Abstract
Objectives
Gait variability is an important indicator of impaired mobility in older adults; however, little is known about the meaning of change in gait variability over time. This study estimated clinically meaningful change in measures of gait variability using both distribution-and anchor-based approaches.
Design
Community-based observational cohort study.
Setting
Bronx County and the research center at Albert Einstein College of Medicine.
Participants
Of 1148 participants in the Einstein Aging Study, 241 had quantitative gait assessments in two consecutive years between 2001 and 2005.
Measurements
Gait variables were collected using a 12-foot instrumented walkway as participants walked at their normal walking speed. Gait variability was defined as the within-person standard deviation (SD) across steps in two 12-foot walks. Distribution-based meaningful change estimates used Cohen’s effect size (0.2 for small and 0.5 for moderate effects). Anchor-based estimates were obtained using dichotomous and ordinal self-reported walking ability ratings as anchors.
Results
Distribution based estimates for small and substantial changes of variability measures were: stance time 0.005 and 0.014 s; swing time 0.003 and 0.009 s; step length 0.24 and 0.61 cm; and step width 0.03 and 0.08 cm. Among those reporting no change in walking ability, measures of gait variability were stable over one year. Among those reporting a decline in walking, stance time and swing time variability increased. Among those reporting an improvement in walking, only step length variability improved.
Conclusion
Preliminary criteria for meaningful change are 0.01 s for stance time and swing time variability and 0.25 cm for step length variability. These estimates may identify important changes over time in both clinical settings and research studies.
Keywords: gait speed, gait variability, meaningful change, aging
INTRODUCTION
Gait variability, or fluctuations in gait characteristics from one step to the next, has recently gained much attention.1–8 Gait variability is as an important indicator of impaired mobility in community-dwelling older adults. Greater gait variability has been related to less confidence in walking and lower levels of daily physical activity.3,9 In addition, gait variability has been shown to be an independent predictor of future falls and incident mobility disability.1,2,4,9 However, little is known regarding the natural history of gait variability or how much change is clinically meaningful. It is necessary to define meaningful change in variability such that interventions aimed at improving gait variability can be evaluated appropriately. Such estimates can be used to determine whether meaningful change in gait variability over time has occurred in a patient in the clinical setting. In addition estimates of meaningful change are used in sample size and power computations when planning studies of mobility in aging populations and in evaluating results of studies with gait outcomes for practical significance independent of statistical significance. The purpose of this study is to estimate clinically meaningful change in measures of gait variability in a sample of community-dwelling older adults using both distribution- and anchor-based approaches.
METHODS
Study Population
Participants included ambulatory older adults who were participating in the Einstein Aging Study (EAS), a community-based observational cohort study.10,11 Eligibility criteria for the EAS were age 70 years and older, community-residing, and English speaking. Exclusion criteria included severe audiovisual disturbances and being non-ambulatory, bed bound because of illness. Between 1993 and 2005, 1148 subjects were enrolled. Subjects were enrolled into the study after obtaining informed consent. Subjects were evaluated using study protocols approved by the local institutional review board, at baseline and at annual follow-up visits.10,11 The current study consisted of secondary data analysis of the EAS.
Quantitative gait assessments were started in the EAS in 2001. Of the 510 subjects seen between 2001 and 2005, 427 (84%) had quantitative gait assessment. Reasons for not obtaining assessments included tester unavailability (n=53), subject illness (n=10) or refusal (n=20). Of the 427, 251 (59%) had quantitative gait assessments at two consecutive yearly visits and were eligible for the current study of meaningful change in gait variability. Individuals who walked extremely slowly (< 40 cm/s) or had extreme amounts of gait variability (step length variability > 20 cm and step width variability > 2.0 cm) were considered outliers (i.e. > 2 SD from the mean) with specific and substantial pathology and were removed from the analyses (n=10), leaving a final sample of 241 participants.
