, calculated by the standard addition method, corresponded to a concentration of 457 nM DHN-MA in an aliquot (n = 1) of the same urine sample analyzed using the calibration curve (R2 ≥ 0.996, Fig. 1C). The lower limit of quantitation was determined to be 10 nM (S/N = 10) for these analyses, allowing for detection of 5 nM concentrations of DHN-MA since samples were concentrated 2x during sample preparation.
Stability of Standards
The LPO-MA conjugate solutions used in this study were generally found to be stable over a period of six months when stored at -20 °C. HNA-MA is the one exception since it spontaneously converts to HNAL-MA and should be prepared fresh every few weeks. We found it best to assess the standards and internal standards by LC-MS/MS each time a batch of samples was run to ensure that the levels remained consistent over time, preparing new standards from the LPO product and MA if necessary.
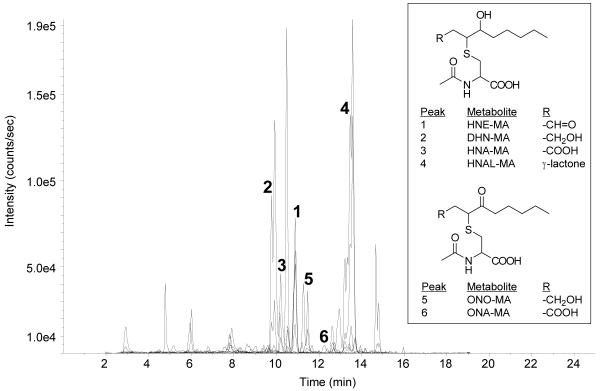
LPO products in human urine
The LPO products, HNE-MA, DHN-MA, HNA-MA, HNAL-MA, ONO-MA, and ONA-MA were all detected in human urine samples (Fig. 2). We were not able to quantify HNA-MA, HNAL-MA, and ONA-MA in human urine. The endogenous amounts of ONA-MA, estimated at < 10 nM, were too small to quantify by isotope-dilution LC-MS/MS with satisfactory precision and accuracy. HNA-MA and HNAL-MA presented a different challenge. Due to the spontaneous conversion of HNA-MA into HNAL-MA under aqueous conditions, we have thus far been unable to obtain homogenous synthetic standards of either material for use in our calibration curves. ONE-MA
Figure 2.
LC-SRM chromatogram of a human urine sample. Key to chromatographic peaks: (1) HNE-MA m/z 318 → 189 and m/z 318 → 171; (2) DHN-MA m/z 320 → 191 and m/z 320 → 143; (3) HNA-MA m/z 334 → 162; (4) HNAL-MA m/z 316 → 162 and m/z 316 → 143; (5) ONO-MA m/z 318 → 162; (6) ONA-MA m/z 332 → 169 and m/z 332 → 162. ONE-MA was not detected.
It should be noted that while we have previously demonstrated the presence of ONE-MA in rat urine [20], it was not detectable in our human urine samples. Its absence in human urine is likely due to preferential phase I metabolism of ONE or ONE-GSH resulting in metabolites ONO-MA and ONA-MA. ONE is also able to covalently modify cysteine, histidine, and lysine residues in proteins [27, 28], making it undetectable by our methods. Moreover, the GSH conjugate of ONE retains its ability to undergo Schiff base formation. It is also possible that thiadiazabicyclo-ONE-glutathione (TOG), a metabolite of ONE in cultured endothelial (EA.hy 926) cells [29], is formed in vivo. The formation of TOG involves cyclization of the γ-glutamic acid residue of ONE-GSH, preventing further metabolism of the GSH moiety to form ONE-MA. TOG formation is likely a minor pathway, however, since we are able to quantify ONO-MA levels and to detect ONA-MA in the human urine samples.
