Abstract
Background
Recent evidence indicates that spontaneous sarcoplasmic reticulum (SR) Ca release underlies the mechanism of sinoatrial node (SAN) acceleration during β-stimulation, indicating the importance of Ca clock in SAN automaticity. Whether or not the same mechanism applies to atrial ectopic pacemakers (AEP) remains unclear.
Objective
The purpose of this study was to assess the mechanism of AEP.
Methods
We simultaneously mapped intracellular calcium (Cai) and membrane potential in 12 isolated canine right atria. The late diastolic Cai elevation (LDCAE) was used to detect the Ca clock activity. Pharmacological interventions with isoproterenol (ISO), ryanodine, and ZD7288, a blocker of the If membrane current, were performed.
Results
Ryanodine, which inhibits SR Ca release, reduced LDCAE in SAN, resulting in an inferior shift of the pacemaking site. Cycle length increased significantly in a dose-dependent way. In the presence of 3-10 μmol/L of ryanodine, ISO infusion consistently induces AEPs from the lower crista terminalis. All ectopic beats continuing over 30 seconds were located at the lower crista terminalis. These AEPs were resistant to ryanodine treatment even at high doses. Subsequent blockade of If inhibited the AEP and resulted in profound bradycardia.
Conclusion
Spontaneous SR Ca release underlies ISO-induced increase of superior SAN activity. As compared with SAN, the AEP is less dependent on the Ca clock and more dependent on the membrane clock for its automaticity. AEPs outside the SAN can effectively serve as backup pacemakers when the Ca clock functionality is reduced.
Keywords: calcium, sinoatrial node, sarcoplasmic reticulum, If current, ectopic beat, Ca clock
Sick sinus syndrome (SSS) refers to a spectrum of cardiac arrhythmias that includes sinus arrest, sinoatrial block, sinus bradycardia or alternating paroxysmal atrial tachyarrhythmias with bradycardia (tachy-brady syndrome). When the sinoatrial node (SAN) fails to initiate the heart beat, other subsidiary sites take over and become the dominant pacemaker. It is generally established that such a hierarchy of pacemakers exists in the heart in order to maintain a robust pacemaking process.1 However, a more local hierarchy of pacemakers may exist in the right atrium (RA) and may be responsible for various atrial arrhythmias. For example, Rozanski et al.2 reported that atrial ectopic pacemakers (AEP) activity emerged after suppression of SAN function with the ligation of sinus node artery at the midportion of the sulcus terminalis, and the AEP was originated at the junction of the inferior RA with the inferior vena cava. The mechanism of atrial ectopic beats is unclear. In the SAN cells, the mechanism of spontaneous diastolic depolarization has traditionally been attributed to a “voltage clock” or “membrane clock” mechanism, mediated primarily by the hyperpolarization-activated pacemaker current (If).3 Recent studies implicate a complementary “Ca clock” mechanism mediated by Ca2+ release from the sarcoplasmic reticulum (SR).4-6 It is still controversial whether sinus rate is controlled by voltage clock, Ca clock, or both.7 We8 recently reported that spontaneous SR Ca release is important in the SAN automaticity in isolated RA during β-adrenergic stimulation. Whether or not the modulation of intracellular Ca2+ dynamics affects AEP in the atrium remains unclear.
We hypothesize that (1) Spontaneous Ca release inhibition can suppress SAN automaticity and uncover AEP; (2) The If current is responsible for the generation of ectopic beats in RA when the spontaneous Ca release is inhibited. To test these hypotheses, we performed dual optical mapping of transmembrane potential (Vm) and intracellular calcium (Cai) in intact canine SAN and RA in response to ryanodine intervention at baseline and during β-adrenergic stimulation.
Methods
Langendorff-perfused canine SAN preparation
This study protocol was approved by the Institutional Animal Care and Use Committee of Indiana University School of Medicine and the Methodist Research Institute, and conforms to the guidelines of the American Heart Association. We studied isolated canine RAs in 12 mongrel dogs (22 to 28 kg). The heart was rapidly excised under general anesthesia and the right coronary artery was perfused with 37°C Tyrode's solution equilibrated with 95% O2 and 5% CO2 to maintain a pH of 7.4. The composition of Tyrode's solution was (in mmol/L): 125 NaCl, 4.5 KCl, 0.25 MgCl2, 24 NaHCO3, 1.8 NaH2PO4, 1.8 CaCl2, and 5.5 glucose. The coronary perfusion pressure was regulated between 50 and 60 mmHg. To ensure adequate atrial perfusion, all ventricular coronary branches were tied off. Both ventricles and left atrium were removed. Because SAN is subepicardial in dogs,9 we mapped the epicardial side of the tissue. The SAN area was located posterior to the sulcus terminalis (gray color area in Figure 2A). Contractility was inhibited by 10-17 μmol/L of blebbistatin and the motion artifact was negligible even after isoproterenol (ISO) infusion. Pseudo-ECG was recorded with widely spaced bipolar RA electrodes.