Measures
Quantitative gait assessment
Gait variables were collected using a 12-foot instrumented walkway with embedded pressure sensors (GAITRite, CIR systems, Havertown, PA). Participants were asked to walk on the mat in a well-lit hallway at their normal walking speed two times. Start and stop points were marked by white lines and included a 3 foot distance each for initial acceleration and terminal deceleration. Excellent reliability and validity for GAITRite assessments have been reported for subjects in EAS12and in other studies.13
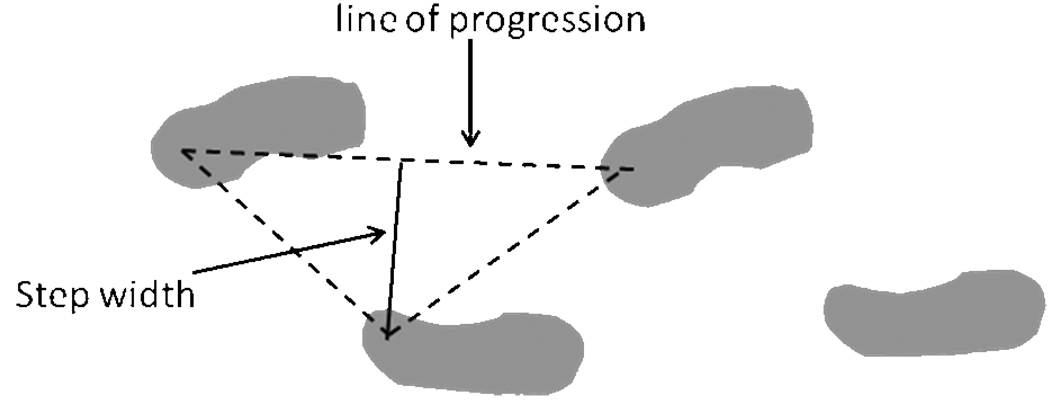
The gait assessment provides many different parameters; however, for this analysis the focus was on gait speed and gait variability. Stance time was recorded as the time elapsed between the first contact and the last contact of a footfall (i.e. the time the foot is in contact with the ground). Swing time was recorded as the time elapsed between the last contact of the current footfall and the first contact of the next footfall on the same foot (i.e. the time the foot is in the air). Step length was measured from the point of initial heel contact of the current foot to the point of initial heel contact of the previous footfall on the opposite side. Step width was measured as the perpendicular distance from heel point of one footfall to the line of progression of the opposite foot (Figure 1). Gait variability in each measure of gait was defined as the within-subject standard deviation derived from all of the right steps recorded over two trials. Greater values of stance time, swing time, and step length variability and lesser values of step width variability are believed to be worse.14 In order to compare our sample to others in the literature we also examined meaningful change in gait speed. Gait speed was calculated as the average velocity across two trials based on distance divided by time and was reported in cm/s.
Figure 1.
Determination of step width.
Self-reported measures of walking ability
Self-report of walking ability was assessed at each assessment. Clinical research assistants asked two questions from a previously validated mobility assessment questionnaire.11 The first was “Do you have any difficulty walking?” with response options “yes” and “no”. The second was “ How far can you walk in an hour on level ground?” with response options “less than a quarter mile”, “a quarter mile”, “a half mile to one mile”, or “greater than a mile”. The change in response to the second question was re-classified as no change, improvement, or decline. The individual questions used from the mobility assessment questionnaire have established item reliability and validity. 11
Health Status
Participants, family members, and caregivers were interviewed at study visits about medical illnesses and activities of daily living. Cognition was assessed using the Blessed Information Memory Concentration test15 and depressive symptoms were assessed using the Geriatric Depression Scale.16 In a subset of participants (n=150), participants were asked to rate their confidence in walking outside alone without falling on a scale from 0 (least confident) to 10 (most confident).