Metabolite confirmation
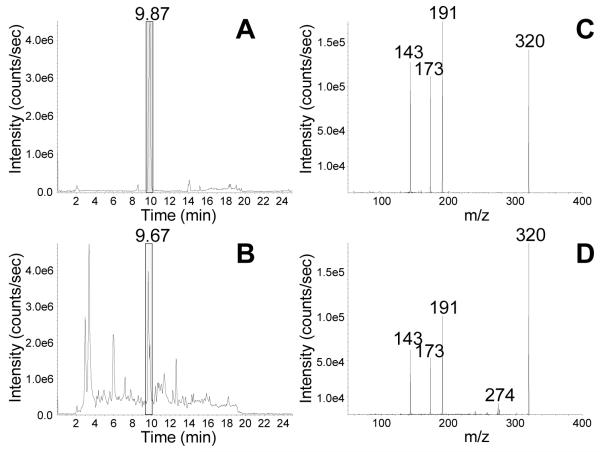
Enhanced product ion (EPI) scanning, along with the comparison of biological samples to synthetic standards prepared in our lab, was used to ensure correct metabolite identification. EPI was performed by selecting a m/z of interest in Q1, inducing fragmentation in Q2, then utilizing the linear ion trap mode to trap these fragments in Q3, followed by Q3 scanning in quadrupole mode. This technique allows for the sensitive MS/MS comparison of analytes in synthetic and biological samples. Figure 3 shows EPI spectra in negative ion mode for synthetic and biological DHN-MA, which eluted at 9.8 min (Fig. 3A, 3C). Both of these samples demonstrate that DHN-MA produces fragments with m/z 191 and m/z 143, both β-elimination fragments and m/z 173, formed via a McLafferty rearrangement (Fig. 3B, 3D). Similar experiments were performed for HNE-MA and ONO-MA in our previous work [20].
Figure 3.
LC-EPI chromatograms of a DHN-MA synthetic standard and a human urine sample. (A) Negative ion electrospray EPI scanning of m/z 320 of a standard reaction mixture of DHN-MA. (B) Negative ion electrospray EPI scanning of m/z 320 of a human urine sample. (C) The EPI spectrum of synthetic DHN-MA shows fragments with m/z 191, m/z 143, and m/z 173. (D) The EPI spectrum of endogenous DHN-MA in a human urine sample shows the same fragments as the synthetic DHN-MA sample, with m/z 191, m/z 143, and m/z 173.
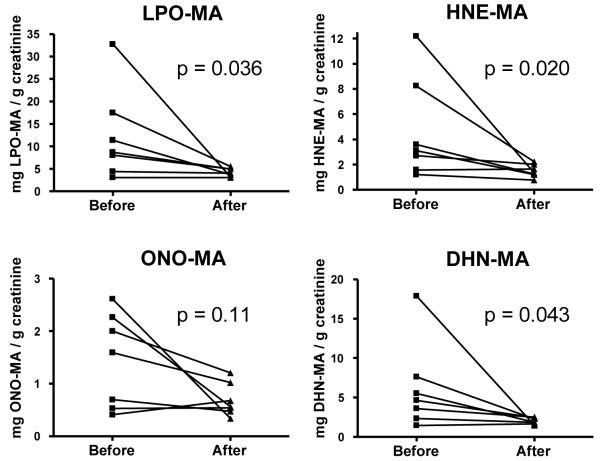
Smoking Cessation
Smoking cessation caused significant decreases in the urinary levels of HNE-MA, DHN-MA, and LPO-MA present in a group of seven human subjects (Fig. 4). There was no significant change in any of these metabolites over the same time period in nonsmoking subjects (paired Student’s t test, p > 0.05). We also did not find any significant differences in metabolite levels between the smoker and nonsmoker groups prior to or following cessation (unpaired Student’s t test, p > 0.05). Neither did we find any significant correlation between LPO-MA and age (p = 0.88) or BMI (p = 0.35). All calculations included normalization for creatinine levels to account for variation in urine concentration between individuals. There was no significant difference in the urinary creatinine levels before and after smoking cessation (p = 0.45).
Figure 4.