Figure 2. The change of activation pattern of SAN by ryanodine.
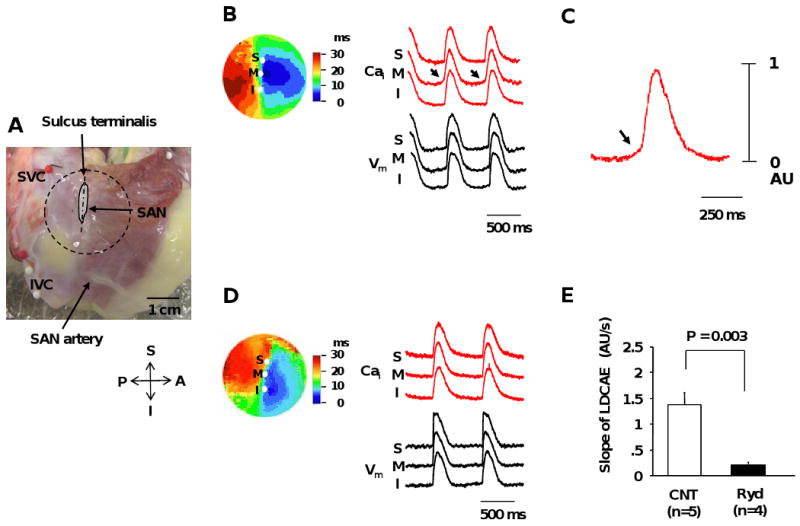
A, Photo of the isolated RA preparation showing the SAN and surrounding RA. The gray shaped area is the SAN. B, Activation pattern of the SAN and surrounding RA under basal condition. The color picture shows isochronal map of Vm propagation. The Cai (red) and Vm (black) recordings from the superior (S), middle (M), and inferior (I) SAN are presented. C, Magnified view of Cai tracing of middle SAN. D, Activation pattern of SAN and surrounding RA during ryanodine infusion of 3 μmol/L. E, Effect of ryanodine (3 μmol/L) on the slope of late diastolic Cai elevation (LDCAE). Arrows point to LDCAE. The activation patterns presented in panels B and D are from the area in the circle in panel A. IVC = inferior vena cava; SAN = sinoatrial node; SVC = superior vena cava. Other abbreviations as in Figure 1.
Assessment of SAN Function
Sinus node recovery time (SNRT) was determined by bipolar pacing with a programmable stimulator (Bloom Associates, Ltd., Reading, PA, USA) for 30 seconds at progressively shorter pacing cycle lengths (400, 350, and 300 msec) with the two electrodes placed near the SAN region. The longest time interval from the last paced atrial depolarization to the first spontaneous sinus beat was recorded as the SNRT. The corrected SNRT (cSNRT) was determined by subtracting an average of 3 sinus cycle lengths prior to the commencement of atrial pacing from the SNRT.
Dual Vm and Cai recordings
Optical mapping analysis was performed as previously described.8 The hearts were stained with Rhod-2 AM and RH237 (Molecular Probes, Eugene, OR, USA) and excited with laser light at 532 nm. Fluorescence was collected using 2 cameras (MiCAM Ultima, Brain Vision, Tokyo, Japan) at 1 ms/frame and 100 × 100 pixels with spatial resolution of 0.35 × 0.35 mm2/pixel. After mapping baseline spontaneous beats, pharmacologic intervention was performed. First, the ryanodine dose responses (0.01 to 30 μmol/L) were evaluated on SAN and surrounding RA (n=4). Next, we examined the response to ISO during baseline and ryanodine infusion (ISO only: n=5, ryanodine + ISO: n=4). In 3 dogs of each group, we examined the response to the If blocker, ZD7288 infusion (30 μmol/L). In addition, we also studied the effects of low-dose ZD7288 (3 μmol/L) on AEP (n=3).
Data Analysis
The Cai and Vm traces were normalized to their respective peak-to-peak amplitude for comparison of timing and morphology. The onset of the late diastolic Cai elevation (LDCAE) was defined as the time of the transition from negative to positive slopes of the dCai/dt curve, whereas the end of LDCAE was defined as the time of an abrupt change of the slope. Student's t-tests were used to compare means between two groups. One-way analyses of variance (ANOVA) followed by Bonferroni-Dunn test were used to compare three or more groups. The effect of ryanodine on cycle length was analyzed with Williams post hoc test. Data was presented as mean ± SEM. A P-value of < 0.05 was considered statistically significant.