Analysis
Following the methodology used by Perera et al17 both distribution- and anchor-based approaches were used to estimate clinically meaningful change in measures of gait variability and gait speed. Values for meaningful change in gait speed have been reported previously.17 We decided to examine meaningful change in gait speed in addition to gait variability so we could compare our sample to previous studies. Effect size (δ) is a distribution-based method for quantifying a difference between two means on a unitless standard scale, so that results from the different measures or studies can be compared. In the setting where longitudinal change is of interest, effect size is defined as δ=(μ1−μ2)/σ1, where μ1 and μ2 respectively are the means at baseline and follow-up, and σ1 is the standard deviation at baseline.18 Guidelines for interpreting an effect size suggest using 0.2 for small, 0.5–0.6 for moderate, and 0.8–1.0 for large changes.19 Distribution-based magnitudes for small and substantial changes in the gait speed and variability measures were estimated as 0.2σ1 and 0.5σ1, respectively.17,19
The comparison of means method is an anchor-based approach that has been widely used in the literature to estimate meaningful differences in a variety of quality of life measures, which involves a simple comparison of mean performance changes between those who do and do not report mobility change according to a gold standard anchor.17,20,21 In the present report, the mean change in gait speed and variability between those who do and do not self-report change in difficulty walking and distance walked in 1 hour were examined separately for decline and improvement. Independent samples t-tests and analysis of variance were used to compare measures of gait variability between groups of individuals (i.e. stable/declined/improved) and paired t-tests were used to compare measures of gait variability between assessments within a group.
Estimated magnitudes of change for the measures of gait variability were inspected for consistency across approaches and anchors. If consistent, the overall criteria for small and substantial change were based on a convenient rounded number near the center of the range of computed estimates.
To further examine change in walking status, stair negotiation and walking confidence were reported with respect to self-reported walking difficulty at baseline and follow-up. The percentage of participants who reported difficulty climbing up stairs and coming down stairs and the mean walking confidence score were examined between those who did and did not self-report change in difficulty walking.
RESULTS
Mean age of the study participants was 80.3 years. Majority of the participants were Caucasian (70%), female (57%) and rated their health as good or better (79%). At baseline, mean gait speed was 96.1 cm/s and 64 participants (27%) reported difficulty walking (Table 1). Hypertension and leg pain during walking were the most prevalent chronic conditions at baseline and the percentage of participants reporting chronic conditions was similar at follow-up (Table 1). Cognitive status (Blessed), depressive symptoms (Geriatric depression scale) and activities of daily living (Lawton-Brody) were relatively unchanged from baseline to follow-up. However, reported falls in the past year and fear of falling increased from baseline to follow-up (Table 1). Mean time between baseline and follow-up visit was 11.8 months (SD= 1.6).
Table 1.
Sample characteristics at baseline and follow-up (n=241)
| Baseline | Follow-up | |
|---|---|---|
| Chronic conditions, n (%) | ||
| Diabetes | 28 (11.6) | 28 (11.6) |
| Hypertension | 108 (45.8) | 108 (45.8) |
| Myocardial infarction | 17 (7.1) | 17 (7.1) |
| Stroke | 19 (7.9) | 19 (7.9) |
| COPD | 6 (2.5) | 6 (2.5) |
| Leg pain during walking | 85 (35.3) | 85 (35.3) |
| Fall past year | 18 (7.5) | 57 (23.7) |
| Fear of falling | 50 (20.7) | 74 (30.7) |
| Blessed, mean (sd) | 2.1 (2.4) | 2.0 (2.6) |
| Geriatric depression scale, mean (sd) | 2.0 (1.8) | 2.2 (2.0) |
| Lawton-Brody, mean (sd) | ||
| Functional status | 2.9 (1.6) | 3.0 (1.4) |
| Physical self-maintenance | 5.7 (0.9) | 5.7 (0.9) |
| Gait characteristic, mean (sd) | ||
| Gait speed, cm/s | 96.1 (20.8) | 93.8 (22.1) |
| Stance time, s | .76 (.10) | .76 (.11) |
| Swing time, s | .42 (.05) | .43 (.05) |
| Step length, cm | 56.48 (9.18) | 55.12 (9.85) |
| Step width, cm | 9.86 (3.77) | 10.17 (4.05) |
| Stance time SD, s | .038 (.027) | .039 (.029) |
| Swing time SD, s | .027 (.016) | .028 (.021) |
| Step length SD, cm | 2.63 (1.22) | 2.86 (1.69) |
| Step width SD, cm | .37 (.16) | .37 (.16) |
| Self-reported walking, n (%) | ||
| Difficulty walking | 64 (26.6) | 88 (36.5) |
| Distance walked in 1 hour, (n=138) | ||
| < ¼ mile | 26 (18.8) | 21 (15.2) |
| ¼ mile | 16 (11.6) | 18 (13.0) |
| ½ to 1 mile | 29 (21.0) | 28 (20.3) |
| >1 mile | 67 (48.6) | 71 (51.4) |
Estimates of meaningful change in gait variability based on a distribution approach
Estimates of meaningful change in standard deviations of stance time, swing time, step length, and step width and gait speed based on the effect size approach are shown in Table 2.