Comparison of LPO metabolites in smokers before and after smoking cessation. HNE-MA, DHN-MA, and LPO-MA were significantly decreased after 12 weeks of smoking cessation in humans with p values of 0.020, 0.043, and 0.036 respectively. LPO-MA is the sum of HNE-MA, DHN-MA and ONO-MA. Data were analyzed on a logarithmic scale.
Discussion
A semi-quantitative method for analysis of HNE-MA and ONE-MA metabolites in the urine of oxidatively stressed rats was previously reported [20]. While this method allowed for the simultaneous analysis of multiple LPO-MA conjugates, the data was not quantitative. Appropriate internal standards for each of our analytes of interest are necessary in order to perform absolute quantitation. We first synthesized MAd3 following the method of Slatter et al. [25]. MAd3 conjugates of HNE, DHN, HNA, ONE, ONO, and ONA, were then prepared as described by Kuiper et al. [20]. Quantitation of endogenous LPO-MA conjugates was subsequently achieved by isotope-dilution LC-MS/MS using SRM. We now demonstrate, for the first time, the quantitative determination of ONO-MA in addition to HNE-MA and DHN-MA in vivo at low mg/g creatinine levels.
In smokers and nonsmokers, we found the urinary levels of ONO-MA to be in the range 0.05-2.26 mg/g creatinine (1.7-177 nM). HNE-MA was present in the range of 0.17-12.19 mg/g creatinine (7.4-225 nM) and DHN-MA at levels of 0.22-17.90 mg/g creatinine (6.6-316 nM). Low LPO-MA conjugate levels in a subgroup of the smokers resulted in the lack of statistical difference between the smoker and nonsmoker groups prior to smoking cessation. Alary et al. [22] also assessed DHN-MA in humans and found production of 5 μg/24 h in seven healthy human volunteers, which corresponds to 2.7 ng/ml (8.4 nM). These levels are comparable to the low levels of DHN-MA in our study. In a study of the urinary excretion of LPO-MA conjugates in rats, Mally et al. [26] measured 113.8 ± 36.8 pmol/mg creatinine for HNE-MA (36 μg/g creatinine) and 1.19 ± 0.33 nmol/mg creatinine for DHN-MA (382 μg/g creatinine). Rathahao et al. [21] and Guéraud et al. [23] found urinary production of DHN-MA in rats to be in the range of 45-230 ng/24 h (equivalent to 8.8-45 nM, assuming a urine production of 16 ml/24 h), with the higher concentrations appearing in BrCCl3 stressed animals. Alary et al. [22] also analyzed rat urine and found DHN-MA production of 10 ng/24 h or 0.8 ng/ml (2.5 nM). Urinary levels of DHN-MA in lean and Zucker obese rats (1.1 μM and 2.9 μM, resp.), reported by Orioli et al. [24], however, are much higher than the other reported values. With the exception of the levels reported by Orioli et al. [24], urinary levels of HNE-MA and DHN-MA in rats seem to be lower than or at the low end of the range of human levels determined in Alary’s study [22] and in our present study. Since the urinary levels of LPO-MA conjugates in rats tend to fall within the same range as the levels we found in human samples, our quantitation method should be applicable to animal investigations as well.
Like HNE and ONE, F2α-isoprostanes are formed from lipid hydroperoxides via radical-mediated pathways. F2α-isoprostanes are generally considered to be the most reliable markers of in vivo oxidative stress [30, 31]. Smoking cessation has been shown to result in significant decreases of urinary F2α-isoprostane levels after one or two weeks [32, 33]. The study conducted by Chehne et al. [33] demonstrated that the decrease of urinary F2α-isoprostane levels upon smoking cessation was similar between patients having clinically manifested atherosclerosis with or without hypercholesterolemia and/or hypertension, indicating that cigarette smoke is a major contributor to in vivo oxidative stress compared to other risk factors of atherosclerosis. Similar to urinary F2α-isoprostane levels, our study of apparently healthy participants showed significant decreases in the levels of MA conjugates of HNE and DHN in the urine upon smoking cessation, reflecting a similar pathway of formation via lipid hydroperoxides.