Results
The effect of SR Ca release inhibitor on sinus node function
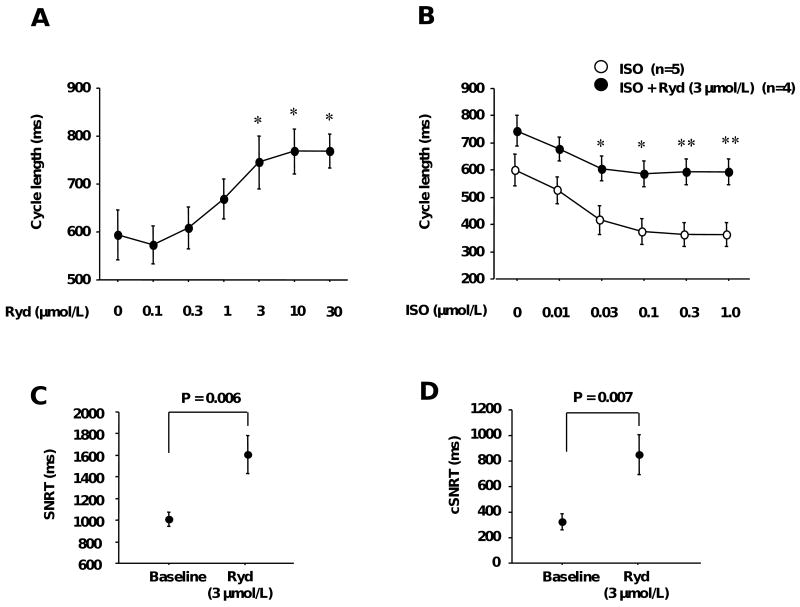
The baseline sinus rate of all intact canine SAN used in the present study was 101 ± 5 bpm (range 80 - 132 bpm, n=12). Figures 1A and B show the changes of cycle length by the SR Ca release inhibitor, ryanodine. Infusion with high dose ryanodine (> 3 μmol/L) often induced ectopic beats. The cycle length, including the sinus and ectopic beats, was analyzed based on pseudo-ECG recordings. Figure 1A shows the dose-response curves of the ryanodine effect on cycle length. Low doses of ryanodine (<0.3 μmol/L), which sensitize ryanodine receptors to Ca2+-induced Ca2+ release, resulted in a slight (<10%) decrease in cycle length. Higher doses of ryanodine, which block ryanodine receptors, caused a dose-dependent suppression of sinus node activity. Cycle length increased significantly in a dose-dependent manner (768.3 ± 47.2 msec at 10 μmol/L of ryanodine, n=4, P=0.013). Figure 1B shows the dose-response curves of cycle length to ISO during ryanodine infusion. ISO dose-dependently decreased cycle length (364.4 ± 42.3 msec at 1.0 μmol/L of ISO, n=5, P=0.001). The ISO-induced decrease of cycle length was suppressed by ryanodine infusion. This suppressive effect was so large that the cycle length in the presence of 3 μmol/L ryanodine and 1.0 μmol/L ISO was similar to the cycle length in the absence of these agents (both about 600 msec).
Figure 1. The effect of ryanodine on intact SAN.
A, Changes of cycle length by ryanodine infusion. * P<0.05 vs. baseline. B, Effects of ryanodine on isoproterenol-induced cycle length decrease. * P<0.05, ** P<0.01 vs. corresponding isoproterenol only group. C, Sinus node recovery time at the baseline and with ryanodine. D, Correct sinus node recovery time at the baseline and with ryanodine. Ryd = ryanodine; ISO = isoproterenol; SNRT = sinus node recovery time; cSNRT = corrected sinus node recovery time.
Ryanodine infusion increased the SNRT (1010 ± 65 to 1607 ± 178 msec, n=4, P=0.006, 1C) and cSNRT (325 ± 64 to 848 ± 156 msec, n=4, P=0.007, Figure 1D).
Effect of SR Ca release inhibitor on activation pattern in SAN and the surrounding RA
Figure 2B shows the activation pattern on SAN and surrounding RA during spontaneous sinus rhythm during baseline recording. The upstrokes of Cai and Vm recordings were nearly simultaneous. Under basal conditions, the leading pacemaker site was located in the middle SAN. Small amplitude LDCAEs were observed in the pacemaking site (Arrows in Figure 2B and C). Ryanodine (3 μmol/L) reduced LDCAE in SAN from 1.38 ± 0.23 to 0.20 ± 0.04 AU (P=0.003, Figure 2E), resulting in an inferior shift of the pacemaking site (Figure 2D).