Table 2.
Effect size based estimates for change in gait characteristics
| Effect Sizea | ||
|---|---|---|
| Small | Moderate | |
| Gait speed (cm/s) | 4.15 | 10.38 |
| Stance time standard deviation (s) | 0.005 | 0.014 |
| Swing time standard deviation (s) | 0.003 | 0.009 |
| Step length standard deviation (cm) | 0.24 | 0.61 |
| Step width standard deviation (cm) | 0.03 | 0.08 |
small effect size = 0.2σ1 ; moderate effect size = 0.5σ1
Meaningful Change Using Anchor-Based Methods
Anchor-based methods for determining meaningful change are presented by direction of change (ie. separately for improvement and decline). Table 3 presents findings based on response to a yes/no question about difficulty walking and Table 4 based on the three-level classification indicating the change in response to the question about how far the participant could walk in an hour on level ground.
Table 3.
Gait characteristics, mean (SD), by self-reported difficulty walking at baseline and follow-up
| No Baseline Walking Difficulty | Had Baseline Walking Difficulty | |||||
|---|---|---|---|---|---|---|
| Follow-up difficulty walking |
No | Yes | No | Yes | ||
| Stable (no difficulty) |
Declined | Meaningful Decline Estimate |
Improved | Stable (difficulty) |
Meaningful Improvement Estimate |
|
| N=127 | N=50 | N=26 | N=38 | |||
| Gait speed (cm/s) | ||||||
| Initial | 101.9 (16.9) | 92.1 (21.3) | 94.8 (19.6) | 83.1 (25.5)b | ||
| Follow-up | 101.2 (19.2) | 86.4 (21.4) | 95.6 (19.9) | 77.6 (22.4) | ||
| Change | −0.7 (11.5) | −5.7 (14.2) | 5.0 | 0.8 (8.5) | −5.4 (9.9) | 6.2 |
| Stance time SD (s) | ||||||
| Initial | .034 (.022) | .047 (.038)a | .034 (.020) | .046 (.025) | ||
| Follow-up | .033 (.020) | .046 (.036) | .042 (.038) | .048 (.033) | ||
| Change | −0.0003 (.029) | −.001 (.030) | 0.001 | .008 (.031) | .002 (.031) | 0.006 |
| Swing time SD (s) | ||||||
| Initial | .019 (.010) | .026 (.022)a | .028 (.020) | .030 (.021)b | ||
| Follow-up | .022 (.013) | .032 (.023) | .027 (.021) | .041 (.031) | ||
| Change | .003 (.015) | .006 (.027) | 0.003 | −0.001 (.021) | .011 (.028) | 0.012 |
| Step length SD (cm) | ||||||
| Initial | 2.40 (1.06) | 2.98 (1.45)a | 3.09 (1.41) | 2.64 (1.12)b | ||
| Follow-up | 2.62 (1.50) | 3.10 (1.80) | 2.89 (1.88) | 3.36 (1.92) | ||
| Change | .22 (1.68) | .13 (1.90) | --- | −.20 (2.20) | .72 (1.94) | 0.92 |
| Step width SD (cm) | ||||||
| Initial | .39 (.15) | .36 (.19) | .33 (.18) | .31 (.16) | ||
| Follow-up | .41 (.13) | .32 (.18) | .36 (.17) | .31 (.17) | ||
| Change | .02 (.12) | -0.04 (.13) | 0.06 | .03 (.17) | .00 (.17) | 0.03 |
SD = standard deviation
p-value < 0.05; comparison of initial values between groups.
p-value <0.05; comparison of initial to follow-up values within group.