The LPO metabolites of our study differ from the F2α-isoprostanes in that they are also products of phase I and phase II metabolism. Thus, the levels of MA conjugates of HNE, DHN, and ONO reflect both formation of HNE and ONE and their subsequent metabolism. Expression levels of GSTs may therefore co-determine urinary levels of HNE-MA, DHN-MA, and ONO-MA. GSTP1, a GST isoenzyme involved in HNE conjugation [34, 35], was induced in lung tissue of smokers whereas other GSTs, GSTA2 and GSTM1, showed no difference in expression levels between smokers and nonsmokers [36]. On the other hand, genetic polymorphism of GST may affect gene expression if the mutation is located in the promoter region. Qian et al. [37] studied single nucleotide polymorphisms (SNPs) of GSTA4, another GST that accepts HNE as a substrate [38]. Qian et al. [37] found that the presence of genotypes TA and AA at locus-1718 of GSTA4 was associated with a 37 % significantly decreased risk of lung cancer compared to the TT genotype. The authors suggested that the TA and AA genotypes, with the SNP in the promoter region, may have increased GSTA4 expression and thus greater capacity to detoxify HNE as compared to the TT genotype. Dwivedi et al. [39] determined that GSTA4 null mice have higher levels of hepatic HNE after CCl4 treatment than wild-type mice, indicating reduced HNE conjugation in GSTA4 null mice. In the study by Qian et al. [40], the TT genotype had a prevalence of 77 % in lung cancer patients (n = 500) and 68 % in cancer-free control subjects (n = 517). The common occurrence of the TT genotype, presumably having reduced GSTA4 expression and reduced capacity to conjugate HNE, may explain the low urinary levels of LPO-MA (< 7 mg/g creatinine) we found in 9 out of 23 smokers.
The low LPO-MA excretion in nine smokers may also be due to smoking-induced phase I metabolism, resulting in enhanced conversion of HNE and ONE into DHN which is not a GST substrate. Aldo-keto reductase 1B10 (AKR1B10) is known to reduce HNE to DHN and to reduce ONE to ONO [41]. Its up-regulation in smokers, shown by Fukumoto et al. [42] and Nagaraj et al. [43], would direct the metabolism of HNE to DHN, resulting in decreased formation of GST-mediated metabolites and MA conjugates (Scheme 1).
We developed a method for the accurate quantitation of ONO-MA, HNE-MA, and DHN-MA in human urine by isotope-dilution LC-MS/MS. We also detected HNA-MA, HNAL-MA, and ONA-MA. The significance of the in vivo detection of ONO-MA and ONA-MA is that these conjugates represent HNE/ONE branching in the breakdown of lipid hydroperoxides as shown in Scheme 1, suggesting that ONO may contribute to the deleterious effects previously ascribed to HNE. Our findings also show that LPO-MA conjugates are elevated in urine obtained from smokers and decrease significantly following smoking cessation, demonstrating the utility of these metabolites as markers of in vivo oxidative stress.
Acknowledgement
We thank pharmacy residents Rachelle Collier and Tiffany Boehland for teaching smoking cessation classes to the smokers enrolled in the study.
Abbreviations
- BMI
body mass index
- DHN
1,4-dihydroxy-2-nonene
- EPI
enhanced product ion
- GSH
glutathione
- GST
glutathione-S-transferase
- HNA
4-hydroxy-2-nonenoic acid
- HNAL
4-hydroxy-2-nonenoic acid lactone
- HNE
4-hydroxy-2-nonenal
- HPLC
high performance liquid chromatography
- LC
liquid chromatography
- LPO
lipid peroxidation
- MA
mercapturic acid
- MAd3
N-(acetyl-d3)-L-cysteine
- MS
mass spectrometry
- ONA
4-oxo-2-nonenoic acid
- ONE
4-oxo-2-nonenal
- ONO
4-oxo-2-nonen-1-ol
- PUFA
polyunsaturated fatty acid
- SNP
single nucleotide polymorphisms
- SRM
selected reaction monitoring
- TOG
thiadiazabicyclo-ONE-glutathione
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This work was supported in part by the National Institutes of Health (R01HL081721, S10RR022589, and P30ES000210), an OSU Center for Healthy Aging Research Fellowship, a grant from the John C. Erkkila, MD, Endowment for Health and Human Performance, and by a donation from the estate of Leland J. Gross.