ISO infusion resulted in an upward (cranial) shift of the pacemaking site to superior SAN (Figure 3A), coincident with the appearance of robust LDCAE (arrows in Figure 3A and B) in this region in all 5 preparations (1.38 ± 0.23 to 3.72 ± 0.58 AU, P=0.016). Ryanodine inhibited ISO-induced effects in superior SAN. In the presence of 10 μmol/L of ryanodine, ISO infusion induced or accelerated the AEPs from the lower crista terminalis. These AEPs became the dominant pacemakers and were highly resistant to ryanodine, because they were not suppressed even at 30 μmol/L. The ectopic beats induced by high dose of ryanodine with or without ISO infusion were located at a total of 7 sites (1-3 sites per RA) in our study. Ectopic beats continuing over 30 seconds originated from 4 sites. All 4 sites were located at the lower crista terminalis (Figure 4A). There were no LDCAE or diastolic depolarization in ectopic beats site in optical mapping pictures from the epicardium (Figure 4B). We also mapped the AEP from the endocardial side of the tissue and observed no LDCAE on the AEP (Figure 4D). However, the Vm traces at the ectopic beat site exhibited a diastolic depolarization that is characteristic of pacemaker activity. The results suggested that AEP site may be located near the endocardial side in the lower crista terminalis, because diastolic depolarization in the Vm traces could not be identified from the epicardial recordings.
Figure 3. Effect of isoproterenol on activation pattern of SAN.
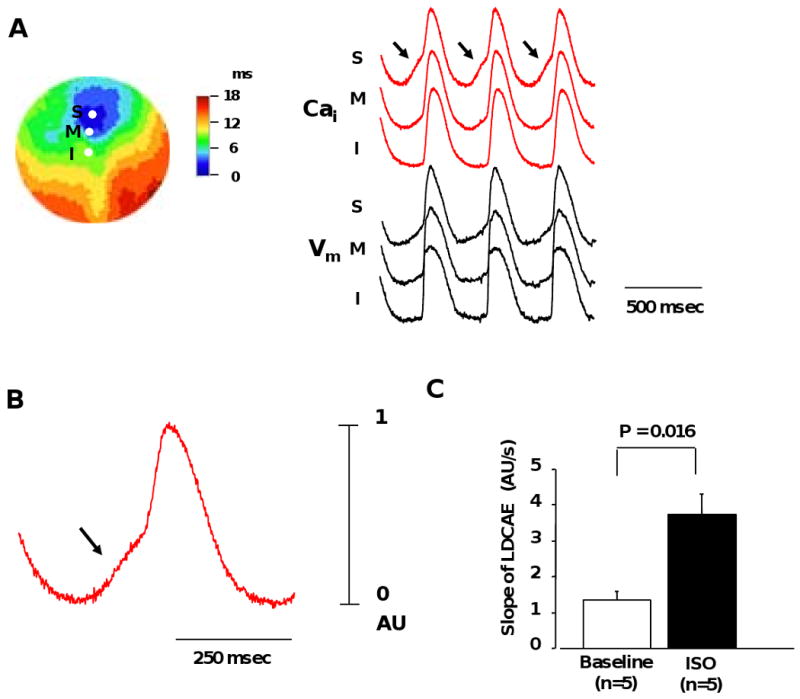
A, Isoproterenol (0.1 μmol/L) -induced activation pattern of SAN. Color picture shows isochronal map of Vm. The Cai (red) and Vm (black) recordings from the superior (S), middle (M), and inferior (I) SAN are presented. B, Magnified view of Cai tracing of superior SAN. C, Effect of isoproterenol (0.1 μmol/L) on the slope of late diastolic Cai elevation (LDCAE). Arrows point to LDCAE.
Figure 4. Activation pattern of AEP.
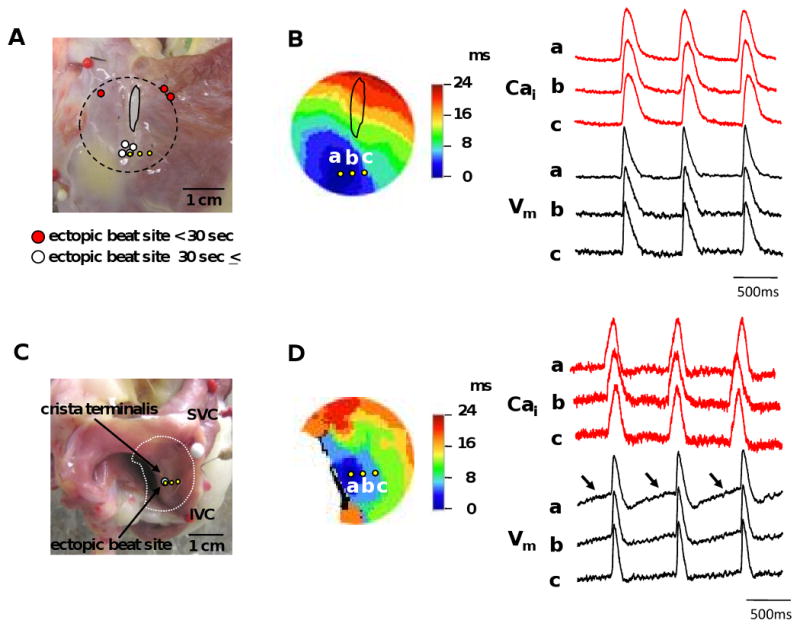
A, Locations of the ectopic beats on the epicardial side. Circles show the sites of ectopic beats. White circles represent ectopic beats continuing over 30 seconds. Red circles represent ectopic beats continuing for less than 30 seconds. Yellow circles correspond with those in B. B, Activation isochrones of an ectopic beat from epicardium. Isochronal map shows epicardial activation pattern of Vm during the ectopic beat. The Cai (red) and Vm (black) recordings from the ectopic pacemaking site (a) and surrounding sites (b and c) are presented. a, b, and c in Vm and Cai recordings correspond with those indicated in the isochronal map. C, Locations of the ectopic beats site from endocardium side. D, Activation isochrones of an ectopic beat from endocardium. Arrows show the diastolic depolarizations.