Table 4.
Gait characteristics, mean (SD), by self-reported walking distance at baseline and follow-up
| Reported no change in walking distance |
Reported walking less at f/u |
Meaningful Decline Estimate |
Reported walking more at f/u |
Meaningful Improvement Estimate |
|
|---|---|---|---|---|---|
| N=67 | N=28 | N=43 | |||
| Gait speed (cm/s) |
|||||
| Initial | 99.6 (20.9) | 92.0 (23.0)a | 90.1 (17.2) | ||
| Follow-up | 98.7 (23.2) | 86.1 (20.9) | 89.6 (19.2) | ||
| Change | −.93 (10.5) | −5.9 (12.4) | 4.97 | −.5 (10.7) | .43 |
| Stance time SD (s) |
|||||
| Initial | .037 (.033) | .034 (.021) a | .047 (.036) | ||
| Follow-up | .035 (.023) | .044 (.031) | .049 (.044) | ||
| Change | −.002 (.026) | .010 (.025) | .012 | .002 (.043) | --- |
| Swing time SD (s) |
|||||
| Initial | .022 (.020) | .022 (.013) a | .027 (.020) | ||
| Follow-up | .026 (.018) | .037 (.031) | .032 (.027) | ||
| Change | .004 (.020) | .015 (.026) | .011 | .004 (.025) | 0 |
| Step length SD(cm) |
|||||
| Initial | 2.56 (1.11) | 2.46 (1.41) | 2.87 (1.32) | ||
| Follow-up | 2.63 (1.42) | 2.77 (1.85) | 2.69 (1.71) | ||
| Change | .07 (1.44) | .31 (1.84) | .24 | −.17 (2.07) | .24 |
| Step width SD (cm) |
|||||
| Initial | 0.36 (.15) | .31 (.15) | .29 (.13) a | ||
| Follow-up | 0.41 (.15) | .32 (.15) | .34 (.13) | ||
| Change | .05 (.14) | .01 (.11) | -- | .05 (.11) | 0 |
SD = standard deviation; f/u = follow-up
p-value <0.05; comparison of initial and follow-up values within group.
Difficulty walking
Fifty participants who reported no difficulty walking at baseline reported difficulty at follow-up and were considered to have declined while 26 who reported difficulty at baseline and no difficulty at follow up and were considered to have improved (Table 3). In those with self-reported decline, compared to those who consistently reported no difficulty at both times (n=127), the difference in gait speed change was 5.0 cm/second and swing time SD change was 0.003 seconds. Effects were not consistent for the other measures (Table 3). Among those who reported no difficulty walking, baseline variability in stance time, swing time and step length were significantly worse among persons who subsequently reported decline compared to those who continued to report no difficulty walking (Table 3; p=0.03, 0.03, and 0.01 respectively).
Among individuals who reported difficulty walking at baseline, 26 reported no difficulty at follow-up and were considered to have improved while the remaining 38 continued to report walking difficulty (Table 3). Compared to persons who reported continued difficulty walking, the majority of gait measures did not improve in the self-reported improvers. Only step length variability decreased (the expected direction for improvement). Individuals who did not report improvement had worse stance variability at baseline compared to those who reported improvement (p=0.06). Participants who reported difficulty walking at both time points had declines in gait speed, swing time variability and step length variability over time (Table 3) (p=0.002, 0.02, and 0.03 respectively).
Distance walked
A sub-sample of the participants (138/241) had self-reported distance walked in one hour at both time points. Based on the four level response to the question “how far can you walk in an hour (on level ground)?” participants who reported the same distance at baseline and follow-up were considered to be stable (n=67). In this group of “stable” participants, measures of gait speed and gait variability were fairly consistent at baseline and follow-up (Table 4) except for step width variability which increased over time (p=0.002).