References
- [1].Benedetti A, Comporti M, Esterbauer H. Identification of 4-hydroxynonenal as a cytotoxic product originating from the peroxidation of liver microsomal lipids. Biochim. Biophys. Acta. 1980;620:281–296. doi: 10.1016/0005-2760(80)90209-x. [DOI] [PubMed] [Google Scholar]
- [2].Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic. Biol. Med. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- [3].Barbin A. Formation of DNA etheno adducts in rodents and humans and their role in carcinogenesis. Acta Biochim. Pol. 1998;45:145–161. [PubMed] [Google Scholar]
- [4].Spiteller G. The important role of lipid peroxidation processes in aging and age dependent diseases. Mol. Biotechnol. 2007;37:5–12. doi: 10.1007/s12033-007-0057-6. [DOI] [PubMed] [Google Scholar]
- [5].Facchinetti F, Amadei F, Geppetti P, Tarantini F, Di Serio C, Dragotto A, Gigli PM, Catinella S, Civelli M, Patacchini R. Alpha,beta-unsaturated aldehydes in cigarette smoke release inflammatory mediators from human macrophages. Am. J. Respir. Cell Mol. Biol. 2007;37:617–623. doi: 10.1165/rcmb.2007-0130OC. [DOI] [PubMed] [Google Scholar]
- [6].Rahman I, van Schadewijk AA, Crowther AJ, Hiemstra PS, Stolk J, MacNee W, De Boer WI. 4-Hydroxy-2-nonenal, a specific lipid peroxidation product, is elevated in lungs of patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2002;166:490–495. doi: 10.1164/rccm.2110101. [DOI] [PubMed] [Google Scholar]
- [7].Butterfield DA, Sultana R. Redox proteomics identification of oxidatively modified brain proteins in Alzheimer’s disease and mild cognitive impairment: insights into the progression of this dementing disorder. J. Alzheimers Dis. 2007;12:61–72. doi: 10.3233/jad-2007-12107. [DOI] [PubMed] [Google Scholar]
- [8].Lovell MA, Markesbery WR. Oxidative DNA damage in mild cognitive impairment and late-stage Alzheimer’s disease. Nucleic Acids Res. 2007;35:7497–7504. doi: 10.1093/nar/gkm821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Picklo MJS, Montine TJ. Mitochondrial effects of lipid-derived neurotoxins. J. Alzheimers Dis. 2007;12:185–193. doi: 10.3233/jad-2007-12209. [DOI] [PubMed] [Google Scholar]
- [10].Mitchell DY, Petersen DR. The oxidation of alpha-beta unsaturated aldehydic products of lipid peroxidation by rat liver aldehyde dehydrogenases. Toxicol. Appl. Pharmacol. 1987;87:403–410. doi: 10.1016/0041-008x(87)90245-6. [DOI] [PubMed] [Google Scholar]
- [11].Doorn JA, Hurley TD, Petersen DR. Inhibition of human mitochondrial aldehyde dehydrogenase by 4-hydroxynon-2-enal and 4-oxonon-2-enal. Chem. Res. Toxicol. 2006;19:102–110. doi: 10.1021/tx0501839. [DOI] [PubMed] [Google Scholar]
- [12].Srivastava S, Dixit BL, Cai J, Sharma S, Hurst HE, Bhatnagar A, Srivastava SK. Metabolism of lipid peroxidation product, 4-hydroxynonenal (HNE) in rat erythrocytes: role of aldose reductase. Free Radic. Biol. Med. 2000;29:642–651. doi: 10.1016/s0891-5849(00)00351-8. [DOI] [PubMed] [Google Scholar]