Effects of SR Ca release Inhibition and If Blockade on ectopic beats rate and its Acceleration by Isoproterenol
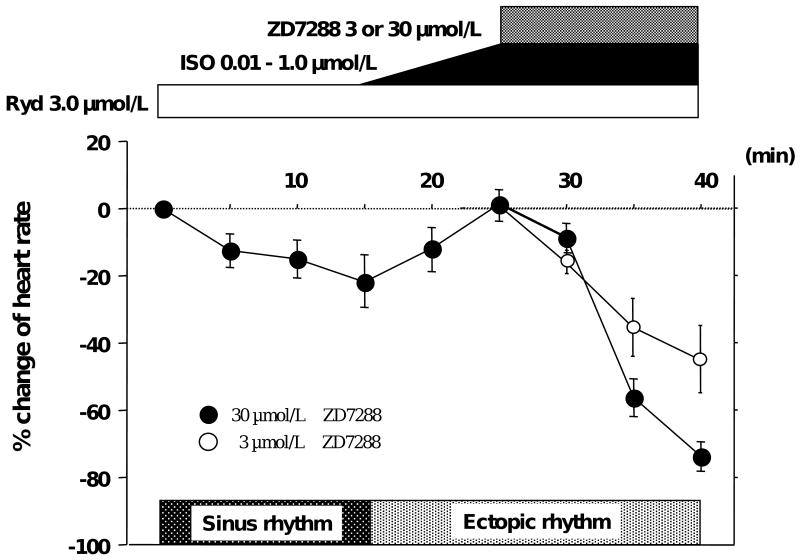
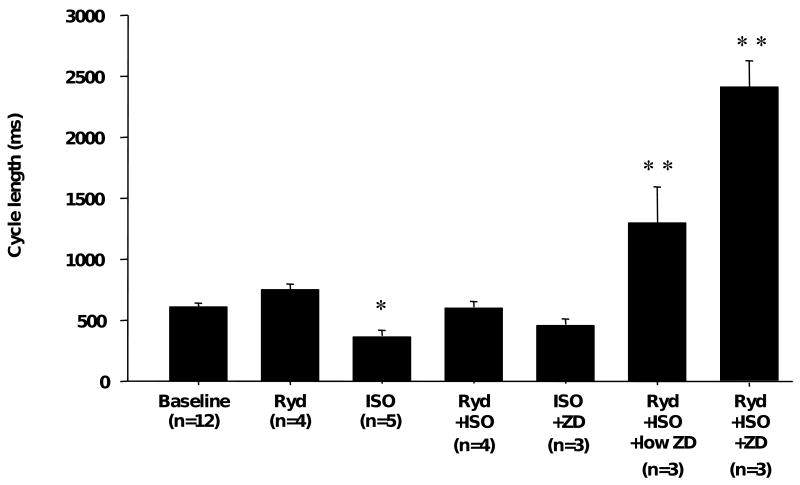
Figure 5 shows typical heart rate changes associated with SR Ca release inhibitor (ryanodine) and If blocker (ZD 7288). Ryanodine decreased heart rate. ISO induced ectopic beats during ryanodine infusion and slightly increased heart rate. However, the excessive increase of rate was inhibited by ryanodine. Afterwards, ZD7288 (30 μmol/L) inhibited AEP. It suppressed the rate of ectopic beats, resulting in profound bradycardia at 25 ± 9 bpm. In addition, we used a lower concentration of ZD7288 (3 μmol/L) as a more specific If blocker. The results (Figure 5) showed that AEP was significantly inhibited even with lower concentration of ZD 7288 (n=3, P=0.045). Figure 6 summarizes cycle lengths induced by various pharmacological agents under basal conditions and during ISO infusion. Under ISO infusion, block of both Ca clock and voltage clock significantly increased cycle length compared to baseline (2411 ± 233 vs. 614 ± 29 msec, P<0.001).