Twenty- eight individuals reported walking a shorter distance at follow-up compared to baseline and were considered to have declined (n=28). Compared to persons reporting stable walking distance, individuals who reported decline, had a decrease in gait speed (5.9 cm/s) and an increase in variability of stance time (0.010 s), swing time (0.15 s), and step length (.31 cm) (Table 4).
Forty-three individuals reported the ability to walk a longer distance at follow-up compared to baseline and were considered to have improved. Compared to those reporting stable walking distance, the self reported improvers did not generally show a significant improvement in gait measures, except for step length variability which improved (decreased) slightly over time (0.17 cm).
Recommended Criterion for Meaningful Change
Because there is no known best statistic or mathematical formula to allow for the combination of the multiple estimates obtained through different distribution- and anchor-based approaches, the recommended criterion for meaningful change were based on the general consistency and tendencies found in the analyses (specifically effect size based estimates, Table 2 and anchor-based methods using self-reported walking distance as the anchor, Table 4), and a preference for rounded numbers was applied for ease and practical use. Preliminary overall estimates for meaningful change in measures of gait variability are 0.01 s for stance time and swing time SD and 0.25 cm for step length SD. The estimates for step width SD were inconsistent across methods, and cannot be summarized to yield an overall recommendation.
Change in Walking Status
In Table 5, stair negotiation and walking confidence are presented with respect to self-reported walking difficulty at baseline and follow-up. Individuals who reported no difficulty walking at baseline or follow-up (no difficulty, stable) remained relatively stable on self-reported stair negotiation and walking confidence. Individuals who declined overtime on self-reported difficulty walking also reported decline on stair negotiation and walking confidence. Individuals who reported difficulty walking at baseline were also more likely to report difficulty with stair negotiation compared to individuals who did not report difficulty walking at baseline.
Table 5.
Stair negotiation and walking confidence by self-reported difficulty walking at baseline and follow-up
| No Baseline Walking Difficulty |
Had Baseline Walking Difficulty |
|||
|---|---|---|---|---|
| Follow-up difficulty walking | No Stable (no difficulty) |
Yes Declined |
No Improved |
Yes Stable (difficulty) |
| N=127 | N=50 | N=26 | N=38 | |
| Difficulty climbing up stairs, % | ||||
| Initial | 28% | 36% | 54% | 63% |
| Follow-up | 27% | 76% | 64% | 82% |
| Difficulty coming down stairs, % | ||||
| Initial | 6% | 8% | 15% | 29% |
| Follow-up | 17% | 52% | 39% | 61% |
| Walking confidence, mean (SD) | N=80 | N=34 | N=11 | N=25 |
| Initial | 9.8 (0.6) | 8.8 (1.6) | 8.8 (1.9) | 7.7 (2.5) |
| Follow-up | 9.4 (1.2) | 7.9 (2.4) | 9.2 (1.5) | 7.3 (2.6) |
DISCUSSION
Based on the general consistency and tendencies found in the distribution- (Table 2) and anchor-based (Table 4) approaches, preliminary overall estimates for meaningful change in measures of gait variability are 0.01 s for stance time and swing time SD and 0.25 cm for step length SD. Our findings regarding change in step width were inconsistent. We treated step width variability as a continuous variable and assumed that reduced step width variability was a negative outcome. There is reason to suggest that both too little or too much step width variability may be undesirable.1 The nonlinear association between step width variability and negative outcomes (i.e. falls) makes it very difficult to determine meaningful change using the traditional statistical methods utilized in this manuscript.
Self-reported decline yielded conflicting results. There was increasing variability in stance time, swing time, and step length with worsening self-report based on the four level question about walking distance but not the dichotomous question about walking difficulty. Interestingly, among persons who initially reported no difficulty walking on the dichotomous question and those who subsequently reported decline had worse variability at baseline in stance time and swing time. It may be that the yes/no format detects an at risk group cross-sectionally while the four level ordinal response provides more information to detect change over time.