- [13].Jian W, Arora JS, Oe T, Shuvaev VV, Blair IA. Induction of endothelial cell apoptosis by lipid hydroperoxide-derived bifunctional electrophiles. Free Radic. Biol. Med. 2005;39:1162–1176. doi: 10.1016/j.freeradbiomed.2005.06.008. [DOI] [PubMed] [Google Scholar]
- [14].Doorn JA, Srivastava SK, Petersen DR. Aldose reductase catalyzes reduction of the lipid peroxidation product 4-oxonon-2-enal. Chem. Res. Toxicol. 2003;16:1418–1423. doi: 10.1021/tx0300378. [DOI] [PubMed] [Google Scholar]
- [15].Blair IA. Endogenous glutathione adducts. Curr. Drug Metab. 2006;7:853–872. doi: 10.2174/138920006779010601. [DOI] [PubMed] [Google Scholar]
- [16].Agianian B, Tucker PA, Schouten A, Leonard K, Bullard B, Gros P. Structure of a Drosophila sigma class glutathione S-transferase reveals a novel active site topography suited for lipid peroxidation products. J. Mol. Biol. 2003;326:151–165. doi: 10.1016/s0022-2836(02)01327-x. [DOI] [PubMed] [Google Scholar]
- [17].Knoll N, Ruhe C, Veeriah S, Sauer J, Glei M, Gallagher EP, Pool-Zobel BL. Genotoxicity of 4-hydroxy-2-nonenal in human colon tumor cells is associated with cellular levels of glutathione and the modulation of glutathione S-transferase A4 expression by butyrate. Toxicol. Sci. 2005;86:27–35. doi: 10.1093/toxsci/kfi171. [DOI] [PubMed] [Google Scholar]
- [18].Gallagher EP, Huisden CM, Gardner JL. Transfection of HepG2 cells with hGSTA4 provides protection against 4-hydroxynonenal-mediated oxidative injury. Toxicol. In Vitro. 2007;21:1365–1372. doi: 10.1016/j.tiv.2007.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Alary J, Bravais F, Cravedi JP, Debrauwer L, Rao D, Bories G. Mercapturic acid conjugates as urinary end metabolites of the lipid peroxidation product 4-hydroxy-2-nonenal in the rat. Chem. Res. Toxicol. 1995;8:34–39. doi: 10.1021/tx00043a004. [DOI] [PubMed] [Google Scholar]
- [20].Kuiper HC, Miranda CL, Sowell JD, Stevens JF. Mercapturic acid conjugates of 4-hydroxy-2-nonenal and 4-oxo-2-nonenal metabolites are in vivo markers of oxidative stress. J. Biol. Chem. 2008;283:17131–17138. doi: 10.1074/jbc.M802797200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Rathahao E, Peiro G, Martins N, Alary J, Guéraud F, Debrauwer L. Liquid chromatography-multistage tandem mass spectrometry for the quantification of dihydroxynonene mercapturic acid (DHN-MA), a urinary end-metabolite of 4-hydroxynonenal. Anal. Bioanal. Chem. 2005;381:1532–1539. doi: 10.1007/s00216-005-3095-6. [DOI] [PubMed] [Google Scholar]
- [22].Alary J, Debrauwer L, Fernandez Y, Cravedi JP, Rao D, Bories G. 1,4-Dihydroxynonene mercapturic acid, the major end metabolite of exogenous 4-hydroxy-2-nonenal, is a physiological component of rat and human urine. Chem. Res. Toxicol. 1998;11:130–135. doi: 10.1021/tx970139w. [DOI] [PubMed] [Google Scholar]