Figure 5. The change of heart rate with drugs treatments.
Heart rate was decreased by ryanodine infusion. Isoproterenol induced ectopic beats from the lower crista terminalis and marginal increase of heart rate. ZD7288 infusion of 3 or 30 μmol/L suppressed the rate of ectopic beats. n=3, respectively.
Figure 6. Spontaneous cycle length with drug treatments.
Bars show cycle lengths induced by different pharmacological interventions. Ryd shows the cycle length under 3.0 μmol/L ryanodine treatment. ISO shows the cycle length during 1.0 μmol/L isoproterenol infusion. Low ZD and ZD show cycle length during plus 3 or 30 μmol/L ZD7288, respectively. *P<0.05 and * *P<0.01 vs. baseline.
Discussion
This study is the first to use dual Ca2+-voltage optical mapping techniques to investigate a local pacemaking hierarchy including ectopy arising from RA. Main findings of the present study are (1) Spontaneous SR Ca release inhibition by ryanodine shifted the pacemaking site downward in SAN and reduced LDCAE at the pacemaking site under basal condition; (2) When SAN automaticity was completely inhibited by ryanodine, AEP occurred as a subsidiary pacemaker. The origin of ectopic sites was in the lower crista terminalis area on the endocardial side. Though a diastolic depolarization was observed at the pacemaking site of AEP in Vm tracings, LDCAE was not observed in Cai recordings. ISO induced only marginal increases of heart rate. The ectopic beats were suppressed by treatment of ZD7288. Our results indicate that If current is intimately involved in the generation of ectopic beats from lower crista terminalis when SAN is impaired, and AEP is more sensitive to If current than Cai cycling. As compared with SAN activations, the AEP is less dependent on the Ca clock and more dependent on the membrane clock for its automaticity.
Spontaneous SR Ca2+ Release and SAN Automaticity
The role of Ca clock in the automaticity of intact SAN remains controversial. Vinogradova et al.5 reported that positive chronotropic effect of β-adrenergic stimulation is the result of increased RyR Ca2+ release. They observed that the chronotropic effect in rabbit isolated SAN cells was abolished or greatly reduced after the suppression of Ca2+ transient by ryanodine. However, Honjo et al.10 observed that ryanodine did not abolish spontaneous activity in intact rabbit SAN and suggested that SR Ca2+ release does not play a dominating role in pacemaking in SAN. Our laboratory8 recently reported that ryanodine prevented ISO-induced LDCAE and blunted sinus rate acceleration, and If blockade with ZD 7288 modestly blunted but did not prevent LDCAE or sinus rate acceleration by ISO in the canine intact RA. The present study demonstrated that inhibition of SR Ca2+ release by ryanodine significantly increased cycle length and suppressed LDCAE at the pacemaking site before the administration of ryanodine. Furthermore, the SNRT and cSNRT were increased with ryanodine treatment. These results suggest that normal SR Ca2+ release contributes to sinus node recovery after rapid pacing. Taken together, spontaneous SR Ca2+ release plays an important role in pacemaking not only during β-adrenergic stimulation, but also under basal condition. Because only low-amplitude LDCAEs were observed in the baseline condition, the Ca clock may be more important under β-adrenergic stimulation than at baseline. An alternative explanation is that our mapping techniques were insufficiently sensitive to detect the Cai waves at baseline. Therefore, the contribution of Ca clock to baseline SAN automaticity could not be determined by this study. The current results are consistent with previous studies showing that β-adrenergic stimulation induced a superior (anterior) shift of pacemaking site in the SAN in intact11 or in Langendorff-perfused canine RAs.8 However, other investigators12 reported that administration of adrenaline causes an inferior shift of the dominant pacemaking in a superfused rabbit SAN preparation. Such a discrepancy could be due to differences in preparation (superfusion versus arterial perfusion), in species used in the study, or differential effects of adrenaline (epinephrine) and a more selective agent (isoproterenol) that exerts only β-adrenergic effects.
Mechanisms of the Ectopic Pacemakers
The ectopic beats initiated from outside the SAN are normally masked by sinus beats except in situations of failed SAN function. It has been demonstrated that tissue peripheral to the SAN structure has the capacity to be the dominant pacemaker when the SAN is surgically isolated.13, 14 Our data demonstrate that impairing spontaneous Ca release in SAN caused ectopic beats to emerge from lower crista terminalis. The ectopic beats were inhibited by the infusion of ZD7288, the If current blocker. These results indicated the ectopic beats from lower crista terminalis depended primarily on If.