Self-reported improvement in walking over a year was only related to improvement in step length variability. Improvements in step length variability may precede improvements in stance time variability. Spatial characteristics of walking, which can be directed by visual cues, are likely to be a more obvious strategy for the walker than monitoring temporal aspects of gait. Thus, perhaps the focus of the older walker who is improving their walking is on consistent step length and not on consistent stance time. However, if walking continues to improve, stance time variability might also improve. Previous investigators have also suggested a differential neural control of spatial and temporal gait variables.3,6,22
Based on the effect size analysis, small gait speed change was 4.15 cm/s and substantial change was 10.38 cm/s. These findings are very close to prior estimates of 5 cm/second for a small meaningful change and 10 cm/second for a substantial change.17 Likewise the small and substantial change estimates reported for stance time variability are consistent with our previous findings.2 In a sample of older adults from the Cardiovascular Health Study we had demonstrated that a 0.01 s increase in stance time variability was associated with a 13% increased risk of mobility disability.2 The consistency of our findings with previous findings supports that our sample is comparative to other research samples of community-dwelling older adults.
When interpreting the results a few factors need to be taken into consideration. First, the study had a relatively small sample of volunteers (n=241) which is not representative of all community-dwelling older persons. Participants with a range of mobility and health status were included in this study thus increasing the variability of the results. In this study change was examined only over a one year time period. The findings should not be generalized to greater follow-up periods. This was a secondary data analysis of an observational cohort study. The cohort study was not specifically designed to examine change in gait characteristics over time. Lastly, change in gait characteristics were examined in relation to change in self-reported walking, information about other factors that may have impacted mobility over time such as hospitalizations or change in health status were not examined. However, self-reported change in walking difficulty was also related to stair negotiation (self-reported) and walking confidence thus providing support that a change in mobility status occurred.
CONCLUSION
Preliminary criteria for meaningful change are 0.01 s for stance time and swing time variability and 0.25 cm for step length variability. Further work is warranted in other preferably larger samples to support our estimates of meaningful change in measures of gait variability.
Acknowledgments
Funding sources and related paper presentations: The Einstein Aging Study is supported by National Institutes on Aging program project grant (AG03949). J. Verghese is supported by a National Institutes on Aging grant (R01 AG025119). University of Pittsburgh Older American's Independence Center grant P30 AG024827-01. JS Brach is supported by a National Institutes on Aging and American Federation of Aging Research Paul Beeson Career Development Award (K23 AG026766).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brach JS, Berlin JE, VanSwearingen JM, Newman AB, Studenski SA. Too much or too little step width variability is associated with a fall history in older persons who walk at or near normal gait speed. J Neuroengineering Rehabil. 2005;2 doi: 10.1186/1743-0003-2-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brach JS, Studenski S, Perera S, VanSwearingen JM, Newman AB. Gait variability and the risk of incident mobility disability. J Gerontol Med Sci. 2007;62A:983–988. doi: 10.1093/gerona/62.9.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brach JS, Studenski S, Perera S, VanSwearingen JM, Newman AB. Stance time and step width variability have unique contributing impairments in older persons. Gait Posture. 2008;27:431–439. doi: 10.1016/j.gaitpost.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maki BE. Gait changes in older adults: predictors of falls or indicators of fear? J Am Geriatr Soc. 1997;45:313–320. doi: 10.1111/j.1532-5415.1997.tb00946.x. [DOI] [PubMed] [Google Scholar]