- [23].Guéraud F, Peiro G, Bernard H, Alary J, Créminon C, Debrauwer L, Rathahao E, Drumare MF, Canlet C, Wal JM, Bories G. Enzyme immunoassay for a urinary metabolite of 4-hydroxynonenal as a marker of lipid peroxidation. Free Radic. Biol. Med. 2006;40:54–62. doi: 10.1016/j.freeradbiomed.2005.08.011. [DOI] [PubMed] [Google Scholar]
- [24].Orioli M, Aldini G, Benfatto MC, Facino RM, Carini M. HNE Michael adducts to histidine and histidine-containing peptides as biomarkers of lipid-derived carbonyl stress in urines: LC-MS/MS profiling in Zucker obese rats. Anal. Chem. 2007;79:9174–9184. doi: 10.1021/ac7016184. [DOI] [PubMed] [Google Scholar]
- [25].Slatter JG, Rashed MS, Pearson PG, Han DH, Baillie TA. Biotransformation of methyl isocyanate in the rat. Evidence for glutathione conjugation as a major pathway of metabolism and implications for isocyanate-mediated toxicities. Chem. Res. Toxicol. 1991;4:157–161. doi: 10.1021/tx00020a006. [DOI] [PubMed] [Google Scholar]
- [26].Mally A, Amberg A, Hard GC, Dekant W. Are 4-hydroxy-2(E)-nonenal derived mercapturic acids and (1)H NMR metabonomics potential biomarkers of chemically induced oxidative stress in the kidney? Toxicology. 2007;230:244–255. doi: 10.1016/j.tox.2006.11.068. [DOI] [PubMed] [Google Scholar]
- [27].Zhang WH, Liu J, Xu G, Yuan Q, Sayre LM. Model studies on protein side chain modification by 4-oxo-2-nonenal. Chem. Res. Toxicol. 2003;16:512–523. doi: 10.1021/tx020105a. [DOI] [PubMed] [Google Scholar]
- [28].Sayre LM, Lin D, Yuan Q, Zhu X, Tang X. Protein adducts generated from products of lipid oxidation: focus on HNE and ONE. Drug Metab. Rev. 2006;38:651–675. doi: 10.1080/03602530600959508. [DOI] [PubMed] [Google Scholar]
- [29].Jian W, Lee SH, Mesaros C, Oe T, Elipe MV, Blair IA. A novel 4-oxo-2(E)-nonenal-derived endogenous thiadiazabicyclo glutathione adduct formed during cellular oxidative stress. Chem. Res. Toxicol. 2007;20:1008–1018. doi: 10.1021/tx700001t. [DOI] [PubMed] [Google Scholar]
- [30].Basu S. F2-isoprostanes in human health and diseases: from molecular mechanisms to clinical implications. Antioxid. Redox Signal. 2008;10:1405–1434. doi: 10.1089/ars.2007.1956. [DOI] [PubMed] [Google Scholar]
- [31].Montuschi P, Barnes P, Roberts LJ., 2nd Insights into oxidative stress: the isoprostanes. Curr. Med. Chem. 2007;14:703–717. doi: 10.2174/092986707780059607. [DOI] [PubMed] [Google Scholar]
- [32].Pilz H, Oguogho A, Chehne F, Lupattelli G, Palumbo B, Sinzinger H. Quitting cigarette smoking results in a fast improvement of in vivo oxidation injury (determined via plasma, serum and urinary isoprostane) Thromb. Res. 2000;99:209–221. doi: 10.1016/s0049-3848(00)00249-8. [DOI] [PubMed] [Google Scholar]
- [33].Chehne F, Oguogho A, Lupattelli G, Palumbo B, Sinzinger H. Effect of giving up cigarette smoking and restarting in patients with clinically manifested atherosclerosis. Prostaglandins Leukot. Essent. Fatty Acids. 2002;67:333–339. doi: 10.1054/plef.2002.0438. [DOI] [PubMed] [Google Scholar]