Previous studies showed that If current is present not only in regions with primary or secondary pacemaker activity, but also in non-pacemaking regions of atrium and ventricle.15-17 Sympathetic nervous system stimulation accelerates cardiac rate via β-adrenergic increases in intracellular cAMP, which shift the voltage-dependence of If activation to more positive potentials.18, 19 These studies suggest that If current activation is able to induce sufficient membrane depolarization for the initiation of ectopic activity in the atrium, and therefore it plays an important role in atrial arrhythmias. Briosci et al.20 showed a quantitative description of the regional distribution and functional properties of HCN4 channels in rabbit SAN by fluorescence and electrophysiological measurements. Their figures indicated that HCN4 expression in cells isolated from crista terminalis is more than that from septum interatrialis. If current was also larger in cells isolated from crista terminalis. However, they simultaneously demonstrated that the expression and If current in the crista terminalis were much less than in the SAN. In spite of a lower level of expression, the ectopic pacemaker seems to rely heavily on the HCN4 for rhythm generation. Consistent with the role of If as a stabilizer of the pacemaker rate,21 one possible explanation is that the If activates upon hyperpolarization. Because the diastolic membrane potential at lower crista terminalis is more negative than the SAN,22 it may generate stronger If than SAN in spite of a lower concentration of HCN4.
Ample structural and functional evidence indicates that intrinsic cardiac autonomic nerve system forms a complex neural network composed of ganglionated plexi concentrated within epicardial fat pads.23 Stimulation of the intrinsic autonomic nervous system is involved in AF initiation and maintenance.24 In the present study, AEP located in crista terminalis was in close proximity to a fat pad (Figure 4A), suggesting that the autonomic nervous system may also be involved in the activation of AEP in lower crista terminalis, especially when the SAN response to autonomic activity is blunted.
Conclusion
If-dependent AEP in the lower crista terminalis emerges as the dominant pacemaker in intact SAN under β-stimulation when the spontaneous Ca release is inhibited. AEP can effectively serve as a backup pacemaker when the Ca clock is disabled.
Acknowledgments
We thank Nicole Courtney and Portia Schmidt for their assistance.
Funding Sources: This study was supported in part by NIH Grants P01 HL78931, R01 HL78932, 71140, a Korean Ministry of Information and Communication (MIC) and Institute for Information Technology Advancement (IITA) through research and develop support project (BJ), a Japan Medtronic fellowship (MM), Piansky Family Endowment (MCF), and Medtronic-Zipes Endowments (PSC) and by an AHA Established Investigator Award (SFL).
Footnotes
This manuscript was processed by a guest editor
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mangoni ME, Nargeot J. Genesis and regulation of the heart automaticity. Physiol Rev. 2008;88:919–982. doi: 10.1152/physrev.00018.2007. [DOI] [PubMed] [Google Scholar]
- 2.Rozanski GJ, Lipsius SL, Randall WC. Functional characteristics of sinoatrial and subsidiary pacemaker activity in the canine right atrium. Circulation. 1983;67:1378–1387. doi: 10.1161/01.cir.67.6.1378. [DOI] [PubMed] [Google Scholar]
- 3.DiFrancesco D. The cardiac hyperpolarizing-activated current, if. Origins and developments. Prog Biophys Mol Biol. 1985;46:163–183. doi: 10.1016/0079-6107(85)90008-2. [DOI] [PubMed] [Google Scholar]
- 4.Maltsev VA, Vinogradova TM, Lakatta EG. The emergence of a general theory of the initiation and strength of the heartbeat. J Pharmacol Sci. 2006;100:338–369. doi: 10.1254/jphs.cr0060018. [DOI] [PubMed] [Google Scholar]
- 5.Vinogradova TM, Bogdanov KY, Lakatta EG. beta-Adrenergic stimulation modulates ryanodine receptor Ca(2+) release during diastolic depolarization to accelerate pacemaker activity in rabbit sinoatrial nodal cells. Circ Res. 2002;90:73–79. doi: 10.1161/hh0102.102271. [DOI] [PubMed] [Google Scholar]
- 6.Vinogradova TM, Lyashkov AE, Zhu W, et al. High basal protein kinase A-dependent phosphorylation drives rhythmic internal Ca2+ store oscillations and spontaneous beating of cardiac pacemaker cells. Circ Res. 2006;98:505–514. doi: 10.1161/01.RES.0000204575.94040.d1. [DOI] [PubMed] [Google Scholar]
- 7.Lakatta EG, DiFrancesco D. What keeps us ticking: a funny current, a calcium clock, or both? J Mol Cell Cardiol. 2009;47:157–170. doi: 10.1016/j.yjmcc.2009.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Joung B, Tang L, Maruyama M, et al. Intracellular calcium dynamics and acceleration of sinus rhythm by beta-adrenergic stimulation. Circulation. 2009;119:788–796. doi: 10.1161/CIRCULATIONAHA.108.817379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Woods WT, Urthaler F, James TN. Spontaneous action potentials of cells in the canine sinus node. Circ Res. 1976;39:76–82. doi: 10.1161/01.res.39.1.76. [DOI] [PubMed] [Google Scholar]
- 10.Honjo H, Inada S, Lancaster MK, et al. Sarcoplasmic reticulum Ca2+ release is not a dominating factor in sinoatrial node pacemaker activity. Circ Res. 2003;92:41–44. doi: 10.1161/01.res.0000055904.21974.be. [DOI] [PubMed] [Google Scholar]
- 11.Boineau JP, Miller CB, Schuessler RB, et al. Activation sequence and potential distribution maps demonstrating multicentric atrial impulse origin in dogs. Circ Res. 1984;54:332–347. doi: 10.1161/01.res.54.3.332. [DOI] [PubMed] [Google Scholar]
- 12.Mackaay AJ, Op't Hof T, Bleeker WK, Jongsma HJ, Bouman LN. Interaction of adrenaline and acetylcholine on cardiac pacemaker function. Functional inhomogeneity of the rabbit sinus node. J Pharmacol Exp Ther. 1980;214:417–422. [PubMed] [Google Scholar]
- 13.Kirchhof CJ, Bonke FI, Allessie MA, Lammers WJ. The influence of the atrial myocardium on impulse formation in the rabbit sinus node. Pflugers Arch. 1987;410:198–203. doi: 10.1007/BF00581916. [DOI] [PubMed] [Google Scholar]
- 14.Kodama I, Boyett MR. Regional differences in the electrical activity of the rabbit sinus node. Pflugers Arch. 1985;404:214–226. doi: 10.1007/BF00581242. [DOI] [PubMed] [Google Scholar]
- 15.Cerbai E, Pino R, Porciatti F, et al. Characterization of the hyperpolarization-activated current, I(f), in ventricular myocytes from human failing heart. Circulation. 1997;95:568–571. doi: 10.1161/01.cir.95.3.568. [DOI] [PubMed] [Google Scholar]
- 16.Hoppe UC, Beuckelmann DJ. Characterization of the hyperpolarization-activated inward current in isolated human atrial myocytes. Cardiovasc Res. 1998;38:788–801. doi: 10.1016/s0008-6363(98)00047-9. [DOI] [PubMed] [Google Scholar]
- 17.Pino R, Cerbai E, Calamai G, et al. Effect of 5-HT4 receptor stimulation on the pacemaker current I(f) in human isolated atrial myocytes. Cardiovasc Res. 1998;40:516–522. doi: 10.1016/s0008-6363(98)00198-9. [DOI] [PubMed] [Google Scholar]
- 18.Bois P, Renaudon B, Baruscotti M, Lenfant J, DiFrancesco D. Activation of f-channels by cAMP analogues in macropatches from rabbit sino-atrial node myocytes. J Physiol. 1997;501:565–571. doi: 10.1111/j.1469-7793.1997.565bm.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DiFrancesco D, Tortora P. Direct activation of cardiac pacemaker channels by intracellular cyclic AMP. Nature. 1991;351:145–147. doi: 10.1038/351145a0. [DOI] [PubMed] [Google Scholar]
- 20.Brioschi C, Micheloni S, Tellez JO, et al. Distribution of the pacemaker HCN4 channel mRNA and protein in the rabbit sinoatrial node. J Mol Cell Cardiol. 2009;47:221–227. doi: 10.1016/j.yjmcc.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 21.Noble D, Denyer JC, Brown HF, DiFrancesco D. Reciprocal role of the inward currents ib, Na and i(f) in controlling and stabilizing pacemaker frequency of rabbit sino-atrial node cells. Proc Biol Sci. 1992;250:199–207. doi: 10.1098/rspb.1992.0150. [DOI] [PubMed] [Google Scholar]
- 22.Feng J, Yue L, Wang Z, Nattel S. Ionic mechanisms of regional action potential heterogeneity in the canine right atrium. Circ Res. 1998;83:541–551. doi: 10.1161/01.res.83.5.541. [DOI] [PubMed] [Google Scholar]
- 23.Yuan BX, Ardell JL, Hopkins DA, Losier AM, Armour JA. Gross and microscopic anatomy of the canine intrinsic cardiac nervous system. Anat Rec. 1994;239:75–87. doi: 10.1002/ar.1092390109. [DOI] [PubMed] [Google Scholar]
- 24.Hou Y, Scherlag BJ, Lin J, et al. Ganglionated plexi modulate extrinsic cardiac autonomic nerve input: effects on sinus rate, atrioventricular conduction, refractoriness, and inducibility of atrial fibrillation. J Am Coll Cardiol. 2007;50:61–68. doi: 10.1016/j.jacc.2007.02.066. [DOI] [PubMed] [Google Scholar]