- 5.Hausdorff JM, Edelberg HK, Mitchell SL, Goldberger AL, Wei JY. Increased gait unsteadiness in community-dwelling elderly fallers. Arch Phys Med Rehabil. 1997;78:278–283. doi: 10.1016/s0003-9993(97)90034-4. [DOI] [PubMed] [Google Scholar]
- 6.Owings TM, Grabiner MD. Step width variability, but not step length variability or step time variability, discriminates gait of healthy young and older adults during treadmill locomotion. Journal of Biomechanics. 2004;37:935–938. doi: 10.1016/j.jbiomech.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 7.Owings TM, Grabiner MD. Variability of step kinematics in young and older adults. Gait Posture. 2004;20:26–29. doi: 10.1016/S0966-6362(03)00088-2. [DOI] [PubMed] [Google Scholar]
- 8.Grabiner MD, Troy KL. Attention demanding tasks during treadmill walking reduce step width variability in young adults. J Neuroengineering Rehabil. 2005;2:25. doi: 10.1186/1743-0003-2-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hausdorff JM, Rios DA, Edelberg HK. Gait variability and fall risk in community-living older adults: a 1-year prospective study. Arch Phys Med Rehabil. 2001;82:1050–1056. doi: 10.1053/apmr.2001.24893. [DOI] [PubMed] [Google Scholar]
- 10.Lipton RB, Katz MJ, Kuslansky G, et al. Screening for demential by telephone using the memory impairment screen. J Am Geriatr Soc. 2003;51:1382–1390. doi: 10.1046/j.1532-5415.2003.51455.x. [DOI] [PubMed] [Google Scholar]
- 11.Verghese J, Katz MJ, Kuslansky G, Hall C, Derby C, Lipton RB. Reliability and validity of a telephone-based mobility assessment questionnaire. Age Aging. 2004;33:628–632. doi: 10.1093/ageing/afh210. [DOI] [PubMed] [Google Scholar]
- 12.Verghese J, Buschke H, Viola L, et al. Validity of divided attention tasks in predicting falls in older individuals: A preliminary study. J Am Geriatr Soc. 2002;50:1572–1576. doi: 10.1046/j.1532-5415.2002.50415.x. [DOI] [PubMed] [Google Scholar]
- 13.Bilney B, Morris M, Webster K. Concurrent related validity of the GAITRite walkway system for quantification of the spatial and temporal parameters of gait. Gait Posture. 2003;17:68–74. doi: 10.1016/s0966-6362(02)00053-x. [DOI] [PubMed] [Google Scholar]
- 14.Brach JS, Perera S, Studenski S. Reliability and validity of measures of gait variability in community-dwelling older adults. Arch Phys Med Rehabil. 2008;89:2293–2296. doi: 10.1016/j.apmr.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blessed G, Tomlinson E, Roth M. The association between quantative measures of dementia and senile change in the cerebral grey matter of elderly subjects. Br J Pscyhiatry. 1968;114:797. doi: 10.1192/bjp.114.512.797. [DOI] [PubMed] [Google Scholar]
- 16.Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 17.Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc. 2006;54:743–749. doi: 10.1111/j.1532-5415.2006.00701.x. [DOI] [PubMed] [Google Scholar]
- 18.Kazis L, Anderson J, Meenan R. Effect sizes for interpreting changes in health status. Medical Care. 1989;27:S178–S189. doi: 10.1097/00005650-198903001-00015. [DOI] [PubMed] [Google Scholar]
- 19.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Hillsdale: Lawrence erlbaum Associates; 1988. [Google Scholar]
- 20.Jaeschke R, Singer J, Guyatt GH. Measurement of health status. Ascertaining the minimally important difference. Control Clin Trials. 1989;10:407–415. doi: 10.1016/0197-2456(89)90005-6. [DOI] [PubMed] [Google Scholar]
- 21.Cella D, Eton DT, Lai JS, et al. Combining anchor and distribution-based methods to derive minimal clinically important differences on the functional assessment of cancer therapy (FACT) anemia and fatigue scales. J Pain Symptom Manage. 2002;24:547–561. doi: 10.1016/s0885-3924(02)00529-8. [DOI] [PubMed] [Google Scholar]
- 22.Rosano C, Aizenstein H, Brach JS, Longerberger A, Studenski S, Newman AB. Gait measures indicate underlying focal gray matter atrophy in the brain of older adults. J Gerontol Med Sci. 2008 doi: 10.1093/gerona/63.12.1380. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]