- [34].Gallagher EP, Gardner JL, Barber DS. Several glutathione S-transferase isozymes that protect against oxidative injury are expressed in human liver mitochondria. Biochem. Pharmacol. 2006;71:1619–1628. doi: 10.1016/j.bcp.2006.02.018. [DOI] [PubMed] [Google Scholar]
- [35].Hayes JD, Flanagan JU, Jowsey IR. Glutathione transferases. Annu. Rev. Pharmacol. Toxicol. 2005;45:51–88. doi: 10.1146/annurev.pharmtox.45.120403.095857. [DOI] [PubMed] [Google Scholar]
- [36].Thum T, Erpenbeck VJ, Moeller J, Hohlfeld JM, Krug N, Borlak J. Expression of xenobiotic metabolizing enzymes in different lung compartments of smokers and nonsmokers. Environ. Health Perspect. 2006;114:1655–1661. doi: 10.1289/ehp.8861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Qian J, Jing J, Jin G, Wang H, Wang Y, Liu H, Wang H, Li R, Fan W, An Y, Sun W, Wang Y, Ma H, Miao R, Hu Z, Jin L, Wei Q, Shen H, Huang W, Lu D. Association between polymorphisms in the GSTA4 gene and risk of lung cancer: a case-control study in a Southeastern Chinese population. Mol. Carcinog. 2009;48:253–259. doi: 10.1002/mc.20478. [DOI] [PubMed] [Google Scholar]
- [38].Hubatsch I, Ridderstrom M, Mannervik B. Human glutathione transferase A4-4: an alpha class enzyme with high catalytic efficiency in the conjugation of 4-hydroxynonenal and other genotoxic products of lipid peroxidation. Biochem. J. 1998;330( Pt 1):175–179. doi: 10.1042/bj3300175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Dwivedi S, Sharma R, Sharma A, Zimniak P, Ceci JD, Awasthi YC, Boor PJ. The course of CCl4 induced hepatotoxicity is altered in mGSTA4-4 null (-/-) mice. Toxicology. 2006;218:58–66. doi: 10.1016/j.tox.2005.10.012. [DOI] [PubMed] [Google Scholar]
- [40].Qian J, Jing J, Jin G, Wang H, Wang Y, Liu H, Wang H, Li R, Fan W, An Y, Sun W, Wang Y, Ma H, Miao R, Hu Z, Jin L, Wei Q, Shen H, Huang W, Lu D. Association between polymorphisms in the GSTA4 gene and risk of lung cancer: a case-control study in a Southeastern Chinese population. Mol. Carcinog. 2009;48:253–259. doi: 10.1002/mc.20478. [DOI] [PubMed] [Google Scholar]
- [41].Martin HJ, Maser E. Role of human aldo-keto-reductase AKR1B10 in the protection against toxic aldehydes. Chem. Biol. Interact. 2009;178:145–150. doi: 10.1016/j.cbi.2008.10.021. [DOI] [PubMed] [Google Scholar]
- [42].Fukumoto S, Yamauchi N, Moriguchi H, Hippo Y, Watanabe A, Shibahara J, Taniguchi H, Ishikawa S, Ito H, Yamamoto S, Iwanari H, Hironaka M, Ishikawa Y, Niki T, Sohara Y, Kodama T, Nishimura M, Fukayama M, Dosaka-Akita H, Aburatani H. Overexpression of the aldo-keto reductase family protein AKR1B10 is highly correlated with smokers’ non-small cell lung carcinomas. Clin. Cancer Res. 2005;11:1776–1785. doi: 10.1158/1078-0432.CCR-04-1238. [DOI] [PubMed] [Google Scholar]
- [43].Nagaraj NS, Beckers S, Mensah JK, Waigel S, Vigneswaran N, Zacharias W. Cigarette smoke condensate induces cytochromes P450 and aldo-keto reductases in oral cancer cells. Toxicol. Lett. 2006;165:182–194. doi: 10.1016/j.toxlet.2006.